Abstract
Purpose
To compare the efficacy of minimally invasive ab-interno canaloplasty (ABiC) vs ab-externo canaloplasty (CP) in reducing intraocular pressure (IOP) and glaucoma medication dependence.
Patients and methods
This nonrandomized, retrospective, single-center, paired eye study assessed the 12-month outcomes of 12 patients with primary open-angle glaucoma who underwent ABiC in one eye and CP in the other eye, either as stand-alone procedures or combined with cataract extraction. Primary endpoints included mean IOP and number of glaucoma medications at 12 months postoperative. Secondary endpoints included surgical complications and secondary interventions.
Results
Four males and eight females with a mean age of 73.8±12.6 years were included. In the CP group, the mean preoperative IOP was 18.1±3.9 mmHg on 2.4±0.5 medications, which reduced to 13.5±2.2 mmHg (P<0.05) on 0.9±0.9 medications (P<0.001). In the ABiC group, the mean preoperative IOP was 18.5±3.4 mmHg on 2.4±0.5 medications and postoperative IOP was 13.8±2.2 mmHg (P<0.05) on 0.8±0.8 medications (P<0.05). There was no significant difference in IOP and medication use between treatment groups at 12 months postoperative. No serious adverse events were recorded in either group, though two patients in the CP group developed pressure spikes 10 mmHg beyond preoperative IOP.
Conclusion
This paired eye study found ABiC to have comparable IOP lowering and glaucoma medication reduction to CP in open-angle glaucoma. This suggests ABiC may be a suitable method for improving aqueous outflow via the trabecular pathway. Further large-scale investigation is needed.
Keywords: ab-interno canaloplasty, intraocular pressure, primary open-angle glaucoma, ab-externo canaloplasty, glaucoma medications, ABiC, MIGS, canaloplasty
Introduction
Treatment for primary open-angle glaucoma (POAG) primarily focuses on lowering intraocular pressure (IOP) and may include medical therapy, laser treatment (argon laser trabeculoplasty, selective laser trabeculoplasty, etc.) as well as more invasive surgical procedures such as trabeculectomy. Recently, minimally invasive glaucoma surgery (MIGS) has become more available and increasingly utilized because of its high safety profile, without any undesirable risks and side effects seen in more aggressive filtration procedures.1–3
There is an expanding array of MIGS options for enhancing outflow through or into the conventional outflow system, the suprachoroidal and subconjunctival space. The proximal portion conventional outflow system lends itself to manipulation to augment aqueous outflow into the distal system by either bypassing with iStent (Glaukos Corp., Laguna Hills, CA) and Hydrus (Ivantis Inc., Irvine, CA) or incising the trabecular meshwork (trabeculotomy and goniotomy). These procedures can be beneficial in that they remove a portion of or bypass the diseased trabecula seen in patients with glaucoma. However, there may be pathophysiological changes to the conventional outflow system beyond the trabecular meshwork. Specifically, in glaucoma patients, additional downstream obstruction in Schlemm’s canal and the collector channels can contribute to resistance.4,5 The impact of these pathological changes is unclear in terms of conventional outflow MIGS procedures but may account for suboptimal IOP lowering in some patients.
Both ab-externo canaloplasty (CP) and ab-interno canaloplasty (ABiC) employ an illuminated microcatheter (iTrack™, Ellex Medical Pty Ltd, Adelaide, Australia) to access, catheterize, and viscodilate the proximal and distal outflow system. Multiple mechanisms of action have been postulated with ABiC and CP. Circumferential (360°) catheterization of Schlemm’s canal with the microcatheter clears mechanical obstructions in the collector channels, providing additional conduits for aqueous outflow. Viscodilation separates the trabecular lamellae and creates microperforations within the inner wall of Schlemm’s canal, allowing for enhanced diffusion of aqueous through the proximal system into the distal system and thereby countering the pathological changes seen in glaucoma.6 Furthermore, viscodilation dilates the canal, the collector channel ostia, and the distal system.7 The main difference between the two procedures is that, unlike CP, ABiC is an ab-interno procedure avoiding scleral incisions and sparing conjunctiva.
