Abstract
Antibiotic allergies are reported by up to 1 in 4 cancer patients, almost 50% of which are considered low risk and precede the cancer diagnosis. We demonstrate the successful and safe implementation of a pilot oral penicillin challenge program for cancer patients with low-risk penicillin allergies, increasing the use of penicillin and narrow-spectrum beta-lactams post-testing.
Keywords: beta-lactam allergy, immunocompromised host, oral challenge, oral provocation, penicillin allergy
Patient-reported antibiotic allergies (so-called antibiotic allergy labels [AALs]) have been reported in up to 1 in 4 cancer patients and have been shown to be associated with inferior patient outcomes: increased use of restricted antimicrobials, poor guideline concordance, and higher re-admission rates [1]. In cancer patients, more than 83% of AALs can be removed via formal antibiotic allergy testing, with such “de-labeling” associated with a 10-fold increase in appropriate antibiotic prescribing [2, 3]. Unfortunately, despite the value of such programs, more than 50% of clinicians report that formal allergy testing services are either not available to them or not accessible in a timely fashion [4, 5]. There has been growing interest in the role of direct oral challenge in patients with low-risk penicillin allergies to help address the obstacles that prevent widespread access to formal skin prick testing programs. However, many of the successful published direct oral provocation papers involve multistep challenges, are restricted to pediatric patients, and exclude immunocompromised hosts [6–9]. In this study, we sought to determine the safety and efficacy of a pilot antimicrobial stewardship (AMS)–led single-dose oral penicillin challenge program in adult cancer patients with low-risk penicillin allergies.
METHODS
Patients were enrolled at 2 tertiary referral centers. Austin Health is a hospital offering specialized cancer services, including allogeneic stem cell transplantation. The Peter MacCallum Cancer Centre is a tertiary referral cancer hospital treating all solid and hematological malignancies and performing autologous stem cell transplantation. Participants were enrolled prospectively between May 31, 2017, and May 31, 2018, at both centers and identified as 2 separate groups: (1) those with an active or recently treated hematological malignancy or solid tumors (study group) and (2) those without a history of hematological or oncological malignancy (control group).
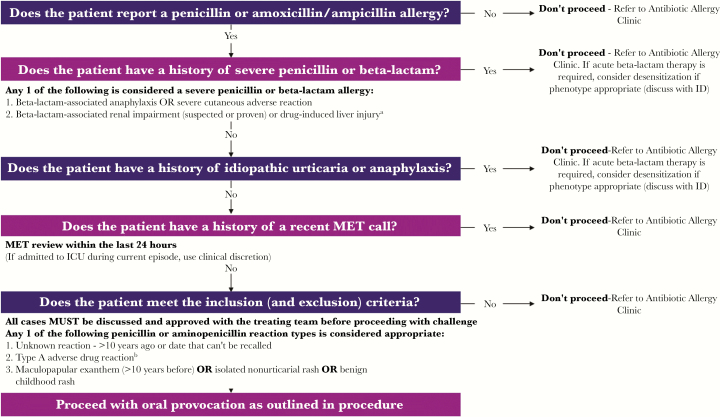
Participants were opportunistically identified by Infectious Diseases and Antimicrobial Stewardship (AMS) services at both sites (inpatients) or by the outpatient AMS-led antibiotic allergy testing service. Inpatients were identified by the Infectious Diseases consult service or the AMS pharmacist from the electronic medical record (EMR). Outpatients were those seen in the conjoint multidisciplinary onsite Antibiotic Allergy Service at Austin Health and the Peter MacCallum Cancer Centre. Identified patients were reviewed for suitability, as per the criteria outlined in Figure 1, as in- or outpatients. This service was provided by an antibiotic allergy nurse and infectious diseases physician at each site. Those reporting penicillin allergy were phenotyped utilizing the previously validated antibiotic allergy assessment tool [10]. A low-risk penicillin allergy was defined as the patient having either (1) an unknown reaction >10 years before, (2) a type A adverse drug reaction (ADR; pharmacologically predictable drug side effect or intolerance), or (3) a history of a benign childhood rash, nonurticarial rash, or maculopapular exanthem more than 10 years ago [11]. Patients with an identified low-risk penicillin allergy had this verified by an infectious diseases physician, and the oral challenge was supervised by a registered nurse working in the antibiotic allergy service.
Figure 1.
