Abstract
Aminorex (5-phenyl-4,5-dihydro-1,3-oxazol-2-amine) and 4-methylaminorex (4-methyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine) are psychostimulants that have long been listed in Schedules IV and I of the UN Convention on Psychotropic Substances of 1971. However, a range of psychoactive analogs exist that are not internationally controlled and therefore often classified as new psychoactive substances (NPS). Aminorex analogs encompass failed pharmaceuticals that reemerged as drugs of abuse, and newly synthesized substances that were solely designed for recreational use by clandestine chemists. NPS, sometimes also referred to as “designer drugs” in alignment with a phenomenon arising in the early 1980s, serve as alternatives to controlled drugs. Aminorex and its derivatives interact with monoaminergic neurotransmission by interfering with the function of monoamine transporters. Hence, these compounds share pharmacological and neurochemical similarities with amphetamines and cocaine. The consumption of aminorex, 4-methylaminorex and 4,4’-dimethylaminorex (4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine) has been associated with adverse events including death, bestowing an inglorious fame on aminorex-derived drugs. In this review, a historical background is presented, as well as an account of the pharmacodynamic and pharmacokinetic properties of aminorex and various analogs. Light is shed on their misuse as drug adulterants of well-established drugs on the market. This review not only provides a detailed overview of an abused substance-class, but also emphasizes the darkest aspect of the NPS market, i.e. deleterious side effects that arise from the ingestion of certain NPS, as knowledge of the pharmacology, the potency or the identity of the active ingredients remains obscure to NPS users.
Keywords: Psychostimulants, designer drugs, new psychoactive substances, aminorex, monoamine transporters, drug abuse
Introduction
New psychoactive substances (NPS) are drugs that are not listed in the United Nations international drug control conventions of 1961 and 1971 but that may pose comparable threats to public health. The NPS term tends to be commonly applied to those substances that have emerged in the last decade.1–3 The market is exceedingly dynamic - on a global level, 803 NPS have been reported to the United Nations Office on Drugs and Crime (UNODC) in the period between 2009–2017.4 Within the European Union, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is monitoring over 670 substances that have appeared over the last two decades5. The drugs encountered on the NPS market are quite diverse in nature but include synthetic cannabinoid receptor agonists affecting the endocannabinoid system, psychostimulants (largest group), hallucinogens, analgesics, central nervous system depressants and others4.
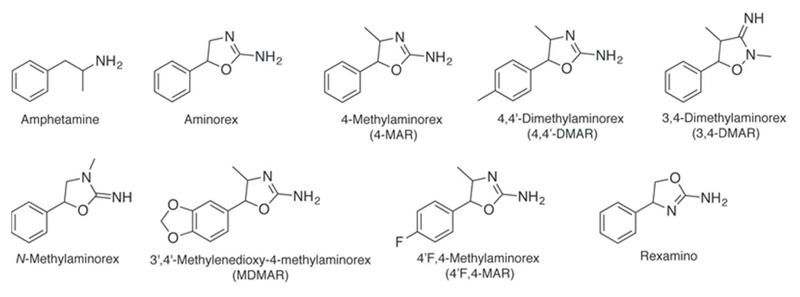
The psychostimulants aminorex (5-phenyl-4,5-dihydro-1,3-oxazol-2-amine) and 4-methylaminorex (4-methyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine, 4-MAR) (see Figure 1) have been listed (Schedules IV and I, respectively) in the UN Convention on Psychotropic Substances of 1971 [6]. Aminorex was originally developed as an appetite suppressant with marketing authorization in Europe but was then removed from the market in the late 1960s due to pronounced adverse effects. In the 1980s, the aminorex analog 4-MAR appeared on the drug market and more recently, 4,4′-dimethylaminorex (4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine, 4,4’-DMAR) made its appearance (see below, Figure 1).
Figure 1.
Chemical structures of amphetamine and known aminorex analogs.
Aminorex and its analogs possess pronounced central nervous system (CNS) effects and, due to similarities in their pharmacological profile, might be classified as amphetamine-type stimulants. Studying the aminorex family of drugs is worthwhile because it highlights the multidisciplinary challenges faced by policymakers, clinicians, law enforcement, users and public health professionals. NPS mimic the effects of controlled drugs (including medicines) but are in many cases not controlled at the time when they are released onto the drug markets7,8. This has led to large-scale manufacturing of many analogs and derivatives in an effort to bypass these controls. As will be detailed below, even some minor changes to established structural templates can significantly change the pharmacological profile, which can result in severe adverse effects including deaths, thus, requiring the need for in-depth research. In addition, the NPS market often lacks control and regulation and consumers are prone to being sold mislabeled or heavily adulterated products, which has also been observed with aminorex analogs. This family of drugs has been around for over 50 years and even though some analogs have been banned, the opportunity for NPS entrepreneurs and organized crime groups to explore new analogs and derivatives has never been greater.
Historical Context
Aminorex (Figure 1) was first described by Poos and colleagues in 1963 as one of several 2-amino-5-aryl-2-oxazolines with anorectic and CNS stimulating properties9. Their employer McNeil Laboratories consequently filed a patent claim, detailing various routes of synthesis and emphasizing the potent CNS activity of aminorex10. The patent claim also accentuates the importance of the introduction of new anorectic drugs to the market as an alternative to the, at the time, widely used amphetamine derivatives. To contextualize: From the year 1944 onwards, amphetamine derivatives, such as desoxyephedrine (methamphetamine), were used in the treatment of obesity in the U.S.A.11 While other amphetamine derivatives were developed for the same indication, concerns with respect to their marked cardiovascular side effects and dependence-producing properties emerged. Aminorex was considered an alternative and, by the name of Menocil and Apiquel, sold over the counter between 1965 and 1968 in Austria, Germany and Switzerland12,13. Initial results suggested that aminorex appeared to be a safe and effective alternative to amphetamine derivatives as anorectic medication14. At the same time, Gurtner et al., amongst others, noticed an increase in the incidence of patients suffering from pulmonary hypertension in Bern, Switzerland15. In the preceding year, Kay and colleagues showed that oral ingestion of a substance can bring about the development of pulmonary hypertension in rats16. Soon, the connection was made that the ingestion of aminorex was responsible for the five- to twenty-fold increase in patients suffering from pulmonary hypertension17–20. The vascular lesions (plexogenic arteriopathy) found in patients who died from the ingestion of aminorex were consistent with those associated with idiopathic pulmonary arterial hypertension12,21. It was reported that, in Bern, 20% of those admitted to the hospital due to aminorex intake died at the time, with 50% dying in the following ten years18,22,23. Still, in general only between 0.2 and 3% of those who had, at the time, ingested aminorex suffered from symptomatic pulmonary arterial hypertension19,24–26. A more recent epidemiological study corroborated that the pulmonary hypertension epidemic had correctly been attributed to aminorex consumption20. Interestingly, the occurrence of pulmonary hypertension, following oral intake of aminorex could not be replicated in animal model studies17,27–34. These two observations suggests that individual genetic predisposition might have played a major role in the etiology of drug-induced pulmonary hypertension19. Current research suggests that the combination of genetic predisposition (mainly a mutation in the bone morphogenetic protein receptor type 2; BMPR2) and the presence of other risk factors (such as the oral intake of aminorex) increases the risk of developing pulmonary arterial hypertension24,35,36. The next steps in the discovery relating to mechanisms of action responsible for aminorex-induced pulmonary arterial hypertension were soon underway37. Weir and colleagues reported an aminorex-induced inhibition of K+ channels in the lung, as well as increased pulmonary artery pressure38,39. Nevertheless, most explanations rather emphasize the drug’s interaction with the serotonergic system and SERT (serotonin transporter; SLC6A4) in particular40. Seiler and colleagues have shown that aminorex causes release of serotonin (5-HT) at SERT, and that it inhibits 5-HT uptake, as well as monoamine oxidases41,42. Rothman et al. could confirm aminorex to be a substrate of SERT, accumulating intracellularly and causing the efflux of 5-HT43. SERT transactivates the platelet-derived growth factor receptor β (PDGFRβ) and SERT, 5-HT and 5-HT receptors (mostly 5-HT1B) regulate the S100A4/Mts1 gene, inducing c-fos and cyclin D1 as well as Rho-kinase expression and activate tyrosine kinases, with each aspect being a contributing factor to pulmonary artery smooth muscle cell proliferation35,40,44–47. It is therefore likely that the interplay of individual genetic predisposition and oral intake of aminorex led to the pulmonary arterial hypertension epidemic, thus, ending the official, over the counter sale of aminorex in 196848. The role of serotonergic mechanisms associated with the proliferation in pulmonary arterial hypertension has been reviewed49.
Although little is known about illicit production and circulation of aminorex during the 1980s, it is interesting to note that this drug emerged decades later in a somewhat surreptitious form, which sparked new interest in the substance. A little backstory is necessary to understand this new development. The anthelmintic drug levamisole is mostly used for veterinary purposes but was originally also intended for use in humans50–52. It is currently still being used for treatment in countries where the incidence of helminthiasis in humans is high across the population51. New indications, such as the treatment of the steroid-sensitive idiopathic nephrotic syndrome, treatment of colorectal cancer, various dermatologic conditions, pulmonary tuberculosis, recurrent aphthous stomatitis and severe aplastic anemia, have been explored53–58. Illicitly, levamisole is being used as an adulterant in street samples of cocaine. This practice has been increasingly reported from the onset of the new millennium onwards both in the U.S.A. and Europe59,60. In the beginning, the use of levamisole as an adulterant was considered enigmatic59. At the same time, a scandal occurred in the field of horseracing: the amphetamine-like drug aminorex was detected in the urine of dozens of horses, implicating the owners in illegal doping61. The common denominator in all the alleged doping cases was that the horses had been given the anthelmintic drug levamisole beforehand61. Barker et al., Bertol et al. and Ho et al. could show that aminorex was indeed formed as a metabolite of levamisole in both horses and humans61–63. This explained the detection of aminorex in drug screenings following the administration of levamisole, both as a drug and as an adulterant of cocaine. Interestingly, Ho et al. also detected the presence of 4-phenyl-4,5-dihydro-1,3-oxazol-2-amine (rexamino, Figure 1) in horses following subcutaneous administration of levamisole63.
Cocaine is one of the most frequently consumed illicit substances and global cocaine production has been increasing during the last couple of years4,64. Common adulterants in cocaine include acetaminophen, acetylsalicylic acid, caffeine, lidocaine, (synthetic) cathinones, hydroxyzine and levamisole65. The latter is currently the most frequently found adulterant, with reports suggesting that between 60% and 80% of all investigated cocaine samples are adulterated with levamisole65–69. The abuse of levamisole-adulterated cocaine has been thoroughly documented and is associated with influenza-like symptoms, ANCA (anti-neutrophil cytoplasmic antibody) positive and negative vasculitis, causing retiform purpura, and glomerulonephritis, pyoderma gangrenosum, cutaneous necrosis (often of the ears, face and/or legs), neutropenia or agranulocytosis, leukocytopenia and multifocal inflammatory leukencephalopathy70–105. The media has picked up this story and reports about “flesh-eating cocaine” surface from time to time106–108.
Two major hypotheses, aiming to explain the widespread use of levamisole as an adulterant in cocaine, are particularly noteworthy70,109. First of all, levamisole is widely used as a prophylactic anthelmintic drug in the livestock industry around the world and prominently so in agricultural societies110. Additionally, levamisole is economically highly viable and resembles cocaine in melting point, look and taste70. The second hypothesis is centered on the effects of cocaine and levamisole although it is unclear whether the decision of drug dealers to adulterate the street drug with levamisole deliberately considered its biological fate and its overall CNS effect on the user. Cocaine is an inhibitor of monoamine (re)uptake, binding to SERT, DAT (dopamine transporter; SLC6A3) and NET (norepinephrine transporter; SLC6A2)111. On the other hand, levamisole is a nicotinic acetylcholine receptor agonist, which can cause the release of monoamines and inhibits monoamine oxidases and catechol-O-methyl-transferase112–116. In itself, it is not a very potent inhibitor of monoamine uptake117. It appears that the levamisole metabolite aminorex plays an additional role in levamisole-mediated effects. As discussed later, aminorex is a potent releasing agent at SERT, DAT and NET, a quality it shares with amphetamine and many amphetamine-type stimulants117. Concerning the biological half-life of levamisole and aminorex, a certain synergism with cocaine is noteworthy. Levamisole and aminorex have a longer half-life than cocaine and can therefore prolong the drug experience117–119. Additionally, levamisole has been shown to potentiate the rewarding and stimulating effects of cocaine in rats68,109,120. It might be the case that the hypotheses are complementary in nature and have both contributed to the fact that levamisole is, at this point in time, the most frequently used adulterant in cocaine. One question that remains to be answered is the role of the metabolite aminorex in the effects (and side-effects) of levamisole-adulterated cocaine. Hess and colleagues have shown in human plasma and urine samples collected from drivers (nota bene: under the influence of cocaine) that levamisole was present in approximately 40% of the plasma samples. In 10% of the samples, aminorex was detected, albeit the concentrations were near the detection limit119. In two post mortem urine samples with very high levamisole concentrations, aminorex quantification was also significant119. Eiden et al. found levamisole in 75% of all analyzed urine samples yet aminorex could not be detected121. In healthy individuals who ingested high doses of pure levamisole, comparatively small amounts of aminorex were found in plasma and urine118,119. It seems to be the case that the conversion rate from levamisole to aminorex might be quite low121,122. Still, Karch et al. describe the case of a deceased long-term poly-drug user with systemically detectable levamisole and aminorex levels who might have suffered from idiopathic pulmonary arterial hypertension and it has been shown that levamisole accumulates in lung tissue123–126. Deaths due to levamisole or aminorex toxicity associated with the consumption of cocaine have rarely been reported126,127. At this point, it is known that only a small fraction of levamisole is metabolized into aminorex and its (side-)effects seem negligible when compared to those observed with levamisole. Future studies should explore the prevalence of pulmonary arterial hypertension in long-term, high-dose users of levamisole-adulterated cocaine.
As mentioned above, there is no evidence of widespread consumption of aminorex in its own right, although Brewster and Davis reported in 1991 that aminorex has been, in at least one instance, misrepresented as 4-MAR to circumvent legal obstacles128. It appears that aminorex is mostly known for its use as an anorectic agent, associated with pulmonary arterial hypertension, and as a metabolite of the currently most widely used cocaine adulterant levamisole.
4-MAR (Figure 1) represents another anorectic substance first published by Poos and colleagues in 19639. Yet, 4-methylaminorex was never marketed as an anorectic drug11. Approximately 25 years later, in the late 1980s, 4-MAR surfaced as a recreational drug and was sold by the street names “U4Euh” “ICE” or “4-MAX” in the United States and in Europe129. One fatality related to the ingestion of 4-MAR has been reported with the autopsy report suggestive of heart and lung failure, including pulmonary and brain edema130. In addition, a case study describes three family members, involved in the manufacturing and consumption of 4-MAR, who suffered from pulmonary hypertension131. This case report suggests that not only aminorex but also its derivative(s) might be appropriate stimuli to cause pulmonary hypertension in susceptible individuals. The substance was swiftly added to the list of controlled substance in the US and some European countries129. As mentioned above, both aminorex and 4-MAR are now controlled internationally and listed in Schedules IV and I of the United Nations Convention on Psychotropic Substances 19716.
Another twenty-five years later, a new aminorex analog appeared on the European drug market: 4,4'-DMAR (Figure 1) was introduced to the European NPS market originally offered for sale under the brand name “Serotoni”132. The compound was first encountered in December 2012 in a customs seizure in the Netherlands133. Thirty-one deaths occurring between June 2013 and February 2014 in Europe were associated with the consumption of 4,4'-DMAR, with the substance claiming eight victims in Hungary, one in Poland and 22 in the United Kingdom133. Clinical notes and autopsy findings reported to the EMCDDA revealed hyperthermia, myoclonus, seizures, hallucinations, disorientation, agitation, internal bleeding and lung and brain edema, as well as lung and heart failure as the major clinical features133. The fatal outcomes were considered consistent with serotonin toxicity and norepinephrine-mediated cardiotoxicity133–136. In addition, the combination of 4,4'-DMAR with other drugs affecting the monoaminergic system might have contributed to the deaths133. 4,4'-DMAR tablets mimicked the style of MDMA (1-(2H-1,3-benzodioxol-5-yl)-N-methylpropan-2-amine, 3,4-methylendioxymethylamphetamine, “ecstasy”/”molly”) tablets with respect to colors, shapes and logos133. Unfortunately, 4,4'-DMAR was seemingly misrepresented as MDMA and used as an adulterant and/or masking agent to hide the lack of the desired drug. It has been shown (see below) that 4,4’-DMAR acted as a 5-10 times more potent non-selective monoamine transporter releasing agent compared to MDMA. From this perspective, it seems likely that users who thought they had procured ecstasy died from acute overdoses as a consequence of norepinephrine, serotonin and dopamine toxicity134,135. These tragic events highlight one of the major problems of an unregulated, illicit NPS market – the misrepresentation of substances and the addition of under-researched adulterants that can lead to adverse effects including fatalities.
