ABSTRACT
Experimental manipulation of the gut microbiome was found to modify emotional and cognitive behavior, neurotransmitter expression and brain function in rodents, but corresponding human data remain scarce. The present double-blind, placebo-controlled randomised study aimed at investigating the effects of 4 weeks’ probiotic administration on behavior, brain function and gut microbial composition in healthy volunteers. Forty-five healthy participants divided equally into three groups (probiotic, placebo and no intervention) underwent functional MRI (emotional decision-making and emotional recognition memory tasks). In addition, stool samples were collected to investigate the gut microbial composition. Probiotic administration for 4 weeks was associated with changes in brain activation patterns in response to emotional memory and emotional decision-making tasks, which were also accompanied by subtle shifts in gut microbiome profile. Microbiome composition mirrored self-reported behavioral measures and memory performance. This is the first study reporting a distinct influence of probiotic administration at behavioral, neural, and microbiome levels at the same time in healthy volunteers. The findings provide a basis for future investigations into the role of the gut microbiota and potential therapeutic application of probiotics.
KEYWORDS: behavior; Emotional decision; fMRI; microbiome; probiotics; recognition, memory; stool
Introduction
The gut microbiota plays an important role not only in gastrointestinal function but also in the regulation of mood, anxiety, and pain via communication with the brain, as evidenced by numerous preclinical studies.1,2 The ‘gut-brain axis‘ had already been studied before this field of research gained new momentum a decade ago with the characterization of the gut microbiome.3 Although the underlying molecular mechanisms remain elusive, the gut microbiota has been shown to modulate behavior and brain processes, including pain perception,2 stress responsiveness,4 prefrontal myelination,5 and brain biochemistry.1 Experimental manipulation of the gut microbial community composition was shown to be able to modify the host's neural function. For example, chronic ingestion of a probiotic Lactobacillus strain by BALB/c mice altered gamma amino butyric acid (GABA) expression in brain regions associated with emotional processing, and this was accompanied by reduced anxiety and depression-like behavior.1 The gut microbiota was found to influence the process of myelination in frontal brain regions, suggesting a role of gut microbiota in higher-order cognitive functions, in addition to emotional processing.5
Most of the evidence for an influence of gut microbiota on behavior is based on animal findings, with only a small number of studies6-8 to support a similar relationship in humans. However, as the psychological assessment was based solely on self-reported measures, firm conclusions regarding the effects of probiotics on human behavior cannot be drawn from these findings. Another study showed that probiotic intake for 4–6 weeks altered neural activity in brain regions that control central processing of emotion and sensation in healthy women but no change in gut microbial composition was detected.6
Recent neuroscientific research has underlined a close connection between emotion and cognition. Emotions determine how an individual perceives the world, organizes the memories and take pertinent decisions.9 Furthermore, structural studies show that the brain areas associated with emotional processing are closely connected to the brain regions responsible for memory formation and decision-making.10 Considering the evidence for an influence of gut microbiota on emotional processing and the connection between emotion, memory and decision-making, we hypothesized that manipulation of gut microbiota by probiotic ingestion can influence the brain mechanisms underlying memory processing and decision-making in an emotional context. Accordingly, we designed a double-blind study investigating the influence of a multi-strain oral probiotic supplement on objective readouts of brain function and self-reported mood and behavior in healthy volunteers (Fig. 1). This was done by functional magnetic resonance imaging (fMRI) to measure brain activation in response to decision-making and memory-based tasks, respectively. To evaluate the implications of gut microbiota in the mechanisms triggered by probiotics, we compared gut microbiome composition before and after probiotic administration.
Figure 1.
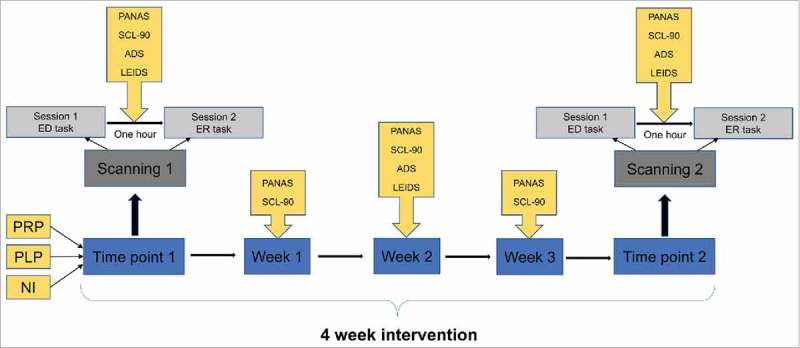
Schematic representation of multiparametric study design.
