Abstract
Study Objectives:
Sleep-disordered breathing (SDB) is highly prevalent in patients with acute stroke. SDB is often underdiagnosed and associated with neurological deterioration and stroke recurrence. Polysomnography or home sleep apnea testing (HSAT) is typically used as the diagnostic modality; however, it may not be feasible to use regularly in patients with acute stroke. We investigated the predictive performance of pulse oximetry, a simpler alternative, to identify SDB.
Methods:
The records of 254 patients, who were admitted to Boston Medical Center for acute stroke and underwent HSAT, were retrospectively reviewed. Oxygen desaturation index (ODI) from pulse oximetry channel were compared to respiratory event index (REI) obtained from HSAT devices. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of ODI were calculated, and different ODI cutoff values to predict SDB were proposed.
Results:
ODI had a strong correlation (r = .902) and agreement with REI. ODI was accurate in predicting SDB at different REI thresholds (REI ≥ 5, REI ≥ 15, and REI ≥ 30 events/h) with the area under the curve (AUC) of .965, .974, and .951, respectively. An ODI ≥ 5 events/h rules in the presence of SDB (specificity 91.7%, PPV 96.3%). An ODI ≥ 15 events/h rules in moderate to severe SDB (specificity 96.4%, PPV 95%) and an ODI < 5 events/h rules out moderate to severe SDB (sensitivity 100%, NPV 100%).
Conclusions:
Nocturnal pulse oximetry has a high diagnostic accuracy in predicting moderate to severe SDB in patients with acute stroke. Oximetry can be a simple modality to rapidly recognize patients with more severe SDB and facilitate the referral to the confirmation sleep study.
Citation:
Lin SH, Branson C, Park L, Leung J, Doshi N, Auerbach SH. Oximetry as an accurate tool for identifying moderate to severe sleep apnea in patients with acute stroke.J Clin Sleep Med. 2018;14(12):2065–2073.
Keywords: early diagnosis, home sleep apnea testing, hospitalized patients, obstructive sleep apnea, oximetry, stroke, oxygen desaturation index, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study evaluates whether pulse oximetry is an effective tool for early detection of sleep apnea in patients hospitalized with acute stroke.
Study Impact: To our knowledge, it is the first study that validates the use of oximetry as a cost-effective and accurate tool to identify moderate to severe sleep apnea and help guide clinical management of sleep-disordered breathing in patients with acute stroke.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common type of sleep-disordered breathing (SDB). OSA is characterized by the intermittent cessation or reduction of airflow during sleep due to complete or partial upper airway obstruction, whereas central sleep apnea (CSA) is characterized by the intermittent absence of both airflow and ventilatory effort. In the general population, the prevalence of OSA ranges from 20% to 30% in men and 10% to 15% in women.1 However, there is a signifi-cant increase in the risk of SDB in patients with acute stroke, irrespective of the type of stroke. One meta-analysis found an estimated 60% to 70% of patients with stroke or transient ischemic attack (TIA) have sleep apnea, with higher frequencies of SDB in men and patients with recurrent strokes. OSA represents most SDB in patients with stroke, as only 7% were found to have CSA-predominant SDB.2
Growing evidence has shown that there is an intimate relationship between OSA and stroke. OSA has been associated with early neurological deterioration, increased stroke recurrence, increased mortality, increased length of hospital stay, and decreased functional recovery. The adverse neurological outcomes correlated directly with the OSA severity based on the apnea-hypopnea index (AHI). Furthermore, OSA may itself be an independent risk factor for stroke.3–6
OSA may increase the risk of the development of stroke by several mechanisms. (1) Acute and chronic hypoxemia caused by OSA may increase sympathetic tone and activate the reninangiotensin-aldosterone system, exacerbating hypertension and arrhythmias including atrial fibrillation. (2) Intermittent hypoxemia generates reactive oxygen species that induce oxidative stress and endothelial dysfunction, causing proinflammatory states and platelet aggregation, eventually promoting atherosclerosis and ischemic damage of the border zone areas and terminal artery territories. (3) Negative intrathoracic pressures during apnea events may increase risk of patent foramen ovale shunting and reduce middle cerebral artery blood flow velocity. (4) An altered cerebrovascular response to hypoxia and carbon dioxide over time contributes to reductions in cerebral blood flow.7–11
Furthermore, OSA can exacerbate early neurologic worsening of a preexisting stroke by a mechanism called reversed Robin Hood syndrome (RRHS). Intermittent exposure to hypercapnia increases carbon dioxide-related vasodilation in unaffected vessels, generating an intracranial steal phenomenon that decreases the blood flow velocity in the ischemic areas of the brain. RRHS may play a key role in clinical deterioration after an acute stroke.11,12 In addition, central sleep apneas are known to appear after stroke, especially in the acute stroke phase, whereas obstructive apneas tend to persist in the chronic stages. As the stroke condition improves, sleep apneas may decrease in intensity.13
Hospitalized patients with acute stroke present a unique opportunity for screening and diagnosing SDB. However, evaluation for OSA is not routinely recommended as part of a stroke workup despite growing evidence that OSA is an independent risk factor for stroke.14 Importantly, clinical signs and symptoms of SDB (eg, sleepiness, body mass index) are not reliable in patients with stroke, and the Berlin Questionnaire has poor positive and negative predictive values among patients with stroke. In-laboratory attended polysomnography (PSG) or home sleep apnea testing (HSAT) is therefore necessary to diagnose SDB.15,16 The American Academy of Sleep Medicine (AASM) practice guideline recommends PSG, rather than HSAT, be used as the preferred modality for the diagnosis of sleep apnea in patients with a history of stroke.17 Often, however, neither PSG nor HSAT are practical for hospitalized patients because of the major costs involved or unavailability of these modalities. Moreover, the clinical condition may evolve over weeks or months poststroke13 and repeating the diagnostic study to reevaluate sleep apnea may be needed but not be feasible in reality. Therefore, several alternatives, including simpler devices for screening for SDB have been proposed to circumvent these barriers of testing.
