Abstract
Objectives
To determine clinical utility of Onclarity human papillomavirus (HPV) assay for atypical squamous cells-undetermined significance (ASC-US) triage, and the value of HPV genotyping within ASC-US.
Methods
Women (n = 33,858; 21 years or older) had HPV testing using Onclarity and Hybrid Capture 2 (HC2). ASC-US individuals (n = 1,960, 5.8%) were referred to colposcopy.
Results
Of ASC-US, 39.1% were HPV positive by Onclarity; HPV 16 was the most prevalent genotype (7.4%). Cervical intraepithelial neoplasia grade 2 (CIN 2) and CIN 3+ prevalences were 4.4% and 2.2%, respectively. Onclarity had sensitivity for CIN 2+ (85.7%) and CIN 3+ (91.4%), and specificities for CIN 2+ (64.1%) and CIN 3+ (62.0%), similar to HC2. Risks for CIN 3+ were 16.1%, 2.8%, 2.5%, and 2.7% with HPV 16, 18, 45, and 11 other genotypes, respectively.
Conclusions
Onclarity is clinically validated for ASC-US triage. Through risk stratification, genotyping could help identify women at highest risk for CIN 3+.
Keywords: Cervical cancer screening, Atypical squamous cells-undetermined significance, Human papillomavirus, Genotype, Cervical intraepithelial neoplasia, Triage
Atypical squamous cells-undetermined significance (ASC-US) is the most common cervical cytologic abnormality. The College of American Pathologists lists the median reporting rate of ASC-US among laboratories in the United States as 5.0% in the 2017 Cytopathology Checklist.1 Fortunately, only a small minority of women with ASC-US have a high-grade cervical cancer precursor (ie, high-grade squamous intraepithelial lesions [HSIL] and adenocarcinoma in situ) as well as invasive cervical cancers. Two recent large US cervical cancer screening trials reported an overall prevalence of high-grade cervical cancer precursors in women with ASC-US of 5.1% (n = 1,578)2 to 9.7% (n = 939)3; no invasive cervical cancers in women with ASC-US were identified in either study. Because most high-grade cervical cancer precursors and invasive cervical cancers are caused by 13 high-risk human papillomavirus (HPV) genotypes,4,5 HPV testing has become the predominant approach to managing women with ASC-US, both in North America and Europe.6 HPV-positive women with ASC-US are referred for colposcopy, whereas HPV-negative women with ASC-US are followed-up with repeat testing in 12 months. This approach is recommended by US clinical management guidelines.7,8
Currently, there are a number of Food and Drug Administration (FDA)-approved HPV assays for use as a triage for women with ASC-US. These include Hybrid Capture 2 (HC2; Qiagen, Gaithersburg, MD), Cervista HPV HR (Hologic, Bedford, MA), cobas HPV (Roche Molecular Systems, Pleasanton, CA), and Aptima HPV (Hologic). Recently, the Onclarity HPV Assay (BD Life Sciences, Sparks, MD) was FDA-approved for use with SurePath liquid-based cytology (LBC) in women, aged 21 years or older, with ASC-US to determine the need for referral to colposcopy. The assay was also approved for detection of HPV genotypes 16, 18, and 45 in this population.9 Onclarity is a polymerase chain reaction (PCR) assay that detects E6/E7 DNA from 13 high-risk HPV types and HPV 66, with simultaneous individual genotyping for HPV 16, 18, 31, 45, 51, and 52, and detection of (33/58), (56/59/66), and (35/39/68) as three separate, pooled groups.10 The performance of this assay in women with ASC-US has been previously evaluated using archived LBC specimens.11 To confirm these findings and obtain regulatory approval by the FDA, the clinical performance of the assay was evaluated in a multicenter US-based clinical study (BD Onclarity trial) that enrolled 33,858 women, aged 21 years or older, undergoing routine screening in the United States. This report describes the assay’s performance characteristics in the subset of women aged 21 years or older with ASC-US, compares the assay’s performance to that of HC2, and investigates the potential clinical utility of genotyping for HPV 16, 18, and 45 in this population.
Materials and Methods
Study Design
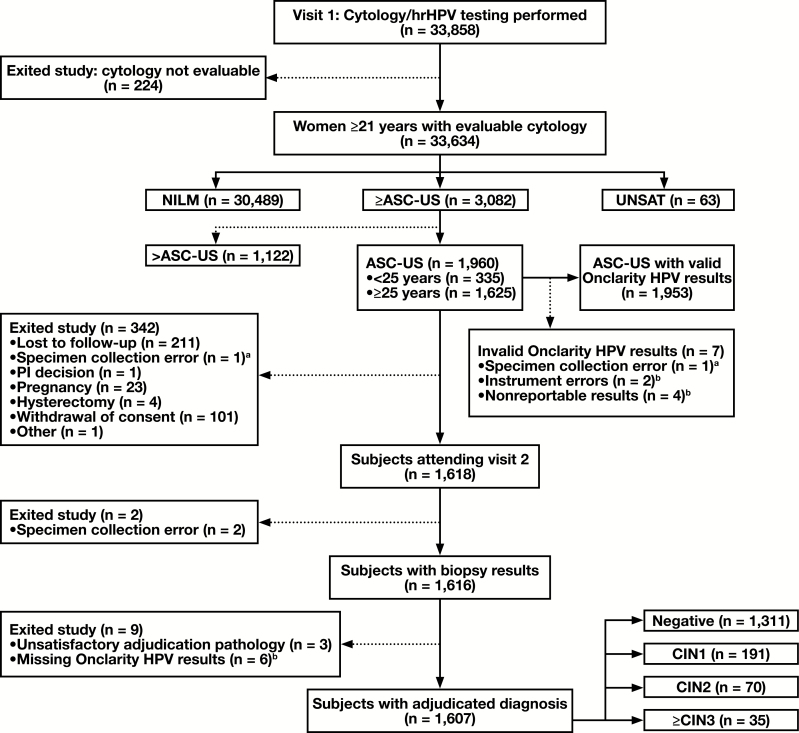
This report includes data from the baseline phase of the ongoing Onclarity trial. The design, screening procedures, inclusion/exclusion criteria, and description of cytology and HPV testing methodology has been previously described in detail.12 The selection algorithm for women in this analysis is shown in Figure 1. The primary study endpoint for disease was identification of a high-grade cervical cancer precursor lesion (CIN 2+), defined as cervical intraepithelial neoplasia grade 2 (CIN 2) or higher lesion (CIN 3, adenocarcinoma in situ, or invasive cervical cancer) by consensus pathology adjudication.
