Abstract
Study Objectives:
At the sleep laboratory, noninvasive positive pressure ventilation titration protocols in patients with neuromuscular disease (NMD) are based on standard pressure cycle devices in a spontaneous/timed mode (BPAP-ST). Experience integrating protocols on average volume-assured pressure support (AVAPS) mode is limited, prompting us to develop a practical single-night titration protocol that provides information to assist clinicians and patients as they decide between BPAP-ST and AVAPS modes.
Methods:
We implemented a sequential titration protocol of BPAP-ST followed by AVAPS during a single-night polysomnography study in patients with NMD and reported polysomnographic and clinical metrics.
Results:
There were 27 patients who completed the protocol: 14 (52%) were male with median and interquartile range (IQR) 64 (59 to 70) years of age and body mass index of 29.6 (25.6–32) kg/m2. They had median (IQR) maximal percent predicted inspiratory and expiratory pressures, and percent vital capacity of 33 (24 to 54), 34 (22 to 47) and 60 (47 to 74), respectively. At final titration of each device, average tidal volume and nadir non-rapid eye movement sleep oxyhemoglobin saturation (SpO2) were higher and respiratory rate/tidal volume, transcutaneous CO2, and arousal index were lower on AVAPS (P < .05) in comparison with BPAP-ST. Full face mask was used in 23 patients (85%). None of the other ventilatory or sleep parameters differed significantly between BPAP-ST and AVAPS (P > .05) sessions.
Conclusions:
A practical single-night split-titration protocol with BPAP-ST and AVAPS can successfully be implemented in patients with NMD, assisting clinicians and patients with the decision on initial treatment modalities and settings.
Citation:
Patel SI, Gay P, Morgenthaler TI, Olson EJ, Shamoun FE, Kashyap R, Herold D, McNamara S, Selim B. Practical implementation of a single-night split-titration protocol with BPAP-ST and AVAPS in patients with neuromuscular disease. J Clin Sleep Med. 2018;14(12):2031–2035.
Keywords: AVAPS, average volume-assured pressure support, bilevel positive airway pressure with backup rate, BPAP-ST, neuromuscular disease
BRIEF SUMMARY
Current Knowledge/Study Rationale: Experience in clinical protocols incorporating average volume- assured pressure support (AVAPS) to standard bilevel positive airway pressure in a spontaneous/timed mode (BPAP-ST) is scant. We evaluated the experience of patients with neuromuscular disease undergoing a polysomnography titration protocol aimed at both BPAP-ST and AVAPS in a single night.
Study Impact: This study describes the successful clinical implementation of a single-night split-titration protocol with BPAP-ST and AVAPS in patients with neuromuscular disease. This study provides a titration model to other sleep centers that seek to offer patients alternative modalities of ventilation such as volume-assured pressure support.
INTRODUCTION
Noninvasive positive pressure ventilation (NPPV) during sleep is used to support patients with chronic alveolar hypoventilation due to a variety of conditions, including neuromuscular disease (NMD).1 NPPV is typically delivered in the NMD population in the form of bilevel positive airway pressure in a spontaneous/timed mode (BPAP-ST).1 Because the actual tidal volume delivered is a function of effort, inspiratory muscle strength, lung compliance, and pressure support delivered, tidal volume will vary across breaths and over time, potentially making it difficult to ensure adequate ventilatory support as patient status changes over time.
In contrast, average volume-assured pressure support (AVAPS) mode (Philips-Respironics, Murrysville, Pennsylvania) is a bilevel servo-controlled noninvasive ventilation system that automatically adjusts inspiratory positive pressure to maintain a consistent tidal volume. By a speed controller that operates on the principle of an error-sensing feedback mechanism (“error signal”), this mode tracks patients' spontaneous respiratory flow, and proportionally adjusts positive pressure during inhalation, to achieve a target respiratory volume threshold. This mode also has a respiratory backup rate system. The intent of AVAPS is to provide greater stability in delivered tidal volume than BPAP-ST with the potential benefit of adapting to the changing respiratory effects of an evolving NMD.
In our sleep laboratory, BPAP-ST has long been the preferred NPPV mode for titration of patients with NMD and chronic respiratory insufficiency. However, AVAPS technology may have an alternative role in ventilatory support of patients with NMD.