Results
The present study used a double-blind, randomized, pre- and post- intervention (4 weeks) assessment design and included three study groups: Probiotic – PRP group (which took the probiotic for 4 weeks), Placebo – PLP group (which took placebo for 4 weeks) and no intervention – NI group (no intervention) with 15 participants each. The participants were similar with respect to mean age and body mass index (Supplementary Table 1, Supplementary file 1). No gastrointestinal symptoms were reported during the study period. Results (except for microbiome analyses) are presented as differences between the pre- (baseline) and post- intervention (after 4 weeks), symbolized by Δ. There was no exclusion of fMRI data and all participants completed both the fMRI sessions (baseline and after 4 weeks). However, stools samples were collected only from PLP and PRP group and not from NI group, due to financial constraints. As some of the participants did not provide stool samples at both the time points, stool sample analysis was performed on 10 participants from PRP group and 13 from PLP group (Supplementary Table 1, Supplementary file 1).
a). Probiotics improved self-reported behavioural measures of positive affect and cognitive reactivity
Probiotic administration influenced the behavioral scores for depression and anxiety questionnaires, significantly increasing positive affect and blunting vulnerability to depression in terms of hopelessness (HOP) and risk aversion (RAV) (for reference, absolute scores are reported in Supplementary Table 2, Supplementary file 1). There was a statistically significant difference for PANAS positive scores between the three groups (ΔNI, ΔPLP, and ΔPRP) as determined by one-way ANOVA (F = 7.45, p=0.002). Post-hoc comparisons using Tukey HSD test indicated that PANAS scores were significantly higher after the 4 weeks probiotic administration as compared to placebo (p = 0.004) and no intervention (p = 0.004). Furthermore, a statistically significant difference was also observed for HOP (F = 6.33, p = 0.004) and RAV (F = 3.57, p = 0.038)) subscales of the LEIDS questionnaire between the groups (ΔNI, ΔPLP, and ΔPRP) after 4 weeks probiotic intervention as determined by one-way ANOVA. Tukey post hoc test revealed that HOP and RAV scores were significantly decreased after 4 weeks probiotic administration as compared to placebo (HOP: p = 0.004, RAV: p = 0.04) and no intervention (HOP: p = 0.004, RAV: p = 0.04). See Fig. 2a, b for details. However, we did not observe any intervention associated differences between the groups in ADS scores (F = 0.044, p = 0.95) and SCL-90 scores (F = 0.390, p = 0.68).
Figure 2.
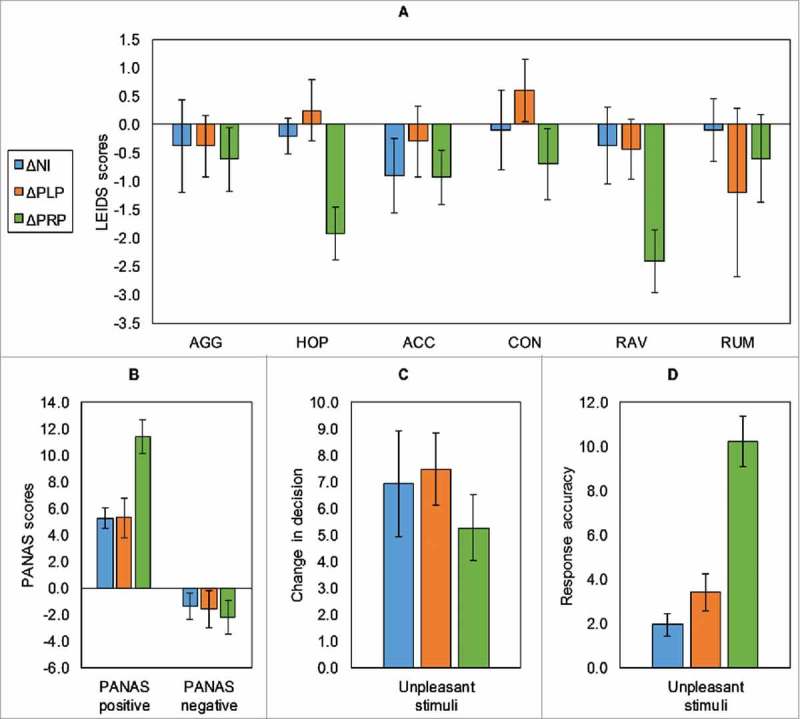
Behavioral results showing, (a) Leiden index of depression severity (LEIDS) scores, (b) PANAS scores (positive and negative), (c) decision change for the emotional decision-making task, (d) the mean response accuracy change (RAU) (for unpleasant stimuli) in the emotional recognition memory task.Error bars indicate for a, b (+/−SE) and for c, d (+/−SD). (AGG – aggression; HOP – hopelessness; ACC – acceptance; CON – control; RAV – risk aversion; RUM – ruminance; *p value ≤ 0.05).
b). Probiotics improved memory performance and altered brain activation patterns
i). Behavioral performance – fMRI tasks
In the emotional decision-making task, probiotic intervention associated changes were observed between the groups (ΔNI, ΔPLP, and ΔPRP) for the decision change for unpleasant stimuli (Fig. 2c) as demonstrated by one-way ANOVA (F = 7.79, p = 0.001). Tukey post-hoc test revealed that probiotic administration was associated with less decision change for unpleasant stimuli as compared to placebo (p = 0.002) and no intervention (p = 0.014). In the emotional recognition memory task, there was a statistically significant difference for response accuracy for unpleasant stimuli (RAU) between the groups ((ΔNI, ΔPLP, and ΔPRP; Fig. 2d) in response to probiotic intervention (F = 24.96, p < 0.001). Post-hoc comparisons demonstrated that probiotic administration significantly increased RAU as compared to placebo (p < 0.001) and no intervention (p < 0.001).
