Abstract
Aim
Lipoprotein(a) [Lp(a)] elevation is a causal risk factor for cardiovascular disease (CVD). It has however been suggested that elevated Lp(a) causes CVD mainly in individuals with high low-density lipoprotein cholesterol (LDL-C) levels. We hypothesized that the risk associated with high Lp(a) levels would largely be attenuated at low LDL-C levels.
Methods and results
In 16,654 individuals from the EPIC-Norfolk prospective population study and in 9,448 individuals from the Copenhagen City Heart Study (CCHS) parallel statistical analyses were performed. Individuals were categorized according to their Lp(a) and LDL-C levels. Cut-offs were set at the 80th cohort percentile for Lp(a). LDL-C cut-offs were set at 2.5, 3.5, 4.5 and 5.5 mmol/L. LDL-C levels in the primary analyses were corrected for Lp(a)-derived LDL-C (LDL-Ccorr). Multivariable-adjusted hazard ratios (HR) were calculated for each category. The category with LDL-Ccorr <2.5 mmol/L and Lp(a) <80th cohort percentile was used as reference category. In the EPIC-Norfolk and CCHS cohorts, individuals with an Lp(a) ≥80th percentile were at increased CVD risk compared to those with Lp(a) <80th percentile for any LDL-Ccorr levels ≥2.5 mmol/L. In contrast, for LDL-Ccorr <2.5 mmol/L, the risk associated with elevated Lp(a) attenuated. However, there was no interaction between LDL-Ccorr and Lp(a) levels on CVD risk in either cohort.
Conclusion
Lp(a) and LDL-C are independently associated with CVD risk. At LDL-C levels below <2.5 mmol/L, the risk associated with elevated Lp(a) attenuates in a primary prevention setting.
Keywords: Lipoprotein(a), LDL-cholesterol, Cardiovascular risk
Introduction
Lipoprotein(a) [Lp(a)] elevation is a strong and independent risk factor for cardiovascular disease (CVD)1. Mendelian randomisation studies support a causal role for Lp(a) in CVD2–4. Despite the fact that Lp(a) measurement is recommended in patients at intermediate risk in the ESC/EAS guidelines5,6, none of the guidelines recommend specific therapeutic interventions in case of high Lp(a) levels due to the absence of evidence for effective therapeutic interventions. Specific Lp(a) lowering strategies are being developed, but have not been tested in clinical outcome studies. In view of a 60% LDL-C lowering7 combined with a 30% Lp(a) lowering8 effect, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been put forward as potential therapeutic strategy in patients with Lp(a) elevation. However, the moderate Lp(a) reduction by PCSK9 inhibitors is clearly insufficient to lower high Lp(a) levels to below the currently accepted Lp(a) threshold9. Interestingly, previous studies suggested that elevated Lp(a) confers risk predominantly in conjunction with elevated levels of low-density lipoprotein cholesterol (LDL-C)10,11. However, it remains a matter of debate whether very low LDL-C levels will result in attenuation of the Lp(a)-mediated CVD risk despite persistent Lp(a) elevation.
Given the proposed potentiation of the CVD risk between LDL-C and Lp(a), we hypothesized that the risk associated with elevated Lp(a) levels would largely be attenuated at lower LDL-C levels. We tested this hypothesis in two large studies corresponding to a primary prevention setting: the European Prospective Investigation of Cancer (EPIC)-Norfolk prospective population study and the Copenhagen City Heart Study prospective population study.
Methods
Study design
The EPIC-Norfolk prospective population study consists of 25,633 individuals recruited from general practices in the Norfolk area, United Kingdom12. Study participants aged between 39 and 79 were enrolled between 1993 and 1997. At baseline, patients completed general health questionnaires and a panel of measurements was performed. During follow-up, all individuals were flagged for mortality at the UK Office of National Statistics, and vital status was ascertained for the entire cohort. Data on all hospital contacts throughout England and Wales were obtained using National Health Service numbers through linkage with the East Norfolk Health Authority (ENCORE) database. Hospital records and death certificates were coded by trained nosologists and categorized according to the International Classification of Disease 10th revision (ICD-10). Death or hospitalizations were attributed to atherosclerotic CVD if the ICD-10 codes I20-I25 or I60-69 were recorded as the underlying cause of death or the reason for hospitalization. These ICD-10 codes represent the ACC/AHA definition of atherosclerotic CVD and include coronary heart disease death, nonfatal myocardial infarction, and fatal or nonfatal stroke13. The study protocol was approved by the Norwich District Health Authority Ethics Committee. All individuals gave written informed consent.
