Abstract
Study Objectives:
To investigate the association between non-apnea sleep disorders (NSD) and subsequent benign paroxysmal positional vertigo (BPPV) risk.
Methods:
This retrospective cohort study was conducted using the Taiwan National Health Insurance Research Database from 2000 to 2013. We established an NSD group (n = 24,624) and an age-, sex- and index year-matched comparison group (n = 98,496). The primary outcome was the occurrence of BPPV. The incidence rates of BPPV in the two cohorts were compared with a 14-year follow-up. Cox proportional hazard regression analysis was used to evaluate the effects of NSD on BPPV risk.
Results:
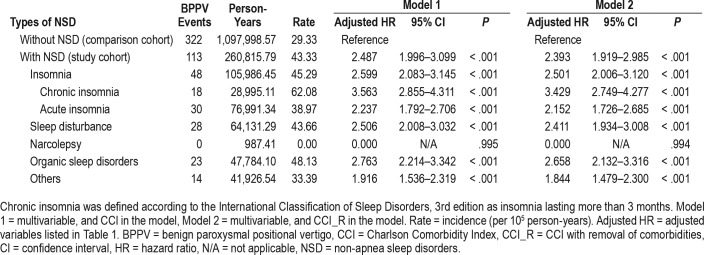
The incidence rate of BPPV was 43.33 per 100,000 person-years for the NSD cohort and 29.33 per 100,000 person-years for the comparison cohort. NSD significantly increased the risk of BPPV (adjusted hazard ratio [HR] = 2.487; 95% confidence interval = 1.996–3.099, P < .001). Subgroup analysis revealed that NSD increase the risk of development of BPPV by 2.357- to 3.658-fold in patients with hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and hyperlipidemia. Furthermore, when comparing different types of NSD, chronic insomnia carries the highest risk of BPPV (adjusted HR = 3.563), followed by organic sleep disorders (adjusted HR = 2.763), sleep disturbance (adjusted HR = 2.506), and acute insomnia (adjusted HR = 2.237).
Conclusions:
We demonstrate that NSD are associated with an increased risk of BPPV. Relative to other types of NSD, patients with chronic insomnia are at the highest risk for development of BPPV.
Citation:
Shih CP, Wang CH, Chung CH, Lin HC, Chen HC, Lee JC, Chien WC. Increased risk of benign paroxysmal positional vertigo in patients with non-apnea sleep disorders: a nationwide, population-based cohort study. J Clin Sleep Med. 2018;14(12):2021–2029.
Keywords: benign paroxysmal positional vertigo, insomnia, non-apnea sleep disorders, vestibular system
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insomnia and sleep disturbance have negative effects on physiology and daytime function. A link between dizziness and sleep disorders has been reported. Nonetheless, no studies have explored the relationship between benign paroxysmal positional vertigo (BPPV) and non-apnea sleep disorders (NSD). This study is the first population-based cohort study to delineate the association between NSD and subsequent risk of BPPV.
Study Impact: This study revealed that the patients with NSD had higher risk of development of BPPV. Among different types of NSD, chronic insomnia conferred the highest BPPV risk, followed by organic sleep disorders, sleep disturbance, and acute insomnia.
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is the most common cause of vertigo and is characterized by experiencing a spinning sensation for periods from a few seconds to 1 minute; these episodes are generally triggered by an abrupt change in head position with respect to gravity.1 The condition mostly occurs in females between 50 and 60 years of age. The prevalence of this condition is between 10.7 and 64.0 per 100,000 population, with a lifetime prevalence of 2.4%.1,2 The pathophysiologic mechanism underlying BPPV involves dislodged otoconia from the utricular macula that enter the semicircular canals. When one or more semicircular canals are inappropriately stimulated by the otoconia moving within the semicircular canals upon a change in head position, episodes of vertigo are induced.1 Another theory explaining the pathophysiology of BPPV is cupulolithiasis.3 Spontaneous remission occurs in some cases; however, the annual rate of recurrence is approximately 15%.1,4 Most cases can be effectively treated with canalith reposition maneuvers. After the treatment, 7% to 23% of cases still show relapse at 1-year follow-up, and long-term relapse may approach 50%.5 The etiology of BPPV in most patients is unclear. Previous studies report that patients with BPPV have higher rates of hearing loss, head trauma, diabetes, hypercholesterolemia, thyroid disease, and allergies than the general population.6,7 Head trauma has been identified to be a mechanical cause of BPPV.8 Aging, osteoporosis, Meniere disease, labyrinthitis, migraine, and sudden sensorineural hearing loss are associated with BPPV.3,5,8 Although benign in its disease course, BPPV can cause physical and psychological distress. Patients with BPPV experience a decrease in health-related quality of life and are at increased risk for falls and impairment of daily activities.8,9 Therefore, the disease has negative effect on social performance and mental health, especially in older patients.