In order to obtain needed information to offer therapy with AVAPS, we implemented a polysomnography-based titration protocol allowing sequential adjustment of both BPAP-ST and AVAPS in a single-night study. This protocol development was the result of a practice improvement activity to converge and standardize our titration protocol for patients with NMD. The subsequent clinical decision as to which form of NPPV to prescribe was based on the polysomnography experience with each mode and expressed patient preference combined with the provider's judgement.
Herein, we present the polysomnographic titration protocol and the data routinely obtained from initial patients with NMD undergoing this evaluation: (1) ventilation parameters (tidal volume, respiratory rate, and transcutaneous carbon dioxide levels [tcCO2]) between both modes at the end of their respective titration periods, (2) differences between final AVAPS tidal volume tolerated by patients during titration to the manufacturer's recommended target tidal volume, and (3) sleep efficiency between the two modes of NPPV.
METHODS
Study Design and Population
Approval was obtained from the Mayo Clinic Institutional Review Board to publish the results of this quality improvement initiative. Adult patients (age 18 years or older) with NMD evaluated at the Center for Sleep Medicine, Mayo Clinic, Rochester, Minnesota presenting for our NMD BPAP-ST/AVAPS polysomnography titration protocol between August 1, 2016 and June 15, 2017 were retrospectively reviewed. Ordering of the titration protocol was at the discretion of the sleep physician and not required, so not all patients with NMD evaluated at the center were enrolled. Patients selected for polysomnography titration possessed symptoms related to sleep-related hypoventilation plus one or more of the following factors: arterial blood gas, overnight oximetry, or pulmonary function test criteria that are required by the Centers for Medicare and Medicaid Services for bilevel positive airway pressure (PAP) reimbursement eligibility in the setting of neuromuscular disease.2 None of the patients had baseline polysomnography sleep data without therapy because all studies were full-night PAP titrations as per our typical standard of care.
Our protocol begins with a consultation with a sleep medicine specialist during which the patient's clinical findings are reviewed and recommendation for polysomnography with BPAP titration is explained. Immediately after that, the patient has a 30-minute BPAP introduction session that includes interface mask fitting (full face mask preferred unless not tolerated by the patient), a recumbent breathing session on minimal BPAP (inspiratory pressure 6 cmH2O; expiratory pressure 4 cmH2O; rate 0), and addressing patient questions/ concerns about BPAP. During the subsequent titration polysomnography, patients are first initiated on BPAP-ST with an initial inspiratory pressure of 6 cmH2O, expiratory pressure 4 cmH2O, and backup rate of 8 breaths per minute. The inspira-tory pressure is then increased as tolerated to maintain mean oxyhemoglobin saturation (SpO2) ≥ 90%. An inspiratory-expiratory pressure difference of at least 5 cmH2O and an inspira-tory pressure minimum of 10 cmH2O are ultimately targeted. In the case of any observed obstructive apnea, inspiratory pressure and expiratory pressure are both increased by 1 cmH2O. If hypopneas are noted, inspiratory pressure is increased by 1 cmH2O. During the first rapid eye movement sleep period the backup breath rate is increased to 10 breaths per minutes and then adjusted for patient comfort up to maximum rate of the patient's spontaneous non-rapid eye movement sleep respiratory rate. If mean SpO2 ≥ 90% is achieved during rapid eye movement sleep (preferably both supine and nonsupine rapid eye movement sleep), patients are switched to AVAPS. The transition of PAP modality is programmed remotely by the attending polysomnography technologist so as to minimize disruption to patient sleep.
For AVAPS titration in ST mode, the initial expiratory pressure, backup breath rate, and estimated average tidal volume are identical to the accepted values obtained during the BPAPST titration. The remaining initial AVAPS settings are: minimum pressure support 4 cmH2O, maximum pressure support 20 cmH2O, maximum pressure 25 cmH2O, rate as established in during BPAP-ST titration, inspiratory time 1.5 seconds, rise time 2 (200 milliseconds) to 3 (300 milliseconds), and AVAPS rate of 5. If the initial programmed tidal volume is lower than the target tidal volume of 8 mL/kg ideal body weight, the tidal volume is subsequently increased in increments of 30 mL every 30 minutes or as tolerated by the patient, to the final target tidal volume or highest tidal volume tolerated by patient, whichever is lower.
Polysomnography Data Collection
Registered polysomnography technologists documented average estimated tidal volume and patient respiratory rate (OmniLab Advanced+ titration system) and a steady state tcCO2 (Sentec Digital Monitor, Missouri) on the final titration settings for each NPPV modality. Immediately following the polysomnography, patient preference for the NPPV mode was recorded.