The Copenhagen City Heart Study (CCHS) consists of 24,260 individuals and is a prospective study of the Danish general population initiated in 1976-1978 with follow-up examinations in 1981-1983, 1991-1994, and 2001-2003. For this study, 9,448 participants from the 1991-1994 and 2001-2003 examinations were included with known Lp(a) and LDL-C levels. Individuals were recruited using the Danish Civil Registration System to best reflect the white Danish general population aged 20-100+ years. Participants filled out a questionnaire including lifestyle, medical- and family history and went through a physical examination including blood sampling, and measurement of blood pressure, height, and weight. In Denmark each individual is assigned a Civil Registration Number at birth and all individuals were followed through the national Danish Patient Registry and the national Danish Causes of Death Registry to ascertain diagnosis of atherosclerotic CVD including nonfatal myocardial infarction, coronary heart disease death, and fatal or nonfatal stroke using ICD-8 codes 410-414 and 430-438, and ICD-10 codes I20-I25 and I60-I68, respectively, and validated as described2,14,15. The CCHS was approved by Danish ethical committees, all individuals gave written informed consent, and the study was conducted according to the declaration of Helsinki.
Biochemical analysis
In EPIC-Norfolk, non-fasting blood16 was drawn at baseline from study individuals, and total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides were determined with the RA1000 analyzer (Bayer Diagnostics, Basingstoke, United Kingdom). The Friedewald formula was used for the calculation of LDL-C levels17. The remaining plasma was stored at -80°C for future analyses. In 2010, samples were thawed and Lp(a) was measured on an Olympos AU640 analyzer with Randox reagents (Randox laboratories Ltd. Crumlin, County Antrim, United Kingdom). The rs10455872 genetic variant was genotyped using Custom TaqMan® SNP Genotyping Assays (Applied Biosystems, Warrington, UK).
For the CCHS, standard hospital assays were used to measure triglycerides, total cholesterol, and HDL-C on fresh nonfasting16 plasma samples at baseline. LDL-C was calculated using the Friedewald equation when plasma triglycerides were <4 mmol/L (352mg/dL), and otherwise measured by a direct assay. Individuals from the CCHS examination in 1991-1994 had Lp(a) total mass measured by an isoform insensitive turbidimetric in-house assay as described previously18.
Statistical analysis
Parallel statistical analyses were performed for EPIC-Norfolk and the CCHS, but independent of each other. For this study, individuals with myocardial infarction or stroke prior to baseline screening were excluded. Also, individuals missing LDL-C or Lp(a) levels, those who were on lipid lowering therapy at baseline, or non-Caucasian individuals were excluded. Two Lp(a) categories were constructed based on the EAS proposed threshold for Lp(a)9, with the cut-off set at the 80th cohort percentile, thereby dividing people in groups with Lp(a) levels <80 and ≥80th cohort percentile in both cohorts. LDL-C was corrected for Lp(a) derived cholesterol (LDL-Ccorr)19 and cut-offs were set at 2.5, 3.5, 4.5 and 5.5 mmol/L, dividing people in groups with LDL-Ccorr levels <2.5, 2.5-3.49, 3.5-4.49, 4.5-5.49 and ≥5.5 mmol/L. Individuals were categorized using these Lp(a) and LDL-Ccorr cut-offs. Multivariable (age-, sex-, smoking-, body mass index-, diabetes mellitus-, systolic blood pressure and glomerular filtration rate- (GFR)) adjusted hazard ratios (HR) were calculated for each category and the category with LDL-C <2.5 mmol/L and Lp(a) <80th cohort percentile was used as reference category. To strengthen clinical applicability of our findings we also calculated multivariable adjusted HR for laboratory measured (non Lp(a) adjusted) LDL-C using the EAS proposed Lp(a) threshold of 50 mg/dL9 and 30 mg/dL20.
Trendlines were constructed for both Lp(a). A Cox-regression analysis was performed to test for interaction between the five LDL-Ccorr groups (<2.5, 2.5-3.49, 3.5-4.49, 4.5-5.49, ≥5.5) and the two Lp(a) groups (<80th versus ≥80th cohort percentile) on the risk of CVD. All statistical analyses were performed using SPSS (Version 24.0, IBM Corporation, Armonk, NY, USA), GraphPad Prism (version 5, GraphPad Software, Inc, California, USA), or STATA (version 13.1, StataCorp, Texas, USA).
Results
Lp(a) and LDL-C levels were available for 26,102 individuals (16,654 from EPIC-Norfolk and 9,448 from the CCHS). Baseline characteristics are presented in Table 1a and 1b for EPIC-Norfolk and the CCHS.
