ABSTRACT
As the use of fecal microbiota transplantation (FMT) has gained momentum, an increasing need for continuous access to healthy feces donors has developed. Blood donors constitute a healthy subset of the general population and may serve as an appropriate group for recruitment. In this study, we investigated the suitability of blood donors as feces donors. In a prospective cohort study, we recruited blood donors onsite at a public Danish blood bank. Following their consent, the blood donors underwent a stepwise screening process: First, blood donors completed an electronic pre-screening questionnaire to rule out predisposing risk factors. Second, eligible blood donors had blood and fecal samples examined. Of 155 blood donors asked to participate, 137 (88%) completed the electronic pre-screening questionnaire, 16 declined, and 2 were excluded. Of the 137 donors who completed the questionnaire, 79 (58%) were excluded mainly due to having an allergy, being overweight, or presenting gastrointestinal complaints. Among the remaining 58 (37%) donors, complete blood and feces screenings were obtained from 46 (79%). Of these 46 donors, 15 (33%) were excluded primarily due to abnormal blood results or the presence of apathogenic intestinal parasites. Overall, 31 (20%; 95% confidence interval 14–27%) of the 155 blood donors qualified as feces donors. In conclusion, blood donors constitute a suitable and motivated population for a continuous recruitment of voluntary feces donors. We found that a stepwise recruitment procedure was feasible and that 20% of the blood donors were eligible for feces donation.
KEYWORDS: blood donors, donor selection, feces donor recruitment, Feces donor epidemiology, feces donor screening, fecal microbiota transplantation
Introduction
Fecal microbiota transplantation (FMT) has emerged as a therapeutic option to treat diseases linked to a disturbed or depleted intestinal microbiome. Currently, FMT is a recommended and highly efficacious treatment for recurrent Clostridium difficile infections (RCDI),1,2 and promising results from previous studies indicate that FMT may have beneficial effects in diseases such as ulcerative colitis (UC), irritable bowel syndrome (IBS), and systemic infections with antibiotic-resistant bacteria (ARB).3-6
An essential component of a functional FMT service is the access to a continuous supply of healthy donor feces. In this regard, both a strict donor selection and an efficient donor recruitment practice are both central components,7 but practical aspects challenge their implementation.8 First, a screening program that minimizes the risk of transferring infectious and non-infectious diseases must be implemented. Detailed screening protocols have been published,7,9-11 but the work required for their implementation is extensive; moreover, despite a scientific consensus for this vigorous screening,12,13 the evidence supporting the screening protocols remains low. Second, as FMT gains momentum, an inherent need for recruiting enough potential feces donors evolves. However, current methods for recruiting new donors lack reproducibility.14
Few previous studies addressed how to perform a standardized donor recruitment in clinical practice.14,15 In studies that advertised study recruitment to the local community, only 6–10% of the volunteers were able to meet all screening criteria.7,14,15 This makes sustaining FMT a time-consuming process and calls for methods to identify eligible donors with a high probability of passing the screening tests. Blood donors represent an ideal, healthy subset of the general population because donating blood requires them to meet strict health and lifestyle criteria that are similar to those applied to feces donors.16,17
In this study, we report our experience using a standardized protocol for recruiting feces donors among a population of voluntary and non-remunerated blood donors. We further relate the willingness and the screening outcome to the potential of blood donors to serve as feces donors.
Results
Study population
A systematic recruitment of feces donors among a blood donor population was carried out from March to December 2016. A total of 155 blood donors were asked to participate in the study (Fig. 1). Sixteen (10%, 95% CI: 6%–16%) of the 155 blood donors declined participation, and two (1% (0.2–5.0%)) were disqualified because of blood donation-related complications or early unrelated discontinuation of the blood donation.
Figure 1.
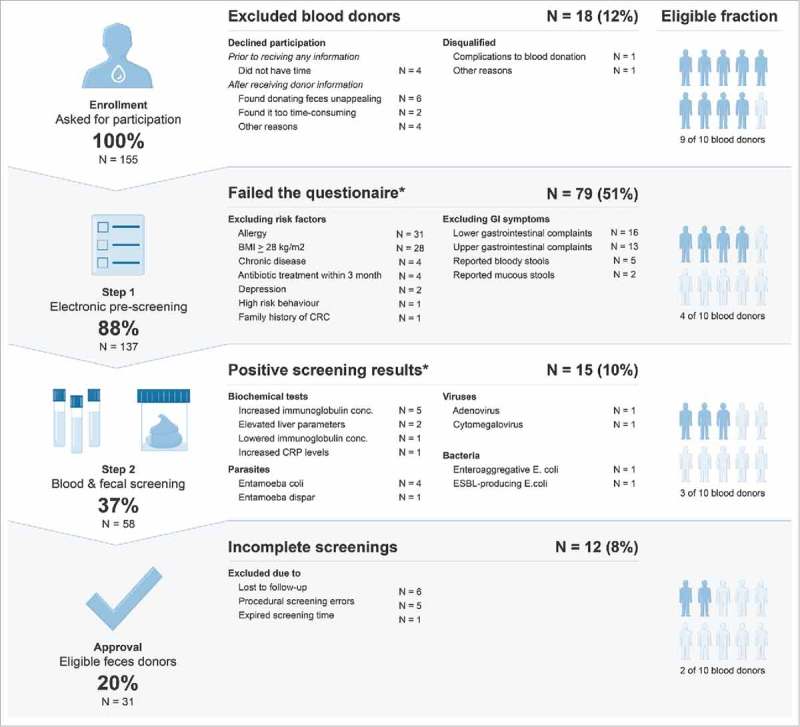
Overall flow of blood donors approached to become feces donors, and outcomes. *Multiple excluding occurrences within the reported group. Abbreviations: BMI: Body mass index, CRC: Colorectal cancer, GI: Gastrointestinal, CRP: C-Reactive protein, ESBL: Extended-spectrum beta-lactamase.