Polysomnography was performed, interpreted, and reported per current American Academy of Sleep Medicine rules.3 We also obtained basic demographic information, pulmonary function values (maximal inspiratory pressure [MIP], maximal expiratory pressure [MEP], vital capacity [VC]), and the neuromuscular disorder diagnosed. For MIP and MEP, the percent of the predicted normal values based on the Black and Hyatt model were recorded.4 For VC, the percent of the predicted normal values based upon the reference values set by the Global Lung Initiative were recorded.5 Respiratory rate/tidal volume was used as an indicator of rapid shallow breathing pattern.
Statistical Analysis
All continuous data distributions were evaluated for normality using the Shapiro-Wilk test. Data are summarized as mean ± standard deviation when normally distributed, or as median and interquartile range [median (Q1, Q3)] when non-normally distributed. Paired t tests were used for parametric comparisons, whereas the Mann-Whitney U test was used for non-parametric comparisons. A value of P < .05 was considered statistically significant. Tests were performed using Wizard version 1.9.18 (233) 2016 and JMP 12.2.0 (SAS Institute, Cary, North Carolina, United States).
RESULTS
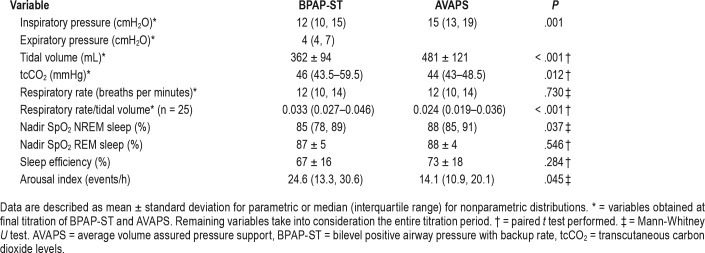
The initial 27 patients undergoing the dual titration protocol were reviewed for this study. Demographic, NMD diagnoses, and pulmonary function data are described in Table 1. Patients were on average 64 (± 5.5) years old, predominantly male, and overweight. Bulbar dysfunction was clearly documented in 13 of 27 patients (48%). The median baseline tcCO2 level on BPAP-ST with inspiratory pressure of 6 cmH2O, expiratory pressure 4 cmH2O, and backup rate of 8 breaths per minute was 46 mmHg (43.5–59.5, P < .012). Ventilatory sleep parameters and device pressure settings at final titration of each device are described in Table 2. Initial full face mask was well tolerated in 23 of 27 patients (85%). The remaining patients (15%) preferred nasal mask. Average inspiratory pressures, average tidal volumes, and nadir SpO2 were higher and respiratory rate/tidal volume and tcCO2 were lower on AVAPS compared to BPAPST. Arousal index was lower on AVAPS when compared to BPAP-ST. All of the following were not significantly different: tcCO2, patient respiratory rate, overall apnea-hypopnea index (AHI), and sleep efficiency. The final asleep tidal volumes on AVAPS prior to study termination were 47.4 mL (with 95% confidence the difference in volume was between 15 to 80 mL) lower than the 8 mL/kg ideal body weight target in the manufacturer's reference table (P = .006).
Table 1.
Demographic variables of patients with NMD undergoing BPAP-ST/AVAPS titration (n = 27).
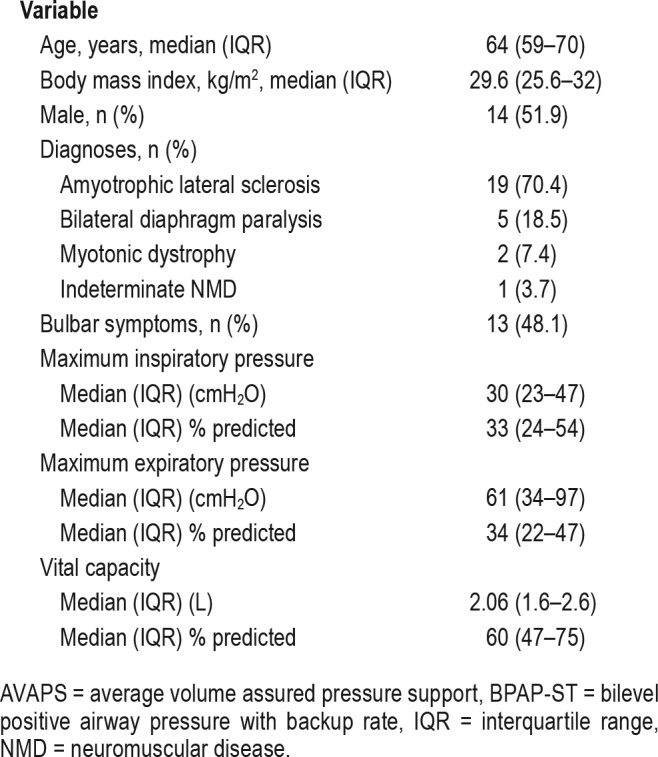
Table 2.