ii). Emotional decision-making task
For the emotional decision-making task, we observed intervention associated significant differences in the brain activation pattern between the three groups (ΔPRP, ΔPLP, ΔNI) in response to the neutral>baseline contrast (N>B) and unpleasant>baseline contrast (U>B). Between group comparisons (ΔPLP>ΔNI, ΔNI>ΔPLP, ΔNI>ΔPRP, ΔPRP>ΔNI, ΔPLP>ΔPRP, ΔPRP>ΔPLP) revealed the following:
N>B contrast: The contrast N>B identified the brain regions which showed enhanced activation in the ΔNI group relative to the ΔPRP group. This contrast revealed significant differences in the precuneus, the mid cingulum, the middle temporal gyrus, the inferior parietal lobule, and the paracentral lobule. In addition, there was a significant increase in brain activity in the left anterior cingulum in the ΔPRP group as compared to the ΔNI group. However, there were no significant BOLD differences between the ΔPLP and ΔNI group and also ΔPLP and ΔPRP group.
U>B contrast: The contrast U>B identified the brain regions which showed enhanced activation in the ΔPLP group relative to the ΔPRP group. This contrast revealed significant differences in the precuneus, the mid cingulum, and the parahippocampal gyrus. However, there were no significant BOLD differences between the ΔPLP and ΔNI group and also ΔPRP and ΔNI group (Supp. Table 3, Supplementary file 1; Fig. 3).
Figure 3.
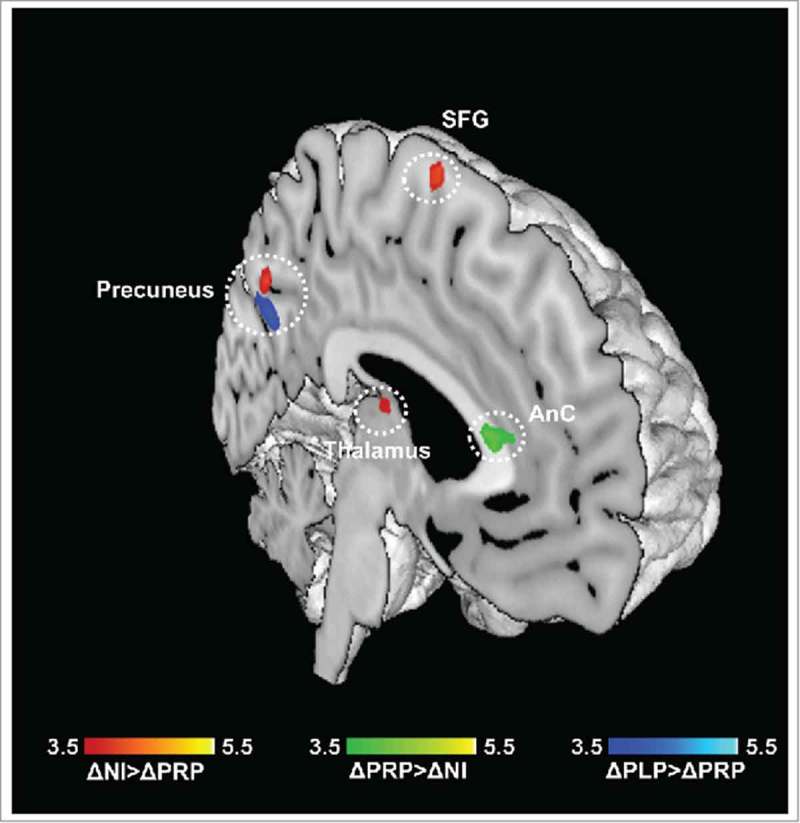
Emotional decision-making (ED) task. Regions showing differences in neural activity in the PRP group as compared to control groups, for N>B contrast (ΔPRP>ΔPLP, ΔNI >ΔPRP), and U>B contrast (ΔPLP>ΔPRP). Results are reported at p < 0.05 corrected for multiple comparisons using Alphasim corrections. (AnC: anterior cingulum; SFG: superior frontal gyrus).
iii). Emotional recognition memory task
Between group comparisons (ΔPLP>ΔNI, ΔNI>ΔPLP, ΔNI>ΔPRP, ΔPRP>ΔNI, ΔPLP>ΔPRP, ΔPRP>ΔPLP) revealed fMRI signal change differences in response to the neutral>baseline contrast (N>B) and unpleasant>baseline contrast (U>B) for the emotional recognition memory task:
N>B contrast: The contrast N>B identified the brain regions which showed enhanced activation in the ΔNI group relative to the ΔPRP group. This contrast revealed significant differences in the lingual gyrus, the calcarine gyrus, and the cerebellum (vermis). In addition, there was a significant increase in BOLD activity in the ΔPLP group relative to the ΔPRP group in the anterior cingulum. However, there were no significant BOLD differences between the ΔPLP and ΔNI group (Supp. Table 4, Supplementary file 1, Fig. 4a).
Figure 4.