The identification of 4,4’-DMAR and its association with deaths occurring between June 2013 and February 2014 in Europe led to swift responses. In Europe, the EMCDDA launched a Joint Report to assess the available information in early 2014 followed by a risk assessment carried out by the EMCDDA’s Scientific Committee in September 2014133,137. Upon reviewing the available information, a decision was made by the European Council to subject 4,4’-DMAR to Europe-wide control measures in 2015138. At a global level, and under the auspices of the World Health Organization, 4,4’-DMAR (amongst other substances) underwent the critical review stage at the thirty-seventh meeting of the Expert Committee on Drug Dependence in November 2015139. It was recommended to place 4,4’-DMAR in Schedule II of the UN 1971 Convention, which was subsequently confirmed by vote by the Commission on Narcotic Drugs in 18 March 2016, thus, placing it under international control140,141. Interestingly, 4,4’-DMAR still appears to be available for purchase from online vendors, mostly based in China e.g.142, although it is unclear whether these vendors are able to supply it. In an Internet snapshot study published in 2014, it was reported that 4-MAR was advertised more frequently than 4,4'-DMAR143.
The 4,4’-DMAR case demonstrated that the NPS market can employ mechanisms of self-correction and that when a substance is noticed to be dangerous, some Internet retailers abstain from selling this substance and shift their focus to other compounds. At the same time, the fact that 4,4’-DMAR was surreptitiously sold in mislabeled forms suggests the potential involvement of organized crime groups that sell NPS on the traditional illicit market as well. However, the discussions surrounding 4,4’-DMAR on user fora remains active to some extent.144 Users seem to be hesitant to consume 4,4'-DMAR because of its association with the fatal intoxication cases but younger users who missed the opportunity to consume 4-MAR when it was uncontrolled in the 1980s, appear to express interest in a revival145–147. NPS users and clandestine chemists turn to various new substituents and derivatives of aminorex with the hope to conserve the desired effects while simultaneously decreasing side-effects and toxicity (see Structural Features).
Structural Features
Aminorex and its derivatives are amphetamine-type (1-phenylpropan-2-amine) stimulants that contain one amino-oxazoline (4,5-dihydro-1,3-oxazol-2-amine) and one phenyl ring (Figure 1). One methyl group is added to the amino-oxazoline ring for 4-MAR (2-amino-4-methyl-5-phenyl-2-oxazoline) and MDMAR (5-(2H-1,3-benzodioxol-5-yl)-4-methyl-4,5-dihydro-1,3-oxazol-2-amine, Figure 1). Additionally, for 4,4′-DMAR (4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine), the phenyl ring is also methylated. Structurally, MDMAR constitutes an interesting MDMA and 4-MAR hybrid, containing the characteristic 1,3-dioxolane ring.148
Aminorex contains one chiral carbon and the available information indicates that it has only been evaluated as a racemic mixture. 4-MAR and other closely related analogs, for example disubstituted at C4 and C5, give rise to four stereoisomers, namely trans-(4R,5R), trans-(4S,5S), cis-(4S,5R) and cis-(4R,5S), and/or to two racemates, (±)-trans and (±)-cis forms. Marked differences in activity and potency between cis- and trans isomers have been reported for some of these compounds.134,148–154 (see Pharmacodynamics & Pharmacokinetics). It has been shown for many amphetamine-type stimulants that isomers with an (S)-configured α-methyl group are more potent pharmacodynamically than the (R)-isomers155–157.
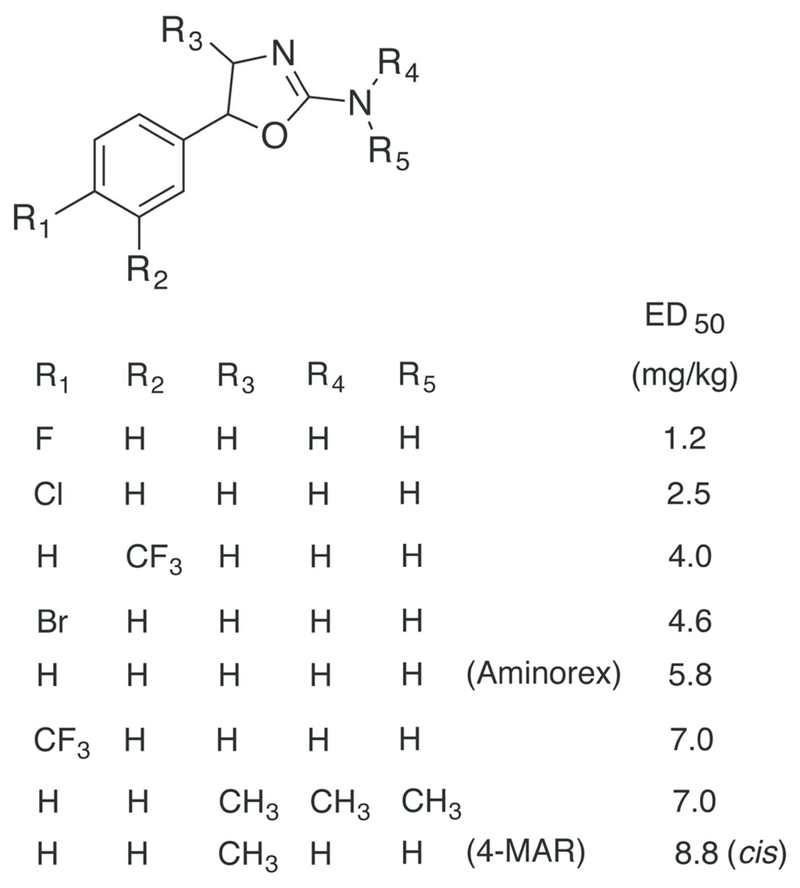
The 4,4′-DMAR isomer 3,4-dimethylaminorex (3,4-MAR, Figure 1) has been considered structurally similar to methamphetamine155. It has first been described by Poos and colleagues9. The analog seems to be a compound that has rarely been reported as a street drug but has rather been experimented with in scientific laboratories153,155,158. However, similar to N-methylaminorex (Figure 1), it has been detected in the European Union in 2015159. Various other, seemingly lesser known derivatives of aminorex have been mentioned in publications or the Internet but have not been investigated in a systematic manner160. Poos et al. have characterized various other locations (on the phenyl and oxazoline ring) and substituents and an overview of aminorex analogs has been published by Trachsel et al.9,161 (Figure 2).
Figure 2.
List of aminorex analogs in decreasing order of potency concerning their anorectic activity in rats (reduction in consumption of beef broth after oral administration)9,162. In comparison, the ED50 value for dextroamphetamine sulfate was 6.8 mg/kg9.
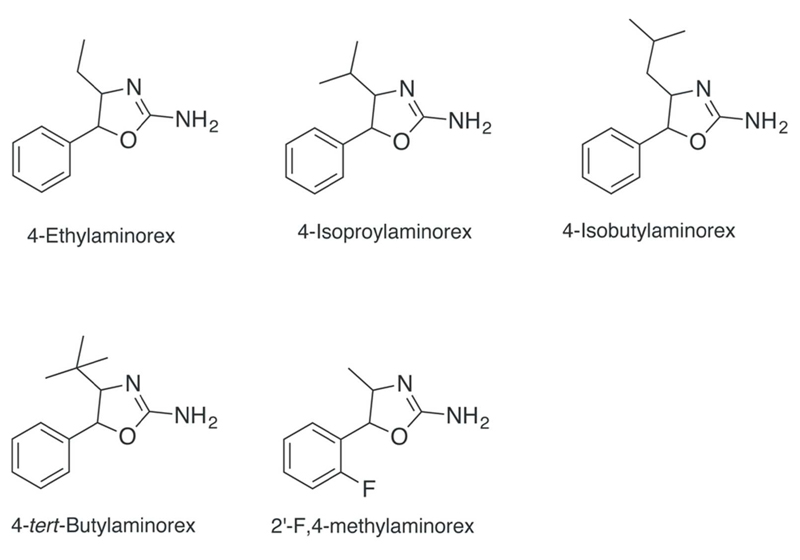
Since the discovery of 4-MAR on the street market in the 1980s it is not surprising that this has caught the attention of law enforcement officials. In a reflective piece on future drugs of abuse published by Cooper as part of an international symposium held in 1988, the author lists aminorex, 4-MAR and six other analogs of particular interest (anorectic potency in rats comparable to amphetamine) (Figure 2) that were based on the work on Poos and colleagues from McNeil Laboratories9. Although not specifically mentioned in this personal communication between Poos and Cooper, the author also suspected the 4-fluoro- and 4-chlorophenyl analogs of 4-MAR and N,N-dimethyl-4-MAR to have “psychotomimetic” properties10,162,163. Other examples disclosed in a patent published by Poos included 4-alkyl analogs of aminorex (Figure 3) and methoxyphenyl-substituted analogs, although detailed information was not provided10. Information about their occurrence on the streets is currently unavailable.
Figure 3.
Aminorex analogs claimed to have CNS stimulant activity10. The synthesis and exploration of effects of 2’-F,4-Methylaminorex has been described by a contributor to an online user forum.
Similarly, the literature based on the work carried out at McNeil Laboratories sparked interest among individuals interested in the chemistry and psychopharmacological exploration of psychoactive substances, collectively interacting through an online discussion forum called The Hive that was in operation between 1997–2004. Many of the posts are still available in archived form online164. The idea of preparing 4,4′-DMAR following established aminorex-type chemistry was proposed in 2003 (the idea apparently emerged during the synthesis of 4-methylmethcathinone that several years later became known as the NPS mephedrone), although it is unclear whether this was taken further by any other Hive member165.
More recently, a user of another online drug forum shared information on a method of synthesis and effects related to 5-(2-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine, i.e. the ortho-fluoro analog of 4-MAR (Figure 3)166,167. It was stated that the fluorinated analog was believed to be safer and less cardiotoxic,166 which illustrates the persistent interest of the NPS community in aminorex derivatives while expressing a cautious approach toward minimizing possible side-effects and toxicity. Still, there seems to be a scientific basis for such an assumption because Rickli and colleagues have shown that 4-methyl and 4-bromo substitutions lead to more potent serotonergic properties than 4-fluoro groups168. It appears that users expressed an interest in the fluoro-substituted analog169 and it was also mentioned that it might be already available for purchase170. A recent report from an online test purchase conducted by the Slovenian National Forensic Laboratory, suggests that the para-fluoro analog of 4-MAR (5-(4-fluorophenyl)-4-methyl-4,5-dihydro-1,3-oxazol-2-amine, 4’F,4-MAR) is also available for purchase from Internet vendors171 (Figure 1). This substance has been offered for sale under the label “4-FPO” and mostly consists of the trans-isomer171. The mislabeled substance is currently being discussed in various user fora and sometimes described as a phenmetrazine derivative, which highlights the potential for confusion172–175. Its synthesis has even been described in detail by a clandestine chemist176. These new developments illustrate the abiding relevance of and interest in aminorex and its analogs in the NPS scene.
Synthesis
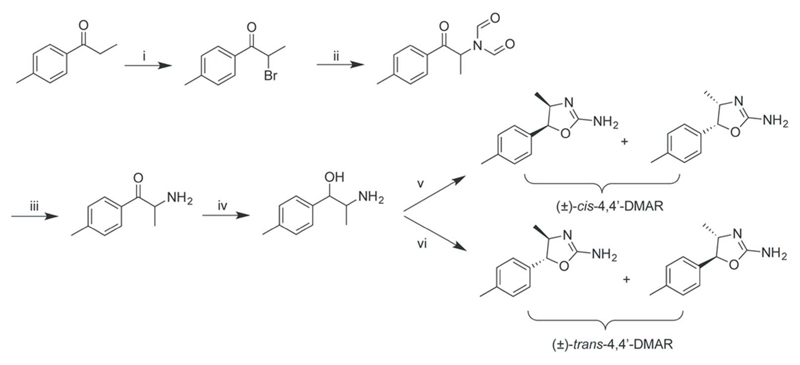
The procedure reported by Poos and co-workers for the preparation of 4-MAR analogs involved the reaction of 2-amino-1-phenylpropan-1-ol precursors with cyanogen bromide followed by cyclization. Correspondingly, the use of 2-amino-1-phenylethanol has been described to provide aminorex9. Furthermore, Poos et al.9 and Klein et al.129 showed that the reaction of cyanogen bromide with the norephedrine led to the formation of the cis-racemate whereas the employment of norpseudoephedrine gave the trans-form of 4-MAR. Interestingly, when investigating a case related to the clandestine synthesis of 4-MAR, it was found that norephedrine could be converted predominantly to the trans-product by reaction with potassium cyanate177, an idea that has been discussed on The Hive forum178. The recent characterization of a 4’F-4-MAR sample obtained from a test purchase revealed the presence of the trans-form and the detection of the cis-form as a minor component171. In analogy to what has been described during the forensic investigation of the trans-4-MAR synthesis177, it would be interesting to consider that the conversion of the 2-amino-1-(4-fluorophenyl)propan-1-ol precursor might have involved potassium cyanate as well. Interestingly, the procedure described for the synthesis of 4’F-4-MAR on the Internet also involved the use of this same reagent although the described procedure did not reveal whether the resulting aminorex analog represented the trans- or cis-form176. The synthesis of racemic cis- and trans-4,4’-DMAR is shown in Figure 4, which was based on the reaction of 2-amino-1-(4-methylphenyl)propan-1-ol with either cyanogen bromide or potassium cyanate. 1-(4-Methylphenyl)propan-1-one served as the starting material for the preparation of the alcohol intermediate (Figure 4)134. Correspondingly, cis- and trans-MDMAR have been prepared via the norepinephrine-type precursor 2-amino-1-(2H-1,3-benzodioxol-5-yl)propan-1-ol148. An interesting observation reported for MDMAR (and not for 4,4’-DMAR) was that the racemic cis-form converted to the trans-form when exposed to water-containing solutions but not when exposed to acetonitrile, which suggested a potential role of water in this conversion process148.
Figure 4.
Synthesis of racemic 4,4’-DMAR134. i) Br2/dichloromethane, 1h at room temperature; ii) sodium diformylamide/acetonitrile, 4 h at reflux; iii) HCl/ethanol, overnight at room temperature; iv) sodium borohydride/methanol, addition over 1.5 h period; v) cyanogen bromide/anhydrous sodium acetate/methanol, 3.5 h on ice, saturated sodium carbonate; vi) potassium cyanate/water, 3 h at reflux, 2 M HCl, 2 h at reflux, saturated aqueous sodium carbonate.
Pharmacodynamics & Mechanisms of Action
It was known to Poos and colleagues that aminorex was not only an anorectic drug but also a drug with CNS stimulating properties9. Another study from the same laboratory revealed that aminorex was a releasing agent of catecholamines179. Rothman and colleagues later confirmed that aminorex was not a mere non-transported uptake inhibitor of monoamine transporters (like cocaine) but rather a releasing agent similar to amphetamine and that its main effects derived from its interaction with the monoamine transporters NET, DAT and SERT180. Since then, various groups have also performed transporter release assays, classifying aminorex and its derivatives as monoamine transporter substrates and releasing agents of monoamines134,135,148.
Brandt et al. and McLaughlin and colleagues have demonstrated that aminorex, 4-MAR, 4,4'-DMAR and MDMAR were potent releasing agents in rat brain synaptosomal preparations at DAT, SERT and NET, with the lowest EC50 (half maximal effective concentration) at DAT, followed closely by NET and SERT134,148 (see Table 1). Furthermore, it has been shown that the presence of additional methyl groups added onto aminorex to form 4-MAR and 4,4'-DMAR have barely changed its potency to cause efflux of monoamines at DAT and NET but have immensely increased its releasing potency at SERT134. The DAT/SERT ratio, with higher values indicating greater selectivity for DAT over SERT, was 45 for aminorex, 31 for 4-MAR and only 2 for 4,4'-DMAR134. In closely related substances, it has been established that substitution of the para-position led to decreased selectivity for DAT over SERT168,181,182. In comparison, MDMA’s DAT/SERT ratio is 0.6 but its EC50 was five to ten times higher than that reported for 4,4'-DMAR148. MDMAR’s potency was slightly lower than that of 4,4'-DMAR but higher than MDMA’s148.
