Abstract
Study Objectives:
To examine the relationship of self-reported sleep during pregnancy with adverse pregnancy outcomes. A secondary objective was to describe the concordance between self-reported and objectively assessed sleep during pregnancy.
Methods:
In this prospective cohort, women completed a survey of sleep patterns at 6 to 13 weeks' gestation (visit 1) and again at 22 to 29 weeks' gestation (visit 3). Additionally, at 16 to 21 weeks (visit 2), a subgroup completed a week-long sleep diary coincident with an actigraphy recording. Weekly averages of self-reported sleep duration and sleep midpoint were calculated. A priori, sleep duration < 7 hours was defined as “short,” and sleep midpoint after 5:00 AM was defined as “late.” The relationship of these sleep abnormalities with hypertensive disorders of pregnancy (HDP) and gestational diabetes mellitus (GDM) was determined.
Results:
Of the 10,038 women enrolled, sleep survey data were available for 7,524 women at visit 1 and 7,668 women at visit 3. A total of 752 women also provided ≥ 5 days of sleep diary data coincident with actigraphy at visit 2. We did not observe any consistent relationship between self-reported short sleep and HDP or GDM. There was an association between self-reported late sleep midpoint and GDM (visit 1 adjusted odds ratio 1.67, 95% confidence interval 1.17, 2.38; visit 2 adjusted odds ratio 1.73, 95% confidence interval 1.23, 2.43). At visit 2, 77.1% of participants had concordance between their diary and actigraphy for short sleep duration, whereas 94.3% were concordant for sleep midpoint.
Conclusions:
Self-reported sleep midpoint, which is more accurate than self-reported sleep duration, is associated with the risk of GDM.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Title: Pregnancy as a Window to Future Cardiovascular Health: Adverse Pregnancy Outcomes as Predictors of Increased Risk Factors for Cardiovascular Disease, Identifier: NCT02231398, URL: https://clinicaltrials.gov/ct2/show/NCT02231398
Citation:
Facco FL, Parker CB, Hunter S, Reid KJ, Zee PC, Silver RM, Haas DM, Chung JH, Pien GW, Nhan-Chang CL, Simhan HN, Parry S, Wapner RJ, Saade GR, Mercer BM, Torres C, Knight J, Reddy UM, Grobman WA. Association of adverse pregnancy outcomes with self-reported measures of sleep duration and timing in women who are nulliparous. J Clin Sleep Med. 2018;14(12):2047–2056.
Keywords: gestational diabetes, hypertension, pregnancy, sleep duration, sleep midpoint
BRIEF SUMMARY
Current Knowledge/Study Rationale: Data, both from nonpregnant and pregnant cohorts, suggest that objectively measured short sleep and late sleep timing in pregnancy are both risk factors for adverse health outcomes. Yet, the use of objectively measured sleep in routine care is limited; thus, it is more practical to use self-reports in pregnancy to screen for at-risk women. Our study examined the relationship of self-reported sleep duration and timing in pregnancy to hypertension and gestational diabetes.
Study Impact: Our data suggest that short sleep duration is underreported and may not be a good marker for adverse pregnancy outcomes. However, we found that self-reports of a sleep midpoint are more accurate, and that self-reported late sleep midpoint (after 5:00 AM) is associated with an increased risk of gestational diabetes mellitus.
INTRODUCTION
Mounting experimental and epidemiologic data suggest that among nonpregnant adults, sleep duration contributes to physical, mental, and emotional well-being.1–4 Short sleep duration has been linked to hypertension and cardiovascular disease.5–8 There are particularly strong data suggesting that short sleep duration is associated with disordered metabolism, specifically an increase in the risk of type 2 diabetes.9–11 In addition to sleep duration, other aspects of sleep, including the timing of sleep and wake cycles and continuity of sleep, have been proposed as cardiometabolic risk factors.12–15
Hypertensive (eg, preeclampsia) and metabolic (eg, gestational diabetes mellitus) complications also can arise during pregnancy, and are associated with both short- and long-term maternal and neonatal morbidity.16,17 Data from studies that have explored the relationship between objectively measured sleep and pregnancy outcomes suggest that short sleep duration and altered sleep patterns are risk factors for adverse outcomes.18–26 Our own data show an association of objectively measured short sleep duration and late sleep midpoint with gestational diabetes.26 Yet, the use of objectively measured sleep as a risk factor in the routine clinical context is limited due to the burden of ascertaining sleep via polysomnography or actigraphy. Therefore, it is more practical to use self-reported accounts of sleep in pregnancy to screen for at-risk women. However, there are conflicting data on whether self-reported sleep correlates well with objectively measured sleep in pregnancy and whether self-reported measures are associated with adverse pregnancy outcomes.27–29 Furthermore, studies that evaluated self-reported sleep and its relationship to adverse pregnancy outcomes have varied widely in definition of sleep variables and timing of sleep ascertainment in relation to obstetric outcomes.