Figure 1 .
Subject reconciliation during baseline enrollment and participation of subjects, aged 21 years or older, with ASC-US cytology, in the trial. aCorresponds to the same specimen. bCorresponds to the same specimens. ASC-US, atypical squamous cells-undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; hrHPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesions or malignancies; PI, principal investigator; UNSAT, unsatisfactory cytology.
Sample size was based on identifying 70 cases of CIN 2+ in women with ASC-US. Initial estimates indicated that, after accounting for all factors including loss to follow-up, this would require enrolling 47,000 subjects aged 21 years or older. However, there was a higher ASC-US and CIN 2+ prevalence than originally projected and this allowed enrollment to be stopped at 33,858 subjects. Women were recruited consecutively during routine cervical cancer screening at 31 clinical sites across 17 states between August 26, 2013, and June 12, 2015. Overall, 1,960 subjects had a cytological diagnosis of ASC-US. Detailed inclusion/exclusion criteria have been previously described.12 The overall HPV vaccination rate (≥one dose) was capped at 10%.
All sites obtained institutional review board approval and written informed consent from all subjects prior to any trial-related procedures. This study was conducted according to those principles outlined by the Declaration of Helsinki and by Good Clinical Practice.
Enrollment Visit (Study Visit 1)
At the enrollment visit, information related to demographics and medical history was recorded. During speculum examination, two cervical samples were collected with either the brush/spatula or a broom-like collection device. The first was placed into BD SurePath media (BD Life Sciences, Burlington, NC) and was used for both cervical cytology (conducted at three clinical laboratories) and HPV testing using the BD Onclarity HPV assay. The second cervical sample was placed into a PreservCyt vial (Hologic) and used for HPV testing using the Onclarity assay and HC2 assay (Qiagen). Cytologic evaluation and results were reported according to the 2001 Bethesda System.13 Computerized imaging was not employed during evaluation of cytology.
HPV Testing
The specific details of the fully automated BD Viper LT system (BD Diagnostics, Sparks, MD) and the Onclarity HPV assay have been published previously.10,12 The Onclarity HPV assay is a real-time PCR assay that utilizes 0.5 mL of SurePath medium. Onclarity is run on the fully integrated BD Viper LT System, which can process up to 120 samples per day in a 9.5-hour period of time (two shifts). The system extracts and purifies DNA, hydrates a dried PCR master mix, and proceeds to automated plate sealing, thermocycling, and results outputting without user intervention. The instrument was designed to reduce the risk of PCR contamination through the incorporation of ready to use reagents and fully automated workflow. In addition to gene-specific targets to E6 and E7 HPV DNA, the assay also detects the human β-globin gene, which acts as an internal control. The clinical cutoff for genotype detection was established using receiver-operator characteristic curve analysis for histologically confirmed CIN 2+ disease.
Colposcopy and Biopsy Visit (Study Visit 2)
All subjects with a cytologic diagnosis of ASC-US were referred to colposcopy. Of the 1,960 subjects referred to colposcopy, 1,953 had valid HPV tests, and a total of 1,607 actually underwent colposcopy and had adjudicated histopathology results and valid HPV results Figure 1. Colposcopy had to be performed within 84 days of Study Visit 1. Both subjects and colposcopists were blinded to cytology and HPV assay results at the time of colposcopy. Colposcopists were instructed to biopsy any lesion or acetowhite area. If no lesions or acetowhite areas were visible during the colposcopy, a random biopsy at the squamocolumnar junction was performed. An endocervical curettage (ECC) was collected from every subject undergoing colposcopy.
Histopathology
Biopsies and ECCs were evaluated independently by at least two of the Central Pathology Review (CPR) gynecological pathologists (Drs Alex Ferenczy, Mark H. Stoler, and Thomas C. Wright, Jr) who were masked to all study information except for the subject’s age. If the two pathologists did not agree, the slides were reviewed by the third CPR pathologist. Consensus was achieved when two of the three pathologists agreed on a diagnosis. In cases where all three diagnoses were discordant, the specimen(s) in question were reviewed together by all three pathologists to achieve a consensus pathology diagnosis. When at least one reviewer identified a specimen as CIN 2 or when one reviewer rated a specimen as CIN 2+, with a second reviewer scoring the same sample as below CIN 2, immunohistochemistry for p16INK4A (p16) (Ventana Medical Systems, Tucson, AZ) was utilized in adjudicating a final diagnosis. For histology, the Lower Anogenital Squamous Terminology standardization and World Health Organization classification, which incorporate Bethesda terminology, is simplified to the CIN grade.14,15 In this report, CIN 1 indicates low-grade squamous intraepithelial lesion (CIN 1), CIN 2 indicates HSIL (CIN 2), and CIN 3 indicates HSIL (CIN 3).