Sleep disturbance can adversely affect physiology, behavior, and daily abilities.10 Non-apnea sleep disorders (NSD) have been recognized to promote the development and exacerbate the severity of several diseases, including diabetes mellitus, hypertension, ischemic stroke, peripheral arterial disease, and kidney injury.11–13 Insomnia is the most common sleep disorder and has been shown to have a detrimental effect on health-related quality of life and to increase the risk of death.10,14 Previous studies report a link between dizziness and sleep disturbance.15,16 The prevalence of sleep disturbance is approximately 65% in patients with chronic dizziness.16 However, no studies investigating the involvement of NSD in increasing subsequent BPPV risk have been conducted. Therefore, this study aimed to determine the risk of subsequent BPPV in patients with NSD by enrolling a NSD cohort from Taiwan's nationwide population-based database. This retrospective cohort study might provide valuable information regarding the demographic risk factors and comorbidities of NSD and their association with BPPV.
METHODS
Data Sources
In this study, we used data from the Taiwan National Health Insurance Research Database (NHIRD) to investigate the risk of having BPPV in patients with NSD over a 14-year period. We reviewed records from the Longitudinal Health Insurance Database (2000–2013), which is a valid representative sample of the total population of 23,000,000 in Taiwan and which includes claims data for 1 million enrollees randomly selected from all beneficiaries of the National Health Insurance (NHI) program. To ensure confidentiality, the enrollees' personal information is scrambled using anonymous identification numbers. The NHI Program was launched in Taiwan in 1995, and as of June 2009, it included contracts with 97% of the medical providers in Taiwan with approximately 23 million beneficiaries, that is, more than 99% of the entire population of Taiwan.17 The NHIRD uses the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to record diagnoses. The Bureau of NHI randomly reviews the records of 1 in 100 ambulatory care visits and 1 in 20 in-patient claims to verify the accuracy of the diagnoses. Several studies have demonstrated the accuracy and validity of the diagnoses in the NHIRD.11,18,19 The Institutional Review Board of Tri-Service General Hospital approved this study and waived the need for individual written informed consent (TSGH IRB No. 2-105-05-082).
Study Design and Sample Participants
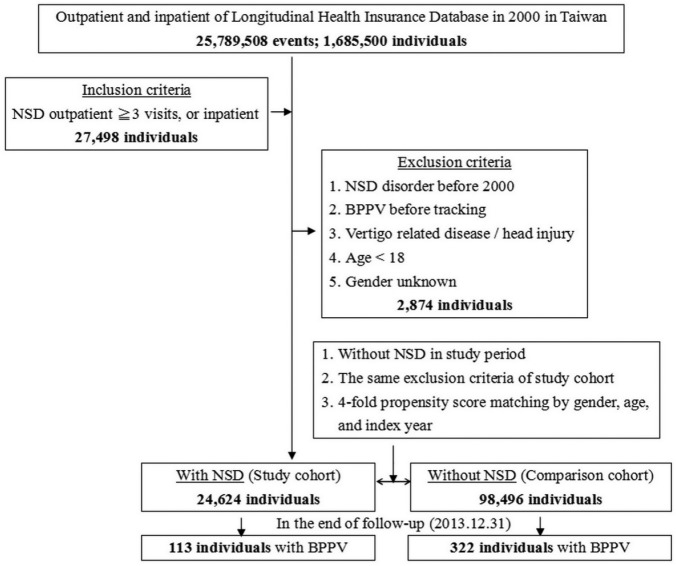
This study was a retrospective matched-cohort design. Among the 1,685,500 individuals recorded in the outpatient and inpatient data from January 1, 2000 to December 31, 2013, 27,498 individuals, including outpatients with at least three diagnoses of NSD (ICD-9-CM codes: 307.4, 327, 347, 780.50, 780.52, 780.54–780.56, 780.58, and 780.59) made at different times and inpatients with diagnoses of NSD were collected. The patients were excluded if they fit into one of the following criteria: NSD diagnosed before 2000, having BPPV (ICD-9-CM code: 386.11) before tracking, having other vertigo-related diagnoses (ICD-9-CM codes: 078.81, 255.1, 386.0–386.10, 