Table 1. In vitro uptake inhibition, release and affinity data for aminorex and its analogs.
| Aminorex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAT | NET | SERT | Reference | Cell system | ||||||
| Inhibition IC50 in nM | Release EC50 in nM | Affinity Ki in nM | Inhibition IC50 in nM | Release EC50 in nM | Affinity Ki in nM | Inhibition IC50 in nM | Release EC50 in nM | Affinity Ki in nM | ||
| -- | 9.1±0.9 | -- | -- | 15.1±3.5 | -- | -- | 414±78 | -- | Brandt et al.134 | Rat brain synaptosomes |
| 710±1050 | -- | -- | 1970±1200 | -- | -- | 26,290 ±1,030 | -- | -- | Hofmaier et al.117 | HEK293 cells |
| [Ki = 216±7] | -- | 784±16 | -- | -- | -- | [Ki = 1,244±106] | -- | 27,700±990 | Rothman et al.43 | Rat brain synaptosomes |
| [Ki = 216±7] | 49.4±7.5 | -- | [Ki = 54.5±4.8] | 26.4±2.8 | -- | [Ki = 1,244±106] | 193±23 | -- | Rothman et al.111,180,185,186 | Rat brain synaptosomes |
| -- | -- | -- | -- | -- | -- | [Ki = 1,380] | [% above basal flow: 51-116] | -- | Tao et al.187 | Rat brain tissue |
| 4-methylaminorex | ||||||||||
| -- | 1.7±0.2 | -- | -- | 4.8±0.9 | -- | -- | 53.2±6.8 | -- | Brandt et al.134 | Rat brain synaptosomes ((±)-cis-4-MAR) |
| 4,4’-dimethylaminorex | ||||||||||
| -- | 8.6±1.1 | -- | -- | 26.9±5.9 | -- | -- | 18.5±2.8 | -- | Brandt et al.134 | Rat brain synaptosomes ((±)-cis-4,4’-DMAR) |
| 1040 (95% CI: 848 to 1282) | -- | 533.8±44.2 | 500 (95% CI: 447 to 553) | -- | 266.8±57 | 1750 (95% CI: 1446 to 2126) | -- | 1881±183.1 | Maier et al.135 | HEK293 cells ((±)-cis-4,4’-DMAR) |
| -- | 10.9±0.7 | -- | -- | 11.8±2.0 | -- | -- | 17.7±2.3 | -- | McLaughlin et al.148 | Rat brain synaptosomes ((±)-cis-4,4’-DMAR) |
| -- | 24.4±2.7 | -- | -- | 31.6±4.6 | -- | -- | 59.9±17.2 | -- | McLaughlin et al.148 | Rat brain synaptosomes ((±)-trans-4,4’-DMAR) |
It has recently been shown that 4,4'-DMAR binds to monoamine transporters with higher affinities compared to monoamine receptors, albeit it has also been shown to bind to hDAT, hNET, hSERT and 5-HT2A and 5-HT2C receptors with relatively low affinities135 (see Table 1). Due to the lack of interaction with the trace amine-associated receptor 1 (TAAR1), 4,4'-DMAR is suspected to be unable to trigger the auto-inhibitory pathway that, for example, MDMA possesses at least in rodents135,183,184.
Poos and colleagues could not notice differences in potency between cis and trans-4-MAR as far as the ability to reduce food intake in rats was concerned9. As previously mentioned, amphetamine-type stimulants that possess an (S)-configured α-methyl group are generally more potent in producing effects in the central nervous system than the respective (R)-isomers188. Correspondingly, it has been shown for 4-MAR that trans-(4S,5S) is more potent than cis-(4S,5R) and cis-(4R,5S) at increasing extracellular dopamine concentrations and thus causing dopamine-related, dose-dependent locomotor activation 149,150,152 (see Table 2). Concerning the compounds’ 5-HT release, it has been shown that cis-(4S,5R) is more potent than trans-(4S,5S) and cis-(4R,5S)149. Glennon and Misenheimer have shown that the trans-(4S,5S) isomer is the most potent isomer at the generalization of (S)-(+)-amphetamine stimuli, followed by the two cis-isomers151. All these studies showed the trans-(4R,5R) isomer have the, by far, weakest potency. Whether a direct relationship between the substance’s potency and its number of (S)-configured methyl groups exists, similar to (S)-amphetamine, remains to be established. While it has been shown for 4-MAR that the (±)-trans racemate is more potent than the (±)-cis racemate to induce stimulus generalization in rats, a study comparing the potency of 4,4'-DMAR and MDMAR racemates in rat brain synaptosomes paints a different picture148,151 (see Table 2). McLaughlin and colleagues point out that the (±)-cis racemates of 4,4'-DMAR and MDMAR seem to be slightly more potent than the respective (±)-trans racemates at evoking efflux at monoamine transporters148.
Table 2. A summary of pharmacodynamic and toxicological studies on aminorex and its analogs.
| Aminorex | ||
|---|---|---|
| Model organism | Results | Study |
| Humans | Toxic doses for humans were determined to be located between 1-2 mg/kg. Symptoms occur between 15 and 120 mins after consumption and are characterised by seizures, respiratory depression, mydriasis, tachycardia, hypertension and hyperpnea. | Borbély et al.189 |
| Hereford calves | An increase in arterial blood pressure but no pulmonary side-effects after daily i.v. injections (0.25 mg/kg) for one month were reported. | Byrne-Quinn & Grover28 |
| Sprague-Dawley rats | A dose of 1.5 μmol/kg, administered i.v., elicited increased motor activity. Stereotypical behaviour was not detected in doses up to 112 μmol/kg. | Costa et al.37 |
| Wistar rats | After administration of 6.5 to 22mg/kg aminorex daily for 118 consecutive days, no anatomical differences of the pulmonary vessels were detectable. | Engelhardt & Hort32 |
| Humans | This retrospective study highlighted that anticoagulant therapy with warfarin in aminorex-caused pulmonary hypertension had beneficial effects on their survival time. | Frank et al.190 |
| Rabbits | Aminorex was found to be an efficient 5-HT releaser in platelets, similar in potency to amphetamine. | Friström et al.191 |
| Human subjects | Two studies (79 and 60 subjects) have proven aminorex (7.5 mg) to be an effective anorectic agent, similar to dextroamphetamine and diethylpropion but more effective than phenmetrazine. Side-effects concerning the CNS, the GI tract and the renal system, as well as leukopenia were reported. | Hadler14 |
| Mongrel cats | 1.0 mg/kg i.p. caused a 90 % decrease in food intake and lead to 81.5 % of a 12 hour session being spent awake, 16.5 % in slow-wave sleep and 2.0 % in paradoxical sleep. | Johnson et al.192 |
| Baboons | Self-injection experiments showed for doses of 0.1 and 0.32 mg/kg/injection cyclical patterns of self-injection, with numbers of injections per day similar to cocaine, suggesting abuse potential. Food intake was decreased significantly at the highest concentration. | Kaminski et al.193 |
| Wistar albino rats; beagle dogs | Treatment with aminorex for 43 (rats) and 20 weeks (dogs) did not reveal evidence of pulmonary vascular disease. | Kay et al.17 |
| Human subjects | Aminorex was shown to be an effective anorectic drug and no relevant side-effects were reported after a daily intake of 10 mg for 120 days (20 patients). | Kew194 |
| Beagle dogs | Treatment of dogs with high doses of aminorex for 13 weeks led to no significant pulmonary and cardiac abnormalities. | Leuschner et al.33 |
| Swine (young) | Aminorex was given for three months but no significant cardiovascular and pulmonary side-effects could be detected. | Mlczoch et al.195 |
| Swine | Up to 15 mg/kg of aminorex per day was fed to swine for four months. No signs of pulmonary arterial hypertension could be detected but a slight decrease in cardiac and pulmonary function was noticeable. | Orr et al.31 |
| Sprague-Dawley rats | Aminorex was shown to be amongst the most potent amphetamine-type stimulants concerning its ability to evoke anorexia and stimulant activity. | Paul et al.196 |
| CHO cells | Aminorex inhibits hKv1.5 channels with a KD > 300 μM. | Perchenet et al.39 |
| Rats | Aminorex was shown to have an ED50 of 5.8 mg/kg. | Poos et al.9 |
| Rats | 3 and 6 mg/kg were administered orally and motor activity was evaluated. Aminorex, when compared to 4-MAR and amphetamine, was shown to reach peak motor activity levels earlier (after 40 minutes) than the other compounds (4-MAR after 80 minutes and amphetamine after 130 minutes). | Poos et al.197 |
| Sprague-Dawley rats | Aminorex was shown to generalise for amphetamine and to have an ED50 of 3.0 μmol/kg. No stimulus generalisation could be obtained with rexamino. | Russell et al.153 |
| Wistar rats | Aminorex induced stereotyped behaviour, lasting three hours at 5.0 mg/kg, administered s.c. in rats pre-treated with α-methyl-p-tyrosine. | Sayers & Handley198 |
| Patas monkeys | Up to 4 mg/kg of aminorex was administered for 347 consecutive days and cardiac catheterization and autopsy results revealed no significant pulmonal and cardiac pathologies. | Smith et al.29 |
| Mongrel dogs | Administration of 1.0 to 1.5 mg/kg aminorex five times per week over two years lead to histologically detectable changes in the pulmonary arteries of 60% of the dogs. The changes were not as severe as those detected in humans suffering from pulmonary arterial hypertension. | Stepanek & Zak34 |
| Chinese hamsters, V79 Chinese hamster cells, Saccharomyces cerevisiae, Escherichia coli, Salmonella typhimurium | In contrast to other amino-oxazoline derivatives, mutagenic effects of aminorex could not be shown in vitro and vivo. Most of the other tested compounds were mutagenic but methylation of the oxazoline ring reduced the detrimental effects. | Suter et al.199 |
| Sprague-Dawley rats | Aminorex caused 5-HT release in rat hypothalamus in a dose-dependent manner. | Tao et al.187 |
| Beagle dogs | 1.5 mg/kg aminorex was orally administered and transient increases of pulmonary and systemic arterial blood pressure but no formation of pulmonary hypertension were noted. | Will & Bisgard30 |
| Rhesus monkeys, CF-1 mice, Sprague-Dawley rats | Aminorex substituted for amphetamine in generalisation tests, served as a positive enforcer (both in monkeys), caused stimulant-mediated locomotor effects at 1.25 mg/kg (mice) and worsened signs of withdrawal (rats). | Woolverton et al.200 |
| Sprague-Dawley rats | Stimulus-generalisation tests revealed that aminorex is recognised by rats as an amphetamine-type stimulant (starting from doses of 0.5 mg/kg). | Young201 |
| Sprague-Dawley rats | 4-MAR (ED50=1.11) was six times less potent than aminorex (ED50=0.22) in cocaine stimulus generalisation experiments. | Young & Glennon202 |
| Sprague-Dawley rats | Aminorex (1 μmol/kg) substituted for S-methcathinone in stimulus generalisation experiments with an ED50 of 0.27 mg/kg. | Young & Glennon203 |
| CBA mice | Between 25 mg/kg were injected intraperitoneally for three times in 24 hours. No significant changes in DOPAC or 5-HIAA, as well as no long-lasting depletion of monoamines could be detected. | Zheng et al.155 |
| 4-methylaminorex | ||
| Sprague-Dawley rats | The trans-4S,5S-isomer was the most potent isomer concerning a suppression of the basal firing rate of spontaneously active dopaminergic neurons (trans-4S,5S > cis-4R,5S = cis-4S,5R >>trans-4R,5R). This effect could be reversed by the addition of haloperidol and the D2 and D3 receptor antagonists eticlopride and sulpiride. The (pre-)treatment with pindolol, fluoxetine, granisetron and phentolamine (amongst others) did not change the effects, while pre-treatment with α-methyl-p-tyrosine and reserpine did. | Ashby et al.150 |
| Sprague-Dawley rats | The isomers of 4-MAR were ranked according to their ability to induce stereotyped behaviour at various doses: trans-4S,5S > cis-4R,5S = cis- 4S,5R >trans-4R,5R. This effect could be attenuated by administration of dopamine receptor antagonists, suggesting a dopaminergic neural causation of 4-MAR’s behavioural effects. | Batsche et al.152 |
| Humans | A case is described where aminorex was misrepresented as 4-MAR. | Brewster & Davis128 |
| Sprague-Dawley rats | Acute changes after administration of a single dose were determined. Between 5 and 20 mg/kg were administered. Tryptophan hydroxylase activity declined in a dose-dependent manner. Locomotor activity was dose-dependent and 20 mg/kg led to clonic seizures, oftentimes fatal. | Bunker et al.204 |
| Humans | A fatality involving 4-MAR is described (blood levels: 21.3 mg/L and urine levels: 12.3 mg/L). | Davis & Brewster130 |
| Humans | A case study is presented where three members of a family that produced and consumed 4-MAR, developed pulmonary hypertension. | Gaine et al.131 |
| Sprague-Dawley rats | Administration of the cis- and trans-racemate led to amphetamine stimulus generalisation with the trans-racemate being three times more potent than the cis-racemate (ED50: trans-4S,5S, 0.25 mg/kg > cis-4S,5R, 1.22 mg/kg = cis-4R,5S, 1.52 mg/kg > trans-4R,5R). | Glennon & Misenheimer151 |
| CF-1 mice | The seizure-causing properties of 4-MAR were evaluated. Its CD50 was determined to be 90μg and its CD97 110 μg, following intracerebroventricular injection. Thus, 4-MAR has a very steep dose-seizure curve. Onset of the seizures was intermediate (between 60-300 sec) and clonus duration was between 30 and 300 sec. After the termination of clonic activity, a second seizure episode followed after a short period of behavioural arrest. Flunarizine and valproate could be shown to be effective in preventing 4-MAR-caused seizures. | Hanson et al.205 |
| Wistar rats | The rank order of potency for elevating extracellular dopamine levels was trans-4S,5S > cis-4S,5R = cis-4R,5S >trans-4R,5R 4-MAR and for elevating 5-HT cis-4S,5R >trans-4S,5S = cis-4R,5S > trans-4R,5R. All isomers caused a decrease of extracellular concentrations of DOPAC and HVA. At lower doses (2.5 and 5.0 mg/kg), the isomers (except for trans-4R,5R) caused rises in locomotor activity and high doses (10 mg/kg) caused biphasic behaviour patterns, with initial rises in locomotor activity being followed by rapid declines and engagement in stereotyped behaviour, ataxia or catatonia. | Kankaanpää et al.149 |
| Baboons, rhesus monkeys | Self-administration experiments highlighted cyclical patterns of self-injection, where days with many injections (0.32 mg/kg/injection) were followed by low-rate days (in baboons). This behaviour lead to agitation, stereotypic movements, hypersensitivity and hallucinations. Rhesus monkeys had unlimited access to the compound for one hour and administered up to 100 injections (0.003 to 0.03 mg/kg/injection). The results were interpreted as 4-MAR having strong potential for abuse. | Mansbach et al.206 |
| Wistar rats | Conditioned place preference tests revealed that all isomers equipotently induced preference. This effect was, for some isomers, attenuated by the administration of dopamine receptor antagonists and lesions in the nucleus accumbens. Rewarding properties of 4-MAR consumption were revealed to be connected to the dopaminergic system. | Meririnne et al.207 |
| Rats | The ED50 of cis-4-MAR was determined to be 8.8 mg/kg. | Poos et al.9 |
| Rats | 3 and 6 mg/kg were administered orally and motor activity was evaluated. 4-MAR caused peak motor activity levels earlier (after 80 minutes) than amphetamine (130 minutes) but later than aminorex (40 minutes). | Poos et al.197 |
| Sprague-Dawley rats | 4S,5S-4-MAR was shown to generalise for amphetamine and to have an ED50 of 1.7 μmol/kg. | Russell et al.153 |
| Mongrel dogs | Sympathomimetic effects of the compound can be attributed to an increased release of catecholamines (published in 1963). | Yelnosky & Katz179 |
| Sprague-Dawley rats | 4-MAR (ED50=1.11) was six times less potent than aminorex (ED50=0.22) in cocaine stimulus generalisation experiments. | Young & Glennon202 |
| Sprague-Dawley rats | Cis-4-MAR (2.3 μmol/kg) substituted for S-methcathinone in stimulus generalisation experiments with an ED50 of 0.49 mg/kg. | Young & Glennon203 |
| CBA mice | Between 5 and 30 mg/kg (depending on the stereoisomer) were injected intraperitoneally for three times in 24 hours. DOPAC levels were increased but no long-lasting depletion of monoamines could be detected. | Zheng et al.155 |
| 4,4’-dimethylaminorex | ||
| Sprague-Dawley rats | An extensive chemical analysis of 4,4'-DMAR was conducted (crystal structure analysis, mass spectrometry, chromatography and spectroscopy). Monoamine transporter assay results are mentioned in Table 1. | Brandt et al.134 |
| -- | A mini-review of 4,4'-DMAR. | Coppola & Mondola208 |
| Humans | 4,4'-DMAR-caused fatalities were examined. Post-mortem concentrations of 4,4'-DMAR were between 0.20 to 3.75 mg/L. Liquid chromatography and mass spectrometry approaches of screening for 4,4'-DMAR were described. | Cosbey et al.209 |
| -- | A mini-review of 4,4'-DMAR. | Glanville et al.210 |
| -- | Drug fora were analysed to paint a picture of the way users discussed 4,4'-DMAR. | Loi et al.144 |
| HEK293 cells, rPC12 cells, human striatal synaptic vesicles | 4,4'-DMAR was classified as a non-selective monoamine releasing agent and binding data was provided. Inhibition of VMAT2 hints at long-term neurotoxic effects in chronic abusers of the substance. | Maier et al.135 |
| Sprague-Dawley rats | An in-depth chemical analysis of MDMAR was provided. In addition, monoamine transporter assays comparing cis-MDMAR, trans-MDMAR, cis-4,4'-DMAR and trans-4,4'-DMAR are portrayed in Table 1. | McLaughlin et al.148 |
| -- | An internet snapshot survey was conducted to analyse the availability of 4,4'-DMAR and 4-MAR in April 2014. | Nizar et al.143 |
Pharmacokinetics
Aminorex, when available as an anorectic drug, existed in the form of 7.5 to 20 mg aminorex fumarate, base or pamoate tablets, to be consumed orally211. 4-MAR seems to mostly have been ingested orally, insufflated or smoked in doses ranging from 5 to >25 mg depending on the routes of administration212. 4,4'-DMAR has mostly been consumed orally or insufflated but has also been inhaled and administered i.v. with doses ranging from 10 to 200 milligrams133,208,210.
