ABSTRACT
Spermidine is a natural polyamine that stimulates cytoprotective macroautophagy/autophagy. External supplementation of spermidine extends lifespan and health span across species, including in yeast, nematodes, flies and mice. In humans, spermidine levels decline with aging, and a possible connection between reduced endogenous spermidine concentrations and age-related deterioration has been suggested. Recent epidemiological data support this notion, showing that an increased uptake of this polyamine with spermidine-rich food diminishes overall mortality associated with cardiovascular diseases and cancer. Here, we discuss nutritional and other possible routes to counteract the age-mediated decline of spermidine levels.
KEYWORDS: Autophagy, cancer, cardiovascular diseases, health span extension, longevity
Spermidine is a natural polyamine present in all living organisms that is critically involved in the maintenance of cellular homeostasis. This chemical affects numerous biological processes, including cell growth and proliferation, tissue regeneration, DNA and RNA stabilization, enzymatic modulation, and regulation of translation, among others [1–3]. Furthermore, spermidine exhibits anti-inflammatory and antioxidant properties, enhances mitochondrial metabolic function and respiration, promotes chaperone activity and improves proteostasis [4]. Intriguingly, external supplementation of spermidine exerts various beneficial effects on aging and age-related disease in a variety of model organisms, including mice [5,6]. For example, spermidine feeding extends lifespan across species [5,7,8], promotes cardio- [5] and neuroprotection [9–11], stimulates antineoplastic immune response [12] and may avoid immunosenescence by stimulating memory T-cell formation [13,14]. Many of these anti-aging properties have been causally linked to the capacity of spermidine to ensure proteostasis through the stimulation of cytoprotective macroautophagy [15–17]. Age-associated conditions including cancer, neurodegeneration and cardiovascular diseases are directly connected to the intracellular accumulation of toxic debris, and its removal by autophagy constitutes a well-documented avenue for protection against age and disease [18–20].
Spermidine induces autophagy through the inhibition of several acetyltransferases [7], including EP300 [21], one of the main negative regulators of autophagy [22]. Its potency has been recently quantified to be equivalent to that of rapamycin [23], an FDA-approved immunosuppressant with protective and autophagy-stimulatory properties [24,25]. Importantly, the genetic impairment of autophagy abrogates the beneficial effects of spermidine on longevity of yeast, flies and worms [7]. Moreover, in mice, the cardioprotective and immunosurveillance-associated anticancer effects of spermidine are lost when autophagy is inhibited in myocardial or cancer cells, respectively [5,12].
In general, polyamine levels in individual organisms are highly diverse. Nevertheless, one commonality is that tissue concentrations of spermidine decline in an age-dependent manner in both model organisms and humans [7,9,26,27]. This may account for decreased autophagy and drive the onset of age-associated diseases. A remarkable exception to this decline in spermidine levels are healthy nonagenarians and centenarians, who retain whole-blood concentrations reminiscent of younger (middle-aged) individuals [26]. The optimal concentration of spermidine in humans to maintain optimal autophagy levels for healthy aging, however, still needs further investigation. The age-induced spermidine decline must involve the alteration of one or several of the distinct factors that determine systemic availability of spermidine (Figure 1). In principle, spermidine bioavailability is determined by the 3 different sources of this polyamine: (i) cellular biosynthesis, (ii) production by intestinal microorganisms and (iii) nutritional supply, as well as (iv) catabolism and (v) urinary excretion.
Figure 1.
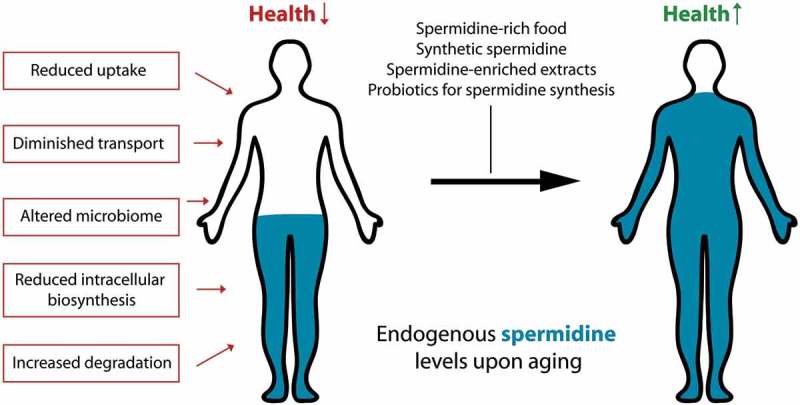
Possible routes to counteract the age-induced decline of spermidine levels. With old age, endogenous spermidine concentrations diminish due to alterations in one or several factors that determine the bioavailability of the substance in the body. This detrimental decline may be counteracted by ingesting polyamine-rich food items, polyamine-enriched plant extracts, synthetic spermidine, or by stimulating polyamine synthesis in the gut microbiome through supplementation of prebiotics or probiotics.