Table 1.
a. EPIC-Norfolk prospective population study: Baseline characteristics
b. Copenhagen City Heart Study prospective population study: Baseline characteristics
| a | Lipoprotein(a) category | |||
|---|---|---|---|---|
| <80th percentile |
≥80th percentile |
<50 mg/dL | ≥50 mg/dL | |
| Number | 11,846 | 2,082 | 13,321 | 3,333 |
| Age, years | 59.0(±9.1) | 58.9(±9.0) | 58.8(±9.1) | 59.0(±9.0) |
| Male sex | 46.3(5,480) | 47.7(993) | 43.0(5,725) | 41.5(1,951) |
| Body mass index, kg/m2 | 26.1(±3.7) | 26.1(±3.7) | 26.1(±3.8) | 26.1(±3.8) |
| Current smoker | 11.5(1,350) | 12.1(249) | 11.4(1,511) | 10.8(357) |
| Diabetes mellitus | 2.9(345) | 2.9(61) | 2.7(364) | 2.9(95) |
| Systolic blood pressure, mmHg | 135(±18) | 135(±18) | 135(±18) | 134(±18) |
| Diastolic blood pressure, mmHg | 82(±11) | 82(±11) | 82(±11) | 82(±11) |
| Glomerular filtration rate, mL/min | 73 (63 – 84) | 72 (63 – 83) | 73 (63 – 84) | 73 (63 – 84) |
| Total cholesterol, mmol/L | 6.1(±1.1) | 6.2(±1.1) | 6.1(±1.1) | 6.4(±1.1) |
| HDL-cholesterol, mmol/L | 1.4(±0.4) | 1.4(±0.4) | 1.4(±0.4) | 1.5(±0.4) |
| LDL-cholesterol, mmol/L | 3.9(±1.0) | 4.0(±1.0) | 3.9(±1.0) | 4.2(±1.0) |
| Corrected LDL-cholesterol, mmol/L | 3.8(±1.0) | 3.6(±0.9) | 3.8(±1.0) | 3.7(±1.0) |
| Triglycerides, mmol/L | 1.5(1.1-2.1) | 1.5(1.1-2.1) | 1.5(1.0-2.1) | 1.5(1.1-2.1) |
| Lipoprotein(a), mg/dL | 9.6(5.6-17.3) | 45.4(35.2–59.1) | 9.0(5.3-15.3) | 53.0(43.2-69.3) |
| b | Lipoprotein(a) category | |||
|---|---|---|---|---|
| <80th percentile |
≥80th percentile |
<50 mg/dL | ≥50 mg/dL | |
| Number | 7,563 | 1,885 | 7,578 | 1,870 |
| Age, years | 58.1(±16) | 59.5(±15) | 58.1(±16) | 59.5(±15) |
| Male sex | 43.7(3,311) | 40.6(760) | 43.7(3,311) | 40.6(760) |
| Body mass index, kg/m2 | 25.4(±4.3) | 25.4(±4.3) | 25.4(±4.3) | 25.4(±4.3) |
| Current smoker | 48.7(3,7) | 47.5(888) | 48.7(3,7) | 47.5(888) |
| Diabetes mellitus | 10.7(812) | 10.3(192) | 10.7(812) | 10.3(192) |
| Systolic blood pressure, mmHg | 138(±23) | 140(±22) | 138(±23) | 140(±22) |
| Diastolic blood pressure, mmHg | 83.7(±12) | 84.7(±12) | 83.7(±12) | 84.7(±12) |
| Glomerular filtration rate, mL/min | 77 (68-91) | 76 (67-90) | 77 (68-91) | 76 (67-90) |
| Total cholesterol, mmol/L | 6.0(±1.2) | 6.7(±1.3) | 6.0(±1.2) | 6.7(±1.3) |
| HDL-cholesterol, mmol/L | 1.6(±0.5) | 1.6(±0.5) | 1.6(±0.5) | 1.6(±0.5) |
| LDL-cholesterol, mmol/L | 3.7(±1.1) | 4.1(±1.2) | 3.7(±1.1) | 4.1(±1.2) |
| Corrected LDL-cholesterol, mmol/L | 3.5(±1.1) | 3.3(±1.1) | 3.5(±1.1) | 3.3(±1.1) |
| Triglycerides, mmol/L | 1.5(1.1-2.1) | 1.7(1.2-2.3) | 1.5(1.1-2.1) | 1.7(1.2-2.3) |
| Lipoprotein(a), mg/dL | 12.0 (4.1-24.4) | 84.0 (63.3-118) | 12.1 (4.1-24.5) | 84.9 (63.5-119) |
Data are presented as mean±standard deviation for continuous variables with a normal distribution, median(interquartile range) for continuous variables with a non-normal distribution, and percentage(number) for categorical variables. LDL=low-density lipoprotein, HDL=high-density lipoprotein. Corrected LDL-cholesterol had cholesterol content of Lp(a) subtracted.
