Abstract
Objectives
Indications for cholecystectomy have changed dramatically over the past three decades. Cystoisospora belli has been reported in cholecystectomy specimens of immunocompetent patients. The present study was designed to determine the prevalence and clinical association of C belli in the gallbladder.
Methods
The study included retrospective review of cholecystectomy specimens (n = 401) removed for various indications, and a prospective cohort of cholecystectomy specimens (n = 22) entirely submitted for histologic evaluation. Correlations of presence of C belli with age, sex, clinical indication, and abnormalities of preoperative laboratory values were assessed by Fisher exact test.
Results
C belli was identified in 39/401 (9.7%) of the retrospective cohort, and 6/22 (27.3%) of the entirely submitted specimens. The presence of C belli showed no correlation with age, sex, clinical indication, or laboratory abnormalities.
Conclusions
C belli resides in a latent state in the gallbladder and may be best considered a commensal organism.
Keywords: Cystoisospora belli, Gallbladder, Cholecystectomy
Biliary pain in the absence of gallstones or sludge presents a complicated and difficult clinical problem, often termed functional gallbladder disorder (FGBD), chronic cholecystitis, acalculous biliary pain, biliary dyskinesia, gallbladder dysmotility, or sphincter of Oddi dysfunction.1 The diagnosis, conceptual classification, and management of FGBD are controversial.
A recent review of the New York State Planning and Research Cooperative System Longitudinal Administrative Database (spanning 1995-2013) revealed that the indications for cholecystectomy have changed dramatically in the past 20 years. By comparison of data from 2010 to that of 1997, calculous cholecystitis declined (–20%; P < .0001), while other indications increased, including acalculous cholecystitis (+94%; P < .0001), biliary dyskinesia (+331%; P < .0001), and biliary colic (+55%; P = .0013).2 There has been a concomitant decrease in the age of patients undergoing cholecystectomy,3 and a shift from open cholecystectomy to laparoscopic cholecystectomy as standard of care. While similar trends have been reported in a survey of the Nationwide Inpatient Sample database, these trends have not been reported outside of the United States, as evidenced by examinations of patient databases in Sweden, Norway, Australia, and Poland.4
While laparoscopic cholecystectomy is associated with shorter postoperative stay and fewer minor complications (eg, wound infections),5 there is a five-fold increase in the incidence of the devastating long-term complication of bile duct injury among patients who undergo laparoscopic cholecystectomy compared to open cholecystectomy.6,7 The sequelae of bile duct injury include serious morbidity,8 reduced quality of life,9 and reduced long-term survival,10 resulting in litigation costs estimated at $1 billion annually in the United States.11 Thus, understanding the etiology of gallbladder disease, particularly in the setting of FGBD, is important from both clinical and pathobiological standpoints.
Cystoisospora are coccidian protozoan parasites of the phylum Apicomplexa. C belli and C natalensis are the only Cystoisospora species known to infect humans, with C belli representing the vast majority of reported cases.12C belli is a poorly understood organism; humans represent the only known host of this parasite and no known reservoir or paratenic hosts exist.13 Symptomatic infections typically result in secretory diarrhea14 and are diagnosed by microscopic examination of small intestinal biopsies for organisms or, more commonly, stool specimens examined for oocysts. C belli initially came to prominent medical attention as an opportunistic infection complicating acquired immunodeficiency syndrome (AIDS), where infection of the small intestine epithelium resulted in severe, secretory diarrhea15 and rarely disseminated extraintestinal infection.16,17 There are also a few reports of HIV-positive patients with chronic cholecystitis who were found to have C belli infection in the resected gallbladder specimen.18,19 Another case report demonstrated biopsy-proven symptomatic C belli infection of the common bile duct in a patient who presented with common bile duct obstruction, and who was concurrently diagnosed with infections by Strongyloides stercoralis and HIV.20 The reported prevalence of C belli in patients with AIDS varies widely, likely due to differences in geographic location and patient populations. Subsequent case reports have documented instances of C belli diarrhea in the setting of other causes of profound immunodeficiency, such as solid organ transplant,21 thymoma,22 inflammatory bowel disease/azathioprine exposure,23 malnutrition,24 and alcohol abuse.25 These studies resulted in the general consensus that C belli is a rare opportunistic infection seen in immunocompromised patients that results in diffuse watery diarrhea, with greater prevalence in tropical climates and in patients with recent travel history.26,27
There have also been case reports of self-limited C belli infection in immunocompetent patients,28 as well as several larger case series suggesting that conventional stool studies performed to detect coccidian oocysts may have low sensitivity,29 raising the possibility that C belli is more prevalent amongst immunocompetent humans than previously recognized. A recent case report of C belli infection in the gallbladder of an immunocompetent patient30 was followed by a retrospective case series describing the clinicopathologic features of 18 cholecystectomies with diagnosed C belli infection.31 This case series found that the majority of gallbladders with reported C belli infection represented acalculous disease in younger patients, but given the limitations of the study could not assess prevalence of C belli. The current study investigates the prevalence of C belli in resected human gallbladders in a larger cohort, and whether its presence is associated with the indication for cholecystectomy and laboratory data.