386.12–386.9, 388.2, and 780.4), having head injury (ICD-9-CM codes: 310.2, 800–804, 850–854, 870–873, 907.0, 907.1, 959.0, and V15.52), age younger than 18 years, and sex unknown. The final study group included 24,624 individuals. The comparison group was based on the same exclusion criteria as the study group but excluded cases of NSD during the study period. We then used propensity score-matching analysis to select four times as many cases matched by index year, sex, and age; that is, the comparison group comprised 98,496 individuals. The study and comparison cohorts were tracked until December 31, 2013 for the occurrence of BPPV. Board-certified otolaryngologists or neurologists who were competent at performing Dix-Hallpike or rollover tests (diagnostic maneuvers of BPPV) confirmed the diagnosis. To increase the diagnostic accuracy, the individuals in whom BPPV was diagnosed in outpatient department visits or who were hospitalized with BPPV as the primary diagnosis by otolaryngologists or neurologists were defined as having BPPV develop (Figure 1). To compare the effects of different types of NSD on subsequent BPPV risk, we further classified NSD into insomnia (ICD-9-CM codes: 307.41, 307.42, and 780.52), sleep disturbance (ICD-9-CM codes: 780.50, 780.54–780.56, 780.58, and 780.59), organic sleep disorders (ICD-9-CM codes: 327), narcolepsy (ICD-9-CM codes: 347), and other sleep disorders (ICD-9-CM code: 307.40, 307.43–307.49). According to the International Classification of Sleep Disorders, Third Edition (ICSD-3), chronic insomnia is defined as insomnia lasting longer than 3 months
Figure 1. Flowchart of the study sample selection from the National Health Insurance Research Database in Taiwan.
BPPV = benign paroxysmal positional vertigo, NSD = non-apnea sleep disorders.
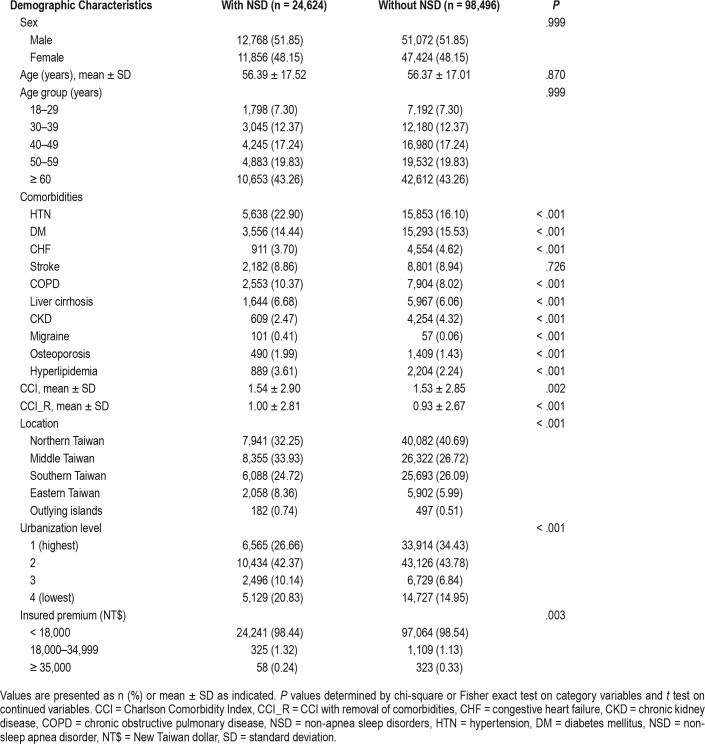
We extracted the demographic information for each individual, including sex, age group (18–29, 30–39, 40–49, 50–59, 60 years or older), geographical area of residence (the north, center, south, and east of Taiwan, and outlying islands), urbanization level of residence (level 1 to 4), and monthly income (consistent with insured premium; in New Taiwan Dollars; < 18,000, 18,000–34,999, ≥ 35,000). The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population > 1,250,000 with a specific designation as a center of political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999 playing an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as populations from 149,999 to 499,999 and < 149,999, respectively.
Baseline comorbidities including hypertension (ICD-9-CM codes: 401–405), diabetes mellitus (ICD-9-CM code: 250), congestive heart failure (ICD-9-CM code: 428), stroke (ICD-9-CM codes: 430–438), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes: 490–496), liver cirrhosis (ICD-9-CM code: 571), chronic kidney disease (ICD-9-CM codes: 585–586), migraine (ICD-9-CM code: 346), osteoporosis (ICD-9-CM code: 733.0), and hyperlipidemia (ICD-9-CM codes: 272.0–272.4) were collected. The Charlson Comorbidity Index (CCI) and the Charlson Comorbidity Index after removal of the aforementioned comorbidities (CCI_R) were used to demonstrate the extent of the comorbidities.