In EPIC-Norfolk, the mean age was 59.0±9.1 and 58.9±9.0 for individuals with an Lp(a) of <80 and ≥80th cohort percentile. Mean LDL-Ccorr levels were 3.8 (±1.0) and 3.6(±0.9) mmol/L for the respective categories. Median [interquartile range] Lp(a) levels were 9.6(5.6–17.3) and 45.4(35.2-59.1) mg/dL for individuals with an Lp(a) <80 and ≥80th cohort percentile. The total number of CVD events was 3,347, namely 1,879 nonfatal myocardial infarctions, 920 deaths due to coronary heart disease, 646 nonfatal strokes and 161 fatal strokes. In CCHS, the mean age was 58.1±16 and 59.5±15 for individuals with an Lp(a) <80 and and ≥80th cohort percentile, respectively. Mean LDL-Ccorr levels were 3.5 (±1.1) and 3.3 (±1.1) mmol/L for the respective categories. Median [interquartile range] Lp(a) levels were 12.0(4.1-24.4) and 84.0(63.3-118) mg/dL for individuals with an Lp(a) <80 and ≥80th cohort percentile. The total number of CVD events was 2,577, namely 1347 nonfatal ischemic heart disease, 393 deaths due to coronary heart disease, 684 nonfatal strokes and, 153 fatal strokes. The CVD risks associated with the Lp(a) category <80 and ≥80th cohort percentile and the five LDL-Ccorr categories are presented in Table 2a and 2b for EPIC-Norfolk and the CCHS. The CVD risk associated with the Lp(a) category <50 and ≥50 mg/dL and the five laboratory-measured LDL-C categories are provided in Supplementary Tables 1a and 1b.
Table 2.
a. EPIC-Norfolk: Cardiovascular risk as a function of Lp(a) and LDL-Ccorr
b. Copenhagen City Heart Study prospective population study: Cardiovascular risk as a function of Lp(a) and LDL-Ccorr
| a | Lp(a) category | |||||||
|---|---|---|---|---|---|---|---|---|
| <80th percentile | ≥80th percentile | |||||||
| LDL-Ccorr | Mean LDL-Ccorr¶ | Median (interquartile range) Lp(a) | Hazard ratio | CVD cases / total number | Median (interquartile range) Lp(a) | Hazard ratio | CVD cases / total number | |
| LDL-Ccorr category, mmol/L | <2.5 | 2.1 | 7.7(4.8-12.7) | 1.00(reference) | 134/1,114 | 53.7(41.8-68.1) | 1.11(0.77-1.59) | 39/279 |
| 2.5 – 3.49 | 3.1 | 8.3(5.0-14.4) | 1.03(0.86-1.25) | 676/4.376 | 50.9(41.8-68.1) | 1.44(1.16-1.78)** | 229/1,182 | |
| 3.5 - 4.49 | 4.0 | 9.4(5.6-15.7) | 1.21(1.01-1.46)* | 960/4,784 | 53.1(43.7-68.1) | 1.64(1.33-2.01)*** | 296/1,187 | |
| 4.5 - 5.49 | 4.9 | 9.9(5.8-16.4) | 1.49(1.23-1.81)*** | 583/2,285 | 56.7(45.8-71.0) | 1.89(1.50-2.39)*** | 158/526 | |
| ≥5.5 | 6.1 | 11.1(6.1-18.0) | 1.61(1.29-2.00)*** | 216/762 | 56.4(46.4-79.0) | 2.17(1.58-2.98)*** | 56/159 | |
| b | Lp(a) category | |||||||
|---|---|---|---|---|---|---|---|---|
| <80th percentile | ≥80th percentile | |||||||
| LDL-Ccorr | Mean LDL-Ccorr¶ | Median (interquartile range) Lp(a) | Hazard ratio | CVD cases / total number | Median (interquartile range) Lp(a) | Hazard ratio | CVD cases / total number | |
| LDL-Ccorr category, mmol/L | <2.5 | 2.0 | 10(3.0-24) | 1.00(reference) | 201/1,358 | 95(65-123) | 1.08(0.85-1.38) | 89/453 |
| 2.5 – 3.49 | 3.0 | 12(4.1-25) | 1.08(0.92-1.26) | 638/2,608 | 83(63-120) | 1.30(1.08-1.58)** | 201/677 | |
| 3.5 - 4.49 | 4.0 | 12(4.5-25) | 1.04(0.89-1.21) | 646/2,207 | 84(63-114) | 1.50(1.24-1.83)*** | 185/503 | |
| 4.5 - 5.49 | 4.9 | 13(4.7-23) | 1.27(1.07-1.50)** | 363/997 | 71(61-110) | 1.45(1.13-1.87)** | 66/186 | |
| ≥5.5 | 6.1 | 15(5.3-27) | 1.42(1.15-1.74)*** | 153/382 | 79(63-119) | 2.34(1.63-3.35)*** | 35/77 | |
Data are presented as age-, sex-, smoking-, body mass index-, diabetes mellitus-, systolic bloodpressure-, glomerular filtration rate-adjusted hazard ratios for the 80th cohort percentile for Lp(a) with corresponding 95% confidence interval limits. LDL-Ccorr=low density lipoprotein cholesterol corrected for Lp(a) derived cholesterol (mmol/L), LDL-C = low density lipoprotein cholesterol, Lp(a) = lipoprotein(a)(mg/dL). ¶=these mean LDL-Ccorr levels apply to both Lp(a) categories, *p<=0.05, **p<0.01, ***=p-value<0.001.