There are a number of studies describing outcomes following CP.8–12 The procedure has been shown to lower IOP both safely and effectively.1,2 There is less published data describing outcomes following ABiC. Körber published findings from a study of 23 patients who had either standalone ABiC or ABiC combined with cataract extraction.13 These results showed reductions in medication dependence and IOP through 12 months postoperative, noting that the magnitude of these reductions was comparable to those typically observed with CP.14 To date, no studies have directly compared ABiC and CP. Consequently, in the present pilot paired-eye study, the 12-month postoperative results compare the IOP lowering and medication reduction of ABiC with those of CP using the patients’ fellow eyes for comparison.
Patients and methods
Subject selection and study design
POAG patients who previously underwent CP in one eye and ABiC in the fellow eye as stand-alone or combined procedures were identified through a systematic chart review. When performed as a stand-alone procedure, fellow eyes in each subject were also treated with a stand-alone procedure. When combined with cataract extraction (three subjects), both eyes had combined procedures. There were two subsets of patients for whom surgery was indicated, the first group were those with IOP greater than the targeted pressure while on maximally tolerated medical therapy with a goal of sufficiently reducing IOP to a range more acceptable for each individual patient. The second subset of patients was those with well-controlled glaucoma but intolerant to medications in both eyes with a treatment goal of reducing medication burden while maintaining IOP. Eyes were not randomized to receive either CP or ABiC, rather the first eye to undergo surgery was treated with CP. When the second eye required surgery, ABiC was used to reach the operative goal of IOP and/or medication burden reduction.
Inclusion criteria included POAG patients with mild-to-severe glaucoma with a predicted mean deviation of 0 to −12.00 dB and worse, no previous angle-based procedures, and presenting with an angle devoid of peripheral anterior synechiae or goniosynechiae. Patients who, in the prior year, had undergone selective laser trabeculoplasty were also excluded to ensure that the results reflected the IOP-lowering effect of ABiC in isolation. Additional exclusion criteria included a history of anatomically narrow angles or angle closure glaucoma. Chart review documenting demographics, IOP, medication list, and visual acuity were performed through to 12 months postoperative.
All procedures were performed by the same surgeon (MJG) between July 2012 and July 2015. The study was performed in accordance with the principles stated in the Declaration of Helsinki and approved by the local Institutional Review Board ([IRB] the Surgical Center of El Paso Medical Executive Committee). Patients’ consent to review their medical records was not required by the IRB as the study was retrospective in nature, and all the data included in the study were nonidentifying.
Primary endpoint was mean IOP at 12 months, and secondary endpoints were number of glaucoma medications, intraoperative and postoperative complications, and secondary interventions.
Canaloplasty
CP was performed via a standard procedure described in previous reports with some modifications.15 A single 9-0 prolene suture was used to serve as a tensioning stent within the canal and viscodilation was performed using Healon GV upon withdrawal of the microcatheter using one click every clock hour. The superficial scleral flap and conjunctiva were closed and secured using fibrin tissue glue. In addition, the eye was pressurized to ~20 mmHg at the end of the procedure to help mitigate the incidence of postoperative hyphema.