Statistical Analysis
Data for prevalence estimates were limited to subjects with an LBC result, key demographic information, and HPV assay results for all genotypes. Prevalence numbers of high-grade cervical lesions were calculated for women that had colposcopy/biopsy results. Performance values (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV], positive likelihood ratio [PLR], and negative likelihood ratio [NLR]), absolute risk, and relative risk were determined using standard statistical tests. The confidence intervals were calculated using standard methods.
Results
Baseline Demographics and Screening Results
Of the 1,960 subjects with ASC-US cytology, 37.3% were younger than 30 years and 62.7% were aged 30 years or older Table 1 . The mean and median ages among the subjects were 36.2 and 34.0 years, respectively. White subjects (73.8%) comprised the majority of participants, with African American subjects (23.4%) constituting the second largest group by race. In the ASC-US population, 11.4% were vaccinated, 1.9% were immunocompromised, and in the 5 years prior to enrollment, 26.5% had abnormal cytology findings and 16.3% had undergone a colposcopy. HPV (any genotype) was detected using Onclarity in 39.1% of subjects. HPV 16 was detected in 7.4% of subjects, and HPV 18 and 45 were detected in 2.5% and 2.4% of subjects, respectively. The prevalence of HPV (any HPV) decreased with increasing age, as did the prevalence of HPV 16, HPV 18, and HPV 45 Table 2 . In the 21 to 29-year age group the overall prevalence of HPV was 54.6% whereas in the 40 years or older group it was only 22.9%. Prevalence, by age group, for HPV 31, (33/58), 51, 52, (35/39/68), and (56/59/66) is shown in Supplemental Table 1 (all supplemental materials can be found at American Journal of Clinical Pathology online).
Table 1 .
Baseline Demographic Information—All ASC-US Subjects
| Characteristic | Total Subjects (n = 1,960), % (No.) |
|---|---|
| Age, y | |
| Mean (SD) | 36.2 ± 11.5 |
| Median | 34.0 |
| 21-29 | 37.3 (732) |
| 30-39 | 26.6 (521) |
| ≥40 | 36.1 (707) |
| Race | |
| Asian | 1.0 (20) |
| African American | 23.4 (459) |
| White | 73.8 (1,446) |
| Othera | 1.8 (35) |
| Ethnicity | |
| Hispanic or Latino | 15.5 (303) |
| Not Hispanic or Latino | 84.5 (1,657) |
| Smoking history | |
| Nonsmoker | 66.5 (1,304) |
| Current | 15.7 (308) |
| Past | 17.8 (348) |
| HPV vaccinated | |
| Yes | 11.4 (224) |
| No | 86.6 (1,698) |
| Unknown | 1.9 (38) |
| Postmenopausal | 12.7 (249) |
| Immunocompromised | 1.9 (37) |
| Abnormal cytology (past 5 y) | 26.5 (519) |
| Colposcopy (past 5 y) | 16.3 (319) |
| HPV status (n = 1,953)b | |
| Any HPV | 39.1 (763) |
| HPV 16 | 7.4 (144) |
| HPV 18c | 2.5 (48) |
| HPV 45c | 2.4 (47) |
| 11 “other” HPV | 32.2 (629) |
| HPV negative | 60.9 (1,190) |
ASC-US, atypical squamous cells-undetermined significance; HPV, human papillomavirus; SD, standard deviation.
aIncludes American Indian, Alaska Native, Native Hawaiian, or other Pacific Islander.
bSeven specimens missing Onclarity HPV results.
cHPV 18 value includes HPV 16; HPV 45 value includes HPV 16 and 18.
Table 2.
Prevalence of HPV by Age in All Evaluable ASC-US Subjects (n = 1,953)
| Age Group, y | Overall HPV+, % (No.) | HPV 16+, % (No.) | HPV 18+, % (No.) | HPV 45+, % (No.) | HPV Other+, % (No.) |
|---|---|---|---|---|---|
| 21-29 (n = 729) | 54.6 (398) | 10.6 (77) | 3.4 (25) | 3.2 (23) | 45.8 (334) |
| 30-39 (n = 521) | 39.2 (204) | 7.7 (40) | 2.5 (13) | 2.1 (11) | 31.1 (162) |
| ≥40 (n = 703) | 22.9 (161) | 3.8 (27) | 1.4 (10) | 1.8 (13) | 18.9 (133) |
ASC-US, atypical squamous cells-undetermined significance; HPV, human papillomavirus.
HPV Prevalence Values by Cervical Disease Status
All subjects with ASC-US were referred to colposcopy; 82.0% (1,607 out of 1,960) underwent the procedure, had a valid HPV result, and had a consensus pathology biopsy result (Figure 1). The prevalence of CIN 2 was 4.4% and the prevalence of CIN 3+ was 2.2% Table 3 . There were two cases of adenocarcinoma in situ (both of whom were HPV 16 positive) and no invasive carcinomas in the 1,607 evaluable women; 82.9% and 91.4%, respectively, of subjects with CIN 2+ and CIN 3+ were HPV positive. The prevalence of HPV 16 increased with increasing severity of cervical disease. HPV 16 prevalence increased from 4.8% in subjects with no CIN to 51.4% in those with CIN 3+. HPV 18 and 45 were less prevalent than HPV 16 in women with CIN 2 or CIN 3+. For HPV 18, the prevalence in CIN 2 and CIN 3+ was 7.1% and 2.9%, respectively, and for HPV 45, prevalence was 2.9% in both CIN 2 and CIN 3+. No cases of CIN 2 or CIN 3+ were detected that involved mixed infection of HPV 16 with either HPV 18 or HPV 45 in this population.