Among individuals in EPIC-Norfolk with an Lp(a) <80th cohort percentile, those with an LDL-Ccorr ≥5.5mmol/L had a CVD risk HR(95% confidence interval;p-value) of 1.61(1.29–2.00;p<0.001) compared with those with an LDL-Ccorr <2.5 mmol/L (Table 2a). In individuals with an Lp(a) ≥80th cohort percentile (and compared with individuals with an Lp(a) <80th percentile and LDL-Ccorr <2.5 mmol/L), those with an LDL-Ccorr ≥5.5 mmol/L had a CVD HR of 2.17 (1.58-2.98;p<0.001) while those with an LDL-Ccorr <2.5 mmol/L had a HR of 1.11(0.77-1.59; p=0.57). Comparable results for the impact of Lp(a) on CVD risk in the various laboratory measured LDL-C categories were obtained using the 50 mg/dL (87th percentile) and 30 mg/dL (77th percentile) cut-off (Supplemental Table 1a and 2a).
Among individuals in the CCHS with Lp(a) <80th cohort percentile, the HR for individuals with a LDL-Ccorr of ≥5.5 mmol/L was 1.42(1.15-1.74;p<0.001), compared with individuals with an LDL-Ccorr of <2.5 mmol/L (Table 2b). In individuals with an Lp(a) ≥80th cohort percentile (and compared with individuals with an Lp(a) <80th percentile and LDL-Ccorr <2.5 mmol/L), those with an LDL-Ccorr ≥5.5 mmol/L had a HR of 2.34(1.63-3.35;p<0.001) while those with an LDL-Ccorr <2.5 mmol/L had a HR of 1.08(0.85-1.38;p= 0.48). Results for laboratory measured LDL-C and Lp(a) cut-off <50 (80th percentile) and ≥50 mg/dL were comparable and can be found in Supplemental Table 1b. Using the 30 mg/dL Lp(a) cut-off, which represents the 67th cohort percentile, resulted in non-significant results in all LDL-C categories <5.5 mmol/L for Lp(a) <30 mg/dL and <4.5 mmol/L for Lp(a) ≥30 mg/dL (Supplemental Table 2b).
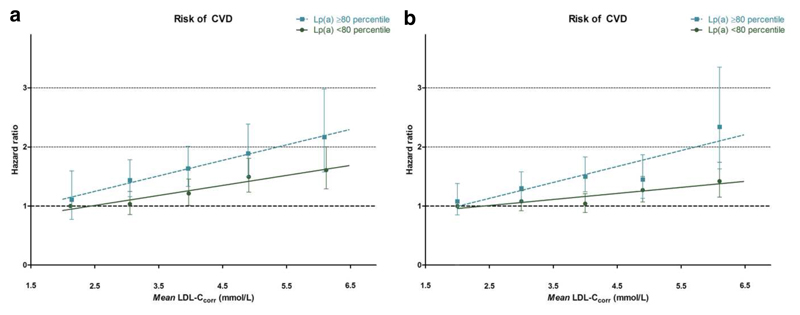
There was no significant interaction between LDL-Ccorr and Lp(a) levels on CVD risk in either cohort (p-value of 0.80 and 0.11 in EPIC-Norfolk and CCHS). The risk of CVD for each Lp(a) category is shown in Figure 1a and 1b. For EPIC-Norfolk, the slope (±SD;r2) of the trend line was 0.170(±0.023;0.95) for the Lp(a) category <80th cohort percentile and 0.263(±0.013;0.99) for the Lp(a) category ≥80th cohort percentile. For CCHS, the slope (±SD;r2) of the trend line was 0.102(±0.025;0.85) for the Lp(a) category <80th cohort percentile and 0.270(± 0.075;0.81) for the Lp(a) category ≥80th cohort percentile.
Figure 1.
a. EPIC-Norfolk prospective population study: CVD risk as a function of LDL-Ccorr and Lp(a)
b. Copenhagen City Heart Study prospective population study: CVD risk as a function of LDL-Ccorr and Lp(a)
Data are presented as age-, sex-, smoking-, body mass index-, diabetes mellitus-, glomerular filtration rate (GFR) and systolic blood pressure adjusted hazard ratios with corresponding 95% confidence intervals and mean LDL-Ccorr levels (mmol/L). Trend lines were constructed based on the mean LDL-Ccorr levels for each category. LDL-Ccorr =low density lipoprotein cholesterol corrected for Lp(a) derived cholesterol, Lp(a)=lipoprotein(a).