Ab-Interno canaloplasty
ABiC was performed via a side port incision ~90° away from the nasal drainage angle, typically at the 12 o’clock position. The microcatheter was then primed with Healon GV and inserted into the anterior chamber with the catheter tip guided toward the nasal angle. Through the temporal clear corneal incision, a small microgoniotomy was created in the nasal trabecular meshwork with either a 25-gauge needle or a straightened Cystotome™ (Cook Medical, Bloomington, IN, USA) under direct visualization with a gonioprism. Micro-surgical forceps were used to grasp the microcatheter at a slightly oblique angle, ~1–2 mm from its distal end. The microcatheter was then gently inserted into Schlemm’s canal via the goniotomy site and circumnavigated 360°; progress and proper microcatheter location was followed by observing the positional fiberoptic red light of the microcatheter. To prevent extension of the goniotomy, a Lester hook was employed to act as a fulcrum; this allowed all microcatheter tension to be placed on the instrument as opposed to the trabecular meshwork. Following complete circumferential intubation of Schlemm’s canal, the microcatheter was slowly withdrawn while steadily injecting two clicks of viscoelastic every clock hour. The dispersive viscoelastic used to maintain the anterior chamber during the procedure was evacuated from the anterior chamber before re-pressurizing with balanced salt solution to ~20 mmHg.
Statistical analysis
Preoperative and postoperative IOP and the number of medications for both the ABiC group and the CP group were compared. Preoperative IOP was determined by the most recent IOP prior to surgery. A between-group analysis was also performed between the ABiC group and the CP group. Because of the small sample size, nonparametric assumptions were made and the Wilcoxon signed rank test was used. Statistical significance was determined as P<0.05.
Results
The study included 24 eyes (12 CP and 12 ABiC) of 12 patients with a mean age of 73.8±12.6 years. Pre- and postoperative IOP and the number of medications for patients in both the CP and ABiC groups are summarized in Tables 1 and 2, respectively. There were no statistically significant differences in preoperative IOP (P>0.05) or medication use (P>0.05) between the CP and ABiC eyes.
Table 1.
Pre- and postoperative* IOP for each patient in the canaloplasty and ABiC groups
| Patient | Canaloplasty | ABiC | ||
|---|---|---|---|---|
| Preop IOP (mmHg) | Postop IOP (mmHg) | Preop IOP (mmHg) | Postop IOP (mmHg) | |
| 1 | 25 | 15 | 18 | 15 |
| 2 | 19 | 15 | 18 | 15 |
| 3 | 16 | 12 | 17 | 16 |
| 4 | 19 | 12 | 17 | 14 |
| 5 | 18 | 15 | 22 | 13 |
| 6 | 12 | 12 | 15 | 13 |
| 7 | 17 | 10 | 23 | 10 |
| 8 | 18 | 18 | 18 | 18 |
| 9 | 12 | 12 | 12 | 12 |
| 10 | 18 | 15 | 20 | 14 |
| 11 | 19 | 14 | 24 | 14 |
| 12 | 24 | 12 | 18 | 11 |
Note:
12 months postoperative.
Abbreviations: ABiC, ab-interno canaloplasty; IOP, intraocular pressure.
Table 2.
Pre- and postoperative* number of medications for each patient in the canaloplasty and ABiC groups
| Patient | Canaloplasty | ABiC | ||
|---|---|---|---|---|
| Preop med (n) | Postop meds (n) | Preop med (n) | Postop meds (n) | |
| 1 | 2 | 2 | 2 | 2 |
| 2 | 3 | 1 | 3 | 1 |
| 3 | 3 | 0 | 3 | 0 |
| 4 | 2 | 0 | 2 | 0 |
| 5 | 2 | 1 | 2 | 1 |
| 6 | 2 | 0 | 2 | 0 |
| 7 | 2 | 2 | 2 | 2 |
| 8 | 3 | 1 | 3 | 1 |
| 9 | 3 | 0 | 3 | 0 |
| 10 | 3 | 2 | 3 | 1 |
| 11 | 2 | 2 | 2 | 2 |
| 12 | 2 | 0 | 2 | 0 |
Note:
12 months postoperative.
Abbreviation: ABiC, ab-interno canaloplasty.