Selection algorithm for oral penicillin rechallenge program. aDrug-induced liver injury was defined as ≥5× the upper limit of normal (ULN) for alanine aminotransferase (ALT), ≥2× the ULN for alkaline phosphatase, or ≥3× the ULN for ALT with bilirubin ≥2 ULN. bA nonimmune adverse drug reaction, such as nausea or vomiting. Abbreviations: ICU, intensive care unit; ID, infectious diseases; MET, medical emergency team.
Patients were excluded if there was (1) pregnancy, (2) cognitive impairment and where a collateral history could not be obtained, (3) history of drug-associated anaphylaxis or angioedema, (4) history of severe cutaneous adverse reactions, and (5) history of acute kidney injury or severe liver impairment associated with antibiotic therapy. Inpatients identified as low risk may also have been excluded due to hemodynamic instability (Figure 1). Other data, collected using a standardized data collection tool, included patient baseline demographics, age-adjusted Charlson Comorbidity Index (CCI), cancer history (if applicable), allergy phenotype, infection history, and antibiotic usage for the 90 days before and 90 days after the oral challenge.
Based on patient primary reported allergy, and upon obtaining informed consent, all participants underwent supervised challenge with either oral penicillin VK 250 mg or amoxicillin 250 mg and were observed for the subsequent 2 hours. The choice of penicillin or amoxicillin was based upon the implicated drug. If the penicillin was “unspecified,” choice was as per the previously published protocol [2]: (1) for patients with a unspecified penicillin allergy that occurred before the advent of amoxicillin release in Australia (1972), penicillin VK challenge only was performed; (2) if penicillin allergy unspecified occurred after amoxicillin release, patient underwent sequential penicillin V then amoxicillin challenge. In patients with a documented history of delayed penicillin or amoxicillin hypersensitivity, a prolonged 5-day challenge (250 mg twice daily) with the same drug was offered. After antibiotic challenge, patient outcome was defined as (1) tolerated oral challenge with no adverse drug reaction or (2) adverse drug reaction. Patients were followed for 5 days after the oral challenge by study investigators either as an inpatient or outpatient (telephone consult), depending on the care setting.
The oral penicillin challenge program was implemented simultaneously on May 31, 2017, at both centers as a hospital clinical guideline, following approval by the local drug and therapeutics committees. Ethics approval was obtained from Austin Health (LNR/17/Austin/259) and the Peter MacCallum Cancer Centre (18/108R). Statistical analyses were performed in Stata 15.0 (StataCorp). Categorical variables were compared using chi-square tests, and continuous variables were compared with the Wilcoxon rank-sum test. A P value <.05 (2-sided) was deemed statistically significant.
RESULTS
One hundred ninety-five patients with a penicillin allergy were reviewed during the study period, 98 (50.2%) with a defined low-risk penicillin allergy. From the 98 patients, 46 patients underwent the penicillin oral challenge; 2 patients refused, and the remaining 50 met an exclusion criterion (Figure 1). Of the 46 patients, 23 (50%) had a cancer diagnosis. The penicillin allergy preceded the cancer diagnosis in all patients. The baseline demographics of the cancer and noncancer patient cohorts are presented in Table 1. Patients in the cancer cohort had a greater CCI (median, 4 vs 2; P = .008) and were more likely to be challenged in the outpatient setting when compared with those without a cancer diagnosis (10/23 vs 19/23; P = .01). Of those with a cancer diagnosis, 5 (21.7%) had a history of hematological malignancy, and 23 (78.2%) had a history of a solid tumor. All patients (23/23, 100%) reported an allergy to penicillin, and 9 (39%) reported both penicillin and cephalosporin allergies. In cancer patients, the predominant AAL phenotype was either unknown (11/23, 47.83%) or that of a childhood rash (7/23, 30.4%), with no significant difference in prevalence noted between cancer and noncancer patients (Table 1). All patients (46/46, 100%) tolerated the oral challenge without adverse drug reactions and were subsequently de-labeled.
Table 1.