Discussion
Our study in two prospective population cohorts corresponding to a primary prevention setting, the EPIC-Norfolk and the Copenhagen City Heart Study, confirms that both LDL-C and Lp(a) are independently associated with CVD risk. Individuals with high Lp(a) levels are characterized by a markedly increased CVD risk compared with individuals with lower Lp(a) levels9. As expected, the absolute CVD risk is substantially lower in subjects with elevated Lp(a) and low LDL-C levels compared with subjects with elevated Lp(a) and high LDL-C levels. More importantly, the CVD risk increase conveyed by Lp(a) appeared to diminish at the lowest LDL-C levels. However, there was no significant interaction between LDL-Ccorr and Lp(a) levels on CVD risk in either cohort, implying that elevated Lp(a) and elevated LDL-C are associated with increased CVD risk independent of the level of the other risk factor.
When evaluating the roles of LDL-C and Lp(a) on CVD-risk, it is essential to first consider the details of these parameters. For LDL-C, recent data have emphasized the contribution of cholesterol in the Lp(a) fraction to the laboratory measured LDL-C19. Although this contribution is negligible in individuals with low Lp(a) levels, it can cumulate up to 50% of the laboratory-measured cholesterol in the LDL-C fraction in individuals with elevated Lp(a)19. To avoid a disproportionate impact of Lp(a)-C particularly in individuals with low LDL-C levels, the Lp(a)-corrected LDL-C was therefore used in our interaction analyses. The threshold used to define Lp(a) elevation is a matter of debate. The absolute cut-off of 50 mg/dL represents different percentile values: the 87th percentile in EPIC-Norfolk and the 80th percentile in CCHS cohort. This discrepancy most likely reflects the difficulties in measuring Lp(a) with marked heterogeneity between different Lp(a) assays21. Hence, inter-assay variation between the EPIC-Norfolk and CCHS may have occurred, which may impact on the interpretation of previous studies22 and therefore hamper the use of absolute cut-offs for Lp(a) in routine clinical practice.
Observational studies on LDL-C and Lipoprotein(a) interaction
Several observational studies support that Lp(a) only increases CVD risk if LDL-C levels exceed a certain threshold. In 500 subjects without CVD in the Bruneck study, elevated Lp(a) (>32 mg/dL) was only a predictor for CVD risk in subjects with LDL-C levels ≥3.3 mmol/L23. In 9,133 participants of the PRIME study, elevated levels of Lp(a) (≥33 mg/dL) were associated with coronary heart disease only in individuals with LDL-C levels higher than 3.7 mmol/L24. Among 27,791 women of the Women’s Health Study elevated Lp(a) levels (≥44 mg/dL) were associated with future CV events only in women with an LDL-C above 3.1 mmol/L11. In a meta-analysis of prospective studies, comprising 126,634 individuals, 1-SD increase of Lp(a) was associated with CVD only in individuals with a non-HDL cholesterol >3.8 mmol/L1; although no interaction was found between the non-HDL cholesterol tertiles, Lp(a) and CVD. Our results substantiate that in a primary prevention setting subjects with elevated Lp(a) levels, not using lipid-lowering therapy, at most have a very modest increase in CV-risk when LDL-C is below 2.5 mmol/L.
Intervention studies on LDL-C and Lipoprotein(a) interaction
In interventional studies, data on the relationship between LDL-C lowering on CVD risk in patients with high Lp(a) is equivocal. In 3,877 individuals of the JUPITER trial treated with rosuvastatin and an achieved median LDL-C of 1.4 mmol/L, elevated Lp(a) (≥50 mg/dL) lost its significance for CVD risk although it still had a nominally high HR(95%CI) of 1.67(0.93-3.02))25. In a post-hoc analysis of the DAL-OUTCOMES study comprising 4,139 acute coronary syndrome patients treated with dalcetrapib or placebo on top of statins and a mean LDL-C of 1.9-2.1 mmol/L, elevated Lp(a) levels (≥50 mg/dL) were also not associated significantly with adverse cardiovascular outcomes although it still had a nominally high HR(95%CI) of 1.16(0.97-1.39)26. Other studies reported a persistent CVD risk increase for individuals with elevated Lp(a), independent of LDL-C lowering interventions. In 411 individuals who underwent a percutaneous coronary intervention and achieved LDL-C levels <2.6 mmol/L, elevated levels of Lp(a) (≥30 mg/dL) were still associated with all-cause mortality and acute coronary syndromes27. When combining data from subpopulations of the JUPITER trial with data from the AIM high and LIPID trials, elevated levels of Lp(a) were also associated with increased CVD risk, independent from achieved LDL-C levels and/or use of lipid-lowering therapy28. However, in the latter analysis, persistent risk increase by Lp(a) elevation was observed particularly in the secondary prevention study (AIM-HIGH) irrespective of placebo or nicotinic acid therapy28,29. When determining the effect of Lp(a) elevation only in the rosuvastatin treated patients, there was no longer a significant effect on CVD risk although it still had a nominally high OR(95%CI) of 1.71(0.99-2.95)25,28.