Materials and Methods
We designed a single-institution, two-arm study to determine the prevalence of C belli infection in the human gallbladder. With institutional research board approval, the laboratory information system of the surgical pathology division at the University of Rochester Medical Center was searched for cholecystectomies, excluding the terms cholelith or calculous (2004-2016). Four hundred and one patients with confirmed absence of gallstones on pathologic review were included in the retrospective review. The original H&E slides were reviewed and the presence or absence of C belli organisms was correlated with age, sex, and the clinical indication for cholecystectomy for all 401 patients, and correlated with available clinical (age, sex, presenting complaint, indication for the procedure, comorbidities, immune status, medication list, and body mass index [BMI]) and laboratory values, including presence/absence of leukocytosis at the time of procedure, peripheral eosinophilia, alkaline phosphatase, bilirubin, medications, and radiologic data (hepatobiliary iminodiacetic acid [HIDA] scan results) for 235 of these patients.
The indication for cholecystectomy was classified into six categories: cholecystitis/abdominal pain, gallstone disease (cholelithiasis/choledocholithiasis, gallstone pancreatitis), biliary dyskinesia, gallbladder polyp, choledochal cyst, and incidental (gallbladders removed for reasons other than symptoms of gallbladder disease). SPSS version 25.0 (IBM, Armonk, NY) was used to query the data for associations between patient demographics and clinical features, with the presence of C belli via Fisher exact test, with a P value less than .05 selected as a threshold for statistical significance.
Because routine sampling of cholecystectomy specimens typically involves histologic evaluation of less than 5% of the specimen, there is a significant potential for underestimating the prevalence of C belli. Given that the previous case series found the majority of patients diagnosed with C belli of the gallbladder were young women with acalculous disease,31 we designed a separate, parallel, prospective study (July 2017-February 2018), in which gallbladders from any patient less than 30 years of age that lacked stones and lacked organisms on the initial routine slide were entirely submitted for histologic evaluation (n = 22).