Table 3 .
Cervical Disease Status by HPV Genotypea
| HPV Assay Result | NEG (n = 1,311), % | CIN 1 (n = 191), % | CIN 2 (n = 70), % | CIN 3+ (n = 35), % | Total (n = 1,607), % |
|---|---|---|---|---|---|
| Any HPV | 32.3 | 60.7 | 82.9 | 91.4 | 39.1 |
| HPV 16 | 4.8 | 8.4 | 21.4 | 51.4 | 7.0 |
| HPV 18 (16 negative) | 1.7 | 4.2 | 7.1 | 2.9b | 2.2 |
| HPV 45 (16 negative) | 2.1 | 5.2 | 2.9 | 2.9b | 2.5 |
| Any HPV 16 or HPV 18 | 6.5 | 12.6 | 28.6 | 54.3 | 9.2 |
| Any HPV 18 or HPV 45 | 4.0 | 11 | 10 | 5.7 | 5.1 |
| Any HPV 16 or HPV 18 or HPV 45 | 8.5 | 17.8 | 31.4 | 57.1 | 11.7 |
| 11 other HPV (16, 18, and 45 negative) | 23.7 | 42.9 | 51.4 | 34.3 | 27.4 |
| HPV negative | 67.7 | 39.3 | 17.1 | 8.6 | 60.9 |
ASC-US, atypical squamous cells-undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; NEG, negative for cervical intraepithelial neoplasia.
aAmong ASC-US subjects with evaluable histology and full genotyping results for all Onclarity testing.
bActual value is 2.85%.
HPV Assay Performance
Performance characteristics (sensitivity, specificity, PPV, NPV, PLR, and NLR) for the detection of CIN 2+ and CIN 3+ were determined for the Onclarity and HC2 HPV assays Table 4 . The performance of the two HPV assays for the detection of CIN 2+ and CIN 3+ was similar, with only minor, nonsignificant differences observed. The sensitivity of Onclarity for CIN 2+ and CIN 3+ was 85.7% and 91.4%, respectively. Specificity for CIN 2+ and CIN 3+ was 64.1% and 62.0%, respectively. NPV for CIN 2+ and CIN 3+ was 98.5% and 99.7%, respectively. Similar performance characteristics were found using HC2. Age had an important impact on the performance characteristics of the two HPV assays (Supplemental Table 2). The sensitivity of Onclarity for CIN 2+ in women 21 to 29 years, 30 to 39 years, and 40 years or older was 93.6%, 83.3%, and 68.8%, respectively. Specificity for CIN 2+ in the three age groups was 49.5%, 63.2%, and 78.2%, respectively.
Table 4 .
Performance of Onclarity vs HC2 for Detection of CINa in Women With ASC-US
| CIN 2+ (n = 105) | CIN 3+ (n = 35) | |||
|---|---|---|---|---|
| Onclarity (95% CI) | HC2 (95% CI) | Onclarity (95% CI) | HC2 (95% CI) | |
| Sensitivity, % | 85.7 (77.8-91.1) | 82.9 (74.5-88.9) | 91.4 (77.6-97.0) | 85.7 (70.6-93.7) |
| Specificity, % | 64.1 (61.6-66.5) | 61.4 (58.9-63.9) | 62.0 (59.6-64.4) | 59.5 (57.1-61.9) |
| PPV, % | 14.4 (13.0-15.6) | 13.1 (11.8-14.3) | 5.1 (4.3-5.6) | 4.5 (3.7-5.0) |
| NPV, % | 98.5 (97.6-99.0) | 98.1 (97.2-98.7) | 99.7 (99.2-99.9) | 99.5 (98.9-99.8) |
| PLR | 2.39 (2.1-2.6) | 2.2 (1.9-2.4) | 2.4 (2.0-2.6) | 2.1 (1.7-2.4) |
| NLR | 0.2 (0.1-0.4) | 0.3 (0.2-0.4) | 0.1 (0.1-0.4) | 0.2 (0.1-0.5) |
ASC-US, atypical squamous cells-undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HC2, Hybrid Capture 2; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
aResults are based on 1,601 women with consensus pathology results and HPV results with both Onclarity and HC2 (paired analysis).
Risk Estimation by HPV Status
Absolute and relative risks for CIN 2+ and CIN 3+ were calculated based on HPV status or HPV genotype Table 5 . Subjects with a positive HPV result (any genotype) had an absolute risk of 14.3% and 5.1% for CIN 2+ and CIN 3+, respectively. For HPV 16-positive subjects, absolute risk increased to 29.5% and 16.1%, respectively, for CIN 2+ and CIN 3+. Absolute risk for CIN 3+ was much less for subjects with HPV 18 (2.8%) or HPV 45 (2.5%), respectively, in the absence of HPV 16. For comparison, the absolute risk for CIN 3+ in subjects with the 11 other HPV genotypes was 2.7%. In HPV negative-subjects, the absolute risk of CIN 3+ was only 0.3%. Further stratification was performed for combinations of HPV 16, 18, and 45. Stratification by HPV 16/18 or HPV 16/18/45 all resulted in risk values for CIN 2+ and CIN 3+ that were elevated compared to either HPV positive (any genotype) or the 11 other HPV positive. Relative risks showed a similar pattern.
Table 5 .