Outcome of the pre-screening questionnaire and blood donor characteristics
Among the 155 blood donors asked to participate in the study, 137 (88% (82%–93%)) consented and were enrolled in the electronic pre-screening. Of these 137 blood donors, 79 (58% (49%–66%)) were excluded mainly due to either having an allergy, a high body mass index (BMI), or excluding GI symptoms (Fig. 1). Five donors reported having had bloody stools within the past 4 weeks in the GI symptoms assessment, and two had clinical signs of depression. Two had high-risk behavior or a family history of predisposing risk factors. Overall, 22 (28% (18%–39%)) of the 79 excluded blood donors had more than one reason for exclusion.
Baseline characteristics of the donors are presented in Table 1. Of the 137 blood donors who consented, 98 (72% (63%–79%)) were male and 39 (28% (21%–37%)) were female, all with a median age of 40 years (interquartile range (IQR): 32–48 years). The median BMI was 25.46 kg/m2 (IQR: 23.7–27.7 kg/m2). Most blood donors reported moderate to high physical activity levels and food frequency scores at 6 (IQR: 5–8), reflecting healthy dietary habits. The blood donors consumed alcohol regularly and 12 (9% (5%–15%)) smoked.
Table 1.
Baseline characteristics of 137 healthy blood donors enrolled in screening as potential feces donors and stratified by gender.
| All | Men | Women | ||
|---|---|---|---|---|
| (n = 137) | (n = 98) | (n = 39) | P-value | |
| Number of participants | 137 (100%) | 98 (72%) | 39 (28%) | |
| Age | 40 (32; 48) | 42 (29; 50) | 39 (33; 47) | 0.62 |
| BMI kg/m2 | 25.46 (23.7; 27.7) | 25.72 (23.8; 28.7) | 25.46 (23.7; 27.7) | 0.42 |
| Smoking status | ||||
| Non-smoker | 125 (91%) | 34 (87.2%) | 91 (92.9%) | 0.29 |
| Current smoker | 12 (9%) | 5 (13%) | 7 (7%) | |
| Pack-years per year | 0.4 (0.18; 0.63) | 0.4 (0.2; 0.5) | 0.4 (0.15; 0.75) | 0.57 |
| Alcohol intake | ||||
| Yes | 118 (86%) | 31 (79%) | 87 (89%) | 0.16 |
| No | 19 (14%) | 8 (21%) | 11 (11%) | |
| Units per week | 3.5 (2; 7) | 2 (1;4) | 4 (2; 8) | 0.003 |
| Food frequency score | 6 (5; 8) | 6 (4; 8) | 6 (5; 8) | 0.53 |
| IPAQ | ||||
| Low | 21 (15%) | 7 (18%) | 14 (14%) | 0.80 |
| Medium | 58 (42%) | 17 (44%) | 41 (42%) | |
| High | 58 (42%) | 15 (38%) | 43 (44%) |
Numbers with percentages or medians with interquartile ranges are presented. P-values measure the differences between women and men.
Abbreviations: IPAQ: International Physical Activity Questionnaire.
Measured as exposure within the last year at the current smoking status.
One missing value in the reported alcohol intake and two incomplete responses in the food frequency questionnaire.
Outcome of blood and fecal screenings
In total, 58 (37% (30%–46%)) of the 155 donors had blood drawn and received a fecal screening kit for further screening. Of these, 52 (90% (79%–96%)) delivered fecal samples, which were tested within a median time of 9 days (IQR 7–9 days). Fifteen (26% (15%–39%)) donors were excluded because of a positive screening result in the blood or microbial screenings (Fig. 1). Donors were most frequently excluded due to either the presence of apathogenic parasites (four had Entamoeba coli, and one had Entamoeba dispar) or biochemical tests outside of the reference range. Two had an enteroaggregative or ESBL-producing strain of Escherichia coli, one had active adenovirus replication, and one had serological results consistent with acute cytomegalovirus (CMV) infection. Five (9% (3%–19%)) donors were rejected due to errors in the procedural handling that resulted in an incomplete screening. Six (10% (4%–21%)) donors did not ship the fecal sample and were considered lost to follow-up.