Oximetry is widely available, inexpensive, and simple to use, making it an attractive alternative in situations where the burden of undiagnosed sleep apnea is high. Oxygen desatu-ration index (ODI) generated by oximetry monitoring is reasonably reproducible if recorded and analyzed using the same device and software. Different parameters from oximetry have been previously investigated as a predictor for AHI, including the Delta index (the average of the absolute differences of oxygen saturation between successive 12-second intervals). Both the Delta index and ODI provided similar levels of diagnostic accuracy.18 Other proposed parameters require more complicated analysis of oximetry recording, including nonlinear analysis (central tendency measure, approximate entropy), and spectral methods (Welch transform, wavelet transform).19–22 We focused our study on the predictive performance of ODI for three reasons. First, ODI is the most widely used measurement and can be easily generated with commercially available programs. Second, compared to other common oximetry indices including CT90, ODI demonstrated a superior accuracy in predicting SDB. Third, the definition of desaturation in ODI is similar to the oxygen desaturation requirement for scoring a hypopnea episode.
The objective of this study was to evaluate the predictive performance of oximetry in detecting SDB in the acute stroke population. We hypothesized that an overnight oximetry test is an accurate method to identify SDB.
METHODS
Study Population
This retrospective study was conducted at Boston Medical Center (BMC). The study was approved by the Institutional Review Board of BMC. The records of 271 patients, who were admitted to BMC with acute stroke and underwent home sleep apnea testing (HSAT) between January 2015 to October 2017, were retrospectively reviewed. The HSAT data had been continuously collected since January 2015 in order to facilitate any potential research regarding acute stoke and sleep apnea, and was utilized for our study as well as other ongoing research at BMC. The inclusion criteria for implementing HSAT were: (1) age 18 years and older, (2) admitted to BMC due to acute stroke (stroke symptom onset < 72 hours) with a National Institutes of Health Stroke Scale (NIHSS) score ≥ 1. Diagnosis of a stroke was confirmed by neurologists based on a history of sudden onset of a neurological deficit lasting > 24 hours and a brain lesion compatible with the neurological deficit under neuroimaging. Exclusion criteria were (1) a previous diagnosis of SBD or currently treated for SDB with positive airway pressure therapy; (2) concomitant severe central nervous system diseases; (3) oxygen requirement with > 2 L oxygen/min; (4) intensive care unit admission.
There were 17 cases excluded from our data analysis due to inadequate oximetry data (poor signals, artifacts, or oximetry recording < 100 minutes) that impaired interpretation, leaving 254 patients in the final analysis. Each stroke was also classified by its subtype (ischemic or hemorrhagic) and location (supratentorial or infratentorial).
Data Collection
The ResMed ApneaLink Air (ResMed, Poway, California, United States) HSAT device with a built-in oximetry sensor was distributed to neurology resident physicians at night. The resident physicians were instructed on how to connect and disconnect the device onto the patient and the device was retrieved the following morning. The data were downloaded and processed with ResMed ApneaLink software system version 10.2 (ResMed, Poway, California, United States) for raw data analysis. The chart review of baseline demographics was performed by a sleep physician.
Oximetry
The built-in pulse oximetry sensor of ApneaLink Air was utilized as our source of oximetry signal. The pulse oximetry used in ApneaLink was Xpod 3012 Nonin (Nonin Medical, Plymouth, Minnesota, United States). Its sampling frequency is 1 Hz and it has an averaging time of 3 seconds with a resolution of 1% oxygen saturation (SpO2). The raw oximetry data were automatically scored by computerized analysis, then visually checked by the sleep technician and obvious artifacts in the oximetry signal were excluded. The ODI is the average number of desaturation episodes per hour, in which each episode has a ≥ 3% decrease in pulse oxyhemoglobin saturation (SpO2) from the average and lasts at least 10 seconds. The cumulative percentages of time at saturations below 90% (CT90), lowest SpO2 %, and average SpO2 % were also analyzed.
Home Sleep Apnea Testing
ApneaLink Air is a type III HSAT device, with a finger probe recording oxygen saturation and pulse rate, a nasal airflow with a nasal pressure cannula, and a respiratory effort sensor belt. The raw recording was manually scored by certified sleep technicians following the scoring guidelines published by the American Academy of Sleep Medicine (AASM). Additionally, the data were reviewed and interpreted by a board-certified sleep medicine physician utilizing ResMed AirView platform. Apnea was defined as a ≥ 90% reduction in airflow from baseline for ≥ 10 seconds. Apneas were further classified as obstructive apnea if respiratory effort was present, central apnea if respiratory effort was absent, or mixed apnea if features of both obstructive and central apnea were present. Hypopnea was defined as a ≥ 30% reduction in airflow for ≥ 10 seconds and was associated with a decrease of ≥ 3% in oxygen saturation. The respiratory event index (REI) was defined as the number of episodes of apnea and hypopnea per hour of monitoring. The REI is used as a surrogate for AHI, because HSAT uses monitoring time in place of total sleep time.