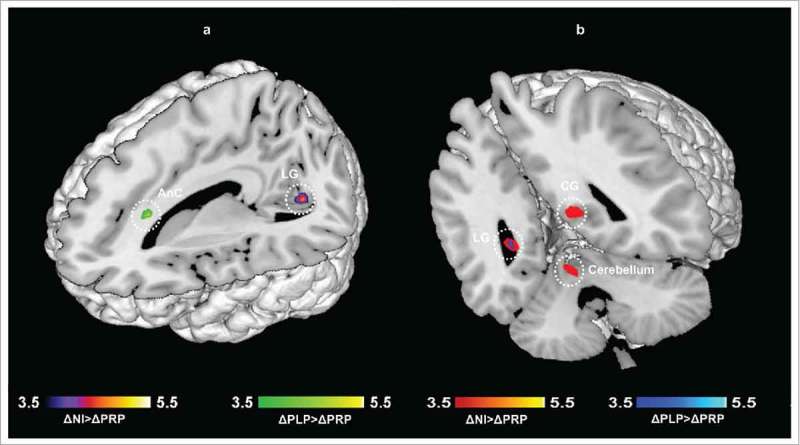
Emotional recognition memory (ER) task. Regions showing differences in neural activity in the PRP group as compared to control groups, a) N>B contrast, b) U>B contrast. Results are reported at p < 0.05 corrected for multiple comparisons using Alphasim corrections. (LG: Lingual gyrus; CG: Calcarine gyrus; AnC: anterior cingulum).
U>B contrast: The contrast U>B identified the brain regions which showed enhanced activation in the ΔNI group relative to the ΔPRP group. This contrast revealed significant differences in the lingual gyrus, the calcarine gyrus, and the anterior cingulum. In addition, there was a significant increase in BOLD activity in the ΔPLP group relative to the ΔPRP group) in the posterior cingulum. However, there were no significant BOLD differences between the ΔPLP and ΔNI group (Supp. Table 4, Supplementary file 1, Fig. 4b).
c). Self-reported behavioral measures correlated with BOLD signal change in the probiotic group
BOLD contrast estimates extracted from the cerebellum and cingulum for the ER task for each participant related to probiotic intervention were correlated significantly with their PANAS positive scores (r = -0.534, p = 0.04). No significant correlations between behavioral parameters and signal change in fMRI tasks were observed in the NI and PLP groups.
d). Probiotics administration was associated with subtle, but significant changes in gut microbial community composition
Gut microbial community composition was analyzed from baseline and 4-week stool samples collected from the probiotic and placebo groups. Supplementary details on results, including retrieved p-values and corresponding methods, are given in Supplementary file 1.
Compared to the baseline, alpha diversity analyses did not reveal any differences in stool samples from PLP and PRP group (after intervention) with respect to microbial diversity and evenness (Supplementary Fig. 1, Supplementary file 1). Neither probiotic nor placebo significantly altered the gut microbial community composition at operational taxonomic unit (OTU) or genus level, with samples grouping mostly according to subject rather than by study group (Supplementary Fig. 2, Supplementary file 1; Fig. 5a). However, LEfSe analyses identified two OTUs specifically associated ( = increased) with the probiotic group, namely Bacteroides sp. (OTU135) and Alistipes sp. (HQ763196_1_1438).
Figure 5.
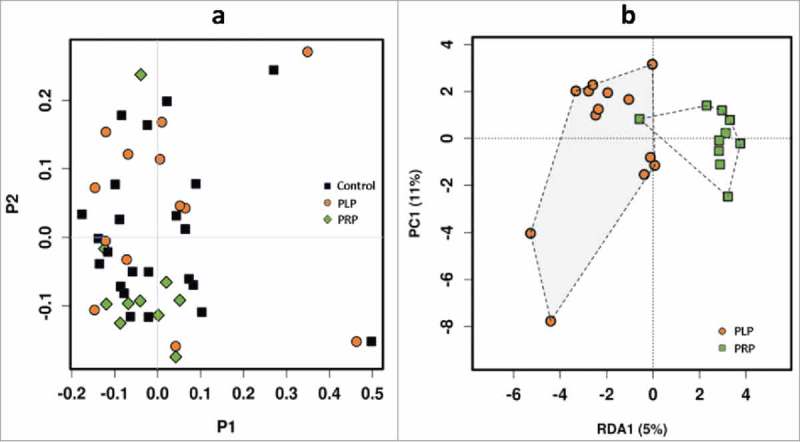
Microbiome analysis showing, (a) PCoA (Principal Coordinates Analysis) plot (to explore and visualize similarities and dissimilarities of the microbiome data of different samples) based on microbial OTUs of control group, placebo group (PLP) and product group (PRP), Bray-Curtis distance, (b) RDA (Redundancy Analysis) plot of PLP (placebo group) versus PRP (product group) after administration.
Multivariate analyses revealed a trend towards a difference between the placebo (n = 13) and probiotic (n = 10) group with regard to microbial community composition after 4 weeks of probiotics intake (Fig. 5b). LEfSe analyses identified 10 specific features for the probiotic group (Supplementary Table 5, Supplementary file 2). Although probiotic administration did not lead to a significant overall shift in microbiome composition (Fig. 5a), LEfSe analyses identified specific features for the group before probiotic administration and afterwards (full details are given in Supplementary Table 5, Supplementary file 2). Based on functional analyses (estimation based on PICRUSt and subsequent LEfSe analyses; all pathways included) the only metabolic pathway found to be associated with probiotic intake was nicotinate and nicotinamide metabolism, which was significantly lower after administration of probiotics.
e). Microbial community composition mirrors behavioral performance in questionnaires and fMRI recognition memory task
Redundancy analysis was performed in order to evaluate whether any variable significantly explains variation in the microbiome composition data file (Supplementary Table 6, Supplementary file 3). Significant correlation with microbiome data was observed for RAN (response accuracy neutral stimuli; p = 0.042), ADS (general depression scale; p = 0.039), and HOP (hopelessness; p = 0.019), with highly significant correlation for RAU (response accuracy unpleasant stimuli; p = 0.004; Supplementary Table 7, Supplementary file 4). The parameters RAU and HOP were also found significantly different for the probiotics group as compared to no intervention and placebo groups.