Overall, 31 (20% (14%–27%)) participants passed all the screenings to qualify as feces donors. Their characteristics are presented in Table 2.
Table 2.
Baseline characteristics of 31 approved and 106 healthy blood donors after the screening.
| Approved feces donors(n = 31) | Rejected feces donors(n = 106) | P-value | |
|---|---|---|---|
| Sex | |||
| Women | 7 (23%) | 32 (30%) | 0.41 |
| Men | 24 (77%) | 74 (70%) | |
| Age | 39 (28; 51) | 40.5 (33; 47) | 0.87 |
| BMI kg/m2 | 24.39 (22.30; 26.29) | 25.78 (23.84; 28.73) | 0.004 |
| Smoking status | |||
| Non-smoker | 26 (84%) | 99 (93%) | 0.099 |
| Current smoker | 5 (16%) | 7 (7%) | |
| Pack-years per year | 0.5 (0.25; 0.75) | 0.4 (0.15; 0.5) | 0.46 |
| Alcohol intake | |||
| No | 4 (13%) | 15 (14%) | |
| Yes | 27 (87%) | 91 (86%) | 0.86 |
| Units per week | 4.5 (2; 10) | 3 (2; 6) | 0.22 |
| Food frequency score | 6 (5; 8) | 6 (5; 7.5) | 0.97 |
| IPAQ physical index | |||
| Low | 5 (16%) | 16 (15%) | 0.90 |
| Medium | 12 (39%) | 46 (43%) | |
| High | 14 (45%) | 44 (42%) |
Numbers with percentages or medians with interquartile ranges are presented. P-values measure the differences between the two groups. Abbreviations: IPAQ: International Physical Activity Questionnaire
Measured as exposure within the last year at the current smoking status.
One missing value in the reported alcohol intake and two incomplete responses in the food frequency questionnaire.
Approved versus rejected donors
Comparison of the baseline characteristics between rejected and enrolled feces donors showed no statistically significant differences, except for BMI, which was explained by a BMI above 28 kg/m2 being an exclusion criterion (Table 2). Of the 31 approved donors, 24 (77% (59%–90%)) were male and 7 (23% (10%–41%)) were female. The median age of the qualified donors was 39 years, while the median age of rejected donors was 41 years (p = 0.87). We observed a statistically non-significant trend (p = 0.099) that blood donors who smoked had an increased likelihood of qualifying as feces donors.
Blood donors declining participation
Among the 155 blood donors asked for participation, 16 blood donors declined participation. Four (3% (0.7%–6%)) declined participation prior to receiving any information about the study. The most common reason for declining was that the blood donors found donating feces unappealing. When grouped by gender (Table 3), women (p = 0.07) tended to decline participation more frequently due to finding donating feces unappealing (p = 0.07).
Table 3.
Overall proportion of possible blood donors declining participation in the feces donor program and their reasons for declining stratified by gender.
| Declined participation (n = 16/155) |
||||
|---|---|---|---|---|
| All | Men | Women | ||
| (16/155) | (8/108) | (8/47) | P-value | |
| No. of declining participants | 16 (10%) | 8 (7%) | 8 (17%) | 0.0706 |
| Declined before receiving information | ||||
| Due to lack of time | 4 (3%) | 2 (2%) | 2 (4%) | 0.585 |
| Declined after receiving information | ||||
| Found donating feces unappealing | 6 (4%) | 2 (2%) | 4 (9%) | 0.065 |
| Other reasons (Not related to feces) | 6 (4%) | 4 (4%) | 2 (4%) | 1 |
Numbers with percentages. All values are derived from the total number of eligible candidates. P-values are derived using the chi-square test when the sample size n is > 5 and using Fisher's exact test when the smallest sample count n is < 5.
Gender-specific proportions were derived from the total number of eligible men (n = 108) or women (n = 47).
Measured according to the total number of participants who received information about the study, n = 152.
Discussion
In this study, we consecutively recruited feces donors among a cohort of blood donors and found that 20% of all blood donors asked to participate passed all fecal screening tests. We found that a stepwise screening approach was feasible and that utilizing the blood bank infrastructure provided a reproducible recruitment basis to meet the continuous need of an FMT service for qualified feces donors.
Our finding of a 20% eligibility rate is high compared with the overall 6–10% rates reported by other studies.7,14,15,18 Still, the recruitment of eligible feces donors remains challenging. Although blood donors represent a healthy subset of the general population, more than half of all included blood donors failed the initial pre-screening. The high exclusion rate in our study is similar to that reported by others,14,15 but unlike the previous studies, we excluded donors that presented any forms of allergy and lowered the upper BMI limit to 28 kg/m2 to further reduce the risk of potential risk factors.19 Correspondingly, including these parameters markedly increased our pre-screening exclusion rate.14,15
The high overall eligibility rate is mainly attributed to fewer donors failing the fecal screenings.14,15 While differences in the recruitment processes between studies limits the comparability of the separate steps, the recruitment steps themselves may be an important factor in increasing the number of donors who pass the later screening steps. The electronic pre-screening in our stepwise screening process rapidly ensured that the donors fulfilled all inclusion criteria and were preliminarily assessed for any influential gastrointestinal symptoms before the blood and fecal screenings were conducted. In doing so, we found an unexpectedly high proportion of donors reporting gastrointestinal symptoms that led to exclusion. By excluding these donors using the questionnaires, we may have decreased the number of donors who would have failed the subsequent screenings. By applying this stepwise approach, we increased the overall cost-effectiveness of the donor recruitment system.