Our objective was to examine the relationship of self-reported sleep duration and timing in pregnancy with adverse pregnancy outcomes in a large cohort of women who were nulliparous and who gave self-reported sleep assessments at multiple timepoints in pregnancy. A secondary objective was to describe the concordance between self-reported and objective measures of sleep in pregnancy.
METHODS
Nulliparous Pregnancy Outcome Study: Monitoring Mothers-to-Be (nuMoM2b)
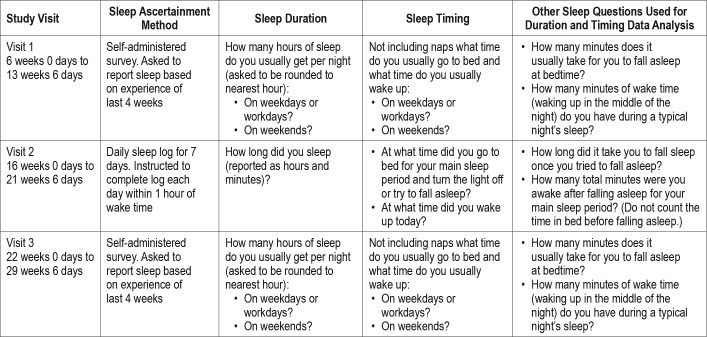
nuMoM2b was a prospective observational cohort study, conducted at eight clinical sites and managed by a central data coordinating and analysis center.30 Women who were nulliparous were screened for eligibility in the first trimester of pregnancy. Women were eligible if they had a viable singleton gestation, were between 6 weeks + 0 days' gestation and 13 weeks + 6 days' gestation. Participants were seen at three study visits during pregnancy and again at delivery. Study visits 1, 2, and 3 occurred during the following gestational age intervals, respectively: 6 weeks 0 days to 13 weeks 6 days; 16 weeks 0 days to 21 weeks 6 days; and 22 weeks 0 days to 29 weeks 6 days. At study visits 1 and 3, women completed a sleep questionnaire that included questions about the duration and timing of their sleep during the past month. At the second study visit, a subset of women was also recruited for the Sleep Duration and Continuity Study, an ancillary study of nuMoM2b. This group of women was asked to objectively measure sleep with actigraphy and to keep a 7-day sleep log while wearing a wrist actigraph. Sleep variables that were collected by study visit are detailed in Table 1. Further details regarding the methods of the actigraphy substudy have been described previously.31
Table 1.
Self-reported sleep measures.
On the visit 1 and 3 sleep questionnaires, women were asked about the timing of their sleep on weekdays/workdays and weekends using the following two questions: “Not including naps, what time do you usually go to bed?” and “Not including naps, what time do you usually wake up?” In addition, women were asked to estimate how many minutes it usually takes for them to fall asleep at bedtime and how many minutes of wake time they typically have during a night's sleep. Calculated sleep duration was estimated as the interval from bedtime to wake time minus the time it takes to fall asleep and the time awake during the night. This was separately calculated for weekdays/workdays and weekends. Quality checks were done on the calculated sleep duration values for weekdays/workdays and weekends using the following question from the sleep questionnaire: “How many hours of sleep do you usually get per night?” If the reported sleep duration from this additional question was not within 2 hours of the calculated sleep duration, then the times used for the calculated sleep duration were run through a series of edit checks to identify and correct potential errors in the selection of am or pm. If the calculated duration was still not within 2 hours of the reported duration after editing potential am/pm errors and recalculating sleep duration, then the calculated duration and the corresponding times were set to missing. Weekly average sleep duration was calculated as a weighted average of the weekday/workday and weekend sleep durations using the following formula: ([week-day/workday duration × 5] + [weekend duration × 2]) / 7. The midpoint of the sleep period for both weekdays and weekends was also calculated, with sleep start time accounting for the duration of time it took to fall asleep after initiation of bedtime. The weekly average sleep midpoint was calculated as a weighted average of the weekday/workday and weekend sleep midpoints using the following formula: ([weekday/workday midpoint × 5] + [weekend midpoint × 2]) / 7.
A subgroup of women was also asked to complete a daily sleep diary for 7 consecutive days near visit 2, coincident with an actigraphy recording. Each day, women were asked to record the time they went to bed, the time they woke up, the time they got out of bed, the amount of time it took to fall asleep, and how many minutes they were awake during the night. Quality checks of the times recorded were performed if the times reported were not relative to each other (eg, wake time was before sleep time). A technician at the central sleep reading center used times recorded by the wrist actigraph to resolve any discrepancies. Sleep duration was estimated as previously described. The calculated sleep duration each day was compared to the sleep duration reported in response to the daily question: “How long did you sleep?” If the calculated and reported sleep durations differed by more than 2 hours, then the calculated sleep duration and the corresponding times were set to missing. After data cleaning, weekly average sleep duration was estimated as the average calculated sleep duration across all available nights. The midpoint of the sleep period, calculated as previously described, was also determined. The weekly average sleep midpoint was calculated as the average sleep midpoint across all available nights. For analyses on the sleep diary data, we included women who provided 5 or more days of sleep diary data coincident with actigraphy data collection.