Absolute and Relative Risks Associated With HPV Status for CIN 2+ and CIN 3+a in Women With ASC-US
| HPV Genotype | CIN 2+ % (95% CI) | CIN 3+ % (95% CI) |
|---|---|---|
| Absolute risk | ||
| HPV positive (n = 629) | 14.3 (13.0-15.5) | 5.1 (4.3-5.6) |
| HPV 16 (n = 112) | 29.5 (22.5-37.0) | 16.1 (11.2-21.2) |
| HPV 18 (n = 36)b | 16.7 (7.9-31.1) | 2.8 (0.5-13.0) |
| HPV 45 (n = 40)b | 7.5 (2.6-19.3) | 2.5 (0.4-11.8) |
| HPV 16 or HPV 18 (n = 148) | 26.4 (20.7-32.5) | 12.8 (9.1-16.7) |
| HPV 16 and/or HPV 18 and/or HPV 45 (n = 188) | 22.3 (17.7-27.3) | 10.6 (7.7-13.6) |
| 11 other HPV genotypes (n = 441) | 10.9 (8.8-13.1) | 2.7 (1.1-4.0) |
| HPV negative (n = 978) | 1.5 (1.0-2.4) | 0.3 (0.1-0.8) |
| Relative risk | ||
| HPV positive vs HPV negative | 9.3 (5.5-15.9) | 16.6 (5.4-50.9) |
| HPV 16 vs HPV negative | 19.2 (10.8-33.9) | 52.4 (16.7-164.3) |
| HPV 18b vs HPV negative | 10.9 (4.5-24.9) | 9.1 (1.3-60.5) |
| HPV 45b vs HPV negative | 4.9 (1.5-14.6) | 8.2 (1.2-54.7) |
| HPV 16/18 positive vs HPV negative | 17.2 (9.8-30.1) | 41.9 (13.4-131.2) |
| HPV 16/18/45 positive vs HPV negative | 14.6 (8.3-25.5) | 34.7 (11.1-108.5) |
| 11 other HPV positive vs HPV negative | 7.1 (4.1-12.5) | 8.9 (2.7-29.1) |
| HPV 16 vs HPV positive without HPV 16 | 2.7 (1.8-3.9) | 5.9 (3.1-11.4) |
ASC-US, atypical squamous cells-undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus.
aDetermined by Onclarity.
bHPV 16 negative.
Discussion
The Onclarity trial enrolled 33,858 subjects, aged 21 years or older, undergoing routine cervical cancer screening. The trial was designed to clinically validate Onclarity performed on SurePath cervical samples for use as a triage test for women, aged 21 years or older, with a cytologic result of ASC-US, as an adjunct to cervical cytology (ie, cotesting) in women aged 30 years or older, and as a stand-alone cervical cancer screening test (ie, HPV primary screening) in women aged 25 years or older. The trial was also designed to clinically validate genotyping for HPV 16, 18, and 45 in each of the clinical situations described above (HPV 16 and 18 only are indicated for primary screening) and to explore the clinical utility of expanded genotyping that would identify genotypes other than HPV 16, 18, and 45. Onclarity is a PCR-based assay that detects the E6/E7 region of the viral DNA of 13 high-risk HPV types and HPV 66 either as individual viral genotypes or as pooled mixtures of two or three genotypes. The current analysis evaluates the performance of Onclarity in the 1960 subjects, aged 21 years or older, with ASC-US who were referred for colposcopy.
The prevalence of ASC-US was 5.8% in the subjects. This is similar to the 5.0% median laboratory percentile-reporting rate of ASC-US for SurePath media used by the College of American Pathologists for the 2017 Cytopathology Checklist. However, it is somewhat higher than the 4.1% ASC-US rate that was reported from the ATHENA study, which used PreservCyt media.2 The overall prevalence of HPV positivity in women with ASC-US (39.1%) is similar to what has been reported in other published regulatory studies of women with ASC-US in the United States Table 6 .2,3,16-18 The CLEAR trial3 studied the performance of the Aptima HPV test and the ATHENA trial2 studied the cobas HPV test in women with ASC-US. Both trials used PreservCyt cytology media and the overall prevalence of HPV was 42.0% and 32.6%, respectively.2,3 Recently, a study from Roche/Kaiser Permanente was used to obtain FDA-approval of the cobas HPV test for use with SurePath media and reported that the prevalence of HPV in women with ASC-US was 45.8%.18 In contrast, the Cervista trial found a much higher prevalence of HPV (all genotypes), as well as HPV 16, compared to any of the other studies.17
Table 6 .