Unlike the other donor recruitment studies,14,15 we actively recruited all our donors by addressing them in person. This method enabled us to assess both a more general perception of donating feces and, importantly, the characteristics of donors who declined participation. To our knowledge, this study is the first to describe persons who declined participation. Almost all blood donors consented to becoming feces donors despite knowing the potential practical implications of becoming long-term feces donors. This high willingness to participate contrasts with a previous report.15 The difference may be partly explained by the highly altruistic nature of blood donors.20 Among the few who declined participation, however, we found that female blood donors tended to be more likely to decline because they found donating feces unappealing. Large-scale recruitment efforts are still needed to determine the exact perception among the declining donors; however, if this tendency to decline among women holds true, then future donor might need to recognize this tendency in the program design.
Blood donors constitute a highly selective population associated with certain characteristics. Men give more blood donations than women because male donors are more likely to meet the minimum hemoglobin and weight criteria to donate blood.16,21 Blood donors are also less likely to be vegans or smokers, but they eat meat and consume alcohol regularly.16,21 Accordingly, blood donors may therefore be expected to have an increased rate of exclusion due to high body weight. The characteristics of included donors in our study did not differ from those reported in the large-scale blood donor cohort studies, except that our study had an increased male ratio. Almost two-thirds of all our participants were male, and this high ratio may in part be due to male donors being booked more frequently due to the above mentioned criteria to donate.21 Besides an expected lower BMI, we did not find that the screening procedures favored certain baseline characteristics between the rejected and qualified donors.
Attention should be given to the required resources for managing the 80% who are excluded during each step of the screening process. Specifically, 36 blood donors reported gastrointestinal symptoms that required a medical evaluation. An FMT service should include medical expertise to help sort out incidental findings that may or may not have clinical relevance to the particular blood donor. We found counseling the rejected blood donors to be time-consuming and challenging.
The evidence supporting the specific parts of the donor screening program is still limited. Currently, the screening guidelines focus on minimizing the potential risk of harm to the recipient. Importantly, we identified blood donors who were colonized with resistant bacterial species including ESBL-producing E. coli. We therefore suggest that tests for these microorganisms are included in future FMT screening programs. We also found that donors who tested positive for intestinal pathogens harbored a variety of different asymptomatic viruses, bacteria, and parasites. Our most common finding in the fecal screening was Entamoeba coli, and despite its apathogenic nature, donors who tested positive were excluded as a precaution. Notably, controversy exists to whether certain protozoa such as Blastocystis species may be more prevalent in healthy individuals and linked to certain beneficial health indices.22,23 For now, however, our data are consistent with the findings of diverse pathogens reported by others14,15 and support the current broad-spectrum screening approach for pathogens.
Most of our donors were excluded due to the presence of risk factors that are potentially transmittable. The causality underlying this still needs to be determined, and to date, only a few case reports have linked potential donor-dependent hazards to an adverse FMT outcome. In two isolated incidences, pronounced weight gain and the development of IBS-like symptoms following FMTs were reported.24,25 In an extensive case series, 31 patients had received feces from a donor who later developed Crohn's disease.26 Fortunately, none of the patients developed clinical sequelae nor microbial signatures of inflammatory bowel disease (IBD).26 Until causality and long-term effects of risk factors can be determined, the reasonable possibility of transfer warrants a cautious and rigorous donor screening with complete traceability of the donor-recipient route.
Standardizing the donor selection refines our future ability to evaluate different donors and the donor-recipient interaction. Systematically increasing the donor availability may also permit us to address how gut-microbiota-shaping factors, such as diet, lifestyle, and living environment, affect the clinical outcome and safety of FMT.27
The study has limitations. First, it is a single-center experience with a limited number of participants to detect potential associations between the specific parameters of the screening program. Associations between certain characteristics and the screening outcome may therefore be plausible but not apparent. Second, a high proportion (10%, 5/51) of the donors were excluded during the blood and fecal screening process because of procedural errors. While this exclusion can be attributed to the novelty of establishing and integrating new procedures, it may underestimate the eligibility rate and distort the screening outcome. Finally, the general lack of uniformity in the design of the screening process between donor recruitment studies weakens the comparability to other recruitment experiences. While no studies have included microbiome analysis of the donor feces, this may prove crucial to the donor-recipient interaction in future studies.
In conclusion, we found that blood donors constitute a suitable, accessible, and motivated population for feces donor recruitment. The recruitment of feces donors among blood donors is associated with high donor eligibility and may serve as an efficient way to ensure a continuous flow of voluntary and unpaid feces donors. Challenges range from counseling blood donors who fail the screening tests, to adjusting the screening program, and to tailoring the donor material to the clinical situations where it is being used.