In both arms of the study, all cases that were positive for C belli were evaluated by at least two GI pathologists, with agreement. Historically, morphologic speciation of Cystoisospora has been based on microscopic examination of oocysts in stool, and thus the lack of stool ova and parasite examinations from these patients precludes definite identification of species. However, human infection by C natalensis has only been reported in a single case report (1953) of a 21-year-old man with concurrent amoebic dysentery and other protozoal and helminthic infections and has not been reported in humans since.12 Therefore, we feel it is reasonable to refer to the organism discussed here as C belli.
Results
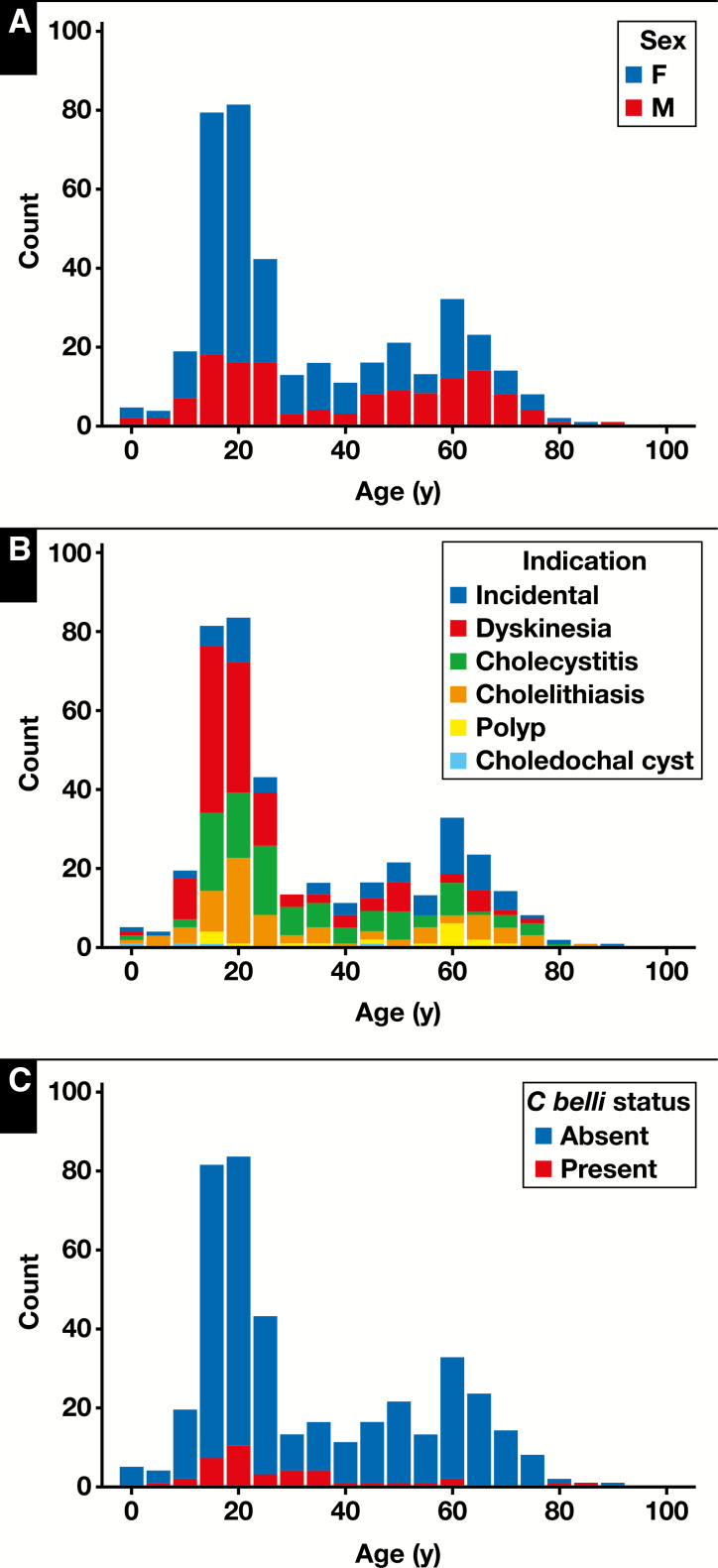
The retrospective cohort of patients who underwent cholecystectomy without gallstones (n = 401) included 265 (66.1%) females and 136 (33.9%) males. The mean age was 33.1 years (± 20.4 years). The population of patients undergoing cholecystectomy showed a bimodal age distribution, with a peak at 13 to 25 years of age and another at 50 to 70 years Figure 1. The latter group had a higher proportion of males, as well as an increased proportion of incidental cholecystectomies performed in the setting of liver transplantation and resections for malignancy Table 1 . Indications for removal were biliary dyskinesia (124/401; 30.9%), abdominal pain/cholecystitis (103/401; 25.7%), gallstone disease (78/401; 19.5%), gallbladder polyp (17/401; 4.2%), choledochal cyst (4/401; 1.0%), and incidental (75/401; 18.7%). Incidental cholecystectomies included gallbladders removed in the setting of trauma (n = 21), liver explant (n = 21), pancreatic/duodenal neoplasm (n = 16), pancreatitis (n = 6), liver mass (n = 7), and other abdominal surgeries (n = 4). The distribution of indication by patient age is also shown in Figure 1. The prevalence of C belli as it relates to age is shown in Figure 1, and the prevalence of C belli as it relates to clinical setting is shown in Table 1.
Figure 1 .
Distribution of patient sex (A), indication for cholecystectomy (B), and Cystoisospora belli infection (C) by age (retrospective cohort; n = 401).
Table 1 .
Patient Demographics of the Entire Cohort Of Patients Included in the Retrospective Review
| Characteristic | Total, No. (%) | C belli Present, No. (%) | C belli Absent, No. (%) | P a |
|---|---|---|---|---|
| (n = 401) | (n = 39) | (n = 362) | ||
| Age <40 | 240 (59.9) | 26 (66.7) | 214 (59.1) | .075 |
| Male | 136 (33.9) | 15 (38.5) | 121 (33.4) | .594 |
| Indication | ||||
| Dyskinesia | 124 (30.9) | 12 (30.1) | 112 (30.9) | .743 |
| Cholecystitis | 103 (25.7) | 8 (20.5) | 95 (26.2) | |
| Gallstone disease | 78 (19.5) | 7 (17.9) | 71 (19.6) | |
| Polyp | 17 (4.3) | 1 (2.6) | 16 (4.4) | |
| Choledochal cyst | 4(1.0) | 0 | 4 (1.1) | |
| Incidental | 75 (18.7) | 11 (28.2) | 64 (17.7) |
C belli, Cystoisospora belli.
aFisher exact test, two-sided.