Data Analysis
Baseline characteristics were summarized using descriptive statistics. Continuous variables were presented as the mean ± standard deviation and categorical variables were presented as a percentage. Different HSAT variables for patients with ODI < 5 and ODI ≥ 5 events/h were compared with Mann-Whitney U test to check the statistical significance of continuous data with skewed distribution. The t test was used for continuous data with normal distribution. Statistical significance was set at .05. A normal distribution of the data was not expected and so the nonparametric Spearman correlation coefficients were utilized between REI and ODI and between REI and CT90. Linear regression analysis was applied. A Bland-Altman plot was used to determine the agreement between REI and ODI.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for different cutoff points for ODI with 95% confidence interval (CI) were calculated. The prevalence of SDB in patients with stroke was set at 70% according to the meta-analysis.2 The optimal cutoff points for the ODI were determined in order to identify patients with SDB with high sensitivity (as an exclusion and screening test, to rule out the presence of OSA), high specificity (as a confirmation test, to rule in the presence of OSA), and, ideally, high accuracy. Receiver operating characteristic (ROC) curves were presented for diagnosing SDB at 3 different REI thresholds (REI ≥ 5, 15, and 30 events/h) using ODI. The area under the curve (AUC) was presented for each REI threshold with the associated 95% CI. All data were entered into an Excel spreadsheet (Microsoft Corp., Redmond, Washington, United States) and statistical analyses were generated using Medcalc (Medcalc Software, Mariakerke, Belgium).
RESULTS
Patient Characteristics and Sleep Apnea Prevalence
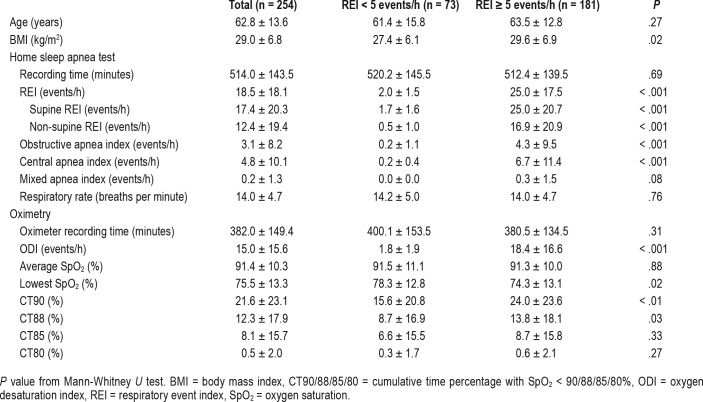
Table 1 summarizes patient baseline characteristics. Of the 254 patients enrolled, 50.7% were males, mean age was 62.8 ± 13.6 years, and mean body mass index was 30.3 ± 8.3 kg/m2. Most of the patients (91.3%) had an ischemic stroke or TIA and 85% had stroke located in the supratentorial region. The most common preexisting comorbid conditions include hypertension (80.7%), hyperlipidemia (51.1%), diabetes mellitus (46.8%), and obesity defined as a body mass index ≥ 30 kg/m2 (36.2%).
Table 1.
Summary of baseline characteristics (n = 254).
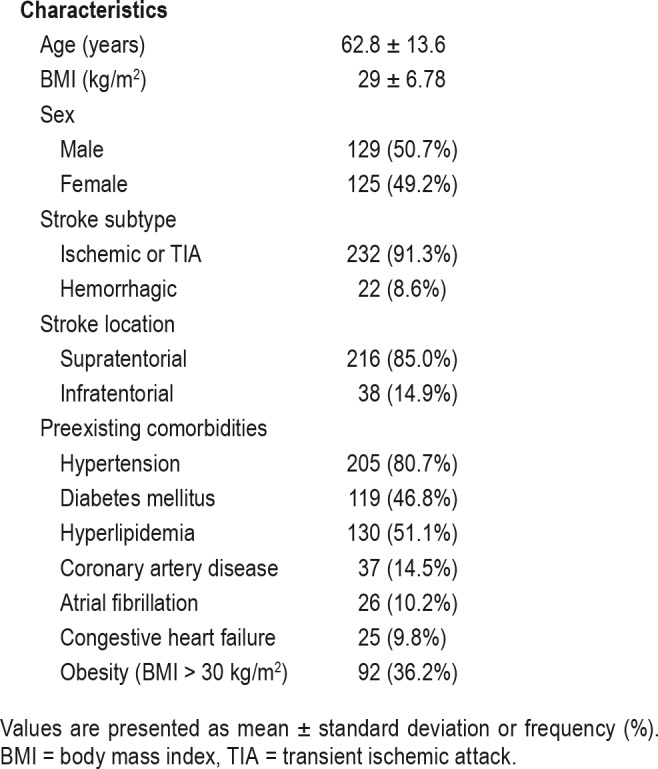
Using ApneaLink as a reference standard, the mean REI of all patients was 18.5 ± 18.1 events/h. SDB was present in 181 (71.3%) based on an REI ≥ 5 events/h criteria. Among those patients, 66 (36.4%) had mild sleep apnea, 62 (34.2%) had moderate sleep apnea, and 53 (29.2%) had severe sleep apnea. A total of 27 patients (14.9%) had predominantly CSA (central events > 50% of the total events). We did not discover a significant difference in the severity of REI between hemorrhagic or ischemic stroke and TIA (21.7 ± 18.5 versus 18.3 ± 18.5, P = .432) or ODI (16 ± 11.5 versus 13.6 ± 16.7, P = .53). However, we observed higher REI in infratentorial stroke than in supratentorial stroke (25.8 ± 20.9 versus 17.3 ± 17.8, P = .010). Similarly, overall ODI was higher in cases with infratentorial stroke (19.2 ± 19.2 versus 12.8 ± 15.6, P = .029). The HSAT results and oximetry results are summarized in Table 2. Those who had SDB (with REI ≥ 5 events/h) did not differ from patients without SDB (REI < 5 events/h) in recording time, oximetry recording time, age, average SpO2%, and respiratory rate. As expected, SDB populations had significantly higher overall REI (including supine REI and nonsupine REI), obstructive and central apnea events, cumulative time percentage with SpO2 < 90 (CT90) and < 88% (CT88).