Sleep Exposure
Based on previous data detailing the relationship between sleep duration in nonpregnant populations and health outcomes, a cutoff of less than 7 hours for sleep duration was defined a priori as short sleep duration.32 A cutoff of later than 5:00 am was defined a priori as a late sleep midpoint.33,34
Primary Outcomes
At least 30 days after delivery, a trained, certified chart abstractor assessed all participants' medical records to record outcomes. The primary outcomes of interest for this analysis were (1) hypertensive disorders of pregnancy (ie, preeclampsia with or without severe features; eclampsia; or antepartum gestational hypertension) and (2) gestational diabetes mellitus (GDM). For any participant with documented hypertension or proteinuria, a detailed chart abstraction was performed that included assessment of blood pressure severity, new-onset neurologic disturbances, epigastric pain or pulmonary edema, and blood and urine laboratory results. Detailed study definitions for types of hypertensive disorders have been previously published.35 Women who presented atypically and were difficult to classify according to study criteria were adjudicated by review of clinical data by the principal investigators and final classification was reached by their consensus judgment.
All glucose tolerance testing (GTT) was performed as part of routine clinical care. A woman was considered to have GDM if she met one of the following GTT criteria: (1) fasting 3-hour 100-gram GTT with two of the following values: fasting ≥ 95 mg/dL, 1-hour ≥ 180 mg/dL, 2-hour ≥ 155 mg/dL, 3-hour ≥ 140 mg/dL; (2) fasting 2-hour 75-gram GTT with one of the following values: fasting ≥ 92 mg/dL, 1-hour ≥ 180 mg/dL, 2-hour ≥ 153 mg/ dL; (3) nonfasting 50-gram GTT with a 1-hour value ≥ 200 mg/ dL if no fasting 3-hour or 2-hour GTT had been performed. In addition to these results, chart abstractors recorded whether a diagnosis of GDM was made during the course of clinical care. If GTT data were available, these data were always used primarily to assign the diagnosis of GDM. If no GTT data were available, an explicit diagnosis of GDM in a patient's chart was used for GDM classification. Women identified as having pregestational diabetes were excluded from all analyses on GDM.
Analytical Methods
Descriptive statistics were used to characterize the study population by dichotomous sleep variables (sleep duration < 7 hours, sleep midpoint > 5:00 am). Exploratory analyses were also performed with sleep duration divided into five categories (< 6 hours, 6 to < 7 hours, 7 to < 8 hours (referent), 8 to < 9 hours and ≥ 9 hours) as well as separately examining long sleep duration (≥ 9 versus < 9 hours). Kruskal-Wallis tests were used to compare the distribution of sleep duration and sleep midpoint for categories of age and body mass index (BMI). Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models to estimate the association of self-reported sleep characteristics at visits 1, 2, and 3 with hypertensive disorders of pregnancy and with GDM. Adjustment covariates chosen a priori were maternal age and prepregnancy BMI, both of which were entered into regression models as continuous variables. The larger number of women who completed visit 1 and visit 3 sleep questionnaires allowed for exploratory analyses, in which adjustment was performed for the additional covariates of race/ethnicity (categorized as white, black, Hispanic, Asian, or other), employment status (categorized as employed or unemployed), and insurance status (categorized as commercial insurance or other insurance). For insurance status, participants could select multiple methods that their health care is paid for from the following choices: government insurance, military insurance, commercial insurance, personal household income, and other. If they indicated commercial insurance alone or in combination with other insurance types, they were categorized as commercial insurance; otherwise if they indicated any other insurance type, they were categorized as other. For all analyses, women with chronic hypertension or pregestational diabetes were excluded.
All tests were two-sided single–degree-of-freedom tests and performed at a nominal significance level of α = .05. No correction was made for multiple comparisons. Analyses were conducted using SAS 9.3/9.4 software (SAS Institute Inc., Cary, North Carolina, United States).