Of the entire retrospective cohort, C belli was identified in 39/401 (9.7%) specimens. C belli was identified in 15/136 (11.0%) of males and 24/265 (9.1%) of females (P = .594); 26/240 (10.8%) of positive cases were from patients younger than 30 years, and 13/161 (8.1%) of positive cases were from patients 30 years or older (P = .395). No significant association with indication for cholecystectomy was identified (P = .743). Two of the 401 patients (both negative for C belli in gallbladder) had stool studies for ova/parasites performed during workup for diarrhea, neither of which were positive. Of the 39 positive patients, 11 had previous duodenal biopsies during their workup, none of which showed definitive C belli infection. In the 235 patients with available clinical and laboratory data Table 2 , the presence of C belli was associated with male gender but not with elevated BMI, leukocytosis, peripheral eosinophilia, elevated alkaline phosphatase, elevated total bilirubin, proton pump inhibitor use, or antibiotic exposure (within 2 weeks prior to surgery).
Table 2 .
Clinical and Laboratory Data for 235 Patients Included in the Retrospective Review
| Characteristic | Total, No. (%) | C belli Present, No. (%) | C belli Absent, No. (%) | P a |
|---|---|---|---|---|
| (n = 235) | (n = 24) | (n = 211) | ||
| Male | 62 (26.4) | 11 (45.8) | 51 (24.2) | .029 |
| Overweight | 111 (47.2) | 7 (29.2) | 104 (49.3) | .13 |
| Leukocytosis | 34 (14.5) | 8 (33.3) | 26 (12.3) | .063 |
| Eosinophilia | 11 (4.7) | 2 (8.3) | 9 (4.3) | .211 |
| Alkaline phosphatase | 16 (6.8) | 2 (8.3) | 14 (6.6) | .827 |
| Total bilirubin | 27 (11.5) | 2 (8.3) | 25 (11.8) | .859 |
| PPI | 63 (26.8) | 6 (25.0) | 57 (27.0) | .612 |
| Antibiotic | 35 (14.9) | 5 (20.8) | 30 (14.2) | .551 |
C belli, Cystoisospora belli; PPI, proton pump inhibitor.
aFisher exact test.
From the cohort of patients in the prospective arm of the study, in which gallbladders were entirely submitted for histologic evaluation, C belli was noted in six of 22 (27.3%). The mean routine slides submitted were 1.14 slides/case; the mean total slides after entire submission of the specimen were 13.3 slides/case. In three cases, C belli was identified on the initial (routine) slide; in three additional cases C belli was identified on subsequent slides that would not have been sampled by routine processing. C belli was identified in the cystic duct margin in one of six (16.7%) and only in the remaining gallbladder sections in five of six (83.3%) positive cases. Of the 22 patients, cholecystectomy was performed for the following indications: eight (36.4%) biliary dyskinesia, six (27.2%) cholelithiasis, five (22.7%) chronic cholecystitis/pain, two (9.1%) for gallbladder polyps, and one (4.5%) was removed incidentally (partial hepatectomy for metastatic colorectal carcinoma) Table 3 . The indications for removal of specimens positive for C belli included biliary dyskinesia (three), cholelithiasis (one), chronic cholecystitis (one), and gallbladder polyp (one). One of the six patients with C belli infection of the gallbladder had duodenal biopsies performed prior to cholecystectomy; on review, C belli organisms were not identified. One patient diagnosed with C belli infection received 2 weeks of antibiotics (trimethoprim/sulfamethoxazole × 1 week, then ciprofloxacin × 1 week) after surgery, and re-presented 4 months later with abdominal discomfort. Stool studies with trichrome stain performed at that time were negative.
Table 3 .
Clinical Indication for the Prospective Cohort
| Indication | Total, No. (%) | C belli Present, No. (%) | C belli Absent, No. (%) | P a |
|---|---|---|---|---|
| (n = 22) | (n = 6) | (n = 16) | ||
| Male | 4 (18.2) | 0 | 4 (25) | |
| Indication | ||||
| Dyskinesia | 8 (36.4) | 3 (50) | 5 (31.3) | .831 |
| Cholecystitis | 5 (22.7) | 1 (16.7) | 4 (25) | |
| Gallstone disease | 6 (27.2) | 1 (16.7) | 5 (31.3) | |
| Polyp | 2 (9.1) | 1 (16.7) | 1 (6.3) | |
| Incidental | 1 (4.5) | 0 | 1 (6.3) |
C belli, Cystoisospora belli.
aFisher exact test.