Comparison of Different Regulatory Trials Involving Women With ASC-US Cytology
| Study | Cervista17 | CLEAR3,16a | ATHENA2 | Kaiser18 | Onclarity |
|---|---|---|---|---|---|
| HPV assay | Cervista | Aptima | cobas | cobas | Onclarity |
| Cytology media | PreservCyt | PreservCyt | PreservCyt | SurePath | SurePath |
| No. of subjects | 1,514 | 912 | 1,578 | 846 | 1,953 |
| Mean age, y | 33.7 | 34.2 | 37.1 | 35.4 | 36.2 |
| HPV positive, % | 57.1 | 38.8 | 32.6 | 45.8 | 39.1 |
| HPV 16 positive, % | 16.8 | 8.1 | 8.2 | 6.5 | 7.4 |
| HPV 18 positive, %b | 4.2 | ND | 2.9 | 2.5 | 2.2 |
| HPV 45 positive, %b | ND | ND | ND | ND | 2.3 |
| HPV 18/45 positive, %b | ND | 5.2 | ND | ND | 4.5 |
| CIN 2 | 3.5 | 5.0 | 2.2 | 5.1 | 4.4 |
| CIN 3+ | 1.6 | 3.6 | 2.9 | 3.8 | 2.2 |
| Invasive cancers, No. | ND | 0 | 0 | 0 | 0 |
| For CIN 2+, % (95% CI) | |||||
| Sensitivity | 92.8 (81.4-96.9) | 86.8 (78.4-92.3) | 90.0 (81.5-94.8) | 82.7 (72.6-89.6) | 85.7 (77.8-91.1) |
| Specificity | 44.2 (41.5-46.9) | 62.9 (59.6-66.0) | 70.5 (68.1-72.7) | 57.5 (53.9-60.9) | 64.1 (61.6-66.5) |
| For CIN 3+, % (95% CI) | |||||
| Sensitivity | 100 (85.1-100) | 90.2 (77.5-96.1) | 93.5 (82.5-97.8) | 87.5 (71.9-95.2) | 91.4 (77.6-97.0) |
| Specificity | 43.0 (40.3-45.7) | 60.2 (57.0-63.4) | 69.3 (66.9-71.5) | 55.5 (52.1-58.9) | 62.0 (59.6-64.4) |
ASC-US, atypical squamous cells-undetermined significance; ATHENA, Addressing the Need for Advanced HPV Diagnostics; CI, confidence interval; CIN, cervical intraepithelial neoplasia; CLEAR, Clinical Evaluation of Aptima mRNA; HPV, human papillomavirus; ND, not determined.
aCastle et al16 used for demographics and prevalence values; Stoler et al3 used for performance values.
bValues for HPV 18 exclude HPV 16-positive specimens; values for HPV 45 exclude HPV 16 and/or 18-positive specimens; values for HPV 18/45 exclude HPV 16-positive specimens.
In the current study, the prevalence of biopsy-confirmed CIN 2 was 4.4% and the prevalence of CIN 3+ was 2.2%. This is similar to what was reported in the Kaiser Permanente study18 and in the CLEAR trial. The prevalence of CIN 2 is approximately twice as high as that found in the ATHENA trial (2.2%).2 It is unlikely that the difference in prevalence of CIN 2 and CIN 3+ in the studies could be due to differences in HPV vaccination rates. In the CLEAR trial, vaccination status was not reported, but enrollment was begun in 2008 at a time when HPV vaccination was just beginning in the United States. In ATHENA, 4.3% of subjects had received at least one dose of an HPV vaccine. This number increased to 11.4% in the current study and 31.8% of the subjects in the Kaiser Permanente study. Interestingly, the Kaiser Permanente study found the highest prevalence of cervical disease among subjects with ASC-US despite the high vaccination rate. Differences in the prevalence of cervical disease between the studies most likely relate to how colposcopy was performed and the biopsies evaluated.3 In CLEAR, ATHENA, and the current study the same panel of pathologists performed the consensus pathology review, whereas in the Kaiser study all pathology was reviewed by Kaiser pathologists. In addition, in the current study adjunctive p16 immunostaining was used when there was a significant discrepancy between the individual pathologists’ diagnoses. Adjunctive p16 immunostaining was not used in CLEAR and ATHENA. Colposcopy was also conducted differently in the four studies. In CLEAR a random biopsy was taken from every cervical quadrant that did not have a lesion whereas in the current study, in ATHENA, and in the Kaiser study a single random biopsy was obtained only if no cervical lesion was identified. The Cervista study allowed biopsies to be obtained at the discretion of the colposcopist. Importantly, an ECC was performed for all subjects in this study. An ECC was performed for all subjects in the CLEAR study, but only performed following unsatisfactory colposcopy findings in the ATHENA and Kaiser studies. No ECC was performed in the Cervista study. The inclusion of a routine ECC for all subjects in the current study likely resulted in a higher rate of detection for high-grade disease compared to studies with no routine ECC. Although the mean age is similar in the five studies (ranges from 33.6 to 37.1 years), the differences in prevalence of high-grade disease in the studies may be due to subtle differences in cervical disease risk profiles or the way cytology was interpreted. It is important to note that no invasive cervical cancers were identified in any of the studies (Table 6).
In the current study, clinical validation of Onclarity as a triage for women with ASC-US was achieved by comparing the performance characteristics of Onclarity for the detection of CIN 2+ and CIN 3+ with those of HC2. For this comparison, Onclarity was performed on the SurePath cytology sample that was always collected first, and HC2 was performed on the ThinPrep cytology sample that was collected second. Because this was a FDA regulatory trial of the performance of Onclarity on SurePath that would always be collected as the first specimen in routine clinical practice, we did not randomly alternate the collection sequence. Sensitivity for CIN 2+ and CIN 3+ was 85.7% and 91.4%, respectively. Specificity for CIN 2+ and CIN 3+ was 64.1% and 62.0%, respectively. These sensitivities and specificities are statistically indistinguishable from those of HC2 in the same population. HC2 is considered to be an established benchmark for comparing HPV tests in the United States.19 Moreover, when comparing the performance of Onclarity using SurePath specimens with the performance of other FDA-approved HPV tests in the four other large US regulatory trials incorporating different HPV assays and/or media, similar sensitivities and specificities are observed for both CIN 2+ and CIN 3+ in all of the trials except for the Cervista trial (Table 6). In the Cervista trial, a higher prevalence of HPV (all genotypes) resulted in a lower specificity than found in the other studies.17 Similar to other reports from the United States, we found that age had an important impact on the performance of the HPV assays. In this study the sensitivity of Onclarity for CIN 2+ decreased from 93.6% in women 21 to 29 years of age to 68.8% in women aged 40 years or older. In contrast, the specificity of Onclarity for CIN 2+ increased from 49.5% in women 21 to 29 years of age to 78.2% in women aged 40 years or older. In the ATHENA trial sensitivity of the cobas HPV assay for CIN 2+ in women with ASC-US decreased from 93.3% in women 21 to 29 years of age to 66.7% in women aged 40 years or older and specificity increased from 49.7% in women 21 to 29 years of age to 85.0% in women aged 40 years or older.20 The current results clinically validate the use of Onclarity using SurePath samples as a triage test for women aged 21 years or older with a cytologic result of ASC-US, because it both meets the benchmarks of sensitivity established by expert consensus as well as performs similarly to other FDA-approved HPV tests.