Modulation of absolute CV-risk in elevated Lp(a)
Although the hazard ratios show an invariable relative risk increase when comparing Lp(a) ≥80th cohort percentile with Lp(a) <80th cohort percentile, the absolute risk characterizing a patient with elevated Lp(a) clearly depends on the LDL-C category of that subject. A low Lp(a) combined with LDL-Ccorr levels shifting from ≥5.5 to <2.5 to, results in a decreased risk (HR) from 1.61(1.29-2.00) to the reference of 1.00 in EPIC-Norfolk and from 1.42(1.15-1.74) to the reference of 1.00 in the CCHS; at high Lp(a) levels (using the same reference group) the corresponding HR decrease was from 2.17(1.58-2.98) to 1.11(0.77-1.59) in EPIC-Norfolk and from 2.34(1.63-3.35) to 1.08(0.85-1.38) in CCHS. In view of the overwhelming evidence on LDL-C change and change in risk independent from treatment-lowering modality7,30–32, our observational data imply that aggressive lowering of LDL-C in individuals with high Lp(a) could divert a substantial part of the adverse effects of Lp(a), thereby markedly reducing the absolute CVD risk at least in a primary prevention setting. The observed CVD risk upon comparing the LDL-C category 4.5-5.49 with 2.5-3.49 mmol/L reveals a lower relative risk of 46% and 22% for the categories with low and high Lp(a) respectively (Table 2a). This translates into CVD risk reduction of respectively 23% and 11% for every mmol/L of LDL-C decrease in the low and high Lp(a) category, which is comparable with the 20% lower risk per mmol/L LDL-C reduction of the CTT Collaboration meta-analysis30. However, combined with our observation that Lp(a) no longer conveys a substantial risk increase at low LDL-C levels in a primary prevention setting, this data supports the concept that potent LDL-C lowering is the primary target to lower absolute risk also in individuals with elevated Lp(a). Compounds offering significant Lp(a) reduction, comprising the PCSK9-antibodies8 and the emerging apo(a)-antisense33, can be considered to further reduce residual Lp(a)-mediated risk. Besides the LDL-C lowering effect of 60-65%, PCSK9 antibodies offer a concomitant reduction of Lp(a) with 25-30%8. Post-hoc analyses in FOURIER34, and ODYSSEY35 trials will reveal to what extent PCSK9-antibody therapy is able to reduce CVD risk in patients with very high Lp(a), which will provide valuable information for the future position of the more potent apo(a) antisense therapy in high-risk patients.
Study limitations
Our study has several limitations, which merit closer consideration. First, the prevalence of subjects with LDL-C levels below 2.5 mmol/L is relatively low resulting in a limited number of elevated Lp(a) subjects in this category (229 and 453 in EPIC-Norfolk and CCHS, respectively), which may have led to loss of power to detect a significant effect of Lp(a) on CVD risk in this category. Second, the cut-off values used in our primary analyses concern the use of Lp(a)-corrected LDL-C combined with the 80th percentile cut-off for Lp(a) elevation. Although this represents the scientifically most sound approach, these values do not resonate with routine clinical practice. When using uncorrected, laboratory measured LDL-C combined with the absolute cut-off of >50mg/dL for Lp(a), the conclusions were however similar. Third, our analysis included mainly primary prevention subjects, with a low to moderate CVD-risk. As a change in CVD-risk following modification of a single risk factor depends heavily on the absolute baseline risk36, we cannot extrapolate our finding of a decreased Lp(a) influence at very low LDL-C levels to the higher risk groups such as patients after an acute coronary syndrome or individuals with familial hypercholesterolemia or diabetes.
In summary, our analyses in the primary prevention setting of EPIC-Norfolk and CCHS general population cohorts substantiate that LDL-C and Lp(a) are independently associated with CVD risk. However, at LDL-C levels <2.5 mmol/L, the CVD risk associated with elevated Lp(a) is attenuated. Prospective evaluation of the impact of robust LDL-C lowering versus routine statin therapy combined with Lp(a) lowering strategies on CVD risk in high-risk patients with elevated Lp(a) levels is warranted to substantiate external validity of our findings in a high-risk secondary prevention setting.
Supplementary Material
Acknowledgements
The authors thank the participants and staff of the EPIC-Norfolk prospective population study and the Copenhagen City Heart Study.