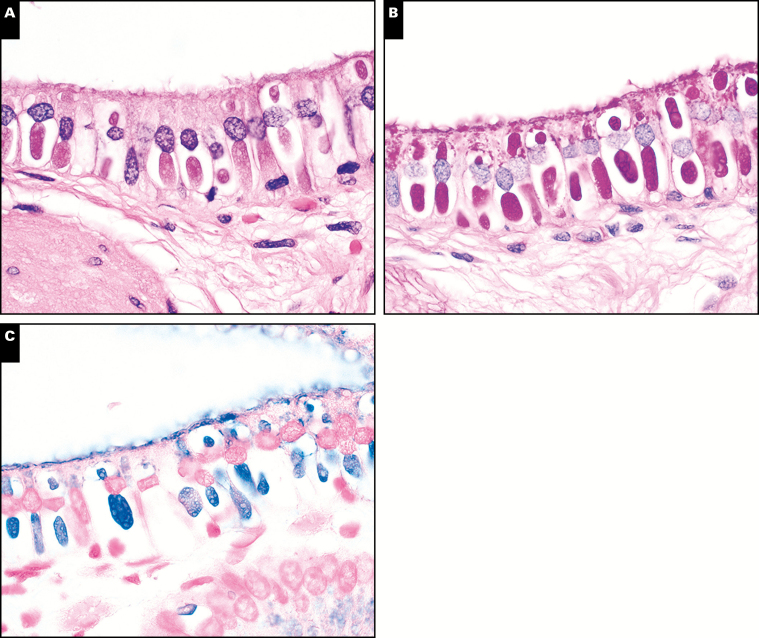
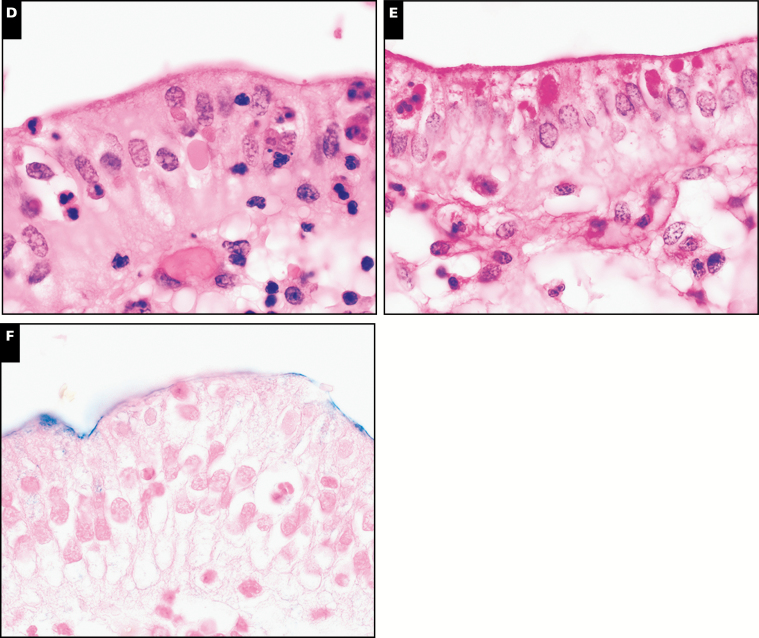
The histology of C belli in the gallbladder is distinctive on H&E staining, featuring a prominent intraepithelial parasitophorous vacuole within the surface epithelium, most often in the absence of inflammation Image 1. In the majority of cases, the parasite burden consisted of scattered inconspicuous clusters of inhabited epithelium; less commonly, there was patchy or moderately diffuse involvement of the epithelium. One potential histologic mimic, hyaline globules (also known as thanatasomes),32 can be distinguished by the presence of epithelial injury with attendant inflammation, lack of a distinct vacuole, and consistent negative staining by Alcian blue (Image 1).32
Image 1 .
Cystoisospora belli is characterized by a distinctive parasitopherous vacuole (A, H&E, ×1,000), and is highlighted by periodic acid-Schiff-diastase (PASD) (B, ×1,000) and Alcian blue histochemical stains (C, ×1,000). Hyaline globules represent one histologic mimic (D, H&E, ×1,000) and also stain positive with PASD (E, ×1,000), but are consistently negative for Alcian blue (F, ×1,000).