Regression analysis (Pearson correlation index; TOP 100 taxa) identified mostly certain OTUs affiliated with the genus Bacteroides to be significantly correlated with RAN and RAU (full details are provided in Supplementary Table 7, Supplementary file 4), indicating a potential link between specific Bacteroides species and brain memory and recognition. Functional capacities of the microbiomes were estimated using PICRUSt. Significant associations of microbial functions were found for RAU (p = 0.024, RDA), and near-significant associations for ADS (p = 0.061; Supplementary Table 8, Supplementary file 5). The strongest positive correlation of individual KEGG pathways with ADS scores was for metabolism of starch and sucrose (p = 0.000034).
Discussion
In the present study, we sought to answer the following questions: (i) Can probiotic-induced changes in self-reported measures of emotional behavior be substantiated by measurement of neural correlates in an emotional context?; (ii) Does probiotic administration influence the neural correlates of emotional decision-making and emotional memory processes?; (iii) Does it change the overall composition of gut microbiome?; (iv) Is there any relationship between gut microbial composition, behavioral scores and imaging measures?
Emotional behaviour and neural correlates in an emotional context: The marked beneficial effects observed in the HOP and RAV subscales of LEIDS in PRP group suggest an influence of probiotic intake on cognitive mechanisms associated with vulnerability to mood disorders. On a similar note, increase in PANAS positive affect indicates improvement in general well-being. Our findings are in line with previous studies showing that manipulation of gut microbiota influences mood and cognition as assessed by psychological questionnaires.7,8
Neural correlates of emotional decision-making and memory processes: Given that emotion and cognition are inseparable and probiotic administration can influence activity in brain areas associated with emotional processing,6 we specifically analysed the effect of the probiotic on cognitive abilities associated with emotional processing, namely, decision-making and recognition memory. Probiotic recipients changed their decision about the selection of the most unpleasant stimuli less frequently than the control subjects (placebo and NI groups) during the ED task, reflecting an improvement in emotional attention. In addition to activations in primary motor and visual areas, we found that the 4 week's probiotic administration was associated with a significant difference in brain activity in cingulum, precuneus, inferior parietal lobule, thalamus, and parahippocampal gyrus. The cingulum receives fibers from the thalamus and is structurally connected to the parahippocampal gyrus, reflecting its influence on emotions and memory processes. The cingulum fibers connect the sites implicated in cognitive control, including reasoning, decision-making and problem- solving.11 The anterior cingulum primarily mediates emotional processes while the middle and posterior divisions specialize in higher order cognitive processes and memory formations.12 The role of the precuneus in decision-making processes is well documented.13 The changes in neural activity in the cingulum and precuneus in ED task indicate an influence of the probiotics on decision-making processes. This influence was stronger for unpleasant, than for neutral stimuli, possibly indicating that unpleasant stimuli elicit a stronger emotional response than do neutral stimuli, leading to greater impact on decisions. Additionally, significant difference in cerebellar activity was observed in ER task in the probiotic group. The cerebellum not only plays a role in motor functions but also influences thoughts and emotions and is important for cognitive processes, especially procedural and episodic memory retrieval.14 In addition, the probiotic group showed significant improvement in response accuracy for unpleasant stimuli. This indicates an improvement of memory performance, especially in the context of emotions. The significant correlations between the self-reported behavioral measures and changes in brain activation in the cingulum and cerebellum provide further evidence for a probiotic influence on decision-making and memory processes.
Probiotic-associated changes in gut microbiota composition: Administration of probiotics did not change the diversity or evenness of the complex microbial communities associated with the healthy subjects. However, a number of OTUs were identified that were indicative for probiotics administration, including several that were affiliated with Bacteroides, which produce succinate, acetate and propionate15 and thus serve as an excellent source of short-chain fatty acids (SCFAs).15 Major local changes in microbiota composition in specific areas of the gastrointestinal tract might not be reflected in faecal samples.16 The probiotics may have changed the microbiota via indirect effects, e.g. provision of important trace nutrients (e.g. vitamin B3) or induction of beneficial strains (i.e. acetate-producing microorganisms) already present. As stool data were not available for NI group, the comparison of gut microbiota profile between no intervention and probiotic/placebo intervention still remains a question for future investigation and is thus, a limitation for the current study.