Methods
Study design and donors
This study was a single-center, prospective, observational cohort study. We consecutively recruited feces donors among a blood donor population to establish a cohort of healthy feces donors that could continuously support the needs of an FMT service.19 The study was conducted at Aarhus University Hospital, Denmark, through a collaboration between the Department of Hepatology and Gastroenterology and the Central Danish Blood Centre at the Department of Clinical Immunology.
All blood donors were recruited onsite at the blood bank. Eligible blood donors were required to be 25–60 years of age and to have passed the initial blood donor screening tests required to donate blood. All eligible blood donors were identified from a daily list of the booked donors and were chosen consecutively to reduce the risk of selection bias. The booking list included the personal identification number, name, gender, and age. If a selected donor failed the blood donor screening on the day of donation or had previously been asked for participation, the next eligible donor was chosen instead.
The blood donors were approached in person and asked to participate during the time from the actual collection of blood to the subsequent resting period. Before entering the study, the blood donors were given oral and written information about FMT, the donation program, and the initial screenings to become a feces donor. All donors who declined participation were systematically interviewed for the reason for dismissal.
Once they consented, all willing blood donors were enrolled in a two-step screening process. First, they completed a self-administered, electronic pre-screening questionnaire to rapidly determine their suitability according to the screening protocols. In the second step, blood donors who met the criteria covered in the questionnaire then completed the remaining blood and fecal screenings. Blood was drawn onsite at the blood bank, and donors were provided with a fecal sampling kit (Easy Sampler®, GP Medical Devices, Denmark) and instructions to perform the fecal screening. The candidates were asked to collect a fecal sample at their next defecation at home and to ship the sample by mail immediately afterwards. The fecal samples were received and tested in accordance with a third-party agreement at a local Department of Microbiology.
To compensate for the time delay in delivering the fecal specimens, all test results had to be available no later than 30 days after inclusion for the screening to be valid. The donor was contacted by telephone after all blood and fecal screening results were assessed and made available. Donors who met all screening criteria and thus qualified as feces donors were booked for a medical consultation to formally enter the actual donation program for active feces donors.
Donor screening
Previous protocols7,9-11 have established standards for the content of feces donor screening. We compiled these screening standards and adapted the content of our screening program accordingly (Fig. 2). As a precautionary measure, we chose to lower the upper BMI limit to 28 kg/m2 and exclude donors with any allergies. In terms of donor management, we extended the screening investigations to comply with the European Tissue and Cell Directive (EUTCD) regulatory standards.19 To fit the content of the donor screening process into a standardized recruitment practice, the donor screenings were organized into three parts: 1) an electronic questionnaire, 2) blood screening, and 3) fecal screening.