Discussion
This study reports the largest sample, to our knowledge, of gallbladders that have been systematically examined for C belli. The principal finding is that this organism is more prevalent among immunocompetent humans than previously recognized. The prevalence of C belli in stool specimens has been evaluated in several different settings. A review of the ledgers of King George Hospital found that 2.6% of 384 patients showed scant C belli oocysts in stool33; nearly all these patients were soldiers (presumably immunocompetent) returning from Egypt and Gallipoli during World War I. In the authors’ opinion, “no ill effect” was attributed to the presence of C belli oocysts in these patients’ stool. Perhaps the best prevalence data reported to date, which have varied widely, are from several large cohort studies of patients with diarrhea in the setting of AIDS, in which symptomatic C belli infection was diagnosed via microscopic examination of stool samples for C belli oocysts. A study of 16,351 patients with HIV/AIDS in Los Angeles, CA (spanning 1985-1992), found an overall prevalence of 1.0%, with highest prevalence among patients from El Salvador (7.4%) and Mexico (5.4%).26 Another study, which utilized the French Hospital Database on HIV, reported 17 cases among 26,815 enrolled, with C belli infection greatest among patients from sub-Saharan Africa.34 That same study compared their findings to a later time period in which combination antiretroviral medications were in use, and found that combination antiretroviral therapy reduced persistent C belli infection on follow-up by 78% (with no difference between the two time periods among patients with CD4 T-cell counts <50/μL). This confirmed that persistent symptomatic infection occurs predominantly in the setting of pronounced immunodeficiency. An interesting study from India included stool studies from patients of unknown immune status who presented with diarrhea (n = 200), and control patients without diarrhea (n = 50), finding a prevalence of 22% and 4% in those with and without diarrhea, respectively, and indicating that, at least in some populations, asymptomatic infection is not uncommon in humans. The variability of prevalence data has been interpreted to reflect differences in patient demographics, with patients from endemic areas having higher rates. Data regarding the prevalence of oocysts in surface water are scarce; one report of potable drinking water from Dakahlia, Egypt identified C belli oocysts in 0.47% of 840 samples,35 suggesting that, at least in some regions, exposure to C belli oocysts may be more common than previously realized.
Our cohort of healthy immunocompetent patients without diarrhea revealed a prevalence of C belli in gallbladder resections that was similar to the prevalence of symptomatic infections in patients with AIDS and diarrhea diagnosed by microscopic examination of stool studies, and greater than the reported prevalence of C belli oocysts in the stool of patients without diarrhea in India. While at first glance these findings may seem unexpected, we believe they make sense in the setting of several observations.
Cryptosporidium parvum is a similar parasite that has caused large outbreaks in the United States when drinking water or recreational water becomes contaminated by bovine feces.36C parvum oocysts are infectious upon excretion in the feces, and very few oocysts are required to initiate symptomatic infection. In contrast, the Cystoisospora oocysts are not infectious at the time of excretion, requiring maturation outside of the body, a process that requires 1 to 2 weeks.11 This presumably decreases the efficiency of infection and the density of infectious oocysts during a typical exposure. Furthermore, humans are the only known host of C belli, making large-scale contamination of drinking water less frequent. Given the low infectious rate and the lack of a nonhuman reservoir, it would seem that the symptomatic infections of (typically immunocompromised) patients would require a population of asymptomatic human carriers that shed oocysts at a (presumably) low rate. Because Cystoisospora species hatch when exposed to bile,37 yet are not commonly appreciated in duodenal biopsies that are examined carefully for subtle abnormalities in routine diagnostic practice, it seems reasonable that C belli may reside in a relatively latent state with low organismal burden in the gallbladder, having been overlooked by surgical pathologists in an organ where clinical management does not typically rely on thorough tissue sampling or high magnification microscopic examination.
The presence of C belli in cholecystectomy specimens has several important differences from symptomatic infections of the biliary tree and small intestine. First, symptomatic infection of the small intestine is invariably associated with secretory diarrhea, often severe enough to require hospitalization. Second, the histology of symptomatic infection by C belli shows numerous organisms accompanied by active inflammation. Stool studies are positive for oocysts. In our cohort of gallbladder resections from immunocompetent patients, the presence of C belli is characterized by a lack of diarrhea, a lack of inflammatory response, and a lack of an association with any of the clinical settings that led to cholecystectomy. While the presence of C belli in human gallbladders does not explain the changing demographics of cholecystectomy, the unexpectedly high prevalence in gallbladder specimens indicates that C belli frequently inhabits humans and may instead be better conceived as a commensal organism with a relatively latent presence in the gallbladder, only giving rise to symptomatic infection in the setting of pronounced immunodeficiency and/or immunosuppression.
There are several limitations to this study. The retrospectively studied population here was biased toward younger patients and excluded cases with cholelithiasis seen on gross examination. However, even with the relative undersampling of patients over 30 years of age, C belli was identified in patients up to 87 years of age, and even this cohort of grossly acalculous gallbladders included 78 gallbladders removed for gallstone disease. A second limitation is the lack of a second, confirmatory method (ie, polymerase chain reaction and/or electron microscopy). However, all of our cases were confirmed on review by at least two GI pathologists, and because the distinctive H&E appearance is sufficient for diagnostic practice to guide clinical decision making, we feel the added expense of these studies is unnecessary.