There were 10 left eyes and 2 right eyes treated with a combination of CP and ABiC. In the CP group, the preoperative IOP ranged from 12 to 25 mmHg vs 10 to 18 mmHg postoperatively. The number of medications ranged from 2 to 3 preoperatively vs 0–2 postoperatively. The mean 12-month postoperative IOP was 13.5±2.2 mmHg on 0.9±0.9 medications vs 18.1± mmHg on 2.4±0.5 medications preoperatively. Reductions in IOP and medication dependence were statistically significant (P<0.05 and P<0.001, respectively).
There were 10 right eyes and 2 left eyes treated with ABiC. The preoperative IOP ranged from 12 to 24 mmHg vs 10 to 18 mmHg postoperatively. The number of medications ranged from 2 to 3 preoperatively vs 0 to 2 postoperatively. The mean 12-month postoperative IOP was 13.8±2.2 mmHg on 0.8±0.8 medications vs 18.5±3.4 mmHg on 2.4±0.5 medications preoperatively. Reductions in IOP and medication dependence were statistically significant (P<0.05 and P<0.001, respectively).
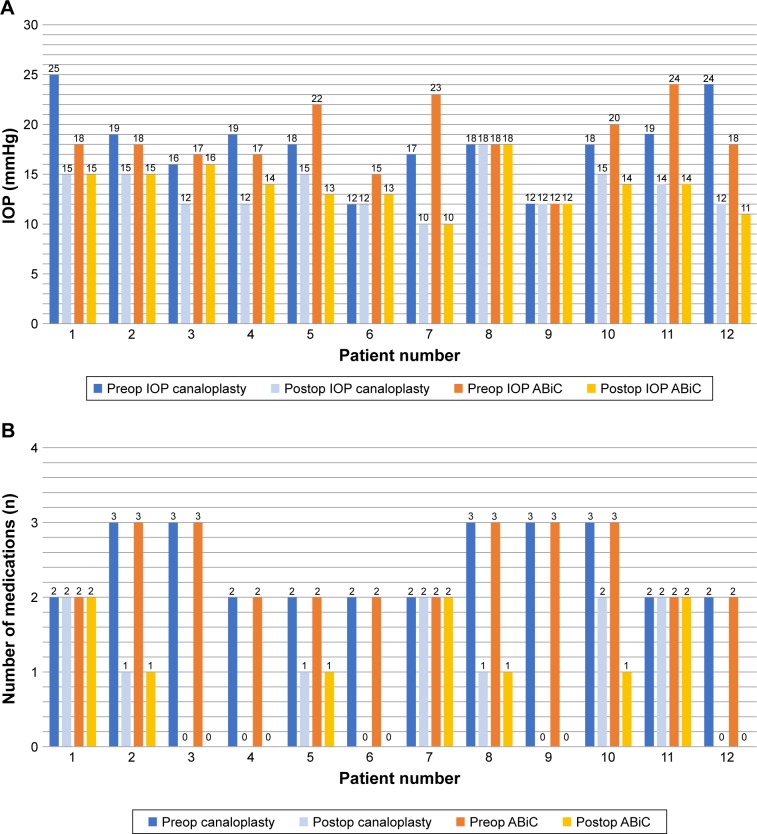
At 12 months postoperative (range: 10–14 months), five subjects in the ABiC group and five subjects in the CP group were on zero anti-glaucoma medications. A comparison of the 12-month reductions in IOP and medication use between ABiC and CP groups showed that there was no significant difference in IOP (P>0.05) and medication use (P>0.05) between treatments at 12 months postoperative. Figure 1A and B shows the pre- and postoperative IOP and medications after 12 months for both ABiC- and CP-treated eyes.
Figure 1.
(A) Comparison of IOP between canaloplasty and ABiC at 12 months postoperative and (B) comparison of number of medications between canaloplasty and ABiC at 12 months postoperative.
Abbreviations: ABic, ab-interno canaloplasty; IOP, intraocular pressure.
Microhyphema formation was seen postoperatively in both groups but resolved without sequelae within ~1 week. Two patients developed pressure spikes 10 mmHg beyond preoperative IOP in the CP group due to peripheral iris obstruction of the Descematic window. Following mechanical goniosynechiolysis and release of the iris band from the window, the patients’ pressure dropped to target IOP. One eye in the CP group also developed a long-standing, trace bleb in the area of the scleral dissection.