Statistical Analysis
Data management and statistical analysis were performed using SPSS software version 22 (SPSS Inc., Chicago, Illinois, United States). χ2 and t tests were used to evaluate the distributions of categorical and continuous variables, respectively. Multivariate Cox proportional hazards regression analysis was used to determine the risk of BPPV, and the results are presented as hazard ratio (HR) with 95% confidence interval (CI). The difference in the risk of BPPV between the study and comparison groups was estimated using the Kaplan-Meier method and the log-rank test. A two-tailed value of P < .05 was considered to indicate statistical significance.
RESULTS
Demographic Characteristics of the Study Population
During the 14-year study period, a total of 123,120 individuals including 24,624 patients with NSD and 98,496 comparison individuals were examined in this study. The demographic characteristics of the NSD cohort and the matched comparison cohort are presented in Table 1. The mean age ± standard deviation (SD) of the NSD cohort was 56.39 ± 17.52 years, and 51.85% were male. The mean age ± SD of the matched comparison cohort was 56.37 ± 17.01 years, and 51.85% were male. Because age and sex were matched between the cohorts, no significant difference in age or sex was found. Compared to the comparison cohort, the NSD cohort had higher rates of hypertension, COPD, liver cirrhosis, migraine, osteoporosis, and hyperlipidemia and lower rates of diabetes mellitus, congestive heart failure, and chronic kidney disease. The level of CCI was higher in the NSD cohort than in the comparison cohort. Patients with NSD lived more frequently in less urbanized areas than did the comparison individuals. In addition, patients with NSD were more frequently located in the middle and eastern areas of Taiwan than were the comparison individuals. The median follow-up duration was 10.42 years for the NSD cohort and 11.25 years for the comparison cohort.
Table 1.
Demographic characteristics of the study cohort at baseline.
Incidence of BPPV Among Patients With NSD
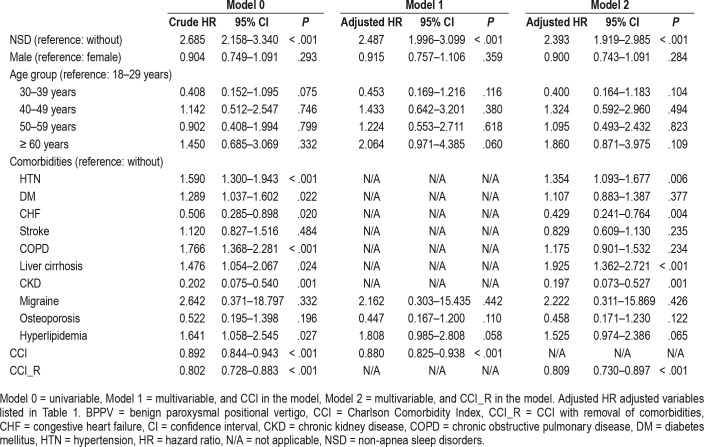
During the follow-up, the cumulative incidence of developing BPPV was 0.46% (113/24,624 individuals) in the NSD cohort and 0.33% (322/98,496 individuals) in the comparison cohort. The incidence of BPPV was 43.33 per 100,000 person-years for the NSD cohort and 29.33 per 100,000 person-years for the comparison cohort. The Kaplan-Meier analysis presented the difference in cumulative risk of the development of BPPV between two cohorts in 14-year follow-up and indicated that patients with NSD were at significantly higher risk of the development of BPPV than were the comparison individuals (log-rank test P < .001) (Figure 2). We analyzed the risk of the development of BPPV among the patients with NSD. The Cox regression analysis of the factors associated with the risk of BPPV showed that the crude HR in the NSD cohort was 2.685 (95% CI = 2.158–3.34) (Table 2). After adjusting for age, sex, comorbidities, geographical area of residence, urbanization level of residence, and monthly income, the adjusted HR in the NSD cohort was 2.487 (95% CI = 1.996–3.099), indicating that patients with NSD had a 2.487-fold higher risk of the development of BPPV than the individuals without NSD. In addition, hypertension (adjusted HR = 1.354; 95% CI = 1.093–1.677) and liver cirrhosis (adjusted HR = 1.925; 95% CI = 1.362–2.721) were independent risk factors that were significantly associated with BPPV.
Figure 2. Kaplan-Meier for the cumulative risk of benign paroxysmal positional vertigo among patients aged 18 and older stratified by NSD based on the log-rank test.