Many facets of the biology of this fascinating organism remain unknown. For instance, does C belli infection cause symptoms when colonization is initially established? Do protozoal infections explain a subset of self-limited diarrheal episodes currently presumed to represent viral gastroenteritis? If the presence of C belli in the gallbladder represents latent infection as we propose, how frequently do these patients shed oocysts? Do paratenic hosts (domesticated cats, dogs, etc) play an unrecognized role in transmission? Further studies of this poorly understood organism are needed.
In conclusion, our data show that C belli frequently inhabits the human gallbladder at a prevalence of at least 9.7% (likely closer to 27%), but the presence of C belli shows no association with the clinical indication for cholecystectomy, and indeed is not increased in the setting of cholecystectomy performed for gallbladder-related symptoms. As such, we would not advise submitting additional sections for the purpose of increasing likelihood of C belli detection for routine patient care. Given the heterogeneous clinical context and the morphologic findings reported here, we propose that the presence of C belli in the human gallbladder represents a latent anatomic reservoir, and may be best classified as a commensal organism in the setting of an immunocompetent patient.
This work was supported by the University of Rochester Medical Center Department of Pathology and Laboratory Medicine Faculty Research Fund.
References
- 1. Cotton PB, Elta GH, Carter CR, et al. Rome IV. Gallbladder and sphincter of Oddi disorders. Gastroenterology. 2016;150:1420-1429. [DOI] [PubMed] [Google Scholar]
- 2. Alli VV, Yang J, Xu J, et al. Nineteen-year trends in incidence and indications for laparoscopic cholecystectomy: the NY state experience. Surg Endosc. 2017;31:1651-1658. [DOI] [PubMed] [Google Scholar]
- 3. Bielefeldt K. The rising tide of cholecystectomy for biliary dyskinesia. Aliment Pharmacol Ther. 2013;37:98-106. [DOI] [PubMed] [Google Scholar]
- 4. Preston JF, Diggs BS, Dolan JP, et al. Biliary dyskinesia: a surgical disease rarely found outside the United States. Am J Surg. 2015;209:799-803. [DOI] [PubMed] [Google Scholar]
- 5. Unger SW, Rosenbaum G, Unger HM, et al. A comparison of laparoscopic and open treatment of acute cholecystitis. Surg Endosc. 1993;7:408-411. [DOI] [PubMed] [Google Scholar]
- 6. The Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med, 1991;324:1073-1078. [DOI] [PubMed] [Google Scholar]
- 7. Pucher PH, Brunt LM, Fanelli RD, et al. SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc. 2015;29:3074-3085. [DOI] [PubMed] [Google Scholar]
- 8. Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg. 1995;130:1123-1128; discussion 1129. [DOI] [PubMed] [Google Scholar]
- 9. Bouras G, Burns EM, Howell AM, et al. Systematic review of the impact of surgical harm on quality of life after general and gastrointestinal surgery. Ann Surg. 2014;260:975-983. [DOI] [PubMed] [Google Scholar]
- 10. Törnqvist B, Zheng Z, Ye W, et al. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin Gastroenterol Hepatol. 2009;7:1013-1018; quiz 915. [DOI] [PubMed] [Google Scholar]
- 11. Berci G, Morgenstern L. Bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2000;14:1091. [DOI] [PubMed] [Google Scholar]
- 12. Lindsay DS, Dubey JP, Blagburn BL. Biology of Isospora spp.from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev. 1997;10:19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkpatrick CE. Animal reservoirs of Cryptosporidium spp. and Isospora belli. J Infect Dis. 1988;158:909-910. [DOI] [PubMed] [Google Scholar]
- 14. Marcial-Seoane MA, Serrano-Olmo J. Intestinal infection with Isospora belli. P R Health Sci J. 1995;14:137-140. [PubMed] [Google Scholar]
- 15. DeHovitz JA, Pape JW, Boncy M, et al. Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:87-90. [DOI] [PubMed] [Google Scholar]
- 16. Frenkel JK, Silva MB, Saldanha J, et al. Isospora belli infection: observation of unicellular cysts in mesenteric lymphoid tissues of a Brazilian patient with AIDS and animal inoculation. J Eukaryot Microbiol. 2003;50:682-684. [DOI] [PubMed] [Google Scholar]