Relationship between gut microbial composition, behavioral scores and imaging measures: Significant correlations between BOLD signal change (cingulum and cerebellum), RA and behavioral measures were observed. Given the roles of cingulum and cerebellum in decision-making and emotional processing, respectively, the present findings further support our hypothesis of probiotic-associated effects on behavior which are reflected in neuroimaging measures. Overall, the microbial community composition mirrored behavioral scores and RA. In particular, RAU, ADS scores, and HOP scores were found to correlate with microbiome composition and abundance data. In addition, high scores in ADS were found to be associated with an inferred higher abundance of metabolic pathways involved in starch and sucrose metabolism, consistent with previous reports of a relationship between sugar consumption and major depression.17
Conclusions
This study provides multidimensional evidence that administration of a multi-strain probiotic and the associated change in gut microbiota composition has a significant interrelated impact on behavioral scores and functional MRI measures in distinct brain areas involved in emotional decision-making and emotional memory processes. The influence of probiotics on human brain metabolism remains an important question for future investigations. A deeper understanding of the underlying mechanisms will help to refine the clinical use of probiotic supplements in the future. The results of the current study are relevant in guiding future clinical studies to determine whether probiotic administration might have potential as an alternative or adjunctive strategy to treat depression and mood disorders. However, as this study is performed on healthy volunteers which possibly react, to the intervention, in a different way than patients, the conclusions from this study cannot be generalized and require future investigations.
Materials and methods
f). Subjects and study design
Forty-five healthy volunteers (20-40yrs) participated in this double-blind, parallel, randomised control study (for details regarding age, body mass index, number of males and females per group, please refer to supplementary Table 1, Supplementary file 1) and were divided equally into three groups: one active intervention group (Probiotic; PRP) and two control groups: Placebo (PLP) and no intervention (NI). The number of male and female participants in each group were not statistically different (X2 (2) = 0.71, p = 70). Exclusion criteria were MR incompatibility, substance abuse, use of antibiotics or probiotics, and CNS trauma/disorders. The study was approved by the local ethics committee of the University of Graz, Austria. This study was conducted in accordance with the principles of the Declaration of Helsinki and written informed consent was obtained from all participants
All participants underwent fMRI scanning at baseline (time point 1) and after 4 weeks (time point 2). Stool samples were collected for the probiotics and placebo group at both time points (and stored immediately after collection at -80°C until analysis).
g). Study product and administration
The probiotic formulation used for this study was Ecologic®825 (manufactured by Winclove Probiotics, The Netherlands, and available commercially as OmniBiotic® Stress Repair, Institut Allergosan, Austria) and was supplied in sachets (one for each day), each containing 3g freeze-dried powder. The probiotic product (7.5*106CFU/g) contained nine bacterial strains, namely, Lactobacillus casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, Lactobacillus plantarum W62 and Bifidobacterium bifidum W23. The placebo formulation, also supplied in 3 g sachets, consisted of the carrier used in the probiotic product (maize starch and maltodextrins) and was matched for color, texture, and odor to the probiotic product but contained no bacteria. Both the placebo and probiotic product sachets were indistinguishable for the study participants and the research personnel. No information about the study product was provided to the participants except that they were being administered a multi strain probiotic product. All participants were instructed to fill in a daily diary about their gastrointestinal symptoms and details about the probiotic/placebo intake. Additionally, participants were contacted via phone every week to check the study compliance.
h). Behavioral assessment
All participants were administered a set of four self-reported questionnaires, namely, PANAS (Positive and negative affect schedule), SCL-90 (Symptoms checklist-90), ADS (Allgemeine Depressionsskala), LEIDS (Leiden index of depression severity) in German to assess their mood and state of well-being before, after, and throughout the course of the study. These questionnaires were incorporated into a daily diary provided to the participants at the beginning of the study (time point 1) for four weeks. For details please refer to Supplementary file 1.
i). fMRI parameters and tasks
MRI data were acquired using a 3 Tesla whole body MRI system (Magnetom Skyra, Siemens, Erlangen, Germany) equipped with a 32 channel receive only head coil. 32 axial slices covering the whole brain using gradient echo- based interleaved EPI sequence (FOV/TE/TR/FA/slice thickness/voxel size = 256mm2/27ms/3s/90°/4mm/4 × 4 × 4mm3) was used to acquire fMRI data. A high-resolution T1-weighted 3D gradient echo (MPRAGE) sequence (FOV/TE/TR/FA/slices/slice thickness/voxel size = 224mm2/1.89ms/1.68s/192/0.88mm/0.9 × 0.9 × 0.9mm3) was acquired coplanar with the EPI scan for anatomical reference.
For this study, two fMRI tasks, emotional decision-making (ED) and emotional recognition memory (ER) were performed by all the participants (Fig. 6a, b). These tasks have been previously validated18,19 and established as measures of emotional decision-making and recognition memory assessment. Please refer to Supplementary file 1 for details on task design and data acquisition.
Figure 6.