One potential advantage of Onclarity is that it provides information on HPV 16, 18, and 45, individually. These three HPV genotypes account for approximately 77% of all invasive cervical cancers globally and are the first, second, and third most common individual genotypes found in invasive cervical cancers.5 In this study we found that specific HPV genotyping has a dramatic impact on the absolute risk and relative risk of having CIN 2+ and CIN 3+. For subjects who are HPV 16 positive, the absolute risk of having CIN 2+ was 29.5%, compared to 14.3% for subjects who were positive for any of the 14 pooled HPV genotypes (inclusive of HPV 16). For CIN 3+, the absolute risk for HPV 16 positive subjects was 16.1% whereas for subjects who were positive for any of the 14 pooled HPV genotypes (inclusive of HPV 16) it was only 5.1%. It is important to point out that the risk for CIN 3+ found in the current study is predominantly the risk for CIN 3 only, because no invasive cancers and only two cases of adenocarcinoma in situ were detected. The relative risk of CIN 2+ for subjects who were HPV 16 positive compared to those who are HPV positive (but not for HPV 16) was 2.7 and for CIN 3+ it was 5.9. Although HPV 18 is associated with 32% of invasive cervical adenocarcinomas and 10% of squamous cell carcinomas, as well as a large percentage of aggressive neuroendocrine carcinomas,5 in this study, of women with ASC-US cytology, the absolute risk and relative risk for CIN 2+ and CIN 3+, when HPV 18 positive, was not increased compared to being positive for the other high-risk HPV genotypes (excluding 16). Similar findings were observed in the ATHENA study.2 In another study of women with ASC-US, the 3-year cumulative risk of CIN 3+ associated with HPV 18 was similar to risk associated with HPV 31 and HPV 33/58 (pooled).21 HPV 45 is genetically similar to HPV 18 and is associated with 5% of squamous cell carcinomas and 12% of adenocarcinomas.5 In this study HPV 45 provided a risk similar to HPV 18 and the other 11 high-risk HPV genotypes. Our study was not designed to measure the risk of adenocarcinoma in women with ASC-US who have HPV 18 or 45. Nevertheless, using a CIN 3+ endpoint, our data suggest that only women with ASC-US, who are HPV 16 positive should be singled out for different management because the risk prediction for HPV 18, 45, and the other 11 HPV genotypes (pooled) are all approximately the same.
Current management guidelines recommend HPV testing as a triage for women, aged 25 years or older, with ASC-US; women who are HPV positive should be referred to colposcopy and those who are HPV negative should be rescreened in 3 years. Current management guidelines do not recommend HPV genotyping for women with ASC-US.7,8 However, because of the very high risk of cervical disease associated with HPV 16, it is widely recognized that there is clinical benefit to knowing if an HPV-positive woman has HPV 16.2 Even if no lesion is found at the time of colposcopy, women who are HPV 16-positive are at higher risk for developing cervical disease in the future.22,23 Although women who are HPV 18/45-positive have a lower risk for CIN 3 than those with HPV 16,16 their risk for invasive cervical cancer (especially adenocarcinoma) is elevated compared to women with the 11 other HPV genotypes (non-16/18/45).5,24,25 Indeed, genotyping guidelines that recommend singling out women with HPV 18, and in some cases HPV 45, when cotesting or using primary screening, are based on the risk of invasive cervical cancer associated with these two genotypes, not the risk of CIN 2 or CIN 3. Because invasive adenocarcinomas typically arise in the endocervical canal and do not arise through progression of a CIN 3 lesion,26 clinicians may want to alter how they perform colposcopy in women positive for HPV 18 or 45, opting to perform an endocervical curettage even if cervical biopsies are not done.
Eventually, as genotyping assays become available that allow the identification of more individual high-risk HPV genotypes, it is likely that risk-based management guidelines will evolve such that women with ASC-US, who have HPV genotypes associated with a low risk of CIN 3+, will not be referred to colposcopy and instead followed up at some interval. The knowledge of past genotypes also permits persistence tracking after a prior ASC-US result at the time of the follow-up testing.
Conclusion
This study contains a number of strengths. A large number of subjects with ASC-US cytology and valid HPV results underwent standardized colposcopies, which included a cervical biopsy and/or ECC if no lesions were visible by colposcopy (n = 1,953). All colposcopy/biopsy procedures were performed in a blinded manner relative to results for cytology and HPV testing (only subject age was known during biopsy review). The results from this study clinically validate the Onclarity assay for use during ASC-US triage for women undergoing cervical cancer screening. Furthermore, they establish the utility of HPV 16/18/45 detection to identify women at the highest risk for developing high-grade cervical disease or cancer in an ASC-US population for which HPV testing is indicated.