Figure 2.
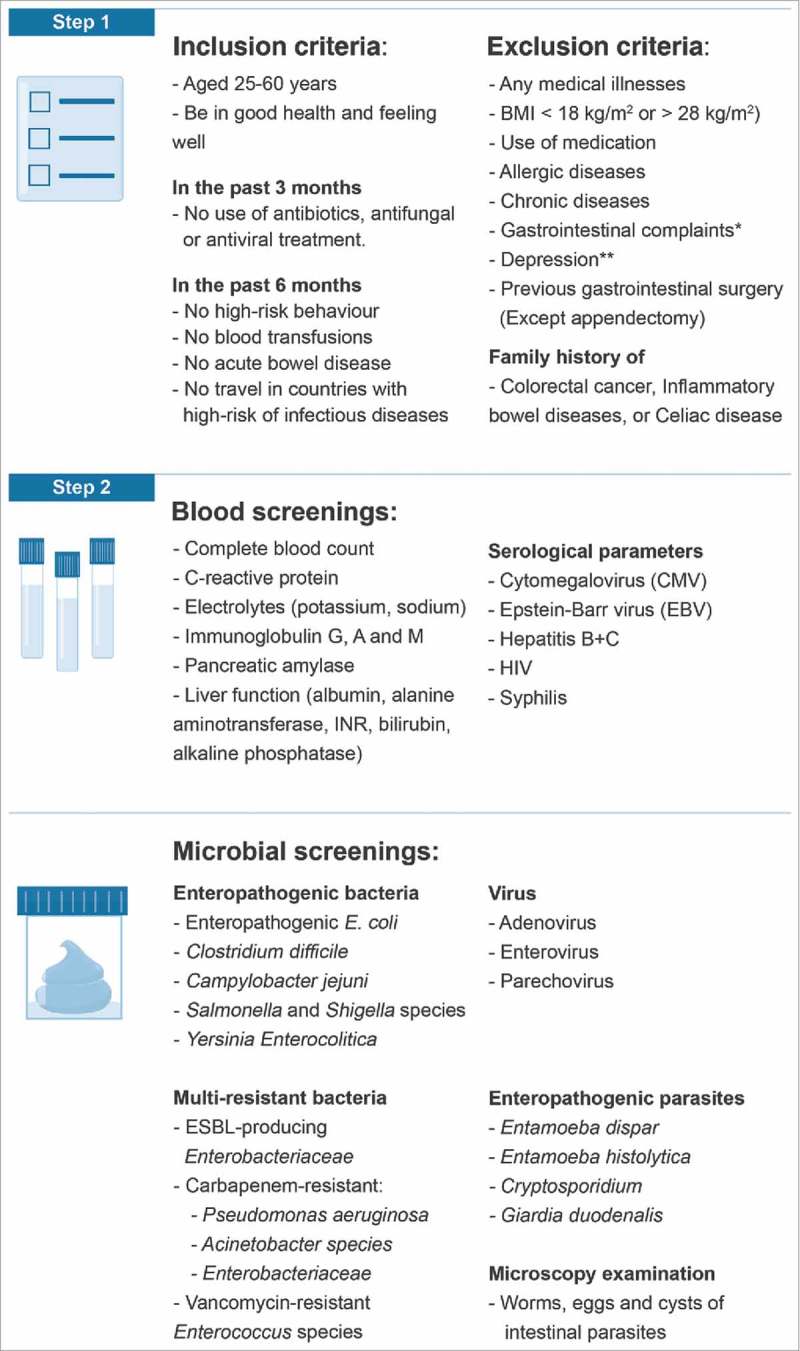
Feces donor screening requirements covered in the stepwise screening process. *Supplementary Fig. 1 **Addressed by Beck Depression Inventory Abbreviations: BMI Body mass index, GI: Gastrointestinal, HIV: Human immunodeficiency virus, ESBL: Extended-spectrum beta-lactamase.
The electronic questionnaire was designed to ascertain the inclusion criteria and to identify any exclusion criteria dictated by the screening protocols. (Fig. 2) To address a preliminary gastrointestinal medical assessment, we added an adapted version of a symptom questionnaire originally designed to systematically address gastrointestinal symptoms in patients referred for Helicobacter pylori breath tests.28 (See supplementary file 1) To evaluate the mental state of the donors, we included the Beck Depression Inventory.29
To characterize the lifestyle habits of potential donors, we added questions about smoking and alcohol use as well as two questionnaires about dietary practices and physical activity. Dietary practices were assessed using a short 8-item single-answer questionnaire validated for type 2 diabetic populations that scored the dietary practices from mostly healthy (0) to mostly unhealthy (16).30 Physical activity was addressed using the short version of the International Physical Activity Questionnaire (IPAQ),31 which groups people into three activity categories (low, moderate or high) based on their reported energy expenditure.
The blood screening included biochemical, hematologic and serological testing to verify the health of donors based on a variety of parameters and to rule out the presence of titers to any transmittable diseases or excluding health traits (Fig. 2).7,9-11
The fecal screening included assessing the presence of commonly known enteropathogenic bacteria, viruses and parasites (Fig. 2).7,9-11 In addition, we screened for the presence of multi-resistant bacteria including extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, vancomycin-resistant Enterococcus (VRE) species, carbapenem-resistant Pseudomonas aeruginosa, Acinetobacter and Enterobacteriaceae.
Statistical analysis
Statistical analysis was performed on the outcomes from the donor recruitment. When applicable, the outcomes and characteristics were stratified by gender and tested for difference. Numerical, non-parametric data were evaluated using Wilcoxon or Mann-Whitney tests. Continuous data are presented as medians with interquartile ranges (IQR), and categorical data are presented as percentages with exact 95% confidence intervals (CI) when reporting the overall study outcomes. Differences were evaluated using either a chi-square test or Fisher's exact test. Data missing from the baseline characteristics were excluded from the overall descriptive analysis, and incomplete screening results were treated as screening failures. Two-tailed p-values below 0.05 were considered statistically significant. All statistical analyses were performed using Stata/MP 13.0 for Windows (StataCorp LP, College Station, Texas, USA).
Ethics statement
Oral and written informed consent were obtained from all approached participants. The study was approved by the Scientific Ethical Committee of Central Denmark (2015–49826). Furthermore, the donor research database was approved by the Danish Data Protection Agency (2012-58-0006).