Table 2.
Summary of home sleep apnea test and oximetry results.
Validity and Accuracy of Oximetry by Spearman Correlation, Bland-Altman Plot, and ROC Curves
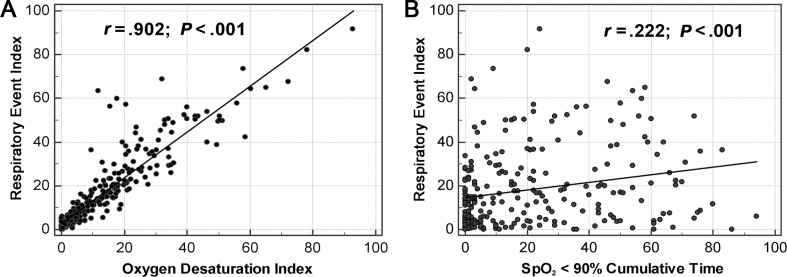
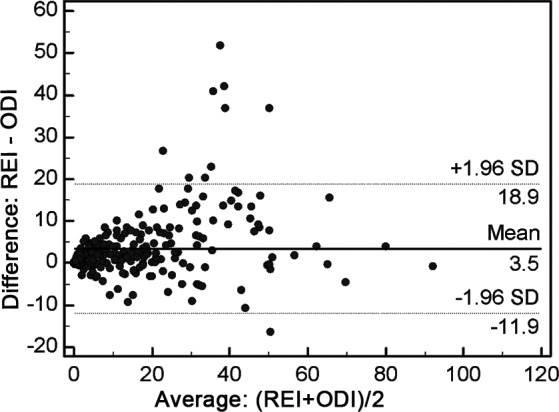
Utilizing Spearman correlation coefficients, we noted there was a strong correlation (r = .902, P < .001) between the ODI and REI. The relationship between the ODI and REI with linear regression is depicted in Figure 1. The resulting regression equation was predicted as REI = 2.79 + 1.047 × ODI. There was a moderate correlation between REI and obstructive apnea index (r = .608, P < .0001), central apnea index (r = .646, P < .0001), and mixed apnea index (r = .417, P < .0001). However, the correlation between ODI and CT90 is weak (r = .222, P < .001, Figure 1), as well as the other indices, including CT88, CT85, and CT80. Table 3 shows the correlation coefficient between REI and different indices. The Bland-Altman plot is displayed in Figure 2, and illustrates the agreement between the REI and the ODI, with the 95% CI between −11.9 to 18.9 events/h. The ODI slightly underestimated REI by an average of 3.5. Furthermore, ODI underestimates REI more in moderate-severe SDB than with mild SDB.
Figure 1. Spearman correlation comparing REI with ODI and REI with CT90.
(A) ODI versus REI, REI = 2.79 + 1.047 × ODI. (B) CT90 versus REI. CT90 = cumulative time percentage with SpO2 < 90%, ODI = oxygen desaturation index, REI = respiratory event index.
Table 3.
Spearman coefficient with REI.
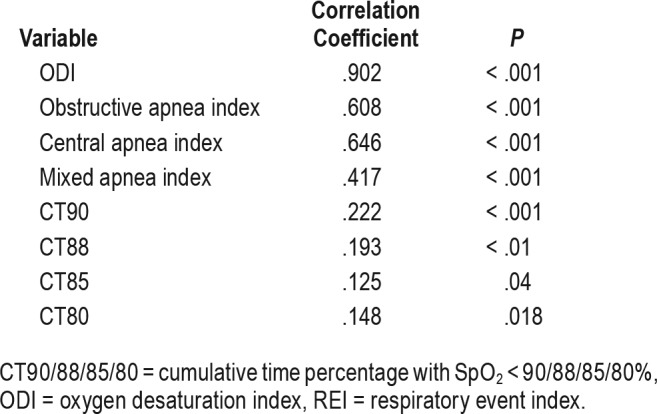
Figure 2. Bland-Altman plots illustrating the agreement between ODI and REI.
ODI = oxygen desaturation index, REI = respiratory event index, SD = standard deviation.
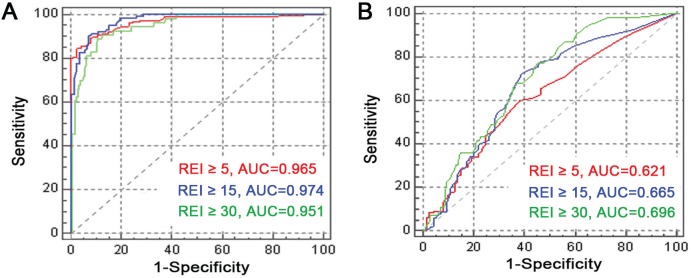
To compare the diagnostic accuracy of the ODI and CT90 to predict SDB at different REI thresholds (REI ≥ 5, REI ≥ 15, REI ≥ 30 events/h), the receiver operator characteristic (ROC) curve is shown in Figure 3. ODI was a good predictor of SDB at the different REI thresholds with the area under ROC curve (AUC) ranging from .951 to .974. Conversely, CT90 has a significantly lower accuracy in predicting REI with an AUC ranging from .621 to .696.