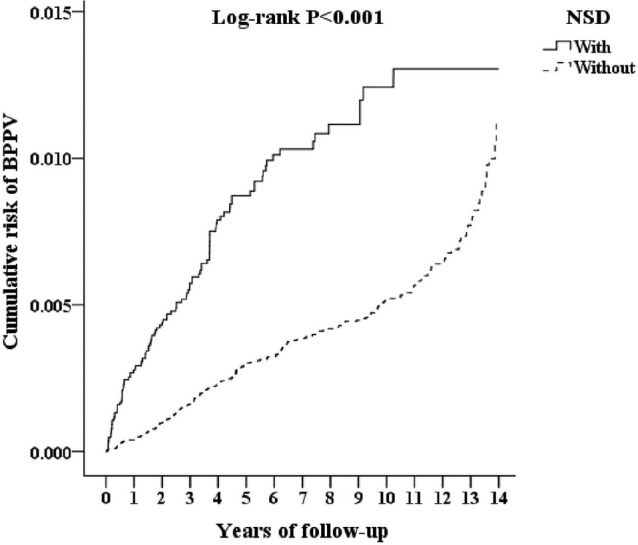
BPPV = benign paroxysmal positional vertigo, NSD = non-apnea sleep disorders.
Table 2.
Cox regression analysis of risk factors for BPPV.
Subgroup Analysis
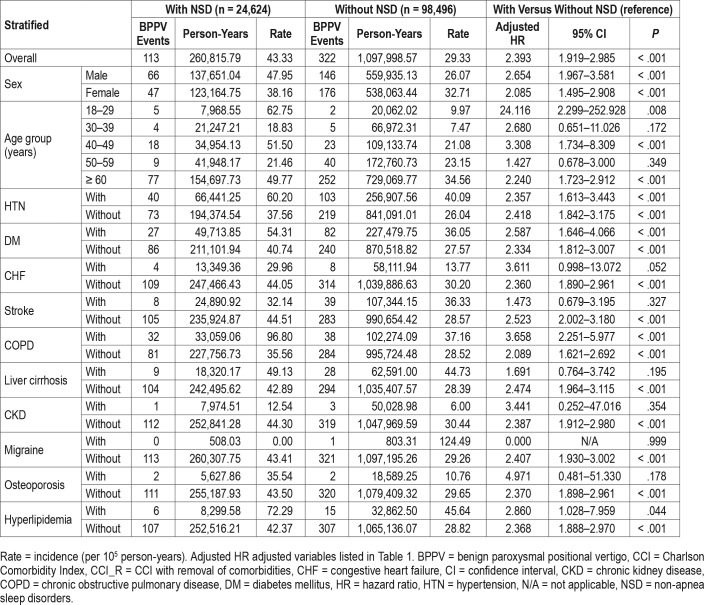
We analyzed the association between NSD and the risk of BPPV as stratified by sex, age, and comorbidities; both the male subgroup with NSD and the female subgroup with NSD had higher incidences of BPPV than the matched subgroup without NSD (47.95 versus 26.07 per 100,000 person-years, and 38.16 versus 32.71 per 100,000 person-years, respectively) (Table 3). The adjusted HR of BPPV related to NSD was 2.654 (95% CI = 1.967–3.581) in the male subgroup and 2.085 (95% CI = 1.495–2.908) in the female subgroup. The effect of NSD on the risk of subsequent BPPV varied among the age subgroups. In all age subgroups (18–29, 40–49 and 60 years or older), NSD increased the risk of developing BPPV more than 2.24-fold. The association between NSD and the risk of BPPV as stratified by comorbidity is shown in Table 3. The adjusted HR was 2.357 (95% CI = 1.613–3.443) in the NSD subgroup with hyper-tension, 2.587 (95% CI = 1.646–4.066) in those with diabetes mellitus, 3.658 (95% CI = 2.251–5.977) in those with COPD, and 2.86 (95% CI = 1.028–7.959) in those with hyperlipidemia. Therefore, among the patients with hypertension, diabetes mellitus, COPD, and hyperlipidemia, NSD increases the risk of the development of BPPV 2.357- to 3.658-fold. In another subgroup analysis, the relationships between the different types of NSD and BPPV were examined (Table 4). The adjusted HRs were 2.237 (95% CI = 1.792–2.706) for acute insomnia; 3.563 (95% CI = 2.855–4.311) for chronic insomnia; 2.506 (95% CI = 2.008– 3.032) for sleep disturbance; 2.763 (95% CI = 2.214–3.342) for organic sleep disorders; and 1.916 (95% CI = 1.536–2.319) for other NSD, indicating that chronic insomnia presented a higher risk of the development of BPPV than the other types of NSD.