Funding:
The EPIC-Norfolk Study was supported by Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143. The Copenhagen City Heart Study is supported by the Danish Heart Foundation, Danish Medical Research Council, and Herlev and Gentofte Hospital, Copenhagen University Hospital. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 667837 (REPROGRAM). GKH is holder of a Vidi grant [016.156.445] from the Netherlands Organisation for Scientific Research (NWO) and is supported by a grant from the European Union [TransCard: FP7-603091-2].
Footnotes
Conflicts of interest:
RV, RH, AL, LS, SV, NJW, KTK: none declared. GKH reports that his institution has received lecturing fees and advisory boards from Amgen, Sanofi-Aventis, Regeneron, Pfizer and the Medicines Company. SMB has participated in advisory boards for Pfizer and Sanofi-Aventis. BGN has received lecture and/or consultancy honoraria from Ionis Pharmaceuticals, Amgen, Sanofi, and Regeneron. ESS reports that his institution has received lecturing fees and advisory board fees from Amgen, Regeneron, Sanofi, Akcea, Novartis and Athera.
References
- 1.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 8.Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): A pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar M, Pilote L, Dufresne L, Engert JC, Thanassoulis G. Lipoprotein(a) Interactions With Low-Density Lipoprotein Cholesterol and Other Cardiovascular Risk Factors in Premature Acute Coronary Syndrome (ACS) J Am Heart Assoc. 2016;5:1–9. doi: 10.1161/JAHA.115.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–1370. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 12.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. Br J Cancer. 1999:95–103. [PubMed] [Google Scholar]
- 13.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S46–8. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011;270:65–75. doi: 10.1111/j.1365-2796.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 15.Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 16.Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Borén J, Chapman MJ, et al. European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federa. Eur Heart J. 2016;37:1944–1958. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 19.Viney NJ, Yeang C, Yang X, Xia S, Witztum JL, Tsimikas S. Relationship between ‘LDL-C’, estimated true LDL-C, apolipoprotein B-100, and PCSK9 levels following lipoprotein(a) lowering with an antisense oligonucleotide. J Clin Lipidol Elsevier Inc. 2018:1933–2874. doi: 10.1016/j.jacl.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J, Grover S, Gupta M, Hegele RA, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 21.Marcovina SM, Albers JJ, Scanu aM, Kennedy H, Giaculli F, Berg K, Couderc R, Dati F, Rifai N, Sakurabayashi I, Tate JR, Steinmetz a. Use of a reference material proposed by the International Federation of Clinical Chemistry and laboratory medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 22.Verbeek R, Boekholdt SM, Stoekenbroek RM, Hovingh GK, Witztum JL, Wareham NJ, Sandhu MS, Khaw K-T, Tsimikas S. Population and Assay Thresholds for the Predictive Value of Lipoprotein(a) for Coronary Artery Disease: The EPIC-Norfolk Prospective Population Study. J Lipid Res. 2016;57:697–705. doi: 10.1194/jlr.P066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, Oberhollenzer F, Egger G, Utermann G, Willeit J. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation. 1999;100:1154–1160. doi: 10.1161/01.cir.100.11.1154. [DOI] [PubMed] [Google Scholar]
- 24.Luc G, Bard J-M, Arveiler D, Ferrieres J, Evans A, Amouyel P, Fruchart J-C, Ducimetiere P. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis. 2002;163:377–384. doi: 10.1016/s0021-9150(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 25.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: An analysis from the JUPITER trial (justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz GG, Ballantyne CM, Barter PJ, Kallend D, Leiter LA, Leitersdorf E, McMurray JJV, Nicholls SJ, Olsson AG, Shah PK, Tardif J-C, et al. Association of Lipoprotein(a) With Risk of Recurrent Ischemic Events Following Acute Coronary Syndrome. JAMA Cardiol. 2017;80220:1–5. doi: 10.1001/jamacardio.2017.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi H, Miyauchi K, Kasai T, Tsuboi S, Ogita M, Naito R, Sai E, Fukushima Y, Katoh Y, Okai I, Tamura H, et al. Impact of lipoprotein(a) as residual risk on long-term outcomes in patients after percutaneous coronary intervention. Am J Cardiol. 2015;115:157–160. doi: 10.1016/j.amjcard.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S. Lipoprotein(a): novel target and emergence of novel therapies to lower cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2016;23:157–164. doi: 10.1097/MED.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 30.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England) 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 32.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet Elsevier Ltd. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 34.Sabatine MS, Giugliano RP, Keech A, Honarpour N, Wang H, Liu T, Wasserman SM, Scott R, Sever PS, Pedersen TR. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94–101. doi: 10.1016/j.ahj.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Robinson JG, Stone NJ. Identifying Patients for Aggressive Cholesterol Lowering: The Risk Curve Concept. Am J Cardiol. 2006;98:1405–1408. doi: 10.1016/j.amjcard.2006.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.