Figure 3. ROC curves for ODI and CT90 to predict REI ≥ 5, REI ≥ 15, and REI ≥ 30 events/h.
(A) ODI to predict REI ≥ 5 (red), REI ≥ 15 (blue), and REI ≥ 30 (green). (B) CT90 to predict REI ≥ 5 (red), REI ≥ 15 (blue), and REI ≥ 30 (green). AUC = area under the ROC curve, CT90 = cumulative time percentage with SpO2 < 90%, ODI = oxygen desaturation index, REI = respiratory event index, ROC = receiver operating characteristic.
Predictive Performance of Oximetry for Diagnosis of SDB
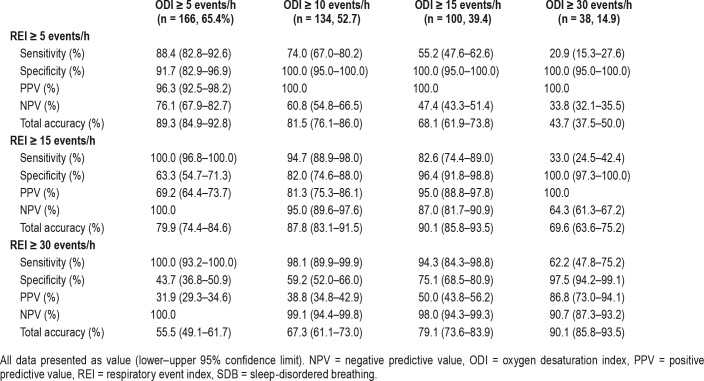
Table 4 presents the predictive performance of ODI for SDB at different REI values with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. The optimal cutoff value was chosen based on maximum accuracy while ensuring high sensitivity and well-preserved specificity. To predict REI ≥ 5, REI ≥ 15, and REI ≥ 30 events/h, the optimal cutoff values are ODI ≥ 5, ODI ≥ 10, and ODI ≥ 20 events/h, respectively. To predict SDB (REI ≥ 5 events/h), utilizing ODI ≥ 5 events/h offers high sensitivity (88.4%), specificity (91.7%), and accuracy (89.3%). Similarly, to predict moderate SDB to severe SDB (REI ≥ 15 events/h), using ODI ≥ 10 events/h can ensure higher sensitivity (94.7%) with a reasonable specificity (82.0%) and accuracy (87.8%). We noted that in severe sleep apnea (REI ≥ 30 events/h), ODI may significantly underestimate REI. Using ODI ≥ 20 events/h as a cutoff can maintain a reasonable sensitivity and specificity while using a higher cutoff of ODI ≥ 25 or ODI ≥ 30 events/h, and sensitivity dropped to 69.8% and 62.2%, respectively.
Table 4.
Predictive value of ODI for SDB at different REI cutoffs.
Since using an ODI ≥ 5 events/h to detect REI ≥ 5 events/h has a specificity of 91.7% and PPV of 96.3%, ODI ≥ 5 events/h may be used to rule in the presence of SDB. In addition, as using ODI ≥ 5 events/h to detect REI ≥ 15 events/h demonstrates a sensitivity of 100% and a NPV of 100%, we would be confident to exclude the existence of moderate to severe SDB if a patient had an ODI < 5 events/h. Using ODI ≥ 15 events/h to predict REI ≥ 15 events/h has a specificity of 96.4% and a PPV of 95%. Therefore, we would also be confident to consider a patient with an ODI ≥ 15 events/h to have moderate to severe SDB.
DISCUSSION
To our knowledge, this is the first study that evaluates the predictive value of oximetry to predict the REI in the setting of acute stroke. One earlier study validating the diagnostic accuracy of oximetry for detection of SDB in a stroke rehabilitation setting concluded that nocturnal oximetry is an accurate diagnostic screening instrument in patients with stroke.23 Although prior studies have proposed HSAT as a reliable screening tool for acute ischemic stroke, widespread implementation of HSAT would require significant resources that smaller community facilities may not have access to.24 As demonstrated in our study, oximetry offers an attractive and accurate alternative to HSAT. Because of its cost effectiveness and lack of extensive training required for acquisition of reliable data, nocturnal oximetry may be more practical to implement on a wider scale. The results acquired from oximetry then can be used for further risk stratification and guide further evaluation, reducing the time delay for confirmatory testing by PSG or HSAT and commencing potential interventions, such as positive airway pressure (PAP) therapy, for patients with clinically significant OSA.
Because there is a high prevalence of sleep apnea in patients with acute stroke, clinicians should maintain awareness for SDB in patients after stroke with a low threshold for pursuing testing. Whether all patients with stroke should undergo screening for sleep apnea—and the optimal timing for testing—however, is still under debate. Decisions to test with oximetry must be individualized and consider patients' preferences, the expected ability to adhere to PAP therapy, overall functional status, quality of life, goals of care, and life expectancy. Additionally, the feasibility of acquiring oximetry equipment and implementing personnel may need to be considered. Furthermore, because oximetry only measures the oxygen saturation change and does not monitor nasal flow and respiratory effort, it is not able to distinguish OSA from CSA. Thus, oximetry is not an acceptable modality for diagnosing sleep apnea and patients still require further sleep study (PSG or HSAT) to confirm the diagnosis.