Cressman and colleagues have tested the change of aminorex fumarate plasma concentration over time in human participants.211 They have shown that aminorex fumarate has a relatively long half-life of 8 hours and effects can even be prolonged and high concentrations maintained by utilizing sustained-release tablets. It has been determined in horses that aminorex is rapidly absorbed (with a half-life of 30 minutes) and that it is eliminated in various steps with a half-life of 24 hours in the slow, extended terminal elimination phase213. This can be pharmacokinetically modelled via a multi-compartment model where the compound is quickly eliminated from plasma and accumulates in deeper compartments and is then slowly metabolized (see Table 3). The accumulation of aminorex and its derivatives might explain the reported cyclical patterns of 4-MAR self-administration in baboons with high rate of self-injection across days alternating with low rates across days206. For (±)-cis-4,4'-DMAR, Lucchetti and colleagues have shown that the compound is rapidly distributed into peripheral tissue, easily passes the blood-brain-barrier and is slowly eliminated with a half-life of five hours214. The compound has been shown to be lipophilic with a brain-to-plasma ratio three times that of (±)-cis-4-MAR154,215.
Table 3. A summary of pharmacokinetic studies on aminorex and its analogs.
| Aminorex | |||
|---|---|---|---|
| Model organism | ADME | Results | Study |
| In vivo: human subjects; in vitro dissolution | A | Aminorex fumarate, as sustained-release tablets in doses of 15 and 20 mg/tablet, was determined to have good absorption rates and allow for prolonged effects. | Cressman et al.211 |
| In vivo: Standardbred gelding horses; in vitro: horse liver microsomes | M | Aminorex and rexamino were discovered to be metabolites of levamisole in horse urine and plasma. | Ho et al.63 |
| In vivo: Thoroughbred horses | A, D, E | Aminorex was administered orally and i.v. Distribution could be described by a three-compartment (with half-lives of 0.04, 2.30 and 18.82 hours) and a two-compartment model, respectively. The substance was renally eliminated and urinary excretion peaked after two hours for the i.v. group and six hours for the p.o. group. | Soma et al.213 |
| 4-methylaminorex | |||
| In vivo: Sprague-Dawley rats | M, E | 4-MAR is mostly eliminated renally (and secondly via the GIT) in its unchanged form (60%). Three metabolites could be identified: 4-MAR is hydrolysed to norephedrine, the phenyl ring can be hydroxylated to form 2-amino-5-[p-hydroxyphenyl]-4-methyl-2-oxazoline and deamination leads to metabolite 5-phenyl-4-methyl-2-oxazolidinone. | Henderson et al.216 |
| In vivo: Wistar rats | E | 4-MAR isomers could be detected by more than half of the tested on-site immunoassays, mostly as (meth-)amphetamine or cocaine. How to detect and quantify 4-MAR in urine using TLC and GC/MS is described in detail. | Kankaanpää et al.217 |
| In vitro | A | Chemical properties of the stereoisomers are described in detail. | Klein et al.129 |
| In vivo: Wistar rats | A, D, M, E | Oral bioavailability of 4-MAR was significantly lower than after i.v. or intraperitoneal administration. Marked differences between the isomers concerning their half-lives were found (for details see main text). Elimination of the trans-4R,5R-isomer was 3 times slower than that of the others. The highest concentrations of 4-MAR were located in the kidney, liver, brain and muscles, suggesting a significant ability to cross the blood-brain-barrier. The metabolites norephedrine (from the cis-isomers) and norpseudoephedrine (from the trans-isomers) were detected in blood and brain. |
Meririnne et al.154 |
| 4,4’-dimethylaminorex | |||
| In vivo: Wistar rats | A, D, E | 1 mg/kg cis-4,4′-DMAR was given i.v. The compound was more rapidly and extensively distributed than 4-MAR and more slowly eliminated (plasma t1/2 of 5.14 ± 0.65 h). I.p. doses of cis-4,4′-DMAR (1, 3 and 10 mg/kg) show a dose-dependent AUC. Data was quantified with HPLC-MS/MS. | Lucchetti et al.214 |
| In vivo: Wistar rats | A, D, M | I.p. injections lead to fast brain absorption (tmax = 30-60 minutes) and high brain concentrations with a brain-to-plasma ratio of 24. The t1/2 was determined to be approximately 50 minutes. Four metabolites, caused by hydroxylation, oxidation, hydrolysis and oxidative deamination of cis-4,4′-DMAR were identified in plasma and in low concentrations also in the brain. Behavioural experiments highlight the rewarding and addictive properties of cis-4,4′-DMAR. | Lucchetti et al.215 |
Concerning the metabolic fate of aminorex, unpublished data from McNeil Laboratories seems to suggest that 31% are excreted non-metabolized and that the amino-oxazoline ring is being hydrolysed to hydroxyphenylurea216. Previously, another study identified hydroxyaminorex as one major metabolite in horses213. Henderson and colleagues have found three metabolites of 4-MAR: (i) the active metabolite norephedrine, caused by the hydrolysis of 4-MAR; (ii) 4-(2-amino-4-methyl-4,5-dihydro-1,3-oxazol-5-yl)phenol, emerging through hydroxylation; and (iii) 4-methyl-5-phenyl-1,3-oxazolidin-2-one, caused by the deamination of 4-MAR216. It was also hypothesized that the methyl group might inhibit the hydrolysis of the amino-oxazoline ring. Lucchetti and colleagues could produce similar results for (±)-cis-4,4'-DMAR215. They discovered four metabolites, caused by hydrolysis, hydroxylation, deamination (which has already been shown for 4-MAR) and oxidation of the compound. The main metabolite located in plasma and brain was the one not detected in 4-MAR, caused by oxidation of 4,4'-DMAR’s para-methyl group215. It has been demonstrated that aminorex is mostly eliminated renally over the course of 72 hours211.
It has been revealed that the concentration of the two trans isomers of 4-MAR in rat brain tissue, 30 minutes after i.p. injection, is significantly higher than that of the cis isomers217. Similarly, the concentration of trans isomers is also increased in dialysate and plasma149. Additionally, the elimination of the trans-(4R,5R) isomer is three times slower than that of the others154. The bioavailability of the trans-(4R,5R) isomer is significantly higher than the other isomers’, indicating protection from pre-systemic metabolic degradation. It has been shown to accumulate in tissue, i.e. mostly in the kidney, liver, brain and muscles, significantly more than the other isomers of 4-MAR. There is a remarkable difference in pharmacokinetic properties between trans-(4S,5S)-, cis-(4S,5R)- and cis-(4R,5S)-isomers, which resemble each other, and the trans-(4R,5R) isomer. It is remarkable that, even though the brain concentration of trans-(4R,5R)-4-MAR is by far the highest, the substance is less potent than the other isomers154.
Behavioral Effects and Dependence Potential
Stimulus generalization tests conducted with rats revealed that aminorex is similar to amphetamine in its effects, albeit slightly less potent151,201. Similar results are available for 4-methylaminorex153,204. Rexamino, detected as a levamisole metabolite in horses, on the other hand, was determined to be inactive in a drug discrimination study in rats trained to discriminate (S)-amphetamine from saline, which was consistent with comments made by Poos who disclosed that rexamino was devoid of CNS stimulant activity10,63,153. Interestingly, derivatives of rexamino have been discovered to be highly potent TAAR1 agonists218. Further investigations have revealed that aminorex and 4-methylaminorex substitute for cocaine stimuli188. These results have been corroborated in experiments with rhesus monkeys, suggesting that aminorex seems to have an abuse liability similar to amphetamine200. Using self-administration paradigms in baboons and rhesus monkeys, Mansbach et al. have shown that 4-MAR displayed reinforcing effects in primates206. The conditioned place preference test has been utilized in rats to assess the rewarding, dopamine-dependent effects of 4-MAR and 4,4'-DMAR consumption207,215.
It has been hypothesized that the psychomotoric effects associated with aminorex involved interactions with brain receptors or releasing dopamine37. The attribution of effects to extracellular dopamine could be confirmed for 4-MAR (for enantiomeric differences see above), even though the cited studies did not include measures of extracellular norepinephrine, that might also be implicated in the behavioral effects in rats149,150,219,220. Batsche and colleagues have considered this option and administered D1 and D2 receptor antagonists in conjunction with 4-MAR and noted an attenuation when compared to the compound alone152. In addition, serotonin and norepinephrine receptor antagonists proved ineffective. These results allude to the possibility that increased locomotor activity in rats could be attributed to high extracellular dopamine levels and that it is dose-dependent. It has been unveiled for (±)-cis-4,4'-DMAR that the substance also increases locomotor activity215.
Drug users have described the effects of aminorex derivatives as feeling euphoric, stimulated, energized and social and the experience of taking the drugs has been described as being similar to both methamphetamine and MDMA144,210,221,222 although further studies are warranted to assess similarity to MDMA’s psychopharmacology in humans. With the animal model results in mind, one can conclude that aminorex and its derivatives are liable to abuse and that they might display dependence potential similar to amphetamine and cocaine.
Adverse Effects and Toxicity
A trial of aminorex as an anorectic drug revealed that it can cause insomnia, restlessness, gastrointestinal, dermatological and cardiovascular side-effects, as well as proteinuria14,189. Acute overdoses (between 1-2 mg/kg for humans) can cause symptoms such as seizures, hyperreflexia, respiratory depression, mydriasis, tachycardia, hypertension and hyperpnea, occurring occur between 15 and 120 mins after consumption189. The fact that aminorex also acts as a substrate and releaser at SERT has been linked to pulmonary arterial hypertension43,185. Drug users have mentioned hand, muscle twitches, numbness, hallucinations, panic attacks, nausea, tachycardia and hyperthermia as unwanted side-effects of the consumption of 4-MAR and 4,4'-DMAR144,221. The clinical and autopsy reports of 4,4'-DMAR patients have described short-term complications such as hyperthermia, seizures, hallucinations, agitation, internal bleeding, edema and heart and lung failure133. These symptoms might be explained as the immediate effects of considerable monoamine release, caused by this particular drug.135
Hallucinations caused by high doses of aminorex or derivatives are difficult to measure in rodents but have been reported for primates subjected to high-dose self-administration regimes206.
Bunker and colleagues have reported that rats receiving 20 mg/kg 4-MAR suffered from clonic seizures in the first hour following the treatment and died in the next two to 17 hours204. Frequent amphetamine-induced seizures can (in rare cases) lead to the development of epilepsy and the formation of epileptogenic brain lesions223. It has been shown that seizures caused by 4-MAR ingestion can be antagonized by the administration of flunarizine, a calcium channel blocker205.
Zheng et al. have examined the neurotoxic properties of several aminorex analogs in comparison to MDMA quantified via long-term depletions in monoamine content 155. They concluded that only the trans-(4S,5S)-isomer of 3,4-dimethylaminorex depletes dopaminergic and serotonergic neurons with a potency similar to MDMA. It has recently been shown that 4,4'-DMAR inhibits the vesicular monoamine transporter 2 (VMAT2; SLC18A2) with a potency similar to that of MDMA135. Perturbed function of VMAT2 has been associated with neurotoxicity224,225. Still, MDMA has been shown to only be neurotoxic when consumed in high doses over a long periods of time226–230. As mentioned before, in contrast to other amphetamine-type stimulants, 4,4'-DMAR does not interact with TAAR1 and therefore lacks the auto-inhibitory pathway that attenuates monoamine release and mediates the neuroprotective effects231,232. It has however been shown that many psychoactive compounds stimulate human TAAR1 less potently than the receptor’s rodent counterparts184. It is currently not confirmed whether aminorex and its derivatives are neurotoxic. The absence of the auto-inhibitory pathway and the inhibition of VMAT2 might be relevant factors in the determination of the potential neurotoxicity of the compound. Current knowledge would suggest that toxic effects might only appear after high dosage consumption over prolonged time periods.
The 5-HT2 receptors (5-HT2A, 5-HT2B and 5-HT2C) are expressed in the endocardium, myocardium and the heart valves233,234. It has been shown for MDMA (but also other psychostimulants) that binding to the 5-HT2B receptor in particular might possibly be associated with valvular heart disease234. On the other hand, these findings could not be replicated in other studies235,236. While it has been shown that 4,4'-DMAR binds to the 5-HT2A and 5-HT2C receptors135, receptor binding assays for 5-HT2B have not been conducted yet. In addition, the interaction of aminorex and other derivatives with the 5-HT2B receptor has not yet been subject of investigation. Hence, concerning the long-term cardiotoxicity of aminorex and derivatives, at this point in time one can only speculate that, because of their similarity to MDMA, a potential interaction with the 5-HT2B receptor might be possibly implicated in cardiotoxic effects.
Concluding Remarks
The historical events linked to aminorex and its derivatives are representative of what has occasionally been termed the NPS/”designer drug” phenomenon8,237. The parental drug aminorex was originally used as approved medication, yet failed to persist on the market when evidence on adverse side effects accumulated. With the help of existing literature describing the properties of a range of closely related drugs, however, new analogs appeared on the streets (e.g. 4-MAR). Because of its sympathomimetic effects, a patent claim has been filed for the usage of 4-MAR as a nasal decongestant238. Other examples exist where an emerging drug that has no history in the scientific (including patent) literature appears for sale on the Internet (e.g. 4,4’-DMAR). Today, (4S,5S)-trans-4,4'-DMAR is mentioned in a patent as a phospholipase A2 inhibitor and therefore claimed as an anti-inflammatory agent133,239. In addition, several isomers of 4,4'-DMAR and related compounds have been patented to be utilized in the treatment of CNS disorders133.
The 4,4’-DMAR case has also illustrated that this drug has escaped the Internet realm predominantly relevant to users who had a specific interest in this substance as it surreptitiously appeared on the traditional illicit street market where it has been supplied to unsuspecting users with tragic consequences. Some Internet suppliers removed the substance from their product catalog once information of the adverse effects emerged. Given the information available on a range of yet unexplored compounds and the interest of the NPS community in the substance group, one might predict further commercial exploitations by NPS entrepreneurs and organized crime groups. The gloomy aspect of the NPS phenomenon is that information on long-term effects, acute toxicity or pharmacology remains limited, combined with the fact that increasingly toxic substances have appeared in recent years5. An appreciable body of scientific studies has aimed to elucidate the pharmacodynamic and pharmacokinetic properties of NPS. However, considering the rate by which NPS are introduced into the markets, the scientific community is constantly trailing and chasing after new developments. Furthermore, scientific literature may serve as a rich source for creative drug dealers to identify preferable adulterants (such as levamisole) for their products. However, the immense amount of data collected on stimulant-type NPS and their structure-activity relationships may help to identify crucial structural determinants to pave the way for the development of improved pharmacotherapies to neuropsychiatric disorders arising from imbalances in monoaminergic neurotransmission.
Funding
Financial support by the Austrian Research Fund/FWF (grants F3506 and W1232 to H.H.S.) is gratefully acknowledged.
Footnotes
Author Contributions
J.M. outlined, wrote and edited the manuscript. F.P.M., S.D.B. and H.H.S. provided guidance and further ideas, contributed written parts and edited the manuscript.
Conflict of Interest
The authors declare no competing financial interest.
References
- (1).What are NPS? [accessed Sept 26, 2018]; https://www.unodc.org/LSS/Page/NPS.