RESULTS
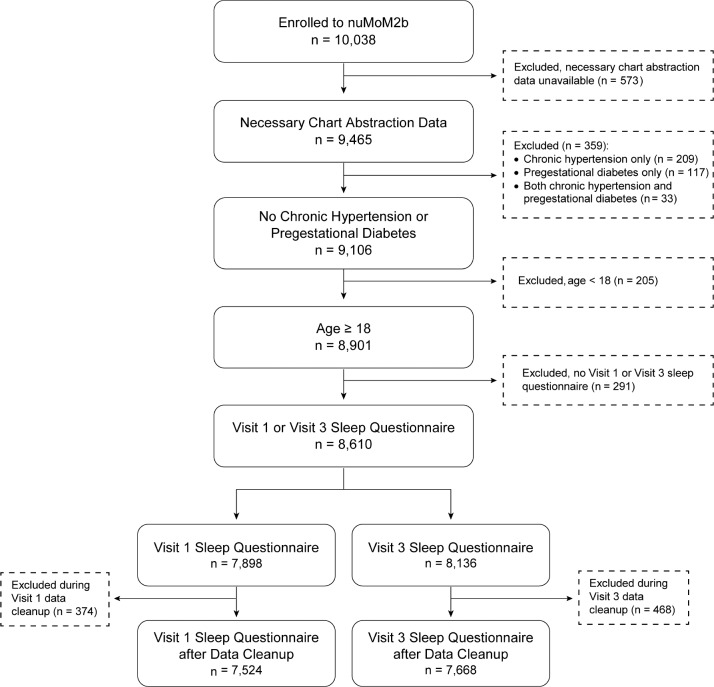
A total of 10,038 women were eligible and enrolled to the nu-MoM2b study. The characteristics of this study population have been previously described.36 After data cleaning, sleep survey data were available on 7,524 women at visit 1, and 7,668 women at visit 3 (Figure 1). A total of 901 women enrolled in the Sleep Duration and Quality ancillary study; 782 had valid actigraphy recordings; 752 of the 782 also provided 5 or more days of sleep diary data coincident with actigraphy data collection.
Figure 1. Enrollment and inclusion in analysis.
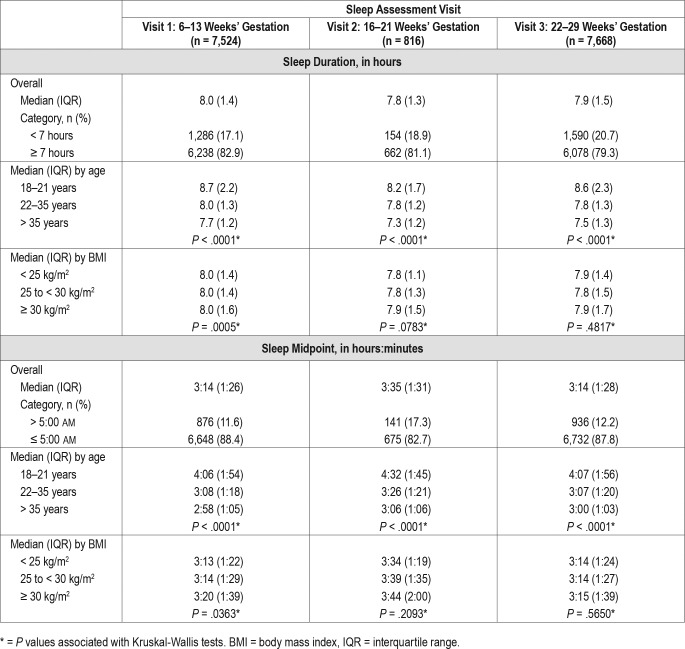
The distribution of sleep duration and sleep midpoint at the 3 different timepoints is presented in Table 2, as is the association between sleep duration and sleep midpoint with age and BMI.
Table 2.
Distribution of sleep duration and sleep midpoint, overall and by age and BMI.
Questionnaire Data at Visit 1 and Visit 3 and Pregnancy Outcomes
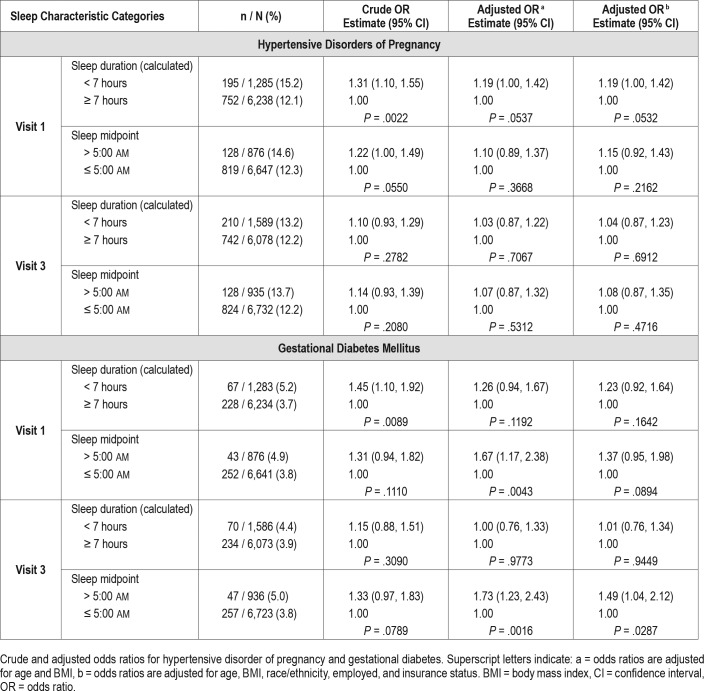
The associations of self-reported short sleep duration and late sleep midpoint ascertained by questionnaire (weighted average of weekday/weekend), with adverse pregnancy outcomes are presented in Table 3.