This retrospective study showed that ODI is strongly correlated with the REI generated from the HSAT. Although ODI slightly underestimated REI by 3.5, ODI had a very high accuracy to predict REI at cutoffs of 5, 15, and 30 events/h with the AUC ranging from .951 to .974. CT90 was shown to have a much weaker correlation with REI and was less accurate in detecting SDB with AUC ranging from .621 to .696. The fact that the same set of oximetry data were utilized for generating both ODI and REI may be a contributing factor to the strong correlation between ODI and REI in our study.
In a previous study using oximetry for detection of SDB in a stroke rehabilitation setting,23 using ODI ≥ 15 events/h to predict REI ≥ 15 events/h exhibited a sensitivity of 77%, specificity of 100%, PPV of 100%, and NPV of 83%. This is similar to our result, with the sensitivity slightly higher in our study (82.6%) but specificity slightly lower (96.4%). Another study compared ODI from ApneaLink versus REI from PSG in the general population25 and found a good sensitivity (> 80% at all REI values) and excellent specificity at REI ≥ 10 events/h and a rather tight correlation between PSG AHI and ODI with AHI < 20 events/h, with a tendency for the ODI to understate the AHI score with AHI ≥ 20 events/h, which is also similar to our findings.
According to the International Classification of Sleep Disorders criteria,26 diagnosis of OSA can be made with an AHI ≥ 5 events/h in patients who have experienced a stroke. To determine a single cutoff value with both adequate sensitivity and specificity with ideally the maximum accuracy, we use ODI ≥ 5, ODI ≥ 10, and ODI ≥ 20 events/h as cutoff values to predict REI ≥ 5, REI ≥ 15, REI ≥ 30 events/h, respectively. ODI cutoff value is lower than REI value because ODI tends to underestimate REI, especially when the sleep apnea is more severe.
Although oximetry has been previously studied as a screening tool in the general population, its greatest value is as a tool to rapidly identify patients with more severe OSA and priori-tize patients for more urgent evaluation.27–31 It seems prudent to differentiate patients with moderate to severe sleep apnea (REI ≥ 15 events/h) from the mild sleep apnea group, because patients with moderate to severe sleep apnea may require more urgent sleep study referral and treatment. Based on the different ODI values obtained from patients with stroke, we propose different management options. For patients with ODI < 5 events/h, we can confidently rule out the presence of moderate to severe sleep apnea (sensitivity of 100%, NPV of 100%), but this does not exclude the possibility of mild sleep apnea because there is a false-negative rate of 23.9% (sensitivity of 88.4%, NPV of 76.1%). Thus, an official sleep study may be considered in selected patients if they are symptomatic. For patients with ODI ≥ 5 to < 15 events/h, we can consider them at least having mild sleep apnea (PPV is 96.3%), and a routine sleep study may be suggested. For patients with ODI ≥ 15 events/h, an urgent referral for sleep study can be considered as it is likely they have moderate to severe SDB (PPV is 95%). Nevertheless, additional studies to determine the appropriate ODI thresholds and proper management options are required.
Limitations
Although pulse oximetry is a convenient and inexpensive way to detect SDB, there are some limitations to our study. First, we acknowledge this is a single-center study in an academic program and further studies are required to confirm its validity in a more generalized setting. In addition, our study was limited to the population with an acute stroke.
Another possible limitation is that we adopted the results from a HSAT (ApneaLink Air) as the standard to evaluate the predictive performance of oximetry. There are several studies that have shown good agreement between the REI from polysomnography and REI recorded by ApneaLink devices, and ApneaLink has been shown to be a reliable method for diagnosing moderate to severe obstructive sleep apnea.25,32,33 Moreover, multiple studies, including the Sleep Apnea Cardiovascular Endpoints (SAVE) study, utilize ResMed ApneaLink device to generate REI result.34 Our pulse oximetry data are derived from ApneaLink using oximetry Xpod 3012 with a sampling frequency of 1 Hz and a resolution 1% SaO2. This raises some concerns about the reproducibility of the oximetry and general-izability to another oximetry system. Indeed, some differences in ODI values were observed between ApneaLink and other oximetry systems. This could be explained in part by the different models of oximeter in oximeter acquisition and processing factors, rather than patient-related factors or differences in proprietary ODI calculation algorithms between different systems.35 A study by Böhning et al. compared five widely used oximeters in clinical practice and found four oximeters had the same sampling frequency (1 Hz) and resolution (1% SaO2) as Xpod 3012 in our study. However, a high dispersion value was found between different oximetry systems and only one device with a signal resolution of 0.1% has a better reproducibility.36 Thus, high-resolution pulse oximetry with a resolution of 0.1% may be a superior modality than regular oximetry.
Clinicians should also acknowledge that sampling frequency and averaging time can influence the performance of the oximetry. Although an optimal sampling frequency has yet to be determined, Nigro et al. suggest that a minimum data storage rate of 0.25 Hz is sufficient to avoid a loss in resolution compromising detection of oxygen desaturation.37 Averaging time refers to the time window used by the oximeter to produce a moving average of the data stream in order to act as a signal filter; this allows for the smoothing out of the short-term fluctuations and artifacts. It should be noted that different averaging times can produce different results with the same oximeter. For recordings of SpO2 in a sleep study, where short desaturations potentially eliciting frequent arousals is relevant, a short averaging time (eg, 3 seconds) may be preferable. Although using a longer averaging time may yield a desirable level of artifact reduction, it progressively reduces the frequency and magnitude of arterial O2 desaturation events.38 Therefore, the corresponding ODI were notably decreased, causing an underestimation of desaturation events. Indeed, The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications suggests that overnight SpO2 data are acceptable with an averaging time of ≤ 3 seconds at a heart rate of 80 beats/min, or approximately 4 beats.39 Further investigation is still needed for determining the optimal oximeter averaging time and data storage rate.