- (2).Tettey JNA, Crean C, Ifeagwu SC, Raithelhuber M. Handbook of Experimental Pharmacology. Springer; Berlin, Heidelberg: 2018. Emergence, Diversity, and Control of New Psychoactive Substances: A Global Perspective; pp. 1–17. [DOI] [PubMed] [Google Scholar]
- (3).Evans-Brown M, Sedefov R. Handbook of Experimental Pharmacology. Springer; Berlin, Heidelberg: 2018. Responding to New Psychoactive Substances in the European Union: Early Warning, Risk Assessment, and Control Measures; pp. 1–47. [DOI] [PubMed] [Google Scholar]
- (4).World Drug Report 2018. [accessed Sept 26, 2018]; https://www.unodc.org/wdr2018/
- (5).Fentanils and synthetic cannabinoids: driving greater complexity into the drug situation — an update from the EU Early Warning System. [accessed Sept 26, 2018]; www.emcdda.europa.eu http://www.emcdda.europa.eu/publications/rapid-communications/fentanils-and-synthetic-cannabinoids-ews-update_en.
- (6).INCB Psychotropics - Green List. [accessed Sept 26, 2018]; https://www.incb.org/incb/en/psychotropics/green-list.html.
- (7).Baumann MH, Volkow ND. Abuse of New Psychoactive Substances: Threats and Solutions. Neuropsychopharmacology. 2016;41(3):663–665. doi: 10.1038/npp.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Huestis MA, Brandt SD, Rana S, Auwärter V, Baumann MH. Impact of Novel Psychoactive Substances on Clinical and Forensic Toxicology and Global Public Health. Clin Chem. 2017;63(10):1564–1569. doi: 10.1373/clinchem.2017.274662. [DOI] [PubMed] [Google Scholar]
- (9).Poos GI, Carson JR, Rosenau JD, Roszkowski AP, Kelley NM, McGowin J. 2-amino-5-Aryl-2-oxazolines. Potent new anorectic agents. J Med Chem. 1963;6(3):266–272. doi: 10.1021/jm00339a011. [DOI] [PubMed] [Google Scholar]
- (10).Poos GI. 2-Amino-5-Aryloxazoline Products. US3161650A. 1964 Dec 15;
- (11).Coleman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143(5):380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- (12).Fishman AP. Primary Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2004;43(12):2–4. doi: 10.1016/j.jacc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- (13).Onakpoya IJ, Heneghan CJ, Aronson JK. Post-Marketing Withdrawal of Anti-Obesity Medicinal Products Because of Adverse Drug Reactions: A Systematic Review. BMC Med. 2016;14(1):191. doi: 10.1186/s12916-016-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hadler AJ. Studies of aminorex, a new anorexigenic agent. J Clin Pharmacol J New Drugs. 1967;7(5):296–302. doi: 10.1002/j.1552-4604.1967.tb00067.x. [DOI] [PubMed] [Google Scholar]
- (15).Gurtner HP, Gertsch M, Salzmann C, Scherrer M, Stucki P, Wyss F. [Are the primary vascular forms of chronic pulmonary heart disease becoming more common?] Schweiz Med Wochenschr. 1968;98(43):1695–1707. [PubMed] [Google Scholar]
- (16).Kay JM, Harris P, Heath D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax. 1967;22:176–179. doi: 10.1136/thx.22.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kay JM, Smith P, Heath D. Aminorex and the pulmonary circulation. Thorax. 1971;26:262–270. doi: 10.1136/thx.26.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gurtner HP. Pulmonary Hypertension, “Plexogenic Pulmonary Arteriopathy” and the Appetite Depressant Drug Aminorex: Post or Propter? Bull Eur Physiopathol Respir. 1979;15(5):897–923. [PubMed] [Google Scholar]
- (19).Fishman AP. Aminorex to fen/phen: an epidemic foretold. Circulation. 1999:156–161. doi: 10.1161/01.cir.99.1.156. [DOI] [PubMed] [Google Scholar]
- (20).Kramer MS, Lane DA. Aminorex, Dexfenfluramine, and Primary Pulmonary Hypertension. J Clin Epidemiol. 1998;51(4):361–364. doi: 10.1016/s0895-4356(97)00289-8. [DOI] [PubMed] [Google Scholar]
- (21).Seferian A, Chaumais M-C, Savale L, Günther S, Tubert-Bitter P, Humbert M, Montani D. Drugs Induced Pulmonary Arterial Hypertension. Presse Médicale. 2013;42(9):e303–e310. doi: 10.1016/j.lpm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- (22).Gurtner HP. Aminorex and Pulmonary Hypertension. A Review Cor Vasa. 1985;27(2–3):160–171. [PubMed] [Google Scholar]
- (23).Widgren S. [Prolonged survey of cases of pulmonary hypertension in relation to consumption of aminorex. Histological, quantitative and morphometric study of 9 cases] Schweiz Med Wochenschr. 1986;116(27–28):918–924. [PubMed] [Google Scholar]
- (24).Souza R, Jardim C, Humbert M. Idiopathic Pulmonary Arterial Hypertension. Semin Respir Crit Care Med. 2013;34(05):560–567. doi: 10.1055/s-0033-1355439. [DOI] [PubMed] [Google Scholar]
- (25).Langleben D. Relearning the Lessons of History: Anorexigens and Pulmonary Hypertension. CHEST. 1998;114(1):55S–57S. doi: 10.1378/chest.114.1_supplement.55s. [DOI] [PubMed] [Google Scholar]
- (26).Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of Drug Therapies Used for Weight Loss and Treatment of Obesity. Drug Saf. 2006;29(4):277–302. doi: 10.2165/00002018-200629040-00001. [DOI] [PubMed] [Google Scholar]
- (27).Fishman AP. Dietary pulmonary hypertension. Circ Res. 1974;35(5):657–660. doi: 10.1161/01.res.35.5.657. [DOI] [PubMed] [Google Scholar]
- (28).Byrne-Quinn E, Grover RF. Aminorex (Menocil) and Amphetamine: Acute and Chronic Effects on Pulmonary and Systemic Haemodynamics in the Calf. Thorax. 1972;27(1):127–131. doi: 10.1136/thx.27.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Smith P, Heath D, Kay JM, Wright JS, McKendrick CS. Pulmonary Arterial Pressure and Structure in the Patas Monkey after Prolonged Administration of Aminorex Fumarate. Cardiovasc Res. 1973;7(1):30–38. doi: 10.1093/cvr/7.1.30. [DOI] [PubMed] [Google Scholar]
- (30).Will JA, Bisgard GE. Haemodynamic Effects of Oral Aminorex and Amphetamine in Unanaesthetized Beagle Dogs. Thorax. 1972;27(1):120–126. doi: 10.1136/thx.27.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Orr JA, Ungerer T, Seavey E, Bisgard GE, Will JA. Hemodynamic Effects of Long Term Feeding of Sympathomimetic Amines to Swine. J Environ Pathol Toxicol. 1978;1(6):911–925. [PubMed] [Google Scholar]
- (32).Engelhardt R, Hort W. [Cardiovascular Effect of Aminorex in Rats after Prolonged Application] Naunyn-Schmiedebergs Arch Für Pharmakol. 1970;266(4):318–319. [PubMed] [Google Scholar]
- (33).Leuschner F, Otto H, Wagener HH. [The Tolerance of Aminorex and Some Other Compounds during Prolonged Administration to Beagle Dogs] Naunyn-Schmiedebergs Arch Für Pharmakol. 1970;266(4):391–392. [PubMed] [Google Scholar]
- (34).Stepanek J, Zak F. [Two-year peroral administration of aminorex in the dog. 2] Z Kardiol. 1975;64(8):768–781. [PubMed] [Google Scholar]
- (35).Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122(12):4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).McGee M, Whitehead N, Martin J, Collins N. Drug-Associated Pulmonary Arterial Hypertension. Clin Toxicol. 2018:1–9. doi: 10.1080/15563650.2018.1447119. [DOI] [PubMed] [Google Scholar]
- (37).Costa E, Naimzada KM, Revuelta A. Effect of Phenmetrazine, Aminorex and (±) p-Chloramphetamine on the Motor Activity and Turnover Rate of Brain Catecholamines. Br J Pharmacol. 1971;43(3):570–579. doi: 10.1111/j.1476-5381.1971.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Weir EK, Reeve HL, Huang JM, Michelakis E, Nelson DP, Hampl V, Archer SL. Anorexic Agents Aminorex, Fenfluramine, and Dexfenfluramine Inhibit Potassium Current in Rat Pulmonary Vascular Smooth Muscle and Cause Pulmonary Vasoconstriction. Circulation. 1996;94(9):2216–2220. doi: 10.1161/01.cir.94.9.2216. [DOI] [PubMed] [Google Scholar]
- (39).Perchenet L, Hilfiger L, Mizrahi J, Clément-Chomienne O. Effects of Anorexinogen Agents on Cloned Voltage-Gated K(+) Channel HKv1.5. J Pharmacol Exp Ther. 2001;298(3):1108–1119. [PubMed] [Google Scholar]
- (40).MacLean MR. The Serotonin Hypothesis in Pulmonary Hypertension Revisited: Targets for Novel Therapies (2017 Grover Conference Series) Pulm Circ. 2018;8(2) doi: 10.1177/2045894018759125. 204589401875912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Seiler KU, Wassermann O, Wensky H. On the Role of Serotonin in the Pathogenesis of Pulmonary Hypertension Induced by Anorectic Drugs; an Experimental Study in the Isolated Perfused Rat Lung, II. Fenfluramine, Mazindol, Mefenorex, Phentermine and R 800. Clin Exp Pharmacol Physiol. 1976;3(4):323–330. doi: 10.1111/j.1440-1681.1976.tb00608.x. [DOI] [PubMed] [Google Scholar]
- (42).Seiler KU, Wasserman O. MAO-Inhibitory Properties of Anorectic Drugs. J Pharm Pharmacol. 1973;25(7):576–578. doi: 10.1111/j.2042-7158.1973.tb09162.x. [DOI] [PubMed] [Google Scholar]
- (43).Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, Fenfluramine, and Chlorphentermine Are Serotonin Transporter Substrates. Implications for Primary Pulmonary Hypertension. Circulation. 1999;100(8):869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- (44).Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt A-M, Lukanidin E, Rabinovitch M. Interdependent Serotonin Transporter and Receptor Pathways Regulate S100A4/Mts1, a Gene Associated with Pulmonary Vascular Disease. Circ Res. 2005;97(3):227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- (45).Simon AR, Severgnini M, Takahashi S, Rozo L, Andrahbi B, Agyeman A, Cochran BH, Day RM, Fanburg BL. 5-HT Induction of c-Fos Gene Expression Requires Reactive Oxygen Species and Rac1 and Ras GTPases. Cell Biochem Biophys. 2005;42(3):263–276. doi: 10.1385/CBB:42:3:263. [DOI] [PubMed] [Google Scholar]
- (46).Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT Transporter Transactivates the PDGFbeta Receptor in Pulmonary Artery Smooth Muscle Cells. FASEB J Off Publ Fed Am Soc Exp Biol. 2007;21(11):2725–2734. doi: 10.1096/fj.06-8058com. [DOI] [PubMed] [Google Scholar]
- (47).Dempsie Y, Maclean MR. Role of the Serotonin Transporter in Pulmonary Arterial Hypertension. Expert Rev Clin Pharmacol. 2008;1(6):749–757. doi: 10.1586/17512433.1.6.749. [DOI] [PubMed] [Google Scholar]
- (48).Follath F, Burkart F, Schweizer W. Drug-Induced Pulmonary Hypertension? Br Med J. 1971;1(5743):265–266. doi: 10.1136/bmj.1.5743.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Adnot S, Houssaini A, Abid S, Marcos E, Amsellem V. Serotonin Transporter and Serotonin Receptors. Handb Exp Pharmacol. 2013;218:365–380. doi: 10.1007/978-3-642-38664-0_15. [DOI] [PubMed] [Google Scholar]
- (50).Janssen PAJ. The Levamisole Story. Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrés des recherches pharmaceutiques. 1976;20:347–383. doi: 10.1007/978-3-0348-7094-8_11. [DOI] [PubMed] [Google Scholar]
- (51).Moser W, Schindler C, Keiser J. Efficacy of Recommended Drugs against Soil Transmitted Helminths: Systematic Review and Network Meta-Analysis. BMJ. 2017;358 doi: 10.1136/bmj.j4307. j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ali MS, Saeed K, Rashid I, Ijaz M, Akbar H, Rashid M, Ashraf K. Anthelmintic Drugs: Their Efficacy and Cost-Effectiveness in Different Parity Cattle. J Parasitol. 2017;104(1):79–85. doi: 10.1645/17-4. [DOI] [PubMed] [Google Scholar]
- (53).Gruppen MP, Bouts AH, Jansen-van der Weide MC, Merkus MP, Zurowska A, Maternik M, Massella L, Emma F, Niaudet P, Cornelissen EAM, et al. A Randomized Clinical Trial Indicates That Levamisole Increases the Time to Relapse in Children with Steroid-Sensitive Idiopathic Nephrotic Syndrome. Kidney Int. 2018;93(2):510–518. doi: 10.1016/j.kint.2017.08.011. [DOI] [PubMed] [Google Scholar]
- (54).Veenstra CM, Krauss JC. Emerging Systemic Therapies for Colorectal Cancer. Clin Colon Rectal Surg. 2018;31(3):179–191. doi: 10.1055/s-0037-1602238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in Dermatology. Am J Clin Dermatol. 2004;5(2):97–104. doi: 10.2165/00128071-200405020-00004. [DOI] [PubMed] [Google Scholar]
- (56).Shamkuwar CA, Meshram SH, Mahakalkar SM. Levamisole as an Adjuvant to Short-Course Therapy in Newly Diagnosed Pulmonary Tuberculosis Patients. Adv Biomed Res. 2017;6:37. doi: 10.4103/2277-9175.203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Sharda N, Shashikanth MC, Kant P, Jain M. Levamisole and Low-Dose Prednisolone in the Treatment of Reccurent Aphthous Stomatitis. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2014;43(4):309–316. doi: 10.1111/jop.12126. [DOI] [PubMed] [Google Scholar]
- (58).Shao Y, Li X, Shi J, Ge M, Huang J, Huang Z, Zhang J, Nie N, Zheng Y. Cyclosporin Combined with Levamisole for Refractory or Relapsed Severe Aplastic Anaemia. Br J Haematol. 2013;162(4):552–555. doi: 10.1111/bjh.12383. [DOI] [PubMed] [Google Scholar]
- (59).Valentino AM, Fuentecilla K. Levamisole: an analytical profile. Microgram Journal. 2005;3(3–4):134–137. [Google Scholar]
- (60).Fucci N. Unusual Adulterants in Cocaine Seized on Italian Clandestine Market. Forensic Sci Int. 2007;172(2–3):e1. doi: 10.1016/j.forsciint.2007.05.021. [DOI] [PubMed] [Google Scholar]
- (61).Barker SA. The Formation of Aminorex in Racehorses Following Levamisole Administration. A Quantitative and Chiral Analysis Following Synthetic Aminorex or Levamisole Administration vs. Aminorex-Positive Samples from the Field: A Preliminary Report. J Vet Pharmacol Ther. 2009;32(2):160–166. doi: 10.1111/j.1365-2885.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- (62).Bertol E, Mari F, Milia MGD, Politi L, Furlanetto S, Karch SB. Determination of Aminorex in Human Urine Samples by GC–MS after Use of Levamisole. J Pharm Biomed Anal. 2011;55(5):1186–1189. doi: 10.1016/j.jpba.2011.03.039. [DOI] [PubMed] [Google Scholar]
- (63).Ho ENM, Leung DKK, Leung GNW, Wan TSM, Wong ASY, Wong CHF, Soma LR, Rudy JA, Uboh C, Sams R. Aminorex and Rexamino as Metabolites of Levamisole in the Horse. Anal Chim Acta. 2009;638(1):58–68. doi: 10.1016/j.aca.2009.02.033. [DOI] [PubMed] [Google Scholar]
- (64).World Drug Report 2017. [accessed Sept 26, 2018]; https://www.unodc.org/wdr2017/index.html.
- (65).Kudlacek O, Hofmaier T, Luf A, Mayer FP, Stockner T, Nagy C, Holy M, Freissmuth M, Schmid R, Sitte HH. Cocaine Adulteration. J Chem Neuroanat. 2017;83–84:75–81. doi: 10.1016/j.jchemneu.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Nationwide Public Health Alert Issued Concerning Life-Threatening Risk Posed by Cocaine Laced with Veterinary Anti-Parasite Drug. [accessed Sept 26, 2018]; https://www.samhsa.gov/newsroom/press-announcements/200909211245.