Discussion
Numerous studies have demonstrated CP to be efficacious with a low-risk profile.8–12 Notably, a 3-year study undertaken by Lewis et al in 157 eyes showed that CP led to a significant and sustained IOP reduction in adult patients with open-angle glaucoma.8 However, traditional CP can be technically challenging, particularly for surgeons who have never performed viscocanalostomy or nonpenetrating deep sclerectomies. Aside from the laborious dissection, one of the reported challenges associated with the tensioning suture is the need to perfect the amount of tension on the inner wall of the canal.
Irrespective of the added challenge, the role of the tensioning suture in CP remains poorly understood, with some speculating it may stretch the trabecular meshwork and inner Schlemm’s canal.6 However, imaging studies have struggled to demonstrate true correlations between suture distention and IOP reduction.16 Furthermore, subgroup analysis in Lewis et al’s study found that CP without a tensioning suture still successfully lowered IOP, demonstrating a 35.7% reduction in IOP at the 3-year follow-up.8 Together, these findings allude to viscodilation as the primary impetus for IOP reduction, theoretically suggesting ABiC may be as effective as CP. In the present study, we assessed the 12-month outcomes of 12 patients with mild-to-moderate POAG who underwent ABiC in one eye and CP in the other.
Like CP, ABiC addresses all aspects of aqueous outflow including the distal structures such as the collector channels, which have been shown to be an important site of outflow resistance.17 However, in contrast to CP, ABiC does not require conjunctival and scleral dissections or placement of a tensioning suture. Körber demonstrated patients undergoing ABiC alone or combined with cataract surgery have a comparable reduction in IOP and medication use to those who underwent traditional CP.13 In that study of 23 eyes, the group undergoing ABiC experienced a reduction in mean IOP from a baseline of 18.8±5.63 mmHg to 14.73±2.97 mmHg at 1-year follow-up. Mean medication use was reduced from 1.69 to 0.21 at the same point. An additional study from this center has similarly demonstrated a significant reduction in mean baseline IOP and the number of medications used following ABiC at 12 months.18 The results of the present study reaffirm these findings, demonstrating a similar reduction from 18.5±3.4 mmHg and 2.4±0.5 medications preoperatively to 13.8±2.2 mmHg and 0.8±0.8 medications 1 year after surgery.
In both ABiC- and CP-treated eyes, there were statistically significant reductions in IOP and medication use at 12 months postoperative, with both procedures lowering IOP and medication use in similar magnitudes. In addition, two patients in the CP group experienced pressure spikes and had to undergo further surgical procedures in order to reach the target IOP. These findings lend further evidence that ABiC may be as effective as CP, with fewer adverse events. If so, the significant procedural advantages of an ab-interno technique and theoretical reduction of risk by avoiding the need to create a trabeculo-Descemet window, which can inadvertently perforate intraoperatively, make ABiC an attractive alternative to traditional CP. In addition, the conjunctival sparing nature of ABiC offers the potential for earlier intervention, without compromising or limiting the possibilities for future glaucoma surgeries if needed. The benefits provided by ABiC add more versatility to the surgeons’ armamentarium to better adapt the treatment paradigm to individual cases.
Although this is a small pilot study, its paired eye design strengthens the comparison. The main limitations of the present study are the retrospective nature and the small sample size, which also make it difficult to identify low-frequency complications. However, there is a paucity of data on ABiC in the literature, and this is the first study to compare it to traditional CP. Thus, we feel this is an important early study of this novel technique. Future studies should aim to include a larger number of patients prospectively with a longer follow-up period. Nevertheless, these data provide further evidence that isolated viscodilation of the Schlemm’s canal can effectively and sufficiently increase outflow and alleviate IOP elevations in patients with POAG.