Baseline Demographics, Clinical Characteristics, and Antibiotic Usage in Cancer vs Noncancer Patients Undergoing Oral Penicillin Challenge
| Patient Group | ||||
|---|---|---|---|---|
| Patient Characteristic | Cancer (n = 23) |
Noncancer (n = 23) |
Overall (n = 46) |
P Value |
| Age, median (IQR), y | 58 (44–71) | 52 (32–77) | 56 (42–74) | .80 |
| Male sex | 12 (52.17) | 9 (40.91) | 21 (46.67) | .45 |
| Age-adjusted CCI, median (IQR) | 4 (3–7) | 2 (1–4) | 3 (2–6) | .01 |
| Antibiotic allergy labels (n = 54) | 24 (44.4) | 30 (54.54) | 54 | |
| Penicillin | 23 (95.83) | 24 (80.0) | 47 (90.37) | .35 |
| Cephalosporin | 0 | 2 (6.66) | 2 (3.7) | |
| Macrolide | 1 (4.16) | 1 (3.33) | 2 (3.7) | |
| Sulfonamide | 0 | 2 (6.66) | 2 (3.7) | |
| Metronidazole | 0 | 1 (3.33) | 1 (1.85) | |
| Implicated penicillins | ||||
| Penicillin (unspecified) | 19 (82.6) | 15 (65.2) | 34 (74) | .21 |
| Amoxicillin | 4 (17.4) | 8 (34.7) | 39 (69.64) | |
| Amoxicillin clavulanate | 0 (0) | 1 (4.3) | 15 (26.79) | |
| Phenotype of implicated penicillins (n = 48) | ||||
| Childhood rash | 7 (30.43) | 5 (20.83) | 12 (25.53) | .23 |
| MPE <10 y before | 4 (17.39) | 10 (41.67) | 14 (29.79) | |
| Type A ADR | 1 (4.35) | 3 (12.5) | 4 (8.51) | |
| Unknown | 11 (47.83) | 6 (25.0) | 17 (36.17) | |
| Childhood penicillin allergy (age < 18 y) | 12 (52.17) | 9 (30.43) | 19 (41.30) | .31 |
| Avoiding penicillins | 23 (100) | 22 (95.65)a | 45 (97.83) | .31 |
| Avoiding cephalosporins | 9 (39.13) | 9 (39.13) | 18 (39.13) | 1 |
| Inpatient challenge | 10 (43.48) | 19 (82.60) | 29 (63.04) | .01 |
| Challenge antibiotics | ||||
| Penicillin single dose 250 mg | 15 (65.21) | 9 (39.13) | 24 (52.17) | 1 |
| Amoxicillin single dose 250 mg | 8 (34.78) | 16b (69.57) | 24 (52.17) | |
| Prolonged challenge (5 d) | 2 (8.69) | 7 (30.43) | 9 (39.13) | |
| Penicillin and amoxicillin challenge | 1 (4.34) | 0 | 1 (2.17) | |
| >1 antibiotic allergy label (any) | 1 (4.34) | 3 (13.04) | 4 (8.70) | .61 |
| Antibiotics administered | ||||
| 90 d pre | 13 (56.52) | 18 (78.26) | 31 (67.39) | .21 |
| 90 d post | 9 (39.13) | 17 (73.91) | 26 (60.46) | .04 |
| No. of antibiotic courses (> 1 antibiotic dose), median (IQR) | ||||
| 90 d pre (n = 51) | - | - | 1 (0–2) | .47 |
| 90 d post (n = 49) | 1 (0–2) | |||
| Adverse drug reactionsc | ||||
| 90 d post | 0 (0) | 0 (0) | 0 (0) | 1 |
| Penicillin (any) use in patients receiving antibiotics | ||||
| 90 d pre | 0 (0) | 1 (5.56) | 1 (3.2) | 1 |
| 90 d post | 7 (77.78) | 15 (88.24) | 22 (84.61) | .59 |
| Narrow-spectrum β-lactam in patients receiving antibioticsd | ||||
| 90 d pre | 0 (0) | 7 (38.89) | 7 (22.58) | .03 |
| 90 d post | 7 (77.78) | 13 (76.47) | 20 (76.92) | 1 |
| β-lactam/β-lactamase inhibitor use in patients receiving antibiotics | ||||
| 90 d pre | 0 (0) | 1 (5.56) | 1 (3.2) | 1 |
| 90 d post | 5 (55.56) | 8 (47.06) | 13 (0.5) | 1 |
| Third- or fourth-generation cephalosporin use in patients receiving antibiotics | ||||
| 90 d pre | 6 (26.08) | 10 (43.48) | 16 (34.8) | .35 |
| 90 d post | 1 (4.34) | 1 (4.34) | 2 (8.69) | 1 |
| Restricted antibiotic use in patients receiving antibioticse | ||||
| 90 d pre | 5 (38.46) | 12 (66.67) | 17 (54.88) | .15 |
| 90 d post | 4 (44.44) | 2 (11.76) | 6 (23.08) | .14 |
Unless otherwise stated, all values represent the number and proportion (%).