- 17. Michiels JF, Hofman P, Bernard E, et al. Intestinal and extraintestinal Isospora belli infection in an AIDS patient: a second case report. Pathol Res Pract. 1994;190:1089-1093; discussion 1094. [DOI] [PubMed] [Google Scholar]
- 18. Agholi M, Aliabadi E, Hatam GR. Cystoisosporiasis-related human acalculous cholecystitis: the need for increased awareness. Pol J Pathol. 2016;67:270-276. [DOI] [PubMed] [Google Scholar]
- 19. Benator DA, French AL, Beaudet LM, et al. Isospora belli infection associated with acalculous cholecystitis in a patient with AIDS. Ann Intern Med. 1994;121:663-664. [DOI] [PubMed] [Google Scholar]
- 20. Walther Z, Topazian MD. Isospora cholangiopathy: case study with histologic characterization and molecular confirmation. Hum Pathol. 2009;40:1342-1346. [DOI] [PubMed] [Google Scholar]
- 21. Koru O, Araz RE, Yilmaz YA, et al. Case report: Isospora belli infection in a renal transplant recipent. Turkiye Parazitol Derg. 2007;31:98-100. [PubMed] [Google Scholar]
- 22. Meamar AR, Rezaian M, Mirzaei AZ, et al. Severe diarrhea due to Isospora belli in a patient with thymoma. J Microbiol Immunol Infect. 2009;42:526-529. [PubMed] [Google Scholar]
- 23. Stein J, Tannich E, Hartmann F. An unusual complication in ulcerative colitis during treatment with azathioprine and infliximab: Isospora belli as “casus belli.” BMJ Case Rep. 2013;2013:pii: bcr2013009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abhilasha K, Saxena S, Malhotna VL, et al. Isospora belli infection in a malnourished child. J Commun Dis. 2007;39:141-143. [PubMed] [Google Scholar]
- 25. Kim MJ, Kim WH, Jung HC, et al. Isospora belli infection with chronic diarrhea in an alcoholic patient. Korean J Parasitol. 2013;51:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sorvillo FJ, Lieb LE, Seidel J, et al. Epidemiology of isosporiasis among persons with acquired immunodeficiency syndrome in Los Angeles County. Am J Trop Med Hyg. 1995;53:656-659. [DOI] [PubMed] [Google Scholar]
- 27. Goodgame R. Emerging causes of traveler’s diarrhea: Cryptosporidium, Cyclospora, Isospora, and Microsporidia. Curr Infect Dis Rep. 2003;5:66-73. [DOI] [PubMed] [Google Scholar]
- 28. Mirdha BR, Singh S, Anand B. Transient Isospora belli infection in a normal child. Indian J Pediatr. 1993;60:299-301. [DOI] [PubMed] [Google Scholar]
- 29. Ribes JA, Seabolt JP, Overman SB. Point prevalence of Cryptosporidium, Cyclospora, and Isospora infections in patients being evaluated for diarrhea. Am J Clin Pathol. 2004;122:28-32. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi H, Falk GA, Cruise M, et al. Chronic cholecystitis with Cystoisospora belli in an immunocompetent patient. BMJ Case Rep. 2015;2015:pii: bcr2015209966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai KK, Goyne HE, Hernandez-Gonzalo D, et al. Cystoisospora belli infection of the gallbladder in immunocompetent patients: a clinicopathologic review of 18 cases. Am J Surg Pathol. 2016;40:1070-1074. [DOI] [PubMed] [Google Scholar]
- 32. Dikov DI, Auriault ML, Boivin JF, et al. Hyaline globules (thanatosomes) in gastrointestinal epithelium: pathophysiologic correlations. Am J Clin Pathol. 2007;127:792-799. [DOI] [PubMed] [Google Scholar]
- 33. Woodcock HM, Penfold WJ. Further notes on protozoan infections occurring at the King George Hospital. Br Med J. 1916;1:407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guiguet M, Furco A, Tattevin P, et al. ; French Hospital Database on HIV Clinical Epidemiology Group HIV-associated Isospora belli infection: incidence and risk factors in the French Hospital Database on HIV. HIV Med. 2007;8:124-130. [DOI] [PubMed] [Google Scholar]
- 35. Elshazly AM, Elsheikha HM, Soltan DM, et al. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2007;37:51-64. [PubMed] [Google Scholar]
- 36. Mac Kenzie WR, Hoxie NJ, Proctor ME, et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161-167. [DOI] [PubMed] [Google Scholar]
- 37. McKenna PB, Charleston WA. Activation and excystation of Isospora felis and Isospora rivolta sporozoites. J Parasitol. 1982;68:276-286. [PubMed] [Google Scholar]