Within mammalian cells, spermidine is generated from its precursor putrescine (which itself is generated from ornithine) or by oxidative degradation of spermine [2,3,28]. The net cytosolic spermidine content further depends on its catabolism, which is mainly driven by acetylation and subsequent oxidation [29–31], but also on its uptake from the extracellular space and its excretion by the cell. This may be mediated by membrane transporters such as the ones operating in yeast and bacteria [32], but could also involve endocytosis/exocytosis processes. Still, polyamine transport in mammals remains not well understood and needs further examination.
The intestinal microbiota represents another source of spermidine synthesis within our body. In mice, the concentration of spermidine in the gut lumen has been shown to directly depend on the colonic microbiota [33]. Thus, commensal gut bacteria may regulate polyamine concentration in the human intestine [34]. In fact, preclinical studies support the critical involvement of the gut microbiome in generating health-relevant spermidine. Therefore, blood levels of spermidine and spermine can be upregulated through oral administration of the polyamine-producing probiotic Bifidobacterium LKM512, resulting in suppressed inflammation and improved longevity in old mice [35]. Interestingly, these effects can be enhanced, if combined with additional supplementation of arginine, a polyamine precursor [36]. Thus, arginine may be considered as a prebiotic for stimulating intestinal spermidine synthesis. However, other prebiotic strategies may consist of providing agents that favor the selective expansion of polyamine-producing bacteria or the upregulation of spermidine synthesis by the existing gut microbiota.
Dietary spermidine is rapidly resorbed from the intestine and distributed in the body without degradation [37]. Thus, food items with high spermidine content can contribute to raising the bioavailability of this polyamine. Such spermidine-rich food items comprise unprocessed plant-derived foods including the durian fruit, shitake mushrooms, fresh green pepper, wheat germ, amaranth grain, cauliflower and broccoli, just to mention a few, but also products resulting from fermentation processes that involve polyamine-generating bacteria and fungi, e.g. soybean products such as natto or many types of mature cheese [38]. Therefore, the systemic levels of spermidine are influenced by diet either directly, by the ingestion of polyamine-rich food items, or indirectly, by effects of the diet on the spermidine-producing microbiota.
The use of a spermidine-rich diet to elevate systemic spermidine levels in human organs [39] may constitute a promising strategy for promoting healthy aging. Spermidine shows no adverse effects during life-long administration in mice [5], and a currently ongoing clinical trial on supplementation of spermidine-rich plant extracts to elderly persons indicates good safety and tolerability [40]. Recently, for the first time, an epidemiological study has reported a positive association between nutritional spermidine uptake and human health span and lifespan [38]. A total of 829 participants aged 45–84 were observed over a time frame of 15 years, during which 341 became deceased. The spermidine uptake of each participant was calculated based on food-frequency questionnaires every 5 years. Individuals with high total nutritional spermidine uptake displayed a reduced incidence of cancer and cardiovascular diseases, correlating with an overall improved survival. This was even the case after correction for possibly confounding factors such as age, sex, body mass index, alcohol and aspirin consumption, dietary quality, metabolic diseases, physical activity, and socioeconomic status.
Importantly, the association between high dietary spermidine uptake and reduced mortality has been recapitulated in a second, independent cohort of 1770 healthy participants aged 39–67 with a medium follow-up of 13 years [38]. However, one limitation of the aforementioned epidemiological studies resides in the absence of laboratory assessments of spermidine concentrations. It will be important to perform similar observational studies while correlating the plasma spermidine levels of each individual patient with dietary patterns (and other lifestyle factors), ideally in a longitudinal fashion. It would be particularly interesting to correlate these measurements with the quantification of autophagic flux and protein acetylation levels, which can be determined in peripheral blood mononuclear cells [41], hoping to establish an epidemiological triangulation between spermidine, autophagy and health.
The age-protective effects of increased spermidine intake are in line with the paradigm that a decline of spermidine concentration upon aging is not only causally linked to reduced health- and lifespan, but that it might be reversed. Given the factors that determine spermidine levels in the body (Figure 1), what would be the most feasible avenue to reverse this age-dependent decline? Despite its documented low toxicity in mice and humans [5,39], an ‘overdose’ of spermidine might, at least theoretically, compromise cellular homeostasis if supraphysiological concentrations are reached. Instead, it may be a more feasible strategy to restore the levels of spermidine that are declining in the elderly to physiological levels reminiscent of young(er) individuals. This may be achieved by a polyamine-rich diet, as demonstrated by Kiechl et al. [38], with food extracts rich in spermidine, supplementation of synthetic spermidine, or prebiotics and probiotics that drive microbial polyamine synthesis in the intestine.
In sum, in our view, spermidine is synthesized by our organism in sufficient quantities during youth, but not in old age. Thus, one may argue that, as we age, spermidine evolves to the status of a vitamin, and thus has to be supplemented from external sources to secure the maintenance of autophagic flux required for organismal homeostasis.