Supplementary Material
STROBE statement—checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | Reported on page | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract. | 1 |
| (b) Provide, in the abstract, an informative and balanced summary of what was done and what was found. | 3 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported. | 4–5 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses. | 4–5 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper. | 11 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection. | 11–12 |
| Participants | 6 | (a) Provide the eligibility criteria and the sources and methods of case and control selection. Provide the rationale for the choice of cases and controls. | 12–14 + Fig. 2 |
| (b) For matched studies, provide matching criteria and the number of controls per case. | N/A | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. If applicable, provide diagnostic criteria. | 12–14 |
| Data sources/ measurements | 8 | For each variable of interest, provide sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods, if there is more than one group. | 12–14 + Fig. 2 |
| Bias | 9 | Describe any efforts to address potential sources of bias. | 12 |
| Study size | 10 | Explain how the study size was established. | N/A |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. | 14 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding factors. | 14 |
| (b) Describe any methods used to examine subgroups and interactions. | 14 | ||
| (c) Explain how missing data were addressed. | 14 | ||
| (d) If applicable, explain case and control matching was addressed. | N/A | ||
| (e) Describe any sensitivity analyses. | N/A | ||
| Results | |||
| Participants | 13 | (a) Report numbers of individuals at each stage of study, e.g., numbers of individuals who were potentially eligible, examined for eligibility, confirmed as eligible, included in the study, and analyzed and who had completed follow-up. | 5–7 + Fig. 1 |
| (b) Provide reasons for non-participation at each stage. | 5–7 + Fig. 1 | ||
| (c) Consider use of a flow diagram. | Fig. 1 | ||
| Descriptive data | 14 | (a) Provide characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders. | 5 + Table 1 |
| (b) Indicate the number of participants with missing data for each variable of interest. | 5–6 + Fig. 1 | ||
| (c) Summarize follow-up time (e.g., average and total amount) | 5–6 | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time | 5–6 + Fig. 1 + Tables 2–3 |
| Main results | 16 | (a) Provide unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Clarify which confounders were adjusted and why they were included. | 5–6 |
| (b) Report category boundaries when continuous variables were categorized. | 6 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period. | N/A | ||
| Other analyses | 17 | Report other completed analyses, e.g., analyses of subgroups and interactions and sensitivity analyses. | 6–7 |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives. | 7 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias. | 10–11 |
| Interpretations | 20 | Provide a cautious overall interpretation of results by considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence. | 10–11 |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results. | 9 and 11 |
| Other information | |||
| Funding | 22 | Provide the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based. | 1 |
Give information separately for exposed and unexposed groups.
Note: An Explanation and Elaboration article discusses each checklist item and provides methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the following websites: PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Funding Statement
The work was supported by an independent grant from the Danish Regions Medical Funds [j.no. 14/217], which aims to ensure a more precise use of medicine that benefits the patients and the economy.
Abbreviations
- ARB
Antibiotic-resistant bacteria
- BMI
Body mass index
- CRC
Colorectal cancer
- CI
Confidence interval
- CMV
Cytomegalovirus
- CRP
C-reactive protein
- EBV
Epstein-Barr virus
- ESBL
Extended-spectrum beta-lactamase
- EUTCD
European Tissue and Cell Directive
- FMT
Fecal microbiota transplantation
- GI
Gastrointestinal
- HIV
Human immunodeficiency virus
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- IPAQ
International Physical Activity Questionnaire
- IQR
Interquartile ranges
- METS
Metabolic equivalent of task
- UC
Ulcerative colitis
- RCDI
Recurrent Clostridium difficile infection
- VRE
Vancomycin-resistant Enterococcus
Disclosure of potential conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
The authors are grateful to all the blood donors who participated in the study and to the staff at the Blood Bank at Aarhus University Hospital.
References
- 1.Debast SB. Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1-26. doi: 10.1111/1469-0691.12418. PMID:24118601. [DOI] [PubMed] [Google Scholar]
- 2.van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34-39. doi: 10.1097/MOG.0000000000000024. PMID:24241245. [DOI] [PubMed] [Google Scholar]
- 3.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al.. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-9. doi: 10.1136/gutjnl-2015-309990. PMID:26338727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 2016;22:2219-41. doi: 10.3748/wjg.v22.i7.2219. PMID:26900286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RW, Connor S, Ng W, Paramsothy R, et al.. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-28. doi: 10.1016/S0140-6736(17)30182-4. PMID:28214091. [DOI] [PubMed] [Google Scholar]
- 6.Bilinski J, Grzesiowski P, Sorensen N, Madry K, Muszynski J, Robak K, Wroblewska M, Dzieciatkowski T, Dulny G, Dwilewicz-Trojaczek J, et al.. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Infect Dis. 2017;65:364-70. doi: 10.1093/cid/cix252. PMID:28369341. [DOI] [PubMed] [Google Scholar]