Importantly, another limitation of our study is we did not evaluate the benefit of PAP in patients with stroke and sleep apnea. The benefit of PAP in reducing cardiovascular events after stroke remains controversial. Although several studies have suggested that treatment of SDB may improve stroke-related outcomes,40–44 other studies, including a recent randomized controlled trial (SAVE study), reported no difference in the rate of stroke or other cardiovascular events in patients treated with PAP compared with control patients.34,45,46 Nonetheless, the SAVE study demonstrated that PAP not only significantly reduces snoring and daytime sleepiness, but also improves health-related quality of life and mood. Therefore, those who respond satisfactorily to PAP applications should continue treatment. Frequent reassessment is necessary for patients who undergo a trial of PAP therapy, particularly in the early post-stroke setting, due to the theoretical potential for a reduction in blood pressure induced by PAP to impair cerebral perfusion.47 In addition, there is known poor PAP adherence with a mean compliance rate of 37% among stroke populations, which has been attributed to PAP intolerance, poor motivation, and cognitive deficits. This may eventually affect the potential treatment benefit from PAP.48 Treatment decisions regarding PAP therapy must be tailored to the clinical status of the patient and their ability to adhere to PAP therapy.
CONCLUSIONS
In conclusion, ODI derived from oximetry was strongly correlated with the REI measured from the HSAT. It effectively identified patients with moderate to severe OSA with excellent sensitivity and specificity when different cutoff values of ODI were applied. Given that there is a high SDB prevalence in the acute stroke population and SDB is often underdiagnosed, oximetry is proposed as a simple and inexpensive modality to rapidly recognize patients with more severe sleep apnea and facilitate referral to confirmatory sleep study. Applying oximetry testing must be individualized according to the preferences of the patient. Nevertheless, future studies are required to determine the optimal cutoff ODI value, the timing for the testing, and the optimal management of SDB in patients with acute stroke.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Boston Medical Center, Boston, MA. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the help to our BMC sleep lab personnel: Walter Lehan, RPSGT; Denise Catania, RPSGT; and Marianne Kelly, RN, for assisting in securing patient logs, HSAT data collection, downloading, and scoring of recorded data. We are grateful to all patients participating in our study and also extend deepest thanks to the neurology faculty and residents who participated in setting up sleep study equipment.
ABBREVIATIONS
- AHI
apnea hypopnea index
- AUC
area under the ROC curve
- CSA
central sleep apnea
- CT80
time percentage with SpO2 < 80%.
- CT85
time percentage with SpO2 < 85%.
- CT88
time percentage with SpO2 < 88%.
- CT90
time percentage with SpO2 < 90%
- HSAT
home sleep apnea testing
- NPV
negative predictive value
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PSG
polysomnography
- REI
respiratory event index
- ROC
receiver operating curves
- SDB
sleep-disordered breathing
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med. 2009;10(7):717–719. doi: 10.1016/j.sleep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 6.Valham F, Mooe T, Rabben T, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118(9):955. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 7.Culebras A. Sleep apnea and stroke. Curr Neurol Neurosci Rep. 2015;15(1):503. doi: 10.1007/s11910-014-0503-3. [DOI] [PubMed] [Google Scholar]
- 8.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 9.Netzer NC. Impaired nocturnal cerebral hemodynamics during long obstructive apneas: the key to understanding stroke in OSAS patients? Sleep. 2010;33(2):146–147. doi: 10.1093/sleep/33.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojadidi MK, Bokhoor PI, Gevorgyan R, et al. Sleep apnea in patients with and without a right-to-left shunt. J Clin Sleep Med. 2015;11(11):1299. doi: 10.5664/jcsm.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S, Stark CD, Stark RJ. Sleep apnoea and the neurologist. Pract Neurol. 2017;17(1):21–27. doi: 10.1136/practneurol-2016-001524. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrov AV, Nguyen HT, Rubiera M, et al. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke. 2009;40(8):2738–2742. doi: 10.1161/STROKEAHA.109.547950. [DOI] [PubMed] [Google Scholar]
- 13.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 14.Kotzian ST, Schwarzinger A, Haider S, Saletu B, Spatt J, Saletu MT. Home polygraphic recording with telemedicine monitoring for diagnosis and treatment of sleep apnoea in stroke (HOPES Study): study protocol for a single-blind, randomized controlled trial. BMJ Open. 2018;8(1):e018847. doi: 10.1136/bmjopen-2017-018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srijithesh P, Shukla G, Srivastav A, Goyal V, Singh S, Behari M. Validity of the Berlin Questionnaire in identifying obstructive sleep apnea syndrome when administered to the informants of stroke patients. J Clin Neurosci. 2011;18(3):340–343. doi: 10.1016/j.jocn.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Kotzian S, Stanek J, Pinter M, Grossmann W, Saletu M. Subjective evaluation of sleep apnea is not sufficient in stroke rehabilitation. Top Stroke Rehabil. 2012;19(1):45–53. doi: 10.1310/tsr1901-45. [DOI] [PubMed] [Google Scholar]
- 17.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalang UJ, Dmochowski J, Veeramachaneni S, et al. Prediction of the apnea-hypopnea index from overnight pulse oximetry. Chest. 2003;124(5):1694–1701. doi: 10.1378/chest.124.5.1694. [DOI] [PubMed] [Google Scholar]