Funding Statement
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). F.M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20, P29262, P24381, P29203 P27893, I1000, “SFB Lipotox” (F3012), and DKplus Metabolic and Cardiovascular Diseases (W1226), as well as to Bundesministerium für Wissenschaft, Forschung und Wirtschaft and the Karl-Franzens University for grants “Unkonventionelle Forschung”. We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge.”.
Conflict of interest
GK, FM and DC-G are scientific co-founders of Samsara Therapeutics. FM and DC-G have equity interest in The Longevity Labs. GK is a co-founder of everImmune.
Disclosure statement
GK, FM and DC-G are scientific co-founders of Samsara Therapeutics. FM and DC-G have equity interest in The Longevity Labs. GK is a co-founder of everImmune.
References
- [1].Bardócz S, Duguid TJ, Brown DS, et al. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. [DOI] [PubMed] [Google Scholar]
- [2].Igarashi K, Kashiwagi K.. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. [DOI] [PubMed] [Google Scholar]
- [3].Pegg AE. Functions of Polyamines in Mammals. J Biol Chem. 2016;291:14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science. 2018;359(6374):eaan2788. [DOI] [PubMed] [Google Scholar]
- [5].Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eisenberg T, Büttner S, Kroemer G, et al. The mitochondrial pathway in yeast apoptosis. Apoptosis Int J Program Cell Death. 2007;12:1011–1023. [DOI] [PubMed] [Google Scholar]
- [7].Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. [DOI] [PubMed] [Google Scholar]
- [8].Yue F, Li W, Zou J, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res. 2017;77:2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gupta VK, Scheunemann L, Eisenberg T, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. [DOI] [PubMed] [Google Scholar]
- [10].Wang I-F, Guo B-S, Liu Y-C, et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci. 2012;109:15024–15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang Q, Zheng C, Cao J, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23:1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pietrocola F, Pol J, Vacchelli E, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Puleston DJ, Simon AK. New roles for autophagy and spermidine in T cells. Microb Cell. 2015;2:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Puleston DJ, Zhang H, Powell TJ, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. eLife. 2014;3:e03706 https://elifesciences.org/articles/03706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. [DOI] [PubMed] [Google Scholar]
- [16].Carmona-Gutierrez D, Hughes AL, Madeo F, et al. The crucial impact of lysosomes in aging and longevity. Ageing Res Rev. 2016;32:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frake RA, Ricketts T, Menzies FM, et al. Autophagy and neurodegeneration. J Clin Invest. 2015;125:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. [DOI] [PubMed] [Google Scholar]
- [20].Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pietrocola F, Lachkar S, Enot DP, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mariño G, Pietrocola F, Eisenberg T, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014;53:710–725. [DOI] [PubMed] [Google Scholar]
- [23].Du Toit A, De Wet S, Hofmeyr J-HS, et al. The precision control of autophagic flux and vesicle dynamics—a micropattern approach. Cells. 2018;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pucciarelli S, Moreschini B, Micozzi D, et al. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. [DOI] [PubMed] [Google Scholar]
- [27].Scalabrino G, Ferioli ME. Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26:149–164. [DOI] [PubMed] [Google Scholar]
- [28].Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kee K, Foster BA, Merali S, et al. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–40083. [DOI] [PubMed] [Google Scholar]
- [30].Jell J, Merali S, Hensen ML, et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem. 2007;282:8404–8413. [DOI] [PubMed] [Google Scholar]
- [31].Hai Y, Shinsky SA, Porter NJ, et al. Histone deacetylase 10 structure and molecular function as a polyamine deacetylase. Nat Commun. 2017;8:15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nowotarski SL, Woster PM, Casero RA. Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev Mol Med. 2013;15:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Matsumoto M, Kibe R, Ooga T, et al. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep. 2012;2:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Matsumoto M, Benno Y. The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol. 2007;51:25–35. [DOI] [PubMed] [Google Scholar]
- [35].Matsumoto M, Kurihara S, Kibe R, et al. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PloS One. 2011;6:e23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kibe R, Kurihara S, Sakai Y, et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4:4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol. 2001;13:1021–1025. [DOI] [PubMed] [Google Scholar]
- [38].Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018. [DOI] [PubMed] [Google Scholar]
- [39].Soda K, Kano Y, Sakuragi M, et al. Long-term oral polyamine intake increases blood polyamine concentrations. J Nutr Sci Vitaminol (Tokyo). 2009;55:361–366. [DOI] [PubMed] [Google Scholar]
- [40].Schwarz C, Stekovic S, Wirth M, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging. 2018;10:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pietrocola F, Demont Y, Castoldi F, et al. Metabolic effects of fasting on human and mouse blood in vivo. Autophagy. 2017;13:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]