Ventilation and polysomnography parameters on BPAP-ST versus AVAPS.
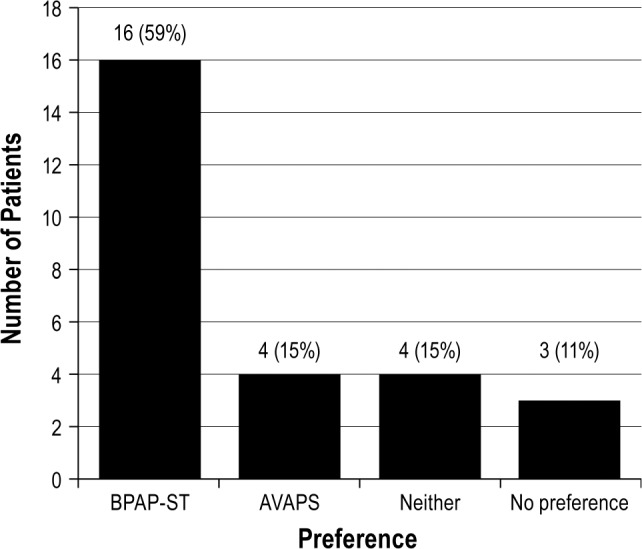
Fourteen patients (52%) were prescribed BPAP-ST, 12 patients (44%) were prescribed AVAPS, and one patient (4%) opted to wait on device prescription until this was discussed further with her neurologist. Patient preference for the BPAP modality is shown in Figure 1.
Figure 1. Patient preference of noninvasive positive airway pressure ventilation mode.
AVAPS = average volume assured pressure support, BPAP-ST = bilevel positive airway pressure with backup rate.
DISCUSSION
Our study describes a “real-world” clinical setting, successfully titrating two modes of pressure support in a single-night polysomnography study. In this cohort of patients with NMD during a single-night polysomnography protocol, AVAPS provided patients with higher tidal volume, higher nadir non-rapid eye movement sleep SpO2, lower arousal index, lower tcCO2, and lower respiratory rate/tidal volume compared to those on BPAP-ST. Other ventilatory and sleep parameters assessed were equivalent during polysomnography. The final tolerated tidal volumes on AVAPS were lower than the manufacturer's recommended 8 mL/kg ideal body weight target. Patients tended to prefer BPAP-ST.
NPPV is the treatment of choice for patients with NMD and chronic alveolar hypoventilation, and BPAP-ST is the most commonly used NPPV modality.1 AVAPS is an alternative form of NPPV that is increasingly available. Although there are theoretical potential benefits to the delivery of targeted tidal volume with AVAPS in the setting of NMD, which may progressively worsen, BPAP-ST is currently the accepted standard mode of NPPV in NMD and the role AVAPS is yet to be fully defined by the current literature.
As observed in the application of our protocol, Nicholson and colleagues have also shown significant improvements in tidal volume and respiratory rate/tidal volume in those using AVAPS when compared to BPAP in patients with NMD.6 That study also showed higher inspiratory pressure, pressure support maximum, and pressure support minimum for those on AVAPS versus BPAP-ST. However, in a study done by Crescimanno and colleagues, volume-guaranteed pressure support and pressure support ventilation revealed similar effects on AHI, SpO2, time spent with SpO2 < 90%, oxygen desaturation index (number of oxygen desaturations of ≥ 4% per hour of total recording time), tidal volume, and respiratory rate on both modes of NPPV for patients with NMD. Arterial blood gases and degree of subjective comfort were also similar.7
Even though our current BPAP-ST titration practice results in a generally satisfactory SpO2, the tidal volumes achieved were significantly lower than the 8 mL/kg target per AVAPS. It is unknown whether this has any clinical relevance for patients going forward. It is possible that lower pressures achieved via BPAP-ST may be the explanation for the patient preference for this NPPV mode over AVAPS.