- (67).Brunt TM, van den Berg J, Pennings E, Venhuis B. Adverse Effects of Levamisole in Cocaine Users: A Review and Risk Assessment. Arch Toxicol. 2017;91(6):2303–2313. doi: 10.1007/s00204-017-1947-4. [DOI] [PubMed] [Google Scholar]
- (68).Tallarida CS, Tallarida RJ, Rawls SM. Levamisole Enhances the Rewarding and Locomotor-Activating Effects of Cocaine in Rats. Drug Alcohol Depend. 2015;149:145–150. doi: 10.1016/j.drugalcdep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Lapachinske SF, Okai GG, dos Santos A, de Bairros AV, Yonamine M. Analysis of Cocaine and Its Adulterants in Drugs for International Trafficking Seized by the Brazilian Federal Police. Forensic Sci Int. 2015;247:48–53. doi: 10.1016/j.forsciint.2014.11.028. [DOI] [PubMed] [Google Scholar]
- (70).Wolford A, McDonald TS, Eng H, Hansel S, Chen Y, Bauman J, Sharma R, Kalgutkar AS. Immune-Mediated Agranulocytosis Caused by the Cocaine Adulterant Levamisole: A Case for Reactive Metabolite(s) Involvement. Drug Metab Dispos Biol Fate Chem. 2012;40(6):1067–1075. doi: 10.1124/dmd.112.045021. [DOI] [PubMed] [Google Scholar]
- (71).Baptiste GG, Alexopoulos A-S, Masud T, Bonsall JM. Systemic Levamisole-Induced Vasculitis in a Cocaine User without Cutaneous Findings: A Consideration in Diagnosis. Case Rep Med. 2015;2015 doi: 10.1155/2015/547023. 547023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Lazareth H, Peytavin G, Polivka L, Dupin N. The Hairy-Print for Levamisole-Induced Vasculitis. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-006602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Farmer RW, Malhotra PS, Mays MP, Egger ME, Smith JW, Jortani SA, Spiller H, Bosse GM, Callen JP, Franklin GA. Necrotizing Peripheral Vasculitis/Vasculopathy Following the Use of Cocaine Laced with Levamisole. J Burn Care Res Off Publ Am Burn Assoc. 2012;33(1):e6–e11. doi: 10.1097/BCR.0b013e318235615a. [DOI] [PubMed] [Google Scholar]
- (74).Fernandez Armenteros JM, Veà Jódar A, Matas Nadal C, Cortés Pinto CP, Soria Gili X, Martí Laborda R-M, Vilardell Villellas F, Casanova Seuma J-M. Severe and Recurrent Levamisole-Induced Cutaneous Vasculopathy. J Cutan Pathol. 2018;45(4):309–311. doi: 10.1111/cup.13105. [DOI] [PubMed] [Google Scholar]
- (75).Roca-Argente L, Moll-Guillen J-L, Espí-Reig J, Blanes-Julia M, García-Martínez A-M, Pujol-Marco C, Hernández-Jaras J. Membranous Glomerulonephritis and Cellular Crescents Induced by Levamisole-Adulterated Cocaine Abuse: A Case Report. Ann Transl Med. 2015;3(18):271. doi: 10.3978/j.issn.2305-5839.2015.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Roberts JA, Chévez-Barrios P. Levamisole-Induced Vasculitis: A Characteristic Cutaneous Vasculitis Associated With Levamisole-Adulterated Cocaine. Arch Pathol Lab Med. 2015;139(8):1058–1061. doi: 10.5858/arpa.2014-0107-RS. [DOI] [PubMed] [Google Scholar]
- (77).Lawrence LA, Jiron JL, Lin H-S, Folbe AJ. Levamisole-Adulterated Cocaine Induced Skin Necrosis of Nose, Ears, and Extremities: Case Report. Allergy Rhinol Provid RI. 2014;5(3):132–136. doi: 10.2500/ar.2014.5.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Liu Y-WJ, Mutnuri S, Siddiqui SB, Weikle GR, Oladipo O, Ganti N, Beach RE, Afrouzian M. Levamisole-Adulterated Cocaine Nephrotoxicity: Ultrastructural Features. Am J Clin Pathol. 2016;145(5):720–726. doi: 10.1093/ajcp/aqw029. [DOI] [PubMed] [Google Scholar]
- (79).Garg L, Gupta S, Swami A, Zhang P. Levamisole/Cocaine Induced Systemic Vasculitis and Immune Complex Glomerulonephritis. Case Rep Nephrol. 2015;2015 doi: 10.1155/2015/372413. 372413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Martinez-Cabriales S, Ocampo-Garza J, Barbosa-Moreno L, Chavez-Alvarez S, Ocampo-Candiani J. Purpura Fulminans 10 Years after Contaminated Cocaine Use. Lancet Lond Engl. 2015;386(10004):e21. doi: 10.1016/S0140-6736(15)60069-1. [DOI] [PubMed] [Google Scholar]
- (81).Muirhead TT, Eide MJ. Images in Clinical Medicine. Toxic Effects of Levamisole in a Cocaine User. N Engl J Med. 2011;364(24):e52. doi: 10.1056/NEJMicm1008722. [DOI] [PubMed] [Google Scholar]
- (82).Nolan AL, Jen K-Y. Pathologic Manifestations of Levamisole-Adulterated Cocaine Exposure. Diagn Pathol. 2015;10:48. doi: 10.1186/s13000-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Veronese FV, Dode RSO, Friderichs M, Thomé GG, da Silva DR, Schaefer PG, Sebben VC, Nicolella AR, Barros EJG. Cocaine/Levamisole-Induced Systemic Vasculitis with Retiform Purpura and Pauci-Immune Glomerulonephritis. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 2016;49(5):e5244. doi: 10.1590/1414-431X20165244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Le Garff E, Tournel G, Becquart C, Cottencin O, Dupin N, Delaporte E, Hedouin V. Extensive Necrotic Purpura in Levamisole-Adulterated Cocaine Abuse - A Case Report. J Forensic Sci. 2016;61(6):1681–1685. doi: 10.1111/1556-4029.13176. [DOI] [PubMed] [Google Scholar]
- (85).Caldwell KB, Graham OZ, Arnold JJ. Agranulocytosis from Levamisole-Adulterated Cocaine. J Am Board Fam Med JABFM. 2012;25(4):528–530. doi: 10.3122/jabfm.2012.04.110177. [DOI] [PubMed] [Google Scholar]
- (86).Dy I, Pokuri V, Olichney J, Wiernik P. Levamisole-Adulterated in Cocaine Causing Agranulocytosis, Vasculopathy, and Acquired Protein S Deficiency. Ann Hematol. 2012;91(3):477–478. doi: 10.1007/s00277-011-1294-0. [DOI] [PubMed] [Google Scholar]
- (87).Álvarez Díaz H, Marińo Callejo AI, García Rodríguez JF, Rodríguez Pazos L, Gómez Buela I, Bermejo Barrera AM. ANCA-Positive Vasculitis Induced by Levamisole-Adulterated Cocaine and Nephrotic Syndrome: The Kidney as an Unusual Target. Am J Case Rep. 2013;14:557–561. doi: 10.12659/AJCR.889731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Chung C, Tumeh PC, Birnbaum R, Tan BH, Sharp L, McCoy E, Mercurio MG, Craft N. Characteristic Purpura of the Ears, Vasculitis, and Neutropenia--a Potential Public Health Epidemic Associated with Levamisole-Adulterated Cocaine. J Am Acad Dermatol. 2011;65(4):722–725. doi: 10.1016/j.jaad.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Gulati S, Donato AA. Lupus Anticoagulant and ANCA Associated Thrombotic Vasculopathy Due to Cocaine Contaminated with Levamisole: A Case Report and Review of the Literature. J Thromb Thrombolysis. 2012;34(1):7–10. doi: 10.1007/s11239-012-0711-0. [DOI] [PubMed] [Google Scholar]
- (90).Keith PJ, Joyce JC, Wilson BD. Pyoderma Gangrenosum: A Possible Cutaneous Complication of Levamisole-Tainted Cocaine Abuse. Int J Dermatol. 2015;54(9):1075–1077. doi: 10.1111/ijd.12212. [DOI] [PubMed] [Google Scholar]
- (91).Carter MR, Amirhaeri S. P-ANCA-Associated Vasculitis Caused by Levamisole-Adulterated Cocaine: A Case Report. Case Rep Emerg Med. 2013;2013:878903. doi: 10.1155/2013/878903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Strazzula L, Brown KK, Brieva JC, Camp BJ, Frankel HC, Kissin E, Mahlberg MJ, Mina MA, Pomeranz MK, Brownell I, et al. Levamisole Toxicity Mimicking Autoimmune Disease. J Am Acad Dermatol. 2013;69(6):954–959. doi: 10.1016/j.jaad.2013.07.037. [DOI] [PubMed] [Google Scholar]
- (93).Ching JA, Smith DJ. Levamisole-Induced Necrosis of Skin, Soft Tissue, and Bone: Case Report and Review of Literature. J Burn Care Res Off Publ Am Burn Assoc. 2012;33(1):e1–5. doi: 10.1097/BCR.0b013e318233fc64. [DOI] [PubMed] [Google Scholar]
- (94).Morris GW, Mason BC, Harris Sprunger R, Hake Harris H, White LA, Patterson DA. Levamisole-Adulterated Cocaine: A Case Series. J Am Board Fam Med JABFM. 2012;25(4):531–535. doi: 10.3122/jabfm.2012.04.110287. [DOI] [PubMed] [Google Scholar]
- (95).Arora NP. Cutaneous Vasculopathy and Neutropenia Associated with Levamisole-Adulterated Cocaine. Am J Med Sci. 2013;345(1):45–51. doi: 10.1097/MAJ.0b013e31825b2b50. [DOI] [PubMed] [Google Scholar]
- (96).Carrara C, Emili S, Lin M, Alpers CE. Necrotizing and Crescentic Glomerulonephritis with Membranous Nephropathy in a Patient Exposed to Levamisole-Adulterated Cocaine. Clin Kidney J. 2016;9(2):234–238. doi: 10.1093/ckj/sfv141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Graf J, Lynch K, Yeh C-L, Tarter L, Richman N, Nguyen T, Kral A, Dominy S, Imboden J. Purpura, Cutaneous Necrosis, and Antineutrophil Cytoplasmic Antibodies Associated with Levamisole-Adulterated Cocaine. Arthritis Rheum. 2011;63(12):3998–4001. doi: 10.1002/art.30590. [DOI] [PubMed] [Google Scholar]
- (98).James KT, Detz A, Coralic Z, Kanzaria H. Levamisole Contaminated Cocaine Induced Cutaneous Vasculitis Syndrome. West J Emerg Med. 2013;14(5):448–449. doi: 10.5811/westjem.2013.3.16184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Srivastava R, Rizwan M, Jamil MO, Kogulan P, Salzman D. Agranulocytosis - Sequelae of Chronic Cocaine Use: Case Series and Literature Review. Cureus. 2017;9(5):e1221. doi: 10.7759/cureus.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Kassim T, Chintalacheruvu L, Bhatty O, Selim M, Diab O, Nayfeh A, Manikkam Umakanthan J, Gbadamosi-Akindele M. A Case of Levamisole-Induced Agranulocytosis. Case Rep Hematol. 2018;2018 doi: 10.1155/2018/7341835. 7341835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Khan MS, Khan Z, Khateeb F, Moustafa A, Taleb M, Yoon Y. Recurrent Levamisole-Induced Agranulocytosis Complicated by Bowel Ischemia in a Cocaine User. Am J Case Rep. 2018;19:630–633. doi: 10.12659/AJCR.908898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Buchanan JA, Oyer RJ, Patel NR, Jacquet GA, Bornikova L, Thienelt C, Shriver DA, Shockley LW, Wilson ML, Hurlbut KM, et al. A Confirmed Case of Agranulocytosis after Use of Cocaine Contaminated with Levamisole. J Med Toxicol Off J Am Coll Med Toxicol. 2010;6(2):160–164. doi: 10.1007/s13181-010-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Vitt JR, Brown EG, Chow DS, Josephson SA. Confirmed Case of Levamisole-Associated Multifocal Inflammatory Leukoencephalopathy in a Cocaine User. J Neuroimmunol. 2017;305:128–130. doi: 10.1016/j.jneuroim.2017.01.018. [DOI] [PubMed] [Google Scholar]
- (104).Hantson P, Di Fazio V, Del Mar Ramirez Fernandez M, Samyn N, Duprez T, van Pesch V. Susac-like Syndrome in a Chronic Cocaine Abuser: Could Levamisole Play a Role? J Med Toxicol Off J Am Coll Med Toxicol. 2015;11(1):124–128. doi: 10.1007/s13181-014-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Dartevel A, Chaigne B, Moachon L, Grenier F, Dupin N, Guillevin L, Bouillet L, Mouthon L. Levamisole-Induced Vasculopathy: A Systematic Review. Semin Arthritis Rheum. 2018 doi: 10.1016/j.semarthrit.2018.07.010. published online Jul 23, 2018. [DOI] [PubMed] [Google Scholar]
- (106).The flesh-eating, bladder-wrecking chemicals hidden in street cocaine. [accessed Jun 28, 2018];The Independent. https://www.independent.co.uk/news/uk/home-news/flesh-eating-cocaine-coke-whats-in-cocaine-heroin-smack-street-drugs-levamisole-cattle-de-wormer-a7305721.html. [Google Scholar]
- (107).Flesh-Eating Cocaine Hits New York, Los Angeles. [accessed Jun 28, 2018];Huffington Post. https://www.huffingtonpost.com/2011/06/29/flesh-eating-cocaine-ny-la_n_886363.html. [Google Scholar]
- (108).Corrupted cocaine sold in Britain making skin rot. [accessed Jun 28, 2018];Telegraph. https://www.telegraph.co.uk/news/health/11887100/Corrupted-cocaine-sold-in-Britain-making-skin-rot.html. [Google Scholar]
- (109).Pope JD, Drummer OH, Schneider HG. The Cocaine Cutting Agent Levamisole Is Frequently Detected in Cocaine Users. Pathology. 2018;50(5):536–539. doi: 10.1016/j.pathol.2018.03.006. [DOI] [PubMed] [Google Scholar]
- (110).Waller PJ. From Discovery to Development: Current Industry Perspectives for the Development of Novel Methods of Helminth Control in Livestock. Vet Parasitol. 2006;139(1–3):1–14. doi: 10.1016/j.vetpar.2006.02.036. [DOI] [PubMed] [Google Scholar]
- (111).Rothman RB, Baumann MH. Monoamine Transporters and Psychostimulant Drugs. Eur J Pharmacol. 2003;479(1–3):23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- (112).Coles GC, East JM, Jenkins SN. The Mechanism of Action of the Anthelmintic Levamisole. Gen Pharmacol Vasc Syst. 1975;6(4):309–313. [Google Scholar]
- (113).Rehni AK, Singh TG. Levamisole-Induced Reduction in Seizure Threshold: A Possible Role of Nicotinic Acetylcholine Receptor-Mediated Pathway. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(3):279–285. doi: 10.1007/s00210-010-0543-4. [DOI] [PubMed] [Google Scholar]
- (114).Szász BK, Mayer A, Zsilla G, Lendvai B, Vizi ES, Kiss JP. Carrier-Mediated Release of Monoamines Induced by the Nicotinic Acetylcholine Receptor Agonist DMPP. Neuropharmacology. 2005;49(3):400–409. doi: 10.1016/j.neuropharm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- (115).Vanhoutte PM, Nueten JMV, Verbeuren TJ, Laduron PM. Differential Effects of the Isomers of Tetramisole on Adrenergic Neurotransmission in Cutaneous Veins of Dog. J Pharmacol Exp Ther. 1977;200(1):127–140. [PubMed] [Google Scholar]
- (116).Shah KK, Gulati OD, Hemavathi KG. Investigation of Some Effects of Levamisole on Dog Blood Pressure. Indian J Physiol Pharmacol. 1986;30(1):55–62. [PubMed] [Google Scholar]
- (117).Hofmaier T, Luf A, Seddik A, Stockner T, Holy M, Freissmuth M, Ecker GF, Schmid R, Sitte HH, Kudlacek O. Aminorex, a Metabolite of the Cocaine Adulterant Levamisole, Exerts Amphetamine like Actions at Monoamine Transporters. Neurochem Int. 2014;73:32–41. doi: 10.1016/j.neuint.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Hess C, Ritke N, Broecker S, Madea B, Musshoff F. Metabolism of Levamisole and Kinetics of Levamisole and Aminorex in Urine by Means of LC-QTOF-HRMS and LC-QqQ-MS. Anal Bioanal Chem. 2013;405(12):4077–4088. doi: 10.1007/s00216-013-6829-x. [DOI] [PubMed] [Google Scholar]
- (119).Hess C, Ritke N, Sydow K, Mehling L-M, Ruehs H, Madea B, Musshoff F. Determination of Levamisole, Aminorex, and Pemoline in Plasma by Means of Liquid Chromatography-Mass Spectrometry and Application to a Pharmacokinetic Study of Levamisole: Determination of Levamisole, Aminorex and Pemoline in Plasma. Drug Test Anal. 2014;6(10):1049–1054. doi: 10.1002/dta.1619. [DOI] [PubMed] [Google Scholar]
- (120).Tallarida CS, Egan E, Alejo GD, Raffa R, Tallarida RJ, Rawls SM. Levamisole and Cocaine Synergism: A Prevalent Adulterant Enhances Cocaine’s Action in Vivo. Neuropharmacology. 2014;79:590–595. doi: 10.1016/j.neuropharm.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Eiden C, Peyrière H, Diot C, Mathieu O. Prevalence of Levamisole and Aminorex in Patients Tested Positive for Cocaine in a French University Hospital. Clin Toxicol. 2015;53(7):604–608. doi: 10.3109/15563650.2015.1054499. [DOI] [PubMed] [Google Scholar]
- (122).Karch SB, Vaiano F, Bertol E. Levamisole, Aminorex, and Pulmonary Arterial Hypertension: A Review. Razavi Int J Med. 2015;3(3):e28277. [Google Scholar]
- (123).Karch SB, Defraia B, Messerini L, Mari F, Vaiano F, Bertol E. Aminorex Associated with Possible Idiopathic Pulmonary Hypertension in a Cocaine User. Forensic Sci Int. 2014;240:e7–e10. doi: 10.1016/j.forsciint.2014.03.028. [DOI] [PubMed] [Google Scholar]
- (124).Karch SB, Mari F, Bartolini V, Bertol E. Aminorex Poisoning in Cocaine Abusers. Int J Cardiol. 2012;158(3):344–346. doi: 10.1016/j.ijcard.2011.06.105. [DOI] [PubMed] [Google Scholar]
- (125).Pawlik E, Mahler H, Hartung B, Plässer G, Daldrup T. Drug-Related Death: Adulterants from Cocaine Preparations in Lung Tissue and Blood. Forensic Sci Int. 2015;249:294–303. doi: 10.1016/j.forsciint.2015.02.006. [DOI] [PubMed] [Google Scholar]
- (126).Indorato F, Romano G, Barbera N. Levamisole-Adulterated Cocaine: Two Fatal Case Reports and Evaluation of Possible Cocaine Toxicity Potentiation. Forensic Sci Int. 2016;265:103–106. doi: 10.1016/j.forsciint.2016.01.005. [DOI] [PubMed] [Google Scholar]
- (127).Larocque A, Hoffman RS. Levamisole in Cocaine: Unexpected News from an Old Acquaintance. Clin Toxicol Phila Pa. 2012;50(4):231–241. doi: 10.3109/15563650.2012.665455. [DOI] [PubMed] [Google Scholar]
- (128).Brewster ME, Davis FT. Appearance of Aminorex as a Designer Analog of Methylaminorex. J Forensic Sci. 1991;36(2):587–592. [Google Scholar]
- (129).Klein RFX, Sperling AR, Cooper DA, Kram TC. The Stereoisomers of 4-Methylaminorex. J Forensic Sci. 1989;34(4):12723J. [Google Scholar]
- (130).Davis FT, Brewster ME. A fatality involving U4Euh, a cyclic derivative of phenylpropanolamine. J Forensic Sci. 1988;33(2):549–553. [PubMed] [Google Scholar]
- (131).Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA. Recreational Use of Aminorex and Pulmonary Hypertension. Chest. 2000;118(5):1496–1497. doi: 10.1378/chest.118.5.1496. [DOI] [PubMed] [Google Scholar]
- (132).EARLY WARNING NOTIFICATION. [accessed Jul 30, 2018]; https://webcache.googleusercontent.com/search?q=cache:216Ek9IMqEsJ:https://www.europol.europa.eu/sites/default/files/documents/ewn_4-methylaminorex_para-methyl-derivative_feb_2014_-_public_.pdf+&cd=1&hl=de&ct=clnk&gl=at.