Table 3.
Visit 1 and visit 3 sleep questionnaire data.
Self-reported short sleep duration at visit 1 was associated with hypertensive disorders (odds ratio [OR] 1.31, 95% confidence interval [CI] 1.10, 1.55, P = .002) and GDM (OR 1.45, 95% CI 1.10, 1.92, P = .009) in univariate analysis. The adjusted analysis showed similar direction of association, but results were not significant: the adjusted OR (adjusting for age and BMI) for hypertension was 1.19 (95% CI 1.00, 1.42, P = .054) and for GDM 1.26 (95% CI 0.94, 1.67, P = .119). Data from visit 3 also does not demonstrate an association between short sleep duration and pregnancy outcomes. In exploratory analyses, we examined sleep duration categorized as < 6 hours, 6 to < 7 hours, 7 to < 8 hours (referent), 8 to < 9 hours and ≥ 9 hours and separately examined long sleep duration (≥ 9 versus < 9 hours). Again, no associations were observed between sleep duration and hypertensive disorders. Long sleep duration was associated with GDM at visit 3 (adjusted OR 1.42, 95% CI 1.03, 1.96, P = .032) but not at visit 1 (supplemental material).
We did not observe a relationship between sleep midpoint and hypertensive disorders of pregnancy at either visit 1 and visit 3. However, there was a consistent association between late sleep midpoint and GDM at both visit 1 and visit 3 time-points. In multivariable analyses with adjustment for age and BMI, the adjusted OR for GDM with late sleep midpoint at visit 1 was 1.67, 95% CI 1.17, 2.38, P = .004. Similarly, at visit 3, the adjusted OR for GDM when late sleep midpoint was reported was 1.73, 95% CI 1.23, 2.43, P = .002.
In exploratory analyses, we considered the additional covariates of race/ethnicity, employed (yes/no), and insurance status (commercial versus government or self-pay). The results are similar with the exception that the P value for the association of sleep midpoint at visit 1 with GDM was no longer statistically significant at .089 (Table 3).
Similar findings regarding short sleep duration and sleep midpoint were present when associations were examined separately for weekend and weekday data (supplemental material).
Concordance Between Actigraphy and Sleep Diary Reported Sleep at Visit 2
Using data from the 752 women with 5 or more days of sleep diary data coincident with 5 or more days of actigraphy data at visit 2, we examined concordance between self-reported and objectively assessed sleep duration (continuous and categorized as < 7 hours / ≥ 7 hours) and sleep midpoint (continuous and categorized > 5:00 am / ≤ 5:00 am). The median (25th, 75th percentiles) difference for sleep duration, diary minus actigraphy, was 23 (−3, 50) minutes; and for sleep midpoint was 2 (−6, 11) minutes. Most participants (77.1%) were concordant between actigraphy and diary for sleep duration categorization. The discordance was unbalanced as 119/206 women (57.8%) with short sleep duration (< 7 hours) on actigraphy had diary data documenting sleep durations of ≥ 7 hours, but only 53/546 (9.7%) with a sleep duration ≥ 7 hours on actigraphy were discordant by diary. In other words, overreporting sleep duration was more common than underreporting. In comparison, 94.3% were concordant for sleep midpoint designation. But similarly, the discordance was unbalanced with 20.1% of women with a late sleep midpoint (> 5:00 am) on actigraphy having a diary midpoint of ≤ 5:00 am, compared to only 2.6% of women with an actigraphy midpoint of ≤ 5:00 am having a discordant diary assessment.