Abbreviations: ADR, adverse drug reaction; CCI, Charlson Comorbidity Index; IQR, interquartile range; MPE, maculopapular exanthema.
aOne patient was avoiding penicillins/aminopenicillins but had tolerated an unknown alternative penicillin.
bIncludes 1 prolonged oral challenge to amoxicillin-clavulanate.
cIncluded any reported adverse drug reaction in the medical record, non-immune- or immune-mediated.
dIncludes penicillin VK, penicillin G, flucloxacillin, amoxicillin, ampicillin, cefazolin, and cefalexin.
eIncludes fluoroquinolone, vancomycin, carbapenem, lincosamide or ≥third-generation cephalosporin
In the 90 days preceding oral challenge, 67.4% (31/46) of patients had received antibiotics compared with 60.5% (26/46) during the 90 days postchallenge (Table 1). Regarding antibiotic prescribing practices, there was a greater likelihood of administration of a penicillin-based antibiotic being prescribed in the 90 days postchallenge (22/26, 84.6%) when compared with the than 90 days prechallenge (1/31, 3.2%; P < .001). These differences remained significant when stratified for either narrow-spectrum beta-lactam (20/26, 76.9%, vs 7/31, 22.58%; P = .001) or beta-lactam/beta-lactamase inhibitor combination penicillins (13/26, 50%, vs 1/31, 3.2%; P = .0001) (Table 1). There was a noted reduction in third- and fourth-generation cephalosporin usage post-testing (2/23, 8.7%, vs 16/23, 69.6%; P = .0001) There were no adverse drug reactions reported in either cohort that received a penicillin or beta-lactam antibiotic in the 90 days after the oral challenge (Table 1).
DISCUSSION
In this study, we have demonstrated the safety and efficacy of a pilot AMS-led oral penicillin challenge program, utilizing a structured selection criterion in a cohort of cancer patients that are often excluded from rechallenge due to active disease and medical complexity. There was no difference in oral challenge outcomes in those with or without cancer, considering that they were equally balanced apart from the expected increased median CCI in those with cancer. This program serves as a future model for an active antibiotic allergy de-labeling strategy, independent of formal allergy skin testing programs, in carefully selected recent or actively treated cancer patients.
Previous authors have demonstrated the utility of oral penicillin challenge programs, in particular among pediatric patients with nonimmediate reactions. Vezir et al. assessed 119 pediatric patients and reported a 96.6% de-labeling success rate after split-dose direct oral beta-lactam provocation. In a large pediatric cohort, Mill et al. reported a success rate of 94.1% with split-dose amoxicillin challenges. In a retrospective review, Tucker and colleagues demonstrated the safety of single-dose direct amoxicillin provocation (250 mg) in adult Marines with no significant medical history, reporting only a 1.5% reaction rate [6]. The safety of a split-dose oral rechallenge was confirmed by Confino-Cohen et al. in 642 adult patients [7]. Further, Iammatteo et al. demonstrated that only 2.6% of 155 patients with non-life-threatening penicillin allergies developed a mild allergic reaction after a 2-step graded challenge to amoxicillin [12]. Although all of these studies indicated that a supervised oral penicillin challenge program was safe and efficacious, the generalizability and widespread applicability of their results were limited by (1) being allergist-led; being confined to (2) outpatients only, (3) pediatric patients, or (4) nonimmunocompromised patients; and/or (5) having a split-dose challenge schedule.