Conclusion
ABiC reduced IOP and dependence on anti-glaucoma medications similarly to CP in open-angle glaucoma patients. Although larger studies are needed to confirm these findings, these data suggest that ABiC can improve aqueous outflow via the trabecular pathway without scleral incision or tensioning suture.
Footnotes
Disclosure
Gallardo is a clinical investigator and speaker for Ellex. Ahmed is a consultant for Ellex. ABiC is a trademark of Ellex Deutcshland GmbH. The authors report no other conflicts of interest in this work.
References
- 1.Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of Canaloplasty versus trabeculectomy with mitomycin C in a single-surgeon series. J Glaucoma. 2013;22(7):577–583. doi: 10.1097/IJG.0b013e318255bb30. [DOI] [PubMed] [Google Scholar]
- 2.Matlach J, Dhillon C, Hain J, Schlunck G, Grehn F, Klink T. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: a prospective randomized clinical trial. Acta Ophthalmol. 2015;93(8):753–761. doi: 10.1111/aos.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borisuth NS, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10(2):112–116. doi: 10.1097/00055735-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ethier CR. The inner wall of Schlemm’s canal. Exp Eye Res. 2002;74(2):161–172. doi: 10.1006/exer.2002.1144. [DOI] [PubMed] [Google Scholar]
- 5.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Comparing two tensioning suture sizes for 360 degrees viscocanalostomy (canaloplasty): a randomised controlled trial. Eye. 2010;24(7):1220–1226. doi: 10.1038/eye.2009.317. [DOI] [PubMed] [Google Scholar]
- 7.Kearney JR, Ball SF, Field MW, Cameron BD. Circumferential viscodilation of Schlemm’s canal with a flexible microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol. 2006;12:1–9. [Google Scholar]
- 8.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: Three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):682–690. doi: 10.1016/j.jcrs.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Koerber NJ. Canaloplasty in one eye compared with viscocanalostomy in the contralateral eye in patients with bilateral open-angle glaucoma. J Glaucoma. 2012;21(2):129–134. doi: 10.1097/IJG.0b013e31820277c0. [DOI] [PubMed] [Google Scholar]
- 10.Bull H, von Wolff K, Körber N, Tetz M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1537–1545. doi: 10.1007/s00417-011-1728-3. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35(5):814–824. doi: 10.1016/j.jcrs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Brusini P. Canaloplasty in open-angle glaucoma surgery: a four-year follow-up. Scient World J. 2014;2014:1–7. doi: 10.1155/2014/469609. Article ID 469609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Körber N. Canaloplasty ab interno – a minimally invasive alternative. Klin Monbl Augenheilkd. 2017;234(8):991–995. doi: 10.1055/s-0042-123829. [DOI] [PubMed] [Google Scholar]
- 14.Cagini C, Peruzzi C, Fiore T, Spadea L, Lippera M, Lippera S. Canaloplasty: current value in the management of glaucoma. J Ophthalmol. 2016;2016:1–6. doi: 10.1155/2016/7080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voykov B, Blumenstock G, Leitritz MA, Dimopoulos S, Alnahrawy O. Treatment efficacy and safety of canaloplasty for open-angle glaucoma after 5 years. Clin Exp Ophthalmol. 2015;43(8):768–771. doi: 10.1111/ceo.12549. [DOI] [PubMed] [Google Scholar]
- 16.Brandao LM, Schotzau A, Grieshaber MC. Suture distension of schlemm’s canal in canaloplasty: an anterior segment imaging study. J Ophthalmol. 2015;7:457605. doi: 10.1155/2015/457605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagdy FM. Canaloplasty versus viscocanalostomy in primary open angle glaucoma. Electron Physician. 2017;9(1):3665–3671. doi: 10.19082/3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;12:2149–2155. doi: 10.2147/OPTH.S177597. [DOI] [PMC free article] [PubMed] [Google Scholar]