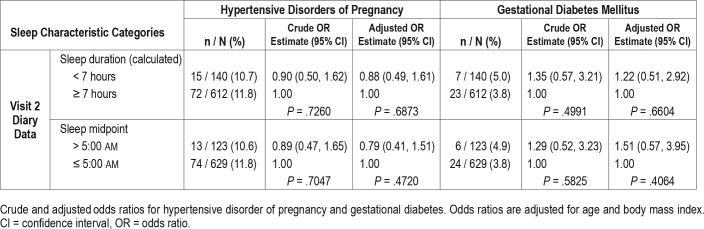
Visit 2 Daily Sleep Diary Data and Pregnancy Outcomes
Sleep diary data from the 752 women in the actigraphy sub-study showed no association of reported short sleep duration or late sleep midpoint with either hypertension or GDM (Table 4). To better understand our findings, given the previously described association between actigraphy-determined short sleep duration and late sleep midpoint at visit 2 and GDM from this cohort,26 we examined the proportion of hypertension and GDM according to actigraphy-diary concordance by sleep duration and midpoint categories (Table 5). When actigraphy and diary results were concordant for sleep duration, the rate of GDM was higher at 5.75% for sleep duration < 7 hours compared to 3.25% for sleep duration ≥ 7 hours. Similarly, when sleep midpoint was concordant, the rate of GDM was higher at 5.61% for late sleep midpoint > 5:00 am versus 3.32% for sleep midpoint ≤ 5:00 am. Women who self-reported a normal sleep duration or sleep midpoint but had an objective actigraphy result that was discordant (ie, showed short sleep or a late sleep midpoint) had higher GDM rates (5.88% and 14.81%) compared to women who self-reported abnormal sleep but had a normal pattern on actigraphy (3.77% and 0%). Similar patterns were seen in rates of hypertensive disorders. In other words, women with short sleep and late sleep timing who misclassified themselves as having a normal sleep had the highest rates of hypertension and GDM.
Table 4.
Visit 2 daily sleep diary data.
Table 5.
Diary-actigraphy concordance and outcome rates.
DISCUSSION
Our objective was to examine the relationship of self-reported sleep duration and timing in pregnancy with adverse pregnancy outcomes in a large cohort of women who were nulliparous and had given self-reported sleep assessments at multiple timepoints in pregnancy. Prior published data from this cohort indicated that objectively determined (using actigraphy) short sleep duration and late sleep midpoint at visit 2 (16 0/7 to 21 6/7 weeks) are positively associated with GDM.26 In contrast, in the current analysis of self-reported sleep duration and timing from the same cohort, we did not observe any consistent relationship between sleep duration and hypertensive disorders of pregnancy or GDM. However, we found an association between self-reported late sleep midpoint and GDM in both early and late pregnancy. Furthermore, after assessing the concordance between daily diary and actigraphy data at visit 2, we noted that self-reported late sleep midpoint ascertainment is more accurate than self-reported short sleep duration.
There are few large studies that have evaluated self-reported sleep measures and pregnancy outcomes, with each using varying definitions of abnormal sleep and adverse outcomes. In a large cross-sectional study from China, 12,506 women at 24 to 28 weeks were asked to answer the following question: “How many hours of sleep do you get during this pregnancy, including both day and night time?”37 They found that women with short (< 7 hours) and long (≥ 9 hours) sleep durations had higher rates of GDM (8.8% and 7.9%, respectively) compared with women who slept ≥ 7 to < 9 h/night. In adjusted analyses, only long sleep duration remained associated with GDM. There are two other large (n > 1,000) prospective cohort studies that examined self-reported sleep duration and pregnancy outcomes. In a single center study in Seattle, Washington, more than 1,200 women at a mean of 14 weeks' gestation were asked: “Since becoming pregnant, how many hours per night do you sleep?”38,39 The referent category for sleep duration in this analysis was ≥ 9 to < 10 hours. They found that both short (≤ 6 hours) and long (≥ 10 hours) sleep durations were associated with higher mean third trimester blood pressure, and that very short sleep duration (< 5 hours) was associated with an increased adjusted odds of preeclampsia (adjusted OR 9.88, 95% CI 1.91–50.80). Similarly, the authors noted a U-shaped relationship between mean glucose concentrations 1 hour after a 50-gram oral glucose challenge and reported sleep duration, and an association between very short sleep duration (≤ 4 hours) and GDM among women who were overweight (adjusted OR 9.83, 95% CI 1.12–86.32). Rawal et al. published a secondary analysis of the Fetal Growth Study-Singleton Cohort.40 They found that first-trimester sleep duration was not associated with GDM risk. In the second trimester, the association between sleep duration and GDM differed by prepregnancy obesity status, with a positive association only noted among women without obesity (5 to 6 hours adjusted OR 2.52, 95% CI 1.27–4.99; 7 hours adjusted OR 2.01, 95% CI 1.09–3.68; ≥ 10 hours adjusted OR 2.17, 95% CI 1.01–4.67).
Evidence from prior studies as well as from our analysis presented here suggests that, on balance, self-reported sleep duration may have an association with hypertensive disorders of pregnancy and GDM, but that these associations are weak. As our data regarding concordance of objective and self-reported sleep demonstrates, the likely reason for the contrast to the associations that have been documented with objectively assessed sleep measures is that many women with short sleep overreport their sleep duration. In fact, in our study population, women with short sleep who misclassified themselves as having normal sleep duration had the highest rates of hyper-tension and GDM.
