ABSTRACT
Mammalian autophagosomes possess the Qa-SNARE STX17 (syntaxin 17) for fusion with lysosomes. However, STX17 is not absolutely required for fusion because STX17 knockout cells partially retain autophagosome-lysosome fusion activity. We recently identified YKT6, an R-SNARE, as another autophagosomal SNARE protein that acts independently of STX17 in mammals. Here, we discuss the features and functions of autophagosomal SNARE proteins by comparing STX17 and YKT6.
Abbreviations: SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; STX17, syntaxin 17.
KEYWORDS: Autophagosome, lysosome, SNARE, syntaxin 17, YKT6
Macroautophagy is achieved by fusion between autophagosomes and lysosomes (Figure 1). STX17 (syntaxin 17) has been identified as an autophagosomal SNARE protein in metazoans such as mammals, Drosophila melanogaster, and Caenorhabditis elegans. However, autophagosome-lysosome fusion is partially retained in STX17 knockout HeLa cells, suggesting that autophagosomes may possess other SNARE proteins besides STX17. By screening approximately 40 human SNARE proteins, we identified YKT6 on autophagosomes [1]. Depletion of both STX17 and YKT6 has an additive inhibitory effect on autophagosome-lysosome fusion (Figure 1). STX17 forms a complex with SNAP29 and lysosomal VAMP7 (or VAMP8), whereas YKT6 forms a complex with SNAP29 and at least lysosomal STX7, which is also required for autophagosomal fusion. Thus, we suggest that autophagosomes have 2 SNARE proteins – STX17 and YKT6 – that function independently.
Figure 1.
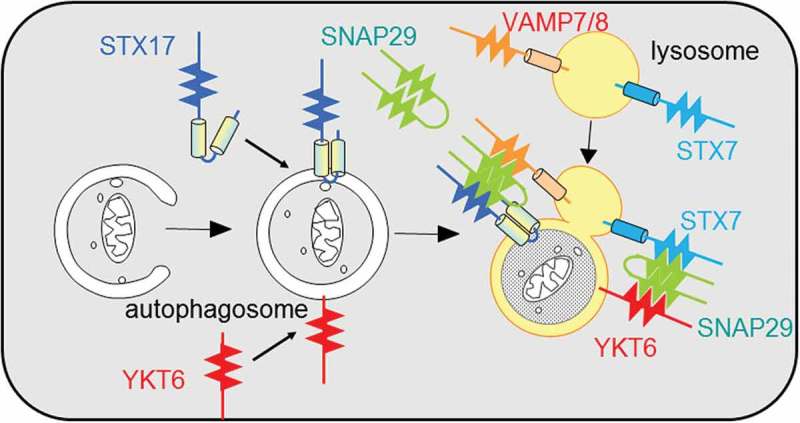
A model of SNARE proteins in autophagosome-lysosome fusion.
YKT6 and STX17 localize to autophagosomes independently of each other. YKT6 and STX17 form a SNARE complex with SNAP29-STX7 and SNAP29-VAMP8, respectively, and mediate autophagosome-lysosome fusion. Cylinders indicate the transmembrane domains. Zigzag lines indicate the SNARE domains.
What are shared features between STX17 and YKT6?
Although most SNARE proteins have a single transmembrane domain, STX17 and YKT6 are both exceptions. STX17 has 2 tandem transmembrane domains at the C terminus that likely form a hairpin-like structure through glycine zipper motifs. YKT6 has no transmembrane domain and is anchored to membranes via palmitoylation and farnesylation of the 2 cysteine residues at the C terminus. Perhaps related to these unique features, these SNARE proteins can be present in the cytosol in soluble form and inserted into autophagosomes immediately before or after closure, but they are not inserted into elongating intermediate structures. This characteristic behavior of the 2 SNARE proteins allows lysosomes to fuse with only mature autophagosomes. Such temporal control of recruitment may be achieved by a change in autophagosomal membrane property as well as a conformational change of the hairpin-like transmembrane domains of STX17 and lipidation of YKT6, but the mechanisms are currently unknown.
What are the differences between STX17 and YKT6?
These SNARE proteins are structurally different: STX17 is a Qa-SNARE whereas YKT6 is an R-SNARE. Thus, they interact with different SNARE proteins on lysosomes; that is, STX17 and YKT6 interact with VAMP7/8 (R-SNARE) and STX7 (Qa-SNARE), respectively. The biological roles of these 2 SNAREs are also different: STX17 is dispensable for cell proliferation whereas YKT6 is essential for growth. Thus, we can knock out the STX17 gene, but only short-term knockdown of the YKT6 gene is feasible. This is because YKT6 is required for multiple, including secretory, pathways.
Is lysosomal function impaired in the absence of STX17 or YKT6?
The answer is ‘no’ for STX17 but ‘yes’ for YKT6. Although STX17 is present in the ER, STX17 knockout cells do not show any defects in lysosomal enzyme sorting or lysosomal function. In contrast, 5-day knockdown of YKT6 impairs lysosomal function and LAMP1 glycosylation. Thus, it is important to differentiate the roles of YKT6 in lysosomal function from those in autophagosome fusion. This can be achieved after short-term knockdown. Upon 3-day knockdown of YKT6, lysosomal function remains intact probably because there are sufficient preexisting enzymes. However, under the same conditions, autophagosome-lysosome fusion is inhibited, suggesting that the role of YKT6 in autophagosome-lysosome fusion is not secondary to lysosomal dysfunction.
Are the roles of STX17 and YKT6 evolutionarily conserved?
STX17 is conserved in metazoans only. By contrast, YKT6 is more widely conserved; for instance, it is present in yeasts and plants. In yeasts, Ykt6 actually acts as an autophagosomal SNARE protein, which was recently published by the groups of Claudine Kraft and Christian Ungermann. Thus, YKT6 could be a prototype of the autophagosomal SNARE, and STX17 may have been acquired later during metazoan evolution. An interesting but unanswered question is why we need STX17 in addition to YKT6. If their partner SNAREs (VAMP7/8 and STX7) exist in distinct populations of lysosomes or related organelles, STX17 and YKT6 may regulate fusion with different degradative organelles.
Do STX17 and YKT6 function independently or cooperatively?
As far as we have found in HeLa cells, STX17 and YKT6 seem to be simply redundant and do not function in the same linear pathway. Recruitment of STX17 and YKT6 to autophagosomes is not interdependent, and, as aforementioned, depletion of either STX17 or YKT6 partially affects autophagosome-lysosome fusion, whereas depletion of both causes an additive effect. However, as both the Qa- and R-SNARE proteins are present on the same autophagosomes, STX17 and YKT6 also interact with each other, which may impede the fusogenic activity of these proteins. Although the significance of this STX17-YKT6 complex is unknown in mammalian cells, Gábor Juhász’s group recently proposed an interesting hypothesis: YKT6 acting upstream of STX17 has a noncanonical regulatory role in forming the STX17-SNAP29-VAMP7 complex. It would therefore be important to reveal the relationship between these 2 different sets of SNARE complexes using an in vitro system.
Funding Statement
This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas (Grant Number 25111005) from Japan Society for the Promotion of Science (JSPS) and Exploratory Research for Advanced Technology (ERATO) (Grant Number JPMJER1702) from Japan Science and Technology Agency (JST) to N.M.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Matsui T, Jiang P, Nakano S, et al. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J Cell Biol. 2018;217(8):2633–2645. pii: jcb.201712058. [DOI] [PMC free article] [PubMed] [Google Scholar]