- 19.Lin CL, Yeh C, Yen CW, Hsu WH, Hang LW. Comparison of the indices of oxyhemoglobin saturation by pulse oximetry in obstructive sleep apnea hypopnea syndrome. Chest. 2009;135(1):86–93. doi: 10.1378/chest.08-0057. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez D, Hornero R, Garcia M, del CF, Zamarron C. Improving diagnostic ability of blood oxygen saturation from overnight pulse oximetry in obstructive sleep apnea detection by means of central tendency measure. Artif Intell Med. 2007;41(1):13–24. doi: 10.1016/j.artmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez D, Hornero R, García M, del Campo F, Zamarrón C. Improving diagnostic ability of blood oxygen saturation from overnight pulse oximetry in obstructive sleep apnea detection by means of central tendency measure. Artif Intell Med. 2007;41(1):13–24. doi: 10.1016/j.artmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Schultheiβ B, Jozefiak-Wesolowska A, Böhning N, Schmittendorf E. Vander Sloten J, Verdonck P, Nyssen M, Haueisen J. 4th European Conference of the International Federation for Medical and Biological Engineering. IFMBE Proceedings. vol 22. Berlin, Heidelberg: Springer; 2009. Spectral Analysis of Overnight Pulse Oximetry Recordings in Sleep Studies. [Google Scholar]
- 23.Aaronson JA, van Bezeij T, van den Aardweg JG, van Bennekom CA, Hofman WF. Diagnostic accuracy of nocturnal oximetry for detection of sleep apnea syndrome in stroke rehabilitation. Stroke. 2012;43(9):2491–2493. doi: 10.1161/STROKEAHA.112.665414. [DOI] [PubMed] [Google Scholar]
- 24.Chernyshev OY, McCarty DE, Moul DE, et al. A pilot study: portable out-of-center sleep testing as an early sleep apnea screening tool in acute ischemic stroke. Nat Sci Sleep. 2015;7:127–138. doi: 10.2147/NSS.S85780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng SS, Chan TO, To KW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39(11):757–762. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 27.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120(2):625–633. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 28.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114(5):993–1000. doi: 10.1213/ANE.0b013e318248f4f5. [DOI] [PubMed] [Google Scholar]
- 29.Dawson A, Loving RT, Gordon RM, et al. Type III home sleep testing versus pulse oximetry: is the respiratory disturbance index better than the oxygen desaturation index to predict the apnoea-hypopnoea index measured during laboratory polysomnography? BMJ Open. 2015;5(6):e007956. doi: 10.1136/bmjopen-2015-007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunisaki KM, Bohn OA, Wetherbee EE, Rector TS. High-resolution wrist-worn overnight oximetry has high positive predictive value for obstructive sleep apnea in a sleep study referral population. Sleep Breath. 2016;20(2):583–587. doi: 10.1007/s11325-015-1251-6. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Mather PJ, Chowdhury A, et al. Sleep overnight monitoring for apnea in patients hospitalized with heart failure (SOMA-HF Study) J Clin Sleep Med. 2017;13(10):1185–1190. doi: 10.5664/jcsm.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15(6):952–960. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 33.Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax. 2011;66(3):213–219. doi: 10.1136/thx.2010.152801. [DOI] [PubMed] [Google Scholar]
- 34.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 35.Ng Y, Joosten SA, Edwards BA, et al. Oxygen desaturation index differs significantly between types of sleep software. J Clin Sleep Med. 2017;13(4):599–605. doi: 10.5664/jcsm.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Böhning N, Schultheiss B, Eilers S, Penzel T, Böhning W, Schmittendorf E. Comparability of pulse oximeters used in sleep medicine for the screening of OSA. Physiol Meas. 2010;31(7):875–888. doi: 10.1088/0967-3334/31/7/001. [DOI] [PubMed] [Google Scholar]
- 37.Nigro CA, Dibur E, Rhodius E. Pulse oximetry for the detection of obstructive sleep apnea syndrome: can the memory capacity of oxygen saturation influence their diagnostic accuracy? Sleep Disord. 2011;2011:427028. doi: 10.1155/2011/427028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross TJ, Keller-Ross M, Issa A, Wentz R, Taylor B, Johnson B. The impact of averaging window length on the “desaturation” indexes obtained via overnight pulse oximetry at high altitude. Sleep. 2015;38(8):1331–1334. doi: 10.5665/sleep.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry RB, Albertario CL, Harding SM, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2018. Version 2.5. [Google Scholar]
- 40.Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37(5):1128–1136. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 41.Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-García MA, Campos-Rodríguez F, Soler-Cataluña JJ, Catalán-Serra P, Román-Sánchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39(4):906–912. doi: 10.1183/09031936.00011311. [DOI] [PubMed] [Google Scholar]
- 43.Khot SP, Davis AP, Crane DA, et al. Effect of continuous positive airway pressure on stroke rehabilitation: a pilot randomized sham-controlled trial. J Clin Sleep Med. 2016;12(7):1019–1026. doi: 10.5664/jcsm.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Shukla G, Afsar M, et al. Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial. J Clin Sleep Med. 2018;14(4):511–521. doi: 10.5664/jcsm.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24(1):47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 46.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 47.Watson NF. Stroke and sleep specialists: an opportunity to intervene? J Clin Sleep Med. 2010;6(2):138–139. [PMC free article] [PubMed] [Google Scholar]
- 48.Birkbak J, Clark AJ, Rod NH. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med. 2014;10(1):103–108. doi: 10.5664/jcsm.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]