However, our data suggest that self-reported sleep timing (used to calculate sleep midpoint) in pregnancy is more accurate than self-reported sleep duration, and is linked to the risk of GDM. These results are consistent with our analysis of objectively measured sleep timing from the same cohort. There is biologic plausibility for this finding, as later sleep timing is associated with circadian misalignment. This misalignment can occur when sleep and wakefulness behaviors do not occur at an appropriate time relative to the timing of the central circadian clock (hypothalamus) and/or relative to the external environment (light-dark cycle). Misalignment can lead to chronodisruption. For example, later sleep timing can lead to increased exposure to artificial light at night, which can suppress melatonin secretion.
It is known that certain forms of shift work may lead to sleep disruption and circadian misalignment. From population studies of nonpregnant individuals, shift work has been associated with poor health outcomes, especially obesity and type 2 diabetes.41–43 Data regarding shift work and pregnancy outcomes are limited. Studies have focused primarily on the outcomes of infertility, miscarriage, preterm birth, and birth weight, and systematic reviews suggest a possible modest increased risk of these complication among shift workers.44,45 Furthermore, epidemiological data on shift work are limited in that many studies have not properly distinguished between different types of shift work. There is clear biologic plausibility that certain types of shift work, particularly rotating shift with work at night, can lead to greater degrees of circadian disruption. Finally, circadian disruption is not limited to shift work. In fact, we have previously shown that women reporting shift work—as well as those who do not have a regular work schedule due to being unemployed—are at the highest risk of having a late sleep midpoint. Specifically, our previously published analysis of objectively measured sleep midpoint in pregnancy found that women who worked regular day shifts had significantly earlier sleep midpoints (n = 441, median [quartile 1: 25th percentile, quartile 3: 75th percentile] sleep midpoint 3:10 am [2:40 am, 3:51 am]) compared to women who reported working some form of shift work (n = 148, median [quartile 1, quartile 3] sleep midpoint 4:14 am [3:22 am, 5:39 am]) or who were unemployed (n = 152, median [quartile 1, quartile 3] sleep midpoint 4:34 am [3:45 am, 5:37 am]), Kruskal-Wallis test P < .0001. Furthermore, we found that late sleep midpoint was more common among young pregnant women (< 22 years), black and Hispanic women, women with obesity, those who smoked, and women with a history of insomnia.26
Our study has several strengths. The nuMoM2b cohort is a large, geographically and demographically diverse study population. We prospectively ascertained self-reported sleep duration and timing variables at three different timepoints in pregnancy, using two different methods (single timepoint questionnaires, multiple day sleep diary). We were also able to report on the concordance between the self-reported sleep and actigraphy-measured sleep. The main limitation of this analysis of self-reported sleep in pregnancy is that by nature of the observational design we are not able to account for all confounding and mediating factors. We recognize that observed effects we reported are likely mediated by medical, social, physiological, and environmental factors.
In conclusion, in this analysis of self-reported sleep duration and timing from a large cohort of nulliparous women, we did not observe any consistent relationship between sleep duration and hypertensive disorders of pregnancy or GDM. We observed an association between questionnaire-based self-reported late sleep midpoint (> 5:00 am) in both early (6 0/7 to 13 6/7 weeks) and late (22 0/7 to 29 6/7 weeks) pregnancy and GDM. Furthermore, after assessing the concordance between daily diary and actigraphy data in a subgroup of the cohort, we found that self-reported sleep midpoint ascertainment is more accurate than self-reported sleep duration. Further research should help to better categorize patterns of sleep timing in pregnancy and their associations with adverse pregnancy outcomes.
DISCLOSURE STATEMENT
All the authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. They have all reviewed and have given approval of the version of the manuscript submitted for review. This study is supported by funding from the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HL119991; U10-HL119989; U10-HL120034; U10-HL119990; U10-HL120006; U10-HL119992; U10-HL120019; U10-HL119993; and U10-HL120018; R01HL105549. Supplemental funding for this analysis was provided by the National Institutes of Health Office of Behavioral and Social Sciences Research through U10-HL119992. In addition, support was provided by the National Institutes of Health National Center for Research Resources and National Center for Advancing Translational Sciences to Clinical and Translational Science Institutes at Indiana University (UL1TR001108) and University of California, Irvine (UL1TR000153). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Phyllis Zee serves as a consult to Philips Respironics, the makers of the actiwatches that were purchased for use in this study. The authors report no conflicts of interest.