- 7.Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant service for the treatment of clostridium difficile infection. Clin Infect Dis. 2016;62:908-14. doi: 10.1093/cid/civ994. PMID:26628567. [DOI] [PubMed] [Google Scholar]
- 8.Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. 2017;8:225-37. doi: 10.1080/19490976.2017.1286006. PMID:28129018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, et al.. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-9. doi: 10.1016/j.cgh.2011.08.014. PMID:21871249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tauxe WM, Dhere T, Ward A, Racsa LD, Varkey JB, Kraft CS. Fecal microbiota transplant protocol for clostridium difficile infection. Lab Med. 2015;46:e19-23. doi: 10.1309/LMCI95M0TWPDZKOD. PMID:25805532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al.. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-15. doi: 10.1056/NEJMoa1205037. PMID:23323867. [DOI] [PubMed] [Google Scholar]
- 12.Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, et al.. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-80. doi: 10.1136/gutjnl-2016-313017. PMID:28087657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konig J, Siebenhaar A, Hogenauer C, Arkkila P, Nieuwdorp M, Noren T, Ponsioen CY, Rosien U, Rossen NG, Satokari R, et al.. Consensus report: faecal microbiota transfer – clinical applications and procedures. Aliment Pharmacol Ther. 2017;45:222-39. doi: 10.1111/apt.13868. PMID:27891639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns LJ, Dubois N, Smith MB, Mendolia GM, Burgess J, Edelstein C, Noh A, Alm E, Kassam Z. 499 Donor recruitment and eligibility for fecal microbiota transplantation: results from an international public stool bank. Gastroenterology. 2015;148:S96-7. doi: 10.1016/S0016-5085(15)30331-0. [DOI] [Google Scholar]
- 15.Paramsothy S, Borody TJ, Lin E, Finlayson S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RW, Connor S, Ng W, et al.. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21:1600-6. doi: 10.1097/MIB.0000000000000405. PMID:26070003. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen CJ, Pedersen OB, Petersen MS, Sorensen E, Kotze S, Thorner LW, Hjalgrim H, Rigas AS, Moller B, Rostgaard K, et al.. Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLoS One. 2014;9:e88196. doi: 10.1371/journal.pone.0088196. PMID:24516611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Moller BK, Juul-Sorensen C, Kotze S, Dinh KM, Erikstrup LT, et al.. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology. 2015;26:580-9. doi: 10.1097/EDE.0000000000000301. PMID:25978794. [DOI] [PubMed] [Google Scholar]
- 18.Khoruts A, Sadowsky MJ, Hamilton MJ. Development of fecal microbiota transplantation suitable for mainstream medicine. Clin Gastroenterol Hepatol. 2015;13:246-50. doi: 10.1016/j.cgh.2014.11.014. PMID:25460566. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen SMD, Hansen MM, Erikstrup C, Dahlerup JF, Hvas CL. Faecal microbiota transplantation: establishment of a clinical application framework. Eur J Gastroenterol Hepatol. 2017;29:e36-45. doi: 10.1097/MEG.0000000000000958. PMID:28863010. [DOI] [PubMed] [Google Scholar]
- 20.Ullum H, Rostgaard K, Kamper-Jorgensen M, Reilly M, Melbye M, Nyren O, Norda R, Edgren G, Hjalgrim H. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion. 2015;55:2479-85. doi: 10.1111/trf.13205. PMID:26098293. [DOI] [PubMed] [Google Scholar]
- 21.Rigas AS, Sorensen CJ, Pedersen OB, Petersen MS, Thorner LW, Kotze S, Sorensen E, Magnussen K, Rostgaard K, Erikstrup C, et al.. Predictors of iron levels in 14,737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion. 2014;54:789-96. doi: 10.1111/trf.12518. PMID:24372094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen LO, Stensvold CR. Blastocystis in health and disease: are we moving from a clinical to a public health perspective? J Clin Microbiol. 2016;54:524-8. doi: 10.1128/JCM.02520-15. PMID:26677249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossen NG, Bart A, Verhaar N, van Nood E, Kootte R, de Groot PF, D'Haens GR, Ponsioen CY, van Gool T. Low prevalence of Blastocystis sp. in active ulcerative colitis patients. Eur J Clin Microbiol Infect Dis. 2015;34:1039-44. doi: 10.1007/s10096-015-2312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang BW, Rezaie A. Irritable bowel syndrome-like symptoms following fecal microbiota transplantation: a possible donor-dependent complication. Am J Gastroenterol. 2017;112:186-7. doi: 10.1038/ajg.2016.472. PMID:28050036. [DOI] [PubMed] [Google Scholar]
- 25.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. PMID:26034755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer M, Bittar M, Papa E, Kassam Z, Smith M. Can you cause inflammatory bowel disease with fecal transplantation? A 31-patient case-series of fecal transplantation using stool from a donor who later developed Crohn's disease. Gut Microbes. 2017;8:205-7. doi: 10.1080/19490976.2017.1283469. PMID:28103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karkman A, Lehtimaki J, Ruokolainen L. The ecology of human microbiota: dynamics and diversity in health and disease. Ann N Y Acad Sci. 2017;1399:78-92. doi: 10.1111/nyas.13326. PMID:28319653. [DOI] [PubMed] [Google Scholar]
- 28.Bovenschen HJ, Janssen MJ, van Oijen MG, Laheij RJ, van Rossum LG, Jansen JB. Evaluation of a gastrointestinal symptoms questionnaire. Dig Dis Sci. 2006;51:1509-15. doi: 10.1007/s10620-006-9120-6. PMID:16927133. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-71. doi: 10.1001/archpsyc.1961.01710120031004. PMID:13688369. [DOI] [PubMed] [Google Scholar]
- 30.Paxton AE, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med. 2011;40:67-71. doi: 10.1016/j.amepre.2010.10.009. PMID:21146770. [DOI] [PubMed] [Google Scholar]
- 31.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al.. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB. PMID:12900694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