In our single-night polysomnography protocol, more patients preferred BPAP-ST than AVAPS. Patient preference may lead to better adherence, which could be more advantageous than ensuring a more physiologically appropriate tidal volume. However, the nonrandomized titration order and the final chosen generally higher tidal volume on AVAPS may have influenced patient preferences. A research study designed to allow for more experience with each modality is likely needed to determine the meaning behind patient preferences more accurately.
A randomized crossover trial utilizing BPAP-ST and AVAPS in obesity hypoventilation syndrome (OHS) over 6 weeks noted mean transcutaneous pCO2 significantly decreased during AVAPS by −12.6 ± 12.2 mmHg. They noted a nonsignificant lower leak on AVAPS.8 Another randomized controlled trial for patients with OHS comparing BPAP-ST to AVAPS outcomes after 3 months of use noted improvements in arterial pCO2 for both the BPAP-ST and AVAPS groups without significant in between group difference. Leak was similar between the two modes of NPPV.9
Data from our titration protocol should not be used to directly compare either the chronic effectiveness of these two modalities or form a firm view of how sleep parameters or ventilation might differ between the modalities in a given patient. There are multiple limitations to this descriptive study including a nonrandomized order of PAP device introduction, a small sample size, and the fact that many of the initial parameters on AVAPS were derived from optimal BPAP-ST titration, which likely accelerated the AVAPS titration and may have increased the likelihood of effective settings reached. The nonrandomized order of the titration protocol makes comparison of sleep stages of limited use, so it was not included. Quantitative mask leak information was not collected as a part of this study; hence, the tidal volume information may not be entirely accurate and falsely elevated for both BPAP-ST and AVAPS titrations. Prior studies done in the NMD population have noted a similar leak between pressure support ventilation and volume-guaranteed pressure support ventilation.6,7
No patient had baseline polysomnography data as all studies were full-night PAP titrations. Patients were on PAP even during the initial patient calibrations so their baseline respiratory system parameters during spontaneous, unassisted breathing are unknown. Prepolysomnography arterial blood gas data were not collected as part of the study and would have been helpful further describing the population. It has not been determined how many patients with NMD who underwent polysomnography in our center over the period retrospectively reviewed were excluded from the protocol, as ordering of the titration protocol was at the discretion of the sleep physician. There is also no long-term outcome data, so the clinical significance of the few differences observed between the modalities is unclear.
Further research is needed utilizing a larger sample size, and randomized introduction to BPAP-ST and AVAPS. Additionally, looking at how each NMD responds to these modes instead of combining them all in one group may be helpful and would be possible with a larger cohort.
CONCLUSIONS
This is a successful clinical implementation of a single-night split-titration protocol with BPAP-ST and AVAPS in patients with NMD. Our experience may provide a titration model to other sleep centers who seek to gain information to assist in offering patients AVAPS.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AVAPS
average volume assured pressure support
- BPAP-ST
bilevel positive airway pressure with backup rate
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- NPPV
noninvasive positive airway pressure ventilation
- NMD
neuromuscular disease
- SpO2
oxyhemoglobin saturation
- tcCO2
transcutaneous carbon dioxide levels
- VC
vital capacity
REFERENCES
- 1.Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6(5):491–509. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare & Medicaid Services. Decision Memo for Noninvasive Positive Pressure RADs for COPD (CAG-00052N) [Accessed July 2, 2018]. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=56&ver=5&viewAMA=Y&bc=AAAAAAAAEAAA&.
- 3.Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual Updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99(5):696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 5.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson TT, Smith SB, Siddique T, et al. Respiratory pattern and tidal volumes differ for pressure support and volume-assured pressure support in amyotrophic lateral sclerosis. Ann Am Thorac Soc. 2017;14(7):1139–1146. doi: 10.1513/AnnalsATS.201605-346OC. [DOI] [PubMed] [Google Scholar]
- 7.Crescimanno G, Marrone O, Vianello A. Efficacy and comfort of volume-guaranteed pressure support in patients with chronic ventilatory failure of neuromuscular origin. Respirology. 2011;16(4):672–679. doi: 10.1111/j.1440-1843.2011.01962.x. [DOI] [PubMed] [Google Scholar]
- 8.Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. 2006;130(3):815–821. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 9.Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67(8):727–734. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]