Table 3.
Risk of BPPV at the end of follow-up as stratified by the variables listed in the table.
Table 4.
Comparison of BPPV occurrence between patients with different types of NSD and the comparison cohort.
DISCUSSION
This nationwide, population-based, cohort study demonstrates that NSD are associated with an increased risk of the development of BPPV that is 2.487 times higher than that of matched comparison cohort. This research is the first study to confirm that NSD are a significant risk factor for BPPV. In addition, the association between NSDs and the risk of subsequent BPPV remained when considering underlying comorbidities, including hypertension, diabetes mellitus, COPD, and hyperlipidemia. Among the patients with these comorbidities, NSD increase the risk of the development of BPPV by 2.357- to 3.658-fold. Patients of both sexes with NSD, but especially males, are at increased risk of the development of BPPV. The incidence was 47.95 per 100,000 person-years in the male patients with NSD as compared with 26.07 in the male individuals without NSD and was 38.16 per 100,000 person-years in the female patients with NSD as compared with 32.71 in the female individuals without NSD. Based on the analysis of NSD subgroups, we found that the effect on subsequent BPPV risk differed among the NSD types. Chronic insomnia conferred the highest BPPV risk (adjusted HR = 3.563; 95% CI = 2.855–4.311), followed by organic sleep disorders (adjusted HR = 2.763; 95% CI = 2.214–3.342), sleep disturbance (adjusted HR = 2.506; 95% CI = 2.008–3.032), and acute insomnia (adjusted HR = 2.237; 95% CI = 1.792–2.706).
BPPV more commonly affects females.1,2,20 A German population study reports that the lifetime prevalence is 3.2% in females, 1.6% in males, and 2.4% overall.2 The sex distribution has a female to male ratio of 2:1.2 Another epidemiological study reveals that the incidence of Japan is about 10.7 per 100,000 persons, and the female to male ratio is 1.46:1.20 In NHIRD, our general population has 20,991 BPPV cases with a female to male ratio of 1.34:1 (see Table S1 in the supplemental material). The sex distribution of our general population is similar to that of previous studies. Interestingly, the number of male BPPV cases was higher than that of female cases in the NSD cohort. The subgroup analysis showed that male patients with NSD were at increased risk of development of BPPV of 2.654-fold, and female patients with NSD had a 2.085-fold higher risk of BPPV. It suggests that males with NSD present with a higher risk of the development of BPPV than females with NSD. This finding can clarify the difference in sex distribution between the NSD cohort and general population.
Fifty percent to 70% of BPPV is idiopathic, and the secondary causes can be identified in the remaining cases.21 Head trauma can displace otoliths due to mechanical stress to the vestibular organ and is regarded as the most common cause of secondary BPPV. Inner ear surgery and dental procedures, two other mechanical factors, have been demonstrated to carry the risk of loosening otoliths and are associated with the occurrence of BPPV.21,22 Other potential factors linked to the etiology of BPPV include previous or preexisting labyrinthine disease, vascular pathology, neural inflammation and degeneration, and neuroendocrine dysfunction.3,8,21,23,24 BPPV can occur following labyrinthine disease resulting from viral infection or ischemia.8 The development of BPPV in patients with Meniere disease may result from hydropically induced damage to the macula of the utricle or partial obstruction of the membranous labyrinth.21 A previous study reported neural pathologic findings in the temporal bone of BPPV cases.23 The major pathological changes in the temporal bone are degeneration of vestibular neuron and significant loss of superior and inferior vestibular ganglion cells.23 Loss of the inhibitory action of otolith organs on canal activation caused by inflammation and the degeneration of otolith neurons is a possible pathophysiological mechanism. Neuroendocrine dysfunction due to the abnormal activation of the hypothalamus-pituitary-adrenal axis can result in inner ear blood flow imbalance, thus influencing otoconial homeostasis, which has been considered part of the pathogenesis of BPPV.24
No direct pathophysiological link between NSD and BPPV has yet been established. Herein, we inferred several potential mechanisms from previous studies that may provide possible explanations. First, sleep deprivation is associated with an increase in vestibulo-ocular reflex asymmetry.25 Thus, NSD may impair the vestibular system. Vestibular dysfunction is regarded as one of the pathogeneses for BPPV.8,21 Second, sleep disturbance and insomnia can induce neuroendocrine dysfunction that is characterized by increased cortisol levels resulting from hypothalamic-pituitary-adrenal axis activation and increased sympathetic activity.26 Neuroendocrine dysfunction is one of the proposed mechanisms for the effect of NSD on the occurrence of BPPV. Third, chronic sleep disturbance triggers an activation of inflammation that influences neural processes and brain function.26 Accordingly, NSD may lead to the inflammation of vestibular neurons, which are suggested to play a crucial role in the pathophysiology of BPPV.23,27
Sleep disorders are known to be associated with medical and psychiatric comorbidities.28 About 40% of individuals with insomnia have a comorbid psychiatric condition.10 Psychiatric diseases, including depressive and anxiety disorders, can lead to insomnia and sleep disturbance.10,28,29 Occupational and environmental problems and stress of lifestyle can be the cause of sleeplessness and inadequate sleep.30 The correlation between insomnia and a variety of medical conditions has been disclosed in the literature.10,31 Individuals with the following medical conditions experience more chronic insomnia than do those without those medical problems: coronary heart disease, congestive heart failure, cancer, high blood pressure, neurologic disease, breathing problems, urinary problems, chronic pain, and various forms of arthritis.10,31,32 Because this study shows that NSD are related to the subsequent development of BPPV, the psychological and emotional stress and medical problems that disturb sleep are potentially cofactors to trigger the development of BPPV.