Supplementary Material
Acknowledgments
Acknowledgments: The authors thank Jeff Andrews, MD, for his thoughtful comments and critique of this manuscript; Devin S. Gary, PhD, for his input on the content of this manuscript and editorial assistance; and Stanley Chao for his statistical support. The individuals acknowledged here are BD Life Sciences employees and have no additional funding or compensation to disclose.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT01944722.
This work was supported by Becton, Dickinson and Company, BD Life Sciences-Diagnostic Systems.
Contributions of authors that are BD Life Sciences employees were: V.P. facilitated data acquisition and revision of the manuscript; K.Y., K.E., and S.K. facilitated conception and design of the study, data acquisition, and interpretation, and drafting and revision of the manuscript; C.K.C. facilitated study conception and design, and manuscript revision. All authors provided final approval of the manuscript and agree to be accountable for the accuracy and integrity of this work.
Conflict of interest statement: T.C.W. is a consultant in clinical trial design and an expert pathologist for HPV vaccine and/or diagnostic trials for BD Life Sciences, Roche, and Inovio Pharmaceuticals and a speaker for Roche and BD Life Sciences. M.H.S. is a consultant in clinical trial design and an expert pathologist for HPV vaccine and/or diagnostic trials for BD Life Sciences, Roche, Inovio Pharmaceuticals, and Merck and a speaker for Roche and BD Life Sciences.
References
- 1. College of American Pathologists. Cytopathology checklist. CAP Number 1870801. Northwestern Memorial Hospital Laboratories. Northfield, IL: College of American Pathologists; 2017. [Google Scholar]
- 2. Stoler MH, Wright TC Jr, Sharma A, et al. . High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011;135:468-475. [DOI] [PubMed] [Google Scholar]
- 3. Stoler MH, Wright TC Jr, Cuzick J, et al. . APTIMA HPV assay performance in women with atypical squamous cells of undetermined significance cytology results. Am J Obstet Gynecol. 2013;208:144.e1-144.e8. [DOI] [PubMed] [Google Scholar]
- 4. Cogliano V, Baan R, Straif K, et al. . Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. [DOI] [PubMed] [Google Scholar]
- 5. de Sanjose S, Quint WG, Alemany L, et al. ; Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. [DOI] [PubMed] [Google Scholar]
- 6. International Agency for Research on Cancer. Human Papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:255-314. [Google Scholar]
- 7. Massad LS, Einstein MH, Huh WK, et al. . 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1-S27. [DOI] [PubMed] [Google Scholar]
- 8. Practice bulletin No. 168 summary: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:923-925. [DOI] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration, Center for Devices and Radiological Health. BD Onclarity HPV Assay (P160037) approval letter, February 12, 2018. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160037a.pdf. Accessed July 12, 2018. [Google Scholar]
- 10. Ejegod DM, Junge J, Franzmann M, et al. . Clinical and analytical performance of the BD Onclarity HPV assay for detection of CIN2+ lesions on SurePath samples. Papillomavirus Res. 2016;2:31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castle PE, Gutierrez EC, Leitch SV, et al. . Evaluation of a new DNA test for detection of carcinogenic human papillomavirus. J Clin Microbiol. 2011;49:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stoler MH, Wright TC Jr, Parvu V, et al. . The Onclarity human papillomavirus trial: design, methods, and baseline results. Gynecol Oncol. 2018;149:498-505. [DOI] [PubMed] [Google Scholar]
- 13. Solomon D, Davey D, Kurman R, et al. . The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119. [DOI] [PubMed] [Google Scholar]
- 14. Kurman R, Carcangiu M, Herrington C, et al. . WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 15. Darragh TM, Colgan TJ, Cox JT, et al. . The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297. [DOI] [PubMed] [Google Scholar]
- 16. Castle PE, Cuzick J, Stoler MH, et al. . Detection of human papillomavirus 16, 18, and 45 in women with ASC-US cytology and the risk of cervical precancer: results from the CLEAR HPV study. Am J Clin Pathol. 2015;143:160-167. [DOI] [PubMed] [Google Scholar]
- 17. Einstein MH, Martens MG, Garcia FA, et al. . Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010;118:116-122. [DOI] [PubMed] [Google Scholar]
- 18. Tewari D, Novak-Weekley S, Hong C, et al. . Performance of the cobas HPV test for the triage of atypical squamous cells of undetermined significance cytology in cervical specimens collected in Surepath. Am J Clin Pathol. 2017;148:450-457. [DOI] [PubMed] [Google Scholar]
- 19. Stoler MH, Castle PE, Solomon D, et al. . The expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assays. Am J Clin Pathol. 2007;127:335-337. [DOI] [PubMed] [Google Scholar]
- 20. Stoler MH, Wright TC Jr, Sharma A, et al. ; ATHENA (Addressing the Need for Advanced HPV Diagnostics) Study Group The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. Am J Clin Pathol. 2012;137:295-303. [DOI] [PubMed] [Google Scholar]
- 21. Schiffman M, Vaughan LM, Raine-Bennett TR, et al. . A study of HPV typing for the management of HPV-positive ASC-US cervical cytologic results. Gynecol Oncol. 2015;138:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kjær SK, Frederiksen K, Munk C, et al. . Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102:1478-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiffman M, Glass AG, Wentzensen N, et al. . A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:1398-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castellsagué X, Díaz M, de Sanjosé S, et al. ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303-315. [DOI] [PubMed] [Google Scholar]
- 25. Dahlström LA, Ylitalo N, Sundström K, et al. . Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer. 2010;127:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix?Int J Cancer. 2016;139:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.