- (133).Report on the risk assessment of 4,4′-DMAR in the framework of the Council Decision on new psychoactive substances. [accessed Sept 26, 2018]; www.emcdda.europa.eu http://www.emcdda.europa.eu/publications/risk-assessment/44-dmar_en.
- (134).Brandt SD, Baumann MH, Partilla JS, Kavanagh PV, Power JD, Talbot B, Twamley B, Mahony O, O’Brien J, Elliott SP, et al. Characterization of a Novel and Potentially Lethal Designer Drug (±)-Cis-Para -Methyl-4-Methylaminorex (4,4’-DMAR, or ‘Serotoni’): Characterization of (±)-Cis- and (±)-Trans-Para -Methyl-4-Methylaminorex (4,4’-DMAR) Drug Test Anal. 2014;6(7–8):684–695. doi: 10.1002/dta.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Maier J, Mayer FP, Luethi D, Holy M, Jäntsch K, Reither H, Hirtler L, Hoener MC, Liechti ME, Pifl C, et al. The Psychostimulant (±)-Cis-4,4’-Dimethylaminorex (4,4’-DMAR) Interacts with Human Plasmalemmal and Vesicular Monoamine Transporters. Neuropharmacology. 2018;138:282–291. doi: 10.1016/j.neuropharm.2018.06.018. [DOI] [PubMed] [Google Scholar]
- (136).Greenier E, Lukyanova V, Reede L. Serotonin Syndrome: Fentanyl and Selective Serotonin Reuptake Inhibitor Interactions. AANA J. 2014;82(5):340–345. [PubMed] [Google Scholar]
- (137).EMCDDA. Europol Joint Report on a new psychoactive substance: 4,4’-DMAR (4-methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine) [accessed Sept 26, 2018]; www.emcdda.europa.eu http://www.emcdda.europa.eu/publications/joint-reports/4-4-DMAR_en.
- (138).Council of the European Union. Council implementing decision (EU) 2015/1873 of 8 October 2015 on subjecting 4-methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine (4,4′-DMAR) and 1-cyclo- hexyl-4-(1,2-diphenylethyl)piperazine (MT-45) to control measures. Off J Eur Union. 2015;L275:32–34. [Google Scholar]
- (139).Thirty-seventh meeting of the Expert Committee on Drug Dependence; WHO; [accessed Aug 14, 2018]. http://www.who.int/medicines/access/controlled-substances/5.5_44_DMAR_CRev.pdf. [Google Scholar]
- (140).Who Expert Committee on Drug Dependence: Thirty-Seventh Report. WHO Expert Committee on Drug Dependence, World Health Organization, WHO; 2016. http://apps.who.int/iris/bitstream/handle/10665/206452/WHO_TRS_998_eng.pdf,jsessionid=D22963786FB9EAAE6C2A69E452143F14?sequence=1. [Google Scholar]
- (141).United Nations Commission on Narcotic Drugs. Report on the fifty-ninth session (11 December 2015 and 14-22 March 2016). Economic and Social Council Official Records. 2016 2016, Supplement No. 8. Decision 59/5. http://www.un.org/ga/search/view_doc.asp?symbol=E/2016/28.
- (142).Products from China - Pharmaceutical Chemistry. [accessed Sep 26, 2018]; https://pharma-chemic.com/
- (143).Nizar H, Dargan PI, Wood DM. Using Internet Snapshot Surveys to Enhance Our Understanding of the Availability of the Novel Psychoactive Substance 4-Methylaminorex and 4,4′-Dimethylaminorex. J Med Toxicol. 2015;11(1):80–84. doi: 10.1007/s13181-014-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Loi B, Zloh M, De Luca MA, Pintori N, Corkery J, Schifano F. 4,4′-Dimethylaminorex (“4,4′-DMAR”, “Serotoni”) Misuse: A Web-Based Study. Hum Psychopharmacol Clin Exp. 2017;32(3):e2575. doi: 10.1002/hup.2575. [DOI] [PubMed] [Google Scholar]
- (145).4-MAR (4-Methylaminorex) [accessed Sept 26, 2018];r/researchchemicals. https://www.reddit.com/r/researchchemicals/comments/8y1810/4mar_4methylaminorex/
- (146).Impressed after trying novel stimulant Cyclazodone, but is it estrogenic? [accessed Sept 26, 2018];r/researchchemicals. https://www.reddit.com/r/researchchemicals/comments/7g44ev/impressed_after_tryiny_novel_stimulant/
- (147).4-Methylaminorex vs 2-FMA. [accessed Sept 26 2018];r/researchchemicals. https://www.reddit.com/r/researchchemicals/comments/53bv1j/4methylaminorex_vs_2fma/
- (148).McLaughlin G, Morris N, Kavanagh PV, Power JD, Twamley B, O’Brien J, Talbot B, Dowling G, Mahony O, Brandt SD, et al. Synthesis, Characterization, and Monoamine Transporter Activity of the New Psychoactive Substance 3′,4′-Methylenedioxy-4-Methylaminorex (MDMAR): Newly Emerging Psychoactive Substances. Drug Test Anal. 2015;7(7):555–564. doi: 10.1002/dta.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Kankaanpää A, Ellermaa S, Meririnne E, Hirsjärvi P, Seppälä T. Acute Neurochemical and Behavioral Effects of Stereoisomers of 4-Methylaminorex in Relation to Brain Drug Concentrations. J Pharmacol Exp Ther. 2002;300(2):450–459. doi: 10.1124/jpet.300.2.450. [DOI] [PubMed] [Google Scholar]
- (150).Ashby CR, Pan H, Minabe Y, Toor A, Fishkin L, Wang RY. Comparison of the Action of the Stereoisomers of the Psychostimulant 4-Methylaminorex (4-MAX) on Midbrain Dopamine Cells in the Rat: An Extracellular Single Unit Study. Synapse. 1995;20(4):351–361. doi: 10.1002/syn.890200408. [DOI] [PubMed] [Google Scholar]
- (151).Glennon RA, Misenheimer B. Stimulus Properties of a New Designer Drug: 4-Methylaminorex (“U4Euh”) Pharmacol Biochem Behav. 1990;35(3):517–521. doi: 10.1016/0091-3057(90)90282-m. [DOI] [PubMed] [Google Scholar]
- (152).Batsche K, Ashby CR, Lee C, Schwartz J, Wang RY. The Behavioral Effects of the Stereoisomers of 4-Methylaminorex, a Psychostimulant, in the Rat. J Pharmacol Exp Ther. 1994;269(3):1029–1039. [PubMed] [Google Scholar]
- (153).Russell BR, Beresford RA, Schmierer DM, McNaughtont N, Clark CR. Stimulus Properties of Some Analogues of 4-Methylaminorex. Pharmacol Biochem Behav. 1995;51(2/3):375–378. doi: 10.1016/0091-3057(94)00407-a. [DOI] [PubMed] [Google Scholar]
- (154).Meririnne E, Ellermaa S, Kankaanpää A, Bardy A, Seppälä T. Pharmacokinetics and Tissue Distribution of the Stereoisomers of 4-Methylaminorex in the Rat. J Pharmacol Exp Ther. 2004;309(3):1198–1205. doi: 10.1124/jpet.103.060053. [DOI] [PubMed] [Google Scholar]
- (155).Zheng Y, Russell B, Schmierer D, Laverty R. The Effects of Aminorex and Related Compounds on Brain Monoamines and Metabolites in CBA Mice. J Pharm Pharmacol. 1997;49(1):89–96. doi: 10.1111/j.2042-7158.1997.tb06758.x. [DOI] [PubMed] [Google Scholar]
- (156).Smith RC, Davis JM. Comparative Effects of d-Amphetamine, l-Amphetamine and Methylphenidate on Mood in Man. Psychopharmacology (Berl.) 1977;53(1):1–12. doi: 10.1007/BF00426687. [DOI] [PubMed] [Google Scholar]
- (157).Pitts EG, Curry DW, Hampshire KN, Young MB, Howell LL. (±)-MDMA and Its Enantiomers: Potential Therapeutic Advantages of R(–)-MDMA. Psychopharmacology (Berl.) 2018;235(2):377–392. doi: 10.1007/s00213-017-4812-5. [DOI] [PubMed] [Google Scholar]
- (158).Noggle FT, Clark CR, Deruiter J. Liquid Chromatographic and Spectral Analysis of the Stereoisomers of Dimethylaminorex. J AOAC Int. 1992;75(3):423–427. [Google Scholar]
- (159).EMCDDA. Europol 2015 Annual Report on the Implementation of Council Decision 2005/387/JHA. Annu Rep. 2015;26 [Google Scholar]
- (160).PiHKAL. [accessed Sept 26, 2018]; https://isomerdesign.com/PiHKAL/explore.php.
- (161).Trachsel D, Lehmann D, Enzensperger C. Phenethylamine: Von Der Struktur Zur Funktion. 1. Auflage. Nachtschatten-Verlag; Solothurn: 2013. [Google Scholar]
- (162).Cooper DA. Future synthetic drugs of abuse. In: Castonguay RT, editor. Proceedings of the international symposium on the forensic aspects of controlled substances; March 28 - April 1, 1988; Washington, D.C: Laboratory Division, Federal Bureau of Investigation, U.S. Dept. of Justice; 1989. pp. 79–103. [Google Scholar]
- (163).Morris H, Wallach J. From PCP to MXE: A Comprehensive Review of the Non-Medical Use of Dissociative Drugs. Drug Test Anal. 2014;6(7–8):614–632. doi: 10.1002/dta.1620. [DOI] [PubMed] [Google Scholar]
- (164).The Hive Forum. [accessed Sept 26, 2018]; the-hive.archive.erowid.org.
- (165).The Hive Archive. [accessed Aug 14, 2018]; https://The-Hive.Archive.Erowid.Org/Forum/Showflat.Pl?Cat=&Number=423945&Search=true& Cat=&Threads=&Search_simple=Simple%20Search&Name_simple=kinetic&Text_simple=4-Methyl%20Methcathinone&Name=&Subject=&Body=&Text=424141&Usertitle=&Signature=&RateRemark=&PostNo=&Limit=20&DateFrom=menu&DateTo=menu&From=all&To=now&FromDate=12-31-97&ToDate=08-09-18%2015%3A03&TypePost=on&TypeDigest=on&RateMinus=&RateNeutral=on&RatePlus=on&Order=date&Sort=DESC&Preview=on&PreviewChar=500&NoHelp=0&SearchID=a7gDPTuZXQbPXWPa&URLForums=All_Forums%3Don&Cache=1&Searchpage=0.
- (166).2’-Fluoro-4-Methylaminorex. He’s done it. [accessed Sept 26, 2018]; r/researchchemicals https://www.reddit.com/r/researchchemicals/comments/7xuj76/2fluoro4methylaminorex_hes_done_it/
- (167).The synthesis of 2’-Fluoro-4-Methylaminorex. [accessed Sept 26, 2018];r/TheeHive. http://www.reddit.com/r/TheeHive/comments/7xvd5d/the_synthesis_of_2fluoro4methylaminorex/
- (168).Rickli A, Hoener MC, Liechti ME. Monoamine Transporter and Receptor Interaction Profiles of Novel Psychoactive Substances: Para-Halogenated Amphetamines and Pyrovalerone Cathinones. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25(3):365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- (169).Anyone here who doesn’t like stims? [accessed Sept 26, 2018]; r/researchchemicals https://www.reddit.com/r/researchchemicals/comments/91tp2s/anyone_here_who_doesnt_like_stims/
- (170).4fluoro-4methylaminorex and 4methyl-4methylaminorex. [accessed Sept 26, 2018];r/researchchemicals. https://www.reddit.com/r/researchchemicals/comments/6fm497/4fluoro4methylaminorex_and_4methyl4methylaminorex/
- (171).Response Project. [accessed Sep 26, 2018]; https://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/pF-4-methylaminorex-ID-1940-18_report.pdf.
- (172).4-FPO : researchchemicals. [accessed Sep 26, 2018]; https://www.reddit.com/r/researchchemicals/comments/89we2s/4fpo/
- (173).4-FPO Experience : researchchemicals. [accessed Sep 26, 2018]; https://www.reddit.com/r/researchchemicals/comments/8awmku/4fpo_experience/
- (174).4-FPO Experience: Part 2! [accessed Sep 26, 2018];r/researchchemicals. https://www.reddit.com/r/researchchemicals/comments/8grt0l/4fpo_experience_part_2/
- (175).4-FPO experiences?? [accessed Sep 26, 2018];r/askdrugs. https://www.reddit.com/r/askdrugs/comments/86szp4/4fpo_experiences/
- (176).Synthesis of para-fluoro-(4-methylaminorex) [accessed Sep 26, 2018]; https://www.designer-drug.com/pte/12.162.180.114/dcd/chemistry/para-fluoro-4-mar.html.
- (177).Rodriguez WR, Allred RA. Synthesis of trans-4-methylaminorex from norephedrine and, potassium cyanate. Microgram Journal. 2005;3(3–4):154. [Google Scholar]
- (178).Hive Novel Discourse. [accessed Sep 26, 2018]; https://the-hive.archive.erowid.org/forum/showflat.pl?static=1&Cat=&Number=212038.