The strengths of this study are its population-based research, use of well-established cohort data with a large sample size and extended follow-up period to identify NSD as a risk factor of the development of BPPV. Nevertheless, there are several limitations to this study. First, the NHIRD does not provide detailed information on several factors that may have been confounding variables in this study, such as tobacco use, alcohol consumption, favored sleeping position, and physical activity. Serum laboratory data are also unavailable because the data in the NHIRD are anonymous. Second, the effect of NSD on the prognosis of BPPV cannot be assessed because detailed clinical data on the severity and outcome of BPPV are unavailable in this database. Third, the presentation of the involved canals in BPPV cases of the NSD cohort cannot be investigated because the information about the involvement of semicircular canals in BPPV patients is unavailable in this database.
In conclusion, this study demonstrates that NSD are a significant and independent risk factor for BPPV. The patients with NSD had a 2.487-fold higher risk of the development of BPPV than the age-, sex- and index year-matched comparison cohort. Furthermore, the patients with chronic insomnia carried the highest risk of incident BPPV. Future studies are needed to clarify the mechanism underlying the effect of NSD on the development of BPPV. Because increasing numbers of patients have NSD, clinicians should be aware of the association between NSD and the subsequent BPPV risk and provide health education regarding vertigo to individuals with sleep disorders.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was supported by grants from the Tri-Service General Hospital Research Foundation (TSGH-C107-026 to CP Shih and TSGH-C107-004 to WC Chien) and by National Defense Medical Research Grants (MAB-105-020 and MAB-107-004 to CP Shih). The authors report no conflicts of interest.
ABBREVIATIONS
- BPPV
benign paroxysmal positional vertigo
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- ICSD
International Classification of Sleep Disorders
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- NSD
non-apnea sleep disorders
- SD
standard deviation
REFERENCES
- 1.Kim JS, Zee DS. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370(12):1138–1147. doi: 10.1056/NEJMcp1309481. [DOI] [PubMed] [Google Scholar]
- 2.Von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zappia JJ. Benign paroxysmal positional vertigo. Curr Opin Otolaryngol Head Neck Surg. 2013;21(5):480–486. doi: 10.1097/MOO.0b013e32836463d6. [DOI] [PubMed] [Google Scholar]
- 4.Furman JM, Cass SP. Benign paroxysmal positional vertigo. N Engl J Med. 1999;341(21):1590–1596. doi: 10.1056/NEJM199911183412107. [DOI] [PubMed] [Google Scholar]
- 5.Fife T, Iverson D, Lempert T, et al. Practice Parameter: Therapies for benign paroxysmal positional vertigo (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70(22):2067–2074. doi: 10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 6.Ogun OA, Janky KL, Cohn ES, Büki B, Lundberg YW. Gender-based comorbidity in benign paroxysmal positional vertigo. PLoS One. 2014;9(9):e105546. doi: 10.1371/journal.pone.0105546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen HS, Kimball KT, Stewart MG. Benign paroxysmal positional vertigo and comorbid conditions. ORL J Otorhinolaryngol Relat Spec. 2004;66(1):11–15. doi: 10.1159/000077227. [DOI] [PubMed] [Google Scholar]
- 8.Nuti D, Masini M, Mandalà M. Benign paroxysmal positional vertigo and its variants. Handb Clin Neurol. 2016;137:241–256. doi: 10.1016/B978-0-444-63437-5.00018-2. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Escamez JA, Gamiz MJ, Fernandez-Perez A, Gomez-Fiñana M. Long-term outcome and health-related quality of life in benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2005;262(6):507–511. doi: 10.1007/s00405-004-0841-x. [DOI] [PubMed] [Google Scholar]