REFERENCES
- 1.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24(5):687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zee PC, Turek FW. Sleep and health: everywhere and in both directions. Arch Intern Med. 2006;166(16):1686–1688. doi: 10.1001/archinte.166.16.1686. [DOI] [PubMed] [Google Scholar]
- 5.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33(8):1037–1042. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 8.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013;8(11):e82305. doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3(1):52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 12.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 14.Carrington MJ, Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep. 2008;31(12):1701–1712. doi: 10.1093/sleep/31.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 17.Gestational diabetes mellitus. Practice Bulletin No. 137. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 18.Beebe KR, Lee KA. Sleep disturbance in late pregnancy and early labor. J Perinat Neonatal Nurs. 2007;21(2):103–108. doi: 10.1097/01.JPN.0000270626.66369.26. [DOI] [PubMed] [Google Scholar]
- 19.Gay CL, Richoux SE, Beebe KR, Lee KA. Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth. 2017;44(2):173–180. doi: 10.1111/birt.12277. [DOI] [PubMed] [Google Scholar]
- 20.Haney A, Buysse DJ, Rosario BL, Chen YF, Okun ML. Sleep disturbance and cardiometabolic risk factors in early pregnancy: a preliminary study. Sleep Med. 2014;15(4):444–450. doi: 10.1016/j.sleep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herring SJ, Nelson DB, Pien GW, et al. Objectively measured sleep duration and hyperglycemia in pregnancy. Sleep Med. 2014;15(1):51–55. doi: 10.1016/j.sleep.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191(6):2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 23.Okun ML, Kline CE, Roberts JM, Wettlaufer B, Glover K, Hall M. Prevalence of sleep deficiency in early gestation and its associations with stress and depressive symptoms. J Womens Health (Larchmt) 2013;22(12):1028–1037. doi: 10.1089/jwh.2013.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharkey KM, Boni GM, Quattrucci JA, Blatch S, Carr SN. Women with postpartum weight retention have delayed wake times and decreased sleep efficiency during the perinatal period: a brief report. Sleep Health. 2016;2(3):225–228. doi: 10.1016/j.sleh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai SY, Lin JW, Wu WW, Lee CN, Lee PL. Sleep disturbances and symptoms of depression and daytime sleepiness in pregnant women. Birth. 2016;43(2):176–183. doi: 10.1111/birt.12215. [DOI] [PubMed] [Google Scholar]
- 26.Facco FL, Grobman WA, Reid KJ, et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017;217(4):447.e1–447.e13. doi: 10.1016/j.ajog.2017.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson DL, Fung A, Walker SP, Barnes M. Subjective reports versus objective measurement of sleep latency and sleep duration in pregnancy. Behav Sleep Med. 2013;11(3):207–221. doi: 10.1080/15402002.2012.670674. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre JP, Ingham CM, Hutchinson BL, et al. A description of sleep behaviour in healthy late pregnancy, and the accuracy of self-reports. BMC Pregnancy Childbirth. 2016;16(1):115. doi: 10.1186/s12884-016-0905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herring SJ, Foster GD, Pien GW, et al. Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breath. 2013;17(4):1323–1327. doi: 10.1007/s11325-013-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Facco FL, Parker CB, Reddy UM, et al. nuMoM2b sleep-disordered breathing study: objectives and methods. Am J Obstet Gynecol. 2015;212(4):542.e1–542.e127. doi: 10.1016/j.ajog.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid KJ, Facco FL, Grobman WA, et al. Sleep during pregnancy: the nuMoM2b pregnancy and sleep duration and continuity study. Sleep. 2017;40(5) doi: 10.1093/sleep/zsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–952. doi: 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas DM, Parker CB, Wing DA, et al. A description of the methods of the nulliparous pregnancy outcomes study: monitoring mothers-to-be (nuMoM2b) Am J Obstet Gynecol. 2015;212(4):539.e1–539.e24. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Leng J, Li W, et al. Sleep duration and quality, and risk of gestational diabetes mellitus in pregnant Chinese women. Diabet Med. 2017;34(1):44–50. doi: 10.1111/dme.13155. [DOI] [PubMed] [Google Scholar]
- 38.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimesterspecific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33(10):1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawal S, Hinkle SN, Zhu Y, Albert PS, Zhang C. A longitudinal study of sleep duration in pregnancy and subsequent risk of gestational diabetes: findings from a prospective, multiracial cohort. Am J Obstet Gynecol. 2017;216(4):399.e1–399.e8. doi: 10.1016/j.ajog.2016.11.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 42.Hansen AB, Stayner L, Hansen J, Andersen ZJ. Night shift work and incidence of diabetes in the Danish Nurse Cohort. Occup Environ Med. 2016;73(4):262–268. doi: 10.1136/oemed-2015-103342. [DOI] [PubMed] [Google Scholar]
- 43.Monk TH, Buysse DJ. Exposure to shift work as a risk factor for diabetes. J Biol Rhythms. 2013;28(5):356–359. doi: 10.1177/0748730413506557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies. BJOG. 2011;118(12):1429–1437. doi: 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stocker LJ, Macklon NS, Cheong YC, Bewley SJ. Influence of shift work on early reproductive outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(1):99–110. doi: 10.1097/AOG.0000000000000321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.