Although this AMS-led oral penicillin challenge pilot program is limited by relatively small study numbers and a short follow-up period, it demonstrates the safety and value of an AMS-led single-dose oral penicillin challenge program in carefully selected immunocompromised patients. The higher number of inpatient challenges reflects the focus of AMS and infectious diseases teams to capture high–antibiotic usage patients. A higher number of inpatient challenges was noted in controls, likely reflective of the larger patient population being at Austin Health rather than the Peter MacCallum Cancer Centre. A further limitation is potential selection bias of patients chosen for oral challenge, although exclusion was primarily based on outlined exclusion criteria. All of our cancer patients had low-risk and remote penicillin allergy labels preceding their cancer diagnosis. Structured programs to address allergy labels in cancer patients—in particular hematology patients, who have the highest antibiotic requirements—are likely to have the greatest benefit, reducing toxicity and antimicrobial resistance associated with penicillin allergy [13–15]. This approach to oral penicillin rechallenge using detailed selection criteria will prove valuable to health care facilities that do not have access to skin testing services and where the prevalence and impact of AALs is high, such as hematology units, where beta-lactam allergy prevalence is upwards of 35% [15]. The ability to identify patients with low-risk penicillin allergies will also have the potential to encourage clinicians to utilize narrow-spectrum cephalosporins in these patients, where the risk of cross-reactivity is less than 2% [16].
Future work lies in potential modifications to the criteria and procedure that enable increased utilization, such as the inclusion of patients with isolated historical urticaria and shortening the duration of observation, aiding case capture and work force implementation, respectively. Larger prospective health services studies are required to validate such low-risk selection criteria and examine the impact on antibiotic appropriateness and health care utilization costs. This will enable the wider implementation of simple point-of-care antibiotic allergy programs in cancer AMS programs.
Acknowledgments
Financial support. J.A.T. is supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (GNT 1139902) and a postgraduate scholarship from the National Centre for Infections in Cancer (NCIC), Peter MacCallum Cancer Centre (Melbourne, Australia). B.L. and M.A.S. are supported by an NHMRC Centres of Research Excellence grant (GNT 1116876). N.E.H. is supported by an NHMRC Early Careers Fellowship (GNT 1073378).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Trubiano JA, Leung VK, Chu MY, et al. . The impact of antimicrobial allergy labels on antimicrobial usage in cancer patients. Antimicrob Resist Infect Control 2015; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trubiano JA, Thursky KA, Stewardson AJ, et al. . Impact of an integrated antibiotic allergy testing program on antimicrobial stewardship: a multicenter evaluation. Clin Infect Dis 2017; 65:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourke J, Pavlos R, James I, Phillips E. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract 2015; 3:365–34.e1. [DOI] [PubMed] [Google Scholar]
- 4. Trubiano JA, Worth LJ, Urbancic K, et al. ; Australasian Society for Infectious Diseases Clinical Research Network; Australasian Society of Clinical Immunology and Allergy Return to sender: the need to re-address patient antibiotic allergy labels in Australia and New Zealand. Intern Med J 2016; 46:1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trubiano JA, Beekmann SE, Worth LJ, et al. . Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the emerging infections network. Open Forum Infect Dis 2016; 3(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017; 5:813–5. [DOI] [PubMed] [Google Scholar]
- 7. Confino-Cohen R, Rosman Y, Meir-Shafrir K, et al. . Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract 2017; 5:669–75. [DOI] [PubMed] [Google Scholar]
- 8. Caubet JC, Kaiser L, Lemaître B, et al. . The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol 2011; 127:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mill C, Primeau MN, Medoff E, et al. . Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016; 170:e160033–41. [DOI] [PubMed] [Google Scholar]
- 10.Devchand M, Urbancic KF, Khumra S, et al. Pathways to improved antibiotic allergy and antimicrobial stewardship practice: The validation of a beta-lactam antibiotic allergy assessment tool. J Allergy Clin Immunol Pract 2018 Aug 29. [DOI] [PMC free article] [PubMed]
- 11. Trubiano JA, Pai Mangalore R, Baey YW, et al. . Old but not forgotten: Antibiotic allergies in General Medicine (the AGM Study). Med J Aust 2016; 204:273–80. [DOI] [PubMed] [Google Scholar]
- 12. Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. . Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol. In press. [DOI] [PubMed] [Google Scholar]
- 13. Blumenthal KG, Lu N, Zhang Y, et al. . Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018; 361:k2400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacFadden DR, LaDelfa A, Leen J, et al. . Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 15. Huang KG, Cluzet V, Hamilton K, Fadugba O. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis 2018; 67:27–33. [DOI] [PubMed] [Google Scholar]
- 16. Romano A, Gaeta F, Arribas Poves MF, Valluzzi RL. Cross-reactivity among beta-lactams. Curr Allergy Asthma Rep 2016; 16:24–26. [DOI] [PubMed] [Google Scholar]