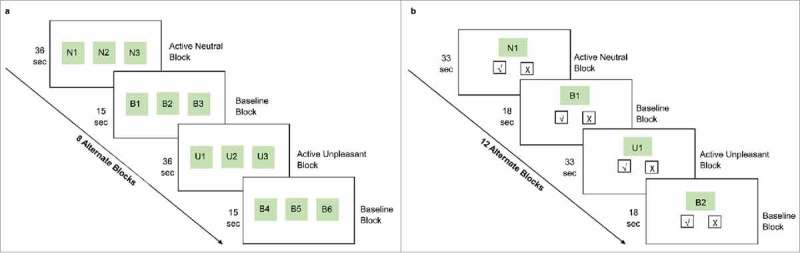
Schematic representation of the experimental fMRI tasks: (a) emotional decision-making task, (b) emotional recognition memory task.
i). Emotional decision-making (ED) task
The ED task employed a block paradigm design with eight alternating activation (A) and baseline (B) blocks (BABABABA….). Five stimuli (7s each) were presented in each activation block preceded by a cue word (1s; N for neutral block, U for unpleasant block). Stimuli were triplets of neutral or unpleasant pictures (either N or U) and the participants were instructed to select the most neutral or most unpleasant picture in the triplet, basing their decision on their personal experiences. 60 neutral and 60 unpleasant pictures were presented in the activation block. The baseline block was a matching task in which the stimuli (7s each) were triplets of geometric pictures and participants were instructed to select a picture with a fixation cross on it. Two stimuli were presented for each baseline block preceded by a cue word (1s; B for baseline block). The total time for the task was 7min 3s.
ii). Emotional recognition memory (ER) task
One hour after the ED task, an ER task was performed to assess the recognition memory for the emotional stimuli presented in the emotional decision-making task. 120 additional pictures (60 neutral and 60 unpleasant) were selected to serve as foils in the memory task. This task also employed a block design with 12 alternating active and baseline blocks. Subjects were instructed to press ‘yes’ or ‘no’ according to whether or not they recalled seeing the picture during the ED task. 10 stimuli (3s each) were presented for each activation block preceded by a cue word (3s; N for neutral block, U for unpleasant block). During the baseline block, participants were presented with geometric pictures with or without fixation cross and were required to press ‘yes’ if they saw a fixation cross and vice versa A total of 5 stimuli were presented for each baseline block preceded by a cue word (3s; B for baseline block). The total time for the task was 10min 30s.
For both the fMRI tasks, the choice of appropriate baseline was based on the factor that it should require similar processing in terms of motor response execution, visual processing, attention, and observation but without the need for decision-making. Furthermore, as both the tasks were performed in an emotional context, it was important that the baseline should not have any emotional content and thereby we chose the geometric figures for the baseline task. In addition, to match the baseline task with active tasks, we introduced the observation and response task (select the picture with fixation) however without any decision-making involved.
j). Data analysis
i). Self-reported questionnaires analysis
The statistical package for the social sciences version 23 (SPSS, Chicago, Illinois) was used for the analysis of self-reported questionnaire data. As we were particularly interested in the intervention related effects on individual level, we calculated difference scores (pre- and post- intervention) for each participant for all the questionnaires and compared the three groups (ΔPLP, ΔNI, and ΔPRP) using one-way ANOVA in SPSS. A p value < 0.05 was considered significant.
ii). fMRI analysis
Behavioral performance for the fMRI tasks was evaluated in all participants using three indices: response accuracy (RA: RAU for unpleasant stimuli, RAN for neutral stimuli), response time (RT) and decision change (see Supplementary file 1 for details). The data (difference scores: pre- and post- intervention) for these parameters was also analysed using one way ANOVA in SPSS software.
fMRI data were pre-processed using SPM12. Preprocessing included slice time correction, fieldmap distortion correction, coregistration of mean fMRI and MPRAGE images, spatial normalization using the DARTEL algorithm and spatial smoothing with an isotropic Gaussian kernel with full-width half-maximum of 6mm. A statistical parametric difference map was generated for each participant between pre- and post- intervention, for both fMRI tasks under each condition (neutral, baseline and unpleasant) by fitting the stimulation paradigm to the functional data, convolved with a hemodynamic response function. Individual first level contrast images (time point 2 – time point 1, symbolized as Δ) were generated for the neutral vs. baseline (N>B) and unpleasant vs. baseline (U>B) contrasts (FWE corrected, p < 0.05). One-sample t-tests in all groups were performed for within-group analysis. For the between- group analysis, one-way ANOVA was performed, followed by 2-sample t-tests for group comparisons (ΔPRP vs ΔPLP, ΔPRP vs ΔNI, ΔNI vs ΔPLP). The results were explored at a significance threshold of p < 0.001, uncorrected for multiple comparisons. A Monte-Carlo simulation (http://www.sciencedirect.com/topics/neuroscience/monte-carlo-method) of the brain volume was employed to establish an appropriate voxel contiguity threshold.20 This correction has the advantage of higher sensitivity, while still correcting for multiple comparisons across the whole brain volume. Assuming an individual voxel type I error of p < 0.001, a cluster extent of 17 contiguous resampled voxels was indicated as necessary to correct for multiple voxel comparisons across the whole brain at p = 0.05 (based on 10,000 simulations). Further, BOLD contrast estimates were also extracted from 8mm ROIs defined on the regions showing significant between-group differences in both fMRI tasks using the MarsBaR toolbox (http://marsbar.sourceforge.net/). These contrast estimates were used later for the correlation analysis with the behavioral data. All the statistical analysis was performed using the statistical package for the social sciences version 23 (SPSS, Chicago, Illinois). A p value<0.05 was considered statistically significant for all the analysis.
iii). Microbiome analyses
Details on DNA extraction, amplicon generation, sequencing and negative controls are given in Supplementary file 1. Raw sequences were submitted to ENA (PRJEB21748) and are publicly available. Almost 13 million raw reads were processed via QIIME following standard operating procedures21 (details in Supplementary file 1). OTU table was processed in Calypso.22 OTUs with <0.01% relative abundance across all samples were removed for analyses.
iv). Availability of materials and data
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Funding Statement
This study was supported by funding from Institut Allergosan (Graz, Austria) and Winclove (Amsterdam, Netherlands). No funding bias has been associated with this study. Funding parties did have no influence on study design, analysis, or interpretation of results.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Andreas Steinwender for technical support and Margit List-Schleich for her help throughout the study. Furthermore, we thank Katharina Gruber and Bhageswar Mohan for their help during the data acquisition and analysis. We would like to thank Julia Balfour of Northstar Medical Writing and Editing Services, Dundee, UK, for providing editorial assistance for the manuscript.