- 10.Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167–174. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin HY, Chang KT, Chang YH, et al. Nonapnea sleep disorders and the risk of acute kidney injury: a nationwide population-based study. Medicine. 2016;95(11):e3067. doi: 10.1097/MD.0000000000003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CH, Huang KY, Chen WC, et al. Sleep disorders in individuals without sleep apnea increase the risk of peripheral arterial disorder: a nationwide population-based retrospective cohort study. Sleep Med. 2015;16(8):966–970. doi: 10.1016/j.sleep.2015.02.538. [DOI] [PubMed] [Google Scholar]
- 13.Huang WS, Tsai CH, Lin CL, Sung FC, Chang YJ, Kao CH. Nonapnea sleep disorders are associated with subsequent ischemic stroke risk: a nationwide, population-based, retrospective cohort study. Sleep Med. 2013;14(12):1341–1347. doi: 10.1016/j.sleep.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CY, Chiou AF. Predictors of sleep quality in community-dwelling older adults in Northern Taiwan. J Nurs Res. 2012;20(4):249–260. doi: 10.1097/jnr.0b013e3182736461. [DOI] [PubMed] [Google Scholar]
- 16.Sugaya N, Arai M, Goto F. The effect of sleep disturbance in patients with chronic dizziness. Acta Otolaryngol. 2017;137(1):47–52. doi: 10.1080/00016489.2016.1213418. [DOI] [PubMed] [Google Scholar]
- 17.Ho Chan WS. Taiwan's healthcare report 2010. EPMA J. 2010;1(4):563–585. doi: 10.1007/s13167-010-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 19.Lin C, Liu T, Lin F, Chung C, Chien WC. Association between sleep disorders and hypertension in Taiwan: a nationwide population-based retrospective cohort study. J Hum Hypertens. 2017;31(3):220–224. doi: 10.1038/jhh.2016.55. [DOI] [PubMed] [Google Scholar]
- 20.Mizukoshi K, Watanabe Y, Shojaku H, Okubo J, Watanabe I. Epidemiological studies on benign paroxysmal positional vertigo in Japan. Acta Otolaryngol Suppl. 1988;447:67–72. doi: 10.3109/00016488809102859. [DOI] [PubMed] [Google Scholar]
- 21.Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) CMAJ. 2003;169(7):681–693. [PMC free article] [PubMed] [Google Scholar]
- 22.Chang TP, Lin YW, Sung PY, Chuang HY, Chung H-Y, Liao WL. Benign paroxysmal positional vertigo after dental procedures: a population-based case-control study. PLoS One. 2016;11(4):e0153092. doi: 10.1371/journal.pone.0153092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gacek RR. Pathology of benign paroxysmal positional vertigo revisited. Ann Otol Rhinol Laryngol. 2003;112(7):574–582. doi: 10.1177/000348940311200702. [DOI] [PubMed] [Google Scholar]
- 24.Monzani D, Genovese E, Rovatti V, Malagoli ML, Rigatelli M, Guidetti G. Life events and benign paroxysmal positional vertigo: a case-controlled study. Acta Otolaryngol. 2006;126(9):987–992. doi: 10.1080/00016480500546383. [DOI] [PubMed] [Google Scholar]
- 25.Lin BY, Young YH. Effect of short-duration sleep deprivation on the vestibuloocular reflex system evaluated by ocular vestibular-evoked myogenic potential test. Acta Otolaryngol. 2014;134(7):698–703. doi: 10.3109/00016489.2014.895039. [DOI] [PubMed] [Google Scholar]
- 26.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada K, Oda M, Yamamoto M, Nomura T, Ohbayashi S, Kitsuda C. A clinical observation of benign paroxysmal positional vertigo (BPPV) after vestibular neuronitis (VN) Acta Otolaryngol Suppl. 1993;503:61–63. doi: 10.3109/00016489309128074. [DOI] [PubMed] [Google Scholar]
- 28.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4):227–247. doi: 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]
- 30.Magnavita N, Garbarino S. Sleep, Health and Wellness at Work: A Scoping Review. Int J Environ Res Public Health. 2017;14(11):1347. doi: 10.3390/ijerph14111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Cormorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12(8 Suppl):S221–S229. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.