Abstract
Study Objectives:
We aimed to determine the association between short telomere length, sleep parameters, and sleep disorders in an adult general population sample.
Methods:
As part of the EPISONO cohort (São Paulo, Brazil), 925 individuals answered questionnaires, underwent a full-night polysomnography and clinical assessment, and had peripheral blood collected for DNA extraction. Insomnia was diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition; and obstructive sleep apnea was defined according to apnea-hypopnea index. For the objective insomnia phenotype, we combined insomnia diagnosis with total sleep time from polysomnography with a cutoff of 360 minutes, allowing the classification of six groups. Self-reported sleep duration was used to classify the individuals as short (< 6 hours), average (6 to 8 hours) and long (> 8 hours) sleepers. The leukocyte telomere length was measured using quantitative real-time polymerase chain reaction. Based on its distribution, we considered leukocyte telomere length < 10th percentile as short telomere and leukocyte telomere length ≥ 10th percentile as non-short telomere.
Results:
After adjusting for sex, age, and body mass index, only insomnia disorder (odds ratio [OR] = 2.654, 95% confidence interval [CI] = 1.025–6.873, P = .044), insomnia disorder total sleep time < 360 minutes (OR = 4.205, 95% CI = 1.097–16.117, P = .036) and long sleepers (OR = 2.177, 95% CI = 1.189– 3.987, P = .012) were associated with short telomere.
Conclusions:
Our findings support the existence of an association among insomnia, insomnia phenotype, and self-reported long sleep duration with the maintenance of telomere length.
Commentary:
A commentary on this article appears in this issue on page 1975.
Citation:
Tempaku P, Hirotsu C, Mazzotti D, Xavier G, Maurya P, Brietzke E, Belangero S, Poyares D, Bittencourt L, Tufik S. Long sleep duration, insomnia, and insomnia with short objective sleep duration are independently associated with short telomere length. J Clin Sleep Med. 2018;14(12):2037–2045.
Keywords: insomnia, long sleep duration, sleep, telomeres
BRIEF SUMMARY
Current Knowledge/Study Rationale: Growing evidence suggests the contribution of sleep in the molecular pathways of aging related to the maintenance of telomere length. In this sense, the study aimed to verify the association between short telomere length with objective and self-reported sleep parameters, as well as sleep disorders in a general population sample.
Study Impact: Our results demonstrate that self-reported long sleep duration (> 8 hours versus 7 to 8 hours) and the presence of insomnia doubled the odds ratio of short telomere length, while the short sleep duration insomnia phenotype led to a fourfold increase in the odds ratio of short telomere length independently of age, sex, and obesity. These results suggest that longer sleepers and insomnia, especially the short sleep phenotype may play a role in the mechanisms related to biological aging.
INTRODUCTION
The telomeric DNA is a sequence of six-nucleotide-unit tandem repeats (TTAGGG(n)) that occurs at the end of chromosomes to protect this terminal region from degradation and prevent the loss of genetic material.1 In human aging, because of the cumulative cell replication throughout lifespan, telomere length tends to diminish and to be strongly correlated with mortality risk,2 being one of the most studied and important biomarkers of aging.3
Short telomeres are commonly found in several pathological conditions as an indicator of disease onset and related outcomes. Studies have found positive association between short telomere and age-related diseases,4 as well as adverse life conditions such as stress and unhealthy behaviors.5 Of note, all of these conditions are bidirectionally related to sleep. It is well established that sleep loss is associated with several alterations in cellular processes6 that can interfere with telomere length maintenance. Indeed, evidence has pointed to an association between short telomeres and short sleep duration,7,8 obstructive sleep apnea (OSA)9–11 and insomnia.12 However, inconsistent findings have also been reported,13,14 indicating that there is a need for a more complete assessment of the association between sleep and short telomeres.15
The biological processes that may explain the association between sleep disturbances and short telomere length are based on the role of sleep in the homeostatic regulation of inflammatory and oxidative pathways. Because telomere length is negatively modulated by oxidative stress and inflammation, we hypothesized that the presence of insomnia, OSA, and poor sleep correlates, such as short and long sleep duration, daytime sleepiness, and low sleep efficiency, would be associated with short telomere length. Thus, to better understand the overall contribution of sleep in the telomere length maintenance, the current study aimed to verify the association between short telomere length with sleep disturbances through objective and self-reported assessment in a general population sample.