- (179).Yelnosky J, Katz R. Sympathomimetic Actions of Cis-2-Amino-4-Methyl-5-Phenyl-2-Oxazoline. J Pharmacol Exp Ther. 1963;141(2):180–184. [PubMed] [Google Scholar]
- (180).Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-Type Central Nervous System Stimulants Release Norepinephrine More Potently than They Release Dopamine and Serotonin. Synap N Y N. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- (181).Mayer FP, Burchardt NV, Decker AM, Partilla JS, Li Y, McLaughlin G, Kavanagh PV, Sandtner W, Blough BE, Brandt SD, et al. Fluorinated Phenmetrazine “Legal Highs” Act as Substrates for High-Affinity Monoamine Transporters of the SLC6 Family. Neuropharmacology. 2018;134:149–157. doi: 10.1016/j.neuropharm.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Solis E, Partilla JS, Sakloth F, Ruchala I, Schwienteck KL, De Felice LJ, Eltit JM, Glennon RA, Negus SS, Baumann MH. N-Alkylated Analogs of 4-Methylamphetamine (4-MA) Differentially Affect Monoamine Transporters and Abuse Liability. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2017;42(10):1950–1961. doi: 10.1038/npp.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu L-H, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological Characterization of Designer Cathinones in Vitro. Br J Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J Pharmacol Exp Ther. 2016;357(1):134–144. doi: 10.1124/jpet.115.229765. [DOI] [PubMed] [Google Scholar]
- (185).Rothman RB, Baumann MH. Therapeutic and Adverse Actions of Serotonin Transporter Substrates. Pharmacol Ther. 2002;95(1):73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- (186).Rothman RB, Blough BE, Baumann MH. Appetite Suppressants as Agonist Substitution Therapies for Stimulant Dependence. Ann N Y Acad Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- (187).Tao R, Fray A, Aspley S, Brammer R, Heal D, Auerbach S. Effects on Serotonin in Rat Hypothalamus of D-Fenfluramine, Aminorex, Phentermine and Fluoxetine. Eur J Pharmacol. 2002;445(1–2):69–81. doi: 10.1016/s0014-2999(02)01751-x. [DOI] [PubMed] [Google Scholar]
- (188).Young R, Glennon RA. Discriminative Stimulus Properties of Amphetamine and Structurally Related Phenalkylamines. Med Res Rev. 1986;6(1):99–130. doi: 10.1002/med.2610060105. [DOI] [PubMed] [Google Scholar]
- (189).Borbély T, Pasi A, Velvart J. Die akute perorale Vergiftung durch 2-Amino-5-Phenyl-Oxazolin-Fumarat beim Menschen anhand von 30 Beobachtungsfällen. Arch Für Toxikol. 1970;26(2):117–124. [PubMed] [Google Scholar]
- (190).Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The Effect of Anticoagulant Therapy in Primary and Anorectic Drug-Induced Pulmonary Hypertension. Chest. 1997;112(3):714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- (191).Friström S, Airaksinen MM, Halmekoski J. Release of Platelet 5-Hydroxytryptamine by Some Anorexic and Other Sympathomimetics and Their Acetyl Derivatives. Acta Pharmacol Toxicol (Copenh) 1977;41(3):218–224. doi: 10.1111/j.1600-0773.1977.tb02142.x. [DOI] [PubMed] [Google Scholar]
- (192).Johnson DN, Funderburk WH, Ward JW. Comparative Effects of Ten Anorectic Drugs on Sleep-Wakefulness Patterns in Cats. Eur J Pharmacol. 1971;15(2):176–179. doi: 10.1016/0014-2999(71)90170-1. [DOI] [PubMed] [Google Scholar]
- (193).Kaminski BJ, Sannerud CA, Griffiths RR. Intravenous Self-Injection of Psychomotor Stimulant--Anorectics in the Baboon. Exp Clin Psychopharmacol. 1996;4(2):141–150. [Google Scholar]
- (194).Kew MC. Aminorex Fumarate: A Double-Blind Trial and Examination for Signs of Pulmonary Arterial Hypertension. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 1970;44(14):421–423. [PubMed] [Google Scholar]
- (195).Mlczoch J, Weir EK, Reeves JT, Grover RF. Long Term Effects of the Anorectic Agent Fenfluramine Alone and in Combination with Aminorex on Pulmonary and Systemic Circulation in the Pig. Basic Res Cardiol. 1979;74(3):313–320. doi: 10.1007/BF01907748. [DOI] [PubMed] [Google Scholar]
- (196).Paul SM, Hulihan-Giblin B, Skolnick P. (+)-Amphetamine Binding to Rat Hypothalamus: Relation to Anorexic Potency for Phenylethylamines. Science. 1982;218(4571):487–490. doi: 10.1126/science.7123250. [DOI] [PubMed] [Google Scholar]
- (197).Poos GI. 2-Amino-5-Aryloxazoline Compositions and Methods of Using Same. US3278382A. 1966 Oct 11;
- (198).Sayers AC, Handley SL. A Study of the Role of Catecholamines in the Response to Various Central Stimulants. Eur J Pharmacol. 1973;23(1):47–55. doi: 10.1016/0014-2999(73)90243-4. [DOI] [PubMed] [Google Scholar]
- (199).Suter W, Jaeger I, Racine RR, Donatsch P, Neumann P, Matter BE. Mutagenicity Evaluation of Amino-Oxazoline Derivatives Using in Vitro and in Vivo Short-Term Tests. Environ Mutagen. 1983;5(4):527–540. doi: 10.1002/em.2860050403. [DOI] [PubMed] [Google Scholar]
- (200).Woolverton WL, Massey BW, Winger G, Patrick GA, Harris LS. Evaluation of the Abuse Liability of Aminorex. Drug Alcohol Depend. 1994;36(3):187–192. doi: 10.1016/0376-8716(94)90144-9. [DOI] [PubMed] [Google Scholar]
- (201).Young R. Aminorex Produces Stimulus Effects Similar to Amphetamine and Unlike Those of Fenfluramine. Pharmacol Biochem Behav. 1992;42(1):175–178. doi: 10.1016/0091-3057(92)90462-o. [DOI] [PubMed] [Google Scholar]
- (202).Young R, Glennon RA. Cocaine-Stimulus Generalization to Two New Designer Drugs: Methcathinone and 4-Methylaminorex. Pharmacol Biochem Behav. 1993;45(1):229–231. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]
- (203).Young R, Glennon RA. Discriminative stimulus effects of S(-)-methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology (Berl.) 1998;140(3):250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
- (204).Bunker CF, Johnson M, Gibb JW, Bush LG, Hanson GR. Neurochemical Effects of an Acute Treatment with 4-Methylaminorex: A New Stimulant of Abuse. Eur J Pharmacol. 1990;180(1):103–111. doi: 10.1016/0014-2999(90)90597-y. [DOI] [PubMed] [Google Scholar]
- (205).Hanson GR, Jensen M, Johnson M, White HS. Distinct Features of Seizures Induced by Cocaine and Amphetamine Analogs. Eur J Pharmacol. 1999;377(2–3):167–173. doi: 10.1016/s0014-2999(99)00419-7. [DOI] [PubMed] [Google Scholar]
- (206).Mansbach RS, Sannerud CA, Griffiths RR, Balster RL, Harris LS. Intravenous Self-Administration of 4-Methylaminorex in Primates. Drug Alcohol Depend. 1990;26(2):137–144. doi: 10.1016/0376-8716(90)90120-4. [DOI] [PubMed] [Google Scholar]
- (207).Meririnne E, Kajos M, Kankaanpää A, Koistinen M, Kiianmaa K, Seppälä T. Rewarding Properties of the Stereoisomers of 4-Methylaminorex: Involvement of the Dopamine System. Pharmacol Biochem Behav. 2005;81(4):715–724. doi: 10.1016/j.pbb.2005.04.020. [DOI] [PubMed] [Google Scholar]
- (208).Coppola M, Mondola R. 4,4′-DMAR: Chemistry, Pharmacology and Toxicology of a New Synthetic Stimulant of Abuse. Basic Clin Pharmacol Toxicol. 2015;117(1):26–30. doi: 10.1111/bcpt.12399. [DOI] [PubMed] [Google Scholar]
- (209).Cosbey S, Kirk S, McNaul M, Peters L, Prentice B, Quinn A, Elliott SP, Brandt SD, Archer RP. Multiple Fatalities Involving a New Designer Drug: Para-Methyl-4-Methylaminorex. J Anal Toxicol. 2014;38(6):383–384. doi: 10.1093/jat/bku031. [DOI] [PubMed] [Google Scholar]
- (210).Glanville J, Dargan PI, Wood DM. 4-Methyl-5-(4-Methylphenyl)-4,5-Dihydrooxazol-2-Amine (4,4′-DMAR, 4,4′-Dimethylaminorex): Availability, Prevalence of Use, Desired Effects and Acute Toxicity: 4,4′-DMAR: Availability, Use and Effects. Hum Psychopharmacol Clin Exp. 2015;30(3):193–198. doi: 10.1002/hup.2472. [DOI] [PubMed] [Google Scholar]
- (211).Cressman WA, Janicki CA, Johnson PC, Doluisio JT, Braun GA. In Vitro Dissolution Rates of Aminorex Dosage Forms and Their Correlation with in Vivo Availability. J Pharm Sci. 1969;58(12):1516–1520. doi: 10.1002/jps.2600581220. [DOI] [PubMed] [Google Scholar]
- (212).Erowid 4-methylaminorex Vault : Dosage. [accessed Sept 26, 2018]; https://erowid.org/chemicals/4_methylaminorex/4_methylaminorex_dose.shtml.
- (213).Soma LR, Rudy JA, Uboh CE, Xu F, Snapp HM. Pharmacokinetics and Effects of Aminorex in Horses. Am J Vet Res. 2008;69(5):675–681. doi: 10.2460/ajvr.69.5.675. [DOI] [PubMed] [Google Scholar]
- (214).Lucchetti J, Marzo CM, Di Clemente A, Cervo L, Gobbi MA. Validated, Sensitive HPLC-MS/MS Method for Quantification of Cis-Para -Methyl-4-Methylaminorex (Cis -4,4’-DMAR) in Rat and Human Plasma: Application to Pharmacokinetic Studies in Rats: A Validated HPLC-MS/MS Method for Cis -4,4’-DMAR Quantification in Plasma. Drug Test Anal. 2017;9(6):870–879. doi: 10.1002/dta.2052. [DOI] [PubMed] [Google Scholar]
- (215).Lucchetti J, Marzo CM, Passoni A, Di Clemente A, Moro F, Bagnati R, Gobbi M, Cervo L. Brain Disposition of Cis- Para -Methyl-4-Methylaminorex (Cis -4,4′-DMAR) and Its Potential Metabolites after Acute and Chronic Treatment in Rats: Correlation with Central Behavioral Effects. J Pharmacol Exp Ther. 2017;361(3):492–500. doi: 10.1124/jpet.117.240788. [DOI] [PubMed] [Google Scholar]
- (216).Henderson GL, Harkey MR, Chueh Y-T. Metabolism of 4-Methylaminorex (“EU4EA”) in the Rat. J Anal Toxicol. 1995;19(7):563–570. doi: 10.1093/jat/19.7.563. [DOI] [PubMed] [Google Scholar]
- (217).Kankaanpää A, Meririnne E, Ellermaa S, Ariniemi K, Seppälä T. Detection and Assay of Cis- and Trans-Isomers of 4-Methylaminorex in Urine, Plasma and Tissue Samples. Forensic Sci Int. 2001;121(1–2):57–64. doi: 10.1016/s0379-0738(01)00453-4. [DOI] [PubMed] [Google Scholar]
- (218).Galley G, Beurier A, Décoret G, Goergler A, Hutter R, Mohr S, Pähler A, Schmid P, Türck D, Unger R, et al. Discovery and Characterization of 2-Aminooxazolines as Highly Potent, Selective, and Orally Active TAAR1 Agonists. ACS Med Chem Lett. 2016;7(2):192–197. doi: 10.1021/acsmedchemlett.5b00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Herman ZS. The Effects of Noradrenaline on Rat’s Behaviour. Psychopharmacologia. 1970;16(5):369–374. doi: 10.1007/BF00404847. [DOI] [PubMed] [Google Scholar]
- (220).Oades RD, Taghzouti K, Rivet JM, Simon H, Le Moal M. Locomotor Activity in Relation to Dopamine and Noradrenaline in the Nucleus Accumbens, Septal and Frontal Areas: A 6-Hydroxydopamine Study. Neuropsychobiology. 1986;16(1):37–42. doi: 10.1159/000118294. [DOI] [PubMed] [Google Scholar]
- (221).4-Methylaminorex (also 4-MAR, U4-E-Uh, Euphoria) : Erowid Exp: Main Index. [accessed Sept 26, 2018]; https://erowid.org/experiences/subs/exp_4Methylaminorex.shtml.
- (222).4,4-DMAR “Serotoni” report. [accessed Sept 26, 2018];r/Drugs. https://www.reddit.com/r/Drugs/comments/1v6hxn/44dmar_serotoni_report/
- (223).Brown JWL, Dunne JW, Fatovic DM, Lee J, Lawn ND. Amphetamine-Associated Seizures: Clinical Features and Prognosis. Epilepsia. 2011;52(2):401–404. doi: 10.1111/j.1528-1167.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- (224).Pifl C, Reither H, Hornykiewicz O. The Profile of Mephedrone on Human Monoamine Transporters Differs from 3,4-Methylenedioxymethamphetamine Primarily by Lower Potency at the Vesicular Monoamine Transporter. Eur J Pharmacol. 2015;755:119–126. doi: 10.1016/j.ejphar.2015.03.004. [DOI] [PubMed] [Google Scholar]
- (225).Lohr KM, Stout KA, Dunn AR, Wang M, Salahpour A, Guillot TS, Miller GW. Increased Vesicular Monoamine Transporter 2 (VMAT2, Slc18a2) Protects against Methamphetamine Toxicity. ACS Chem Neurosci. 2015;6(5):790–799. doi: 10.1021/acschemneuro.5b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Mueller F, Lenz C, Steiner M, Dolder PC, Walter M, Lang UE, Liechti ME, Borgwardt S. Neuroimaging in Moderate MDMA Use: A Systematic Review. Neurosci Biobehav Rev. 2016;62:21–34. doi: 10.1016/j.neubiorev.2015.12.010. [DOI] [PubMed] [Google Scholar]
- (227).Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of Methamphetamine and 3,4-Methylenedioxymethamphetamine. Life Sci. 2014;97(1):37–44. doi: 10.1016/j.lfs.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Garg A, Kapoor S, Goel M, Chopra S, Chopra M, Kapoor A, McCann UD, Behera C. Functional Magnetic Resonance Imaging in Abstinent MDMA Users: A Review. Curr Drug Abuse Rev. 2015;8(1):15–25. doi: 10.2174/1874473708666150303115833. [DOI] [PubMed] [Google Scholar]
- (229).de la Torre R, Farré M. Neurotoxicity of MDMA (Ecstasy): The Limitations of Scaling from Animals to Humans. Trends Pharmacol Sci. 2004;25(10):505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- (230).Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) Neurotoxicity in Rats: A Reappraisal of Past and Present Findings. Psychopharmacology (Berl) 2007;189(4):407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, André CB, Haenggi M, Miss M-T, Galley G, Norcross RD, et al. Brain-Specific Overexpression of Trace Amine-Associated Receptor 1 Alters Monoaminergic Neurotransmission and Decreases Sensitivity to Amphetamine. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37(12):2580–2592. doi: 10.1038/npp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (232).Miner NB, Elmore JS, Baumann MH, Phillips TJ, Janowsky A. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine-Induced Neurotoxicity. NeuroToxicology. 2017;63:57–69. doi: 10.1016/j.neuro.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Lauder JM, Wilkie MB, Wu C, Singh S. Expression of 5-HT2A, 5-HT2B and 5-HT2C Receptors in the Mouse Embryo. Int J Dev Neurosci. 2000;18(7):653–662. doi: 10.1016/s0736-5748(00)00032-0. [DOI] [PubMed] [Google Scholar]
- (234).Baumann MH, Rothman RB. International Review of Neurobiology. New Concepts of Psychostimulant Induced Neurotoxicity. Vol. 88. Academic Press; 2009. Chapter 10 - Neural and Cardiac Toxicities Associated With 3,4-Methylenedioxymethamphetamine (MDMA) pp. 257–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Rickli A, Kopf S, Hoener MC, Liechti ME. Pharmacological Profile of Novel Psychoactive Benzofurans. Br J Pharmacol. 2015;172(13):3412–3425. doi: 10.1111/bph.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME. Pharmacological Profile of Mephedrone Analogs and Related New Psychoactive Substances. Neuropharmacology. 2018;134(Pt A):4–12. doi: 10.1016/j.neuropharm.2017.07.026. [DOI] [PubMed] [Google Scholar]
- (237).Brandt SD, King LA, Evans-Brown M. The New Drug Phenomenon. Drug Test Anal. 2014;6(7–8):587–597. doi: 10.1002/dta.1686. [DOI] [PubMed] [Google Scholar]
- (238).Goodman RM. Method of Decongesting the Nose without Adverse Stimulant Effects. US4980364A. 1990 Dec 25;
- (239).Takagi M, Ishimitsu K, Nishibe T. Oxa(Thia)Zolidine Derivative and Anti-Inflammatory Drug. US6762200B2. 2004 Jul 13;