Author contributions
Conceptualization and Experimental Design: D.B., K.K., J.R., V.S.; Data Acquisition: D.B., C.A.; Microbiome analysis: C.ME., K.K.; fMRI data analysis: D.B., J.R., C.A.; Project supervision: V.S., P.H.; Drafting of the Manuscript: D.B., J.R., V.S., C. M.-E., P.H.; Review & Editing of the Manuscript: D.B., J.R., V.S., P.H., C.ME.
References
- 1.Bravo JA, Forsythe P, Chew M V., Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. PMID:21876150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral FA, Sachs D, Costa V V, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, et al.. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A. 2008;105:2193–7. doi: 10.1073/pnas.0711891105. PMID:18268332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. PMID:16741115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–78. doi: 10.1016/j.psyneuen.2012.03.007. PMID:22483040. [DOI] [PubMed] [Google Scholar]
- 5.Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. PMID:27045844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al.. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–401. doi: 10.1053/j.gastro.2013.02.043. PMID:23474283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2:256–61. doi: 10.4161/gmic.2.4.16108. PMID:21983070. [DOI] [PubMed] [Google Scholar]
- 8.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. doi: 10.1016/j.bbi.2015.04.003. PMID:25862297. [DOI] [PubMed] [Google Scholar]
- 9.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–58. doi: 10.1038/nrn2317. PMID:18209732. [DOI] [PubMed] [Google Scholar]
- 10.Kier EL, Staib LH, Davis LM, Bronen RA. MR Imaging of the Temporal Stem: Anatomic Dissection Tractography of the Uncinate Fasciculus, Inferior Occipitofrontal Fasciculus, and Meyer's Loop of the Optic Radiation. Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 11.Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O'Sullivan MJ. Cingulum Microstructure Predicts Cognitive Control in Older Age and Mild Cognitive Impairment. J Neurosci. 2012;32:17612–19. doi: 10.1523/JNEUROSCI.3299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delano-Wood L, Stricker NH, Sorg SF, Nation DA, Jak AJ, Woods SP, Libon DJ, Delis DC, Frank LR, Bondi MW. Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. J Alzheimers Dis. 2012;29:589–603. PMID:22466061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrigan B, Adlam ALR, Langdon PE. The neural correlates of moral decision-making: A systematic review and meta-analysis of moral evaluations and response decision judgements. Brain Cogn. 2016;108:88–97. doi: 10.1016/j.bandc.2016.07.007. PMID:27566002. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen NC, O'Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, Ponto LL, Hichwa RD. The cerebellum plays a role in conscious episodic memory retrieval. Hum Brain Mapp. 1999;8:226–34. doi: 10.1002/(SICI)1097-0193(1999)8:4%3c226::AID-HBM6%3e3.0.CO;2-4. PMID:10619416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Liu C, Finegold SM, Song Y, Liu C, Finegold SM. Bacteroides. In: Bergey's Manual of Systematics of Archaea and Bacteria. Chichester, UK: John Wiley & Sons, Ltd; 2015. page 1–24. [Google Scholar]
- 16.Gerritsen J, Smidt H, Rijkers GT, de Vos WM, Lapin A, Haslberger A, Parameswaran P, Crowell M, Wing R, Rittmann B, et al.. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–40. doi: 10.1007/s12263-011-0229-7. PMID:21617937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangwisch JE, Hale L, Garcia L, Malaspina D, Opler MG, Payne ME, Rossom RC, Lane D. High glycemic index diet as a risk factor for depression: analyses from the Women's Health Initiative. Am J Clin Nutr. 2015;102:454–63. doi: 10.3945/ajcn.114.103846. PMID:26109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabert MH, Borod JC, Tang CY, Lange G, Wei TC, Johnson R, Nusbaum AO, Buchsbaum MS. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia. 2001;39:556–73. doi: 10.1016/S0028-3932(00)00157-3. PMID:11257281. [DOI] [PubMed] [Google Scholar]
- 19.Shirao N, Okamoto Y, Mantani T, Okamoto Y, Yamawaki S. Gender differences in brain activity generated by unpleasant word stimuli concerning body image: An fMRI study. Br J Psychiatry. 2005;186. doi: 10.1192/bjp.186.1.48. PMID:15630123. [DOI] [PubMed] [Google Scholar]
- 20.Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/S0926-6410(03)00082-X. PMID:12763194. [DOI] [PubMed] [Google Scholar]
- 21.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al.. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. PMID:20383131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion M-J, Berger B, Krause L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics. 2017;33:782–3. PMID:28025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