METHODS
Studied Sample
This work was part of the São Paulo Epidemiologic Sleep Study (EPISONO), a population-based survey conducted in the city of São Paulo (Brazil) in 2007. A total of 1,042 participants of both sexes underwent full-night polysomnography (PSG) and clinical assessment, answered a full set of sleep questionnaires, and had a blood sample collected for biochemical measurements and DNA extraction. Complete rational design, sampling, and procedures have been described elsewhere.16 The study was approved by the Ethics Committee of Universidade Federal de São Paulo (CEP 0593/06). Written informed consent forms were completed and signed by all participants before their inclusion in the study.
Telomere Length Measurement
All volunteers had 10 mL of blood collected in EDTA tubes for DNA extraction from peripheral blood mononuclear cells. The DNA was extracted using the salting-out method, according to the protocol of Miller et al. with some modifications.17 After isolation, the DNA samples were quantified and diluted to 50 ng/μL.
Telomere length measurement was performed by multiplex real-time polymerase chain reaction (PCR), as described by Cawthon.18 The samples and standard curve were all run in triplicate in a ViiA 7 Real-Time PCR System with fast 96-Well Block (Thermo Fisher Scientific, Waltham, Massachusetts, United States). In all runs, the standard curve was used to obtain the quantification of telomere length relative to the albumin gene (also known as the T/S ratio). The T/S ratio is proportional to telomere length and was considered the quantitative measure of mean leukocyte telomere length (LTL).18
Short Telomere Definition
According to the sample distribution of the telomere length (T/S ratio), we categorized the 925 individuals into 2 groups: short telomere were those individuals with a value of T/S ratio equal or below the 10th percentile (T/S = 1.11). Therefore, individuals with T/S ratio above the 10th percentile were included in the non-short telomere group.
Sociodemographic, Lifestyle, and Self-Reported Sleep Assessment
To better evaluate sociodemographic, lifestyle, and self-reported aspects of sleep parameters, we used data from the following structured questionnaires.
Socioeconomic Questionnaire
The Socioeconomic Questionnaire is a structured and validated questionnaire with 15 questions to evaluate the social classes of the Brazilian population.19
Alcohol, Smoking and Substance Involvement Screening Test
The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) is a validated and brief screening questionnaire to identify individuals who use psychoactive substances and their risk of addiction. This questionnaire was used to obtain the frequency of alcohol use and smoking in the past 3 months.20
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) consists of seven domains related to self-reported sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping pills, and daytime sleep dysfunction in the past month. The sum of the values ranges from 0 to 21 in the total questionnaire score, with scores above 5 suggesting poor sleep quality. This questionnaire was additionally used to evaluate the self-reported sleep duration and the presence of insomnia complaints (difficulty to initiate sleep and early awakening).21
UNIFESP Sleep Questionnaire
The UNIFESP Sleep Questionnaire is composed of 59 questions regarding routine, sleep problems, and life habits. This questionnaire was used to assess the presence of insomnia complaints (difficulty to initiate or maintain sleep, or early awakening), in addition to PSQI, as well as the frequency of physical activity.22
Epworth Sleepiness Scale
The Epworth Sleepiness Scale is a validated self-administered questionnaire for the Portuguese language that evaluates the probability of falling asleep in eight situations involving daily activities. The overall score ranges from 0 to 24, with scores above 9 indicating the presence of excessive daytime sleepiness.23,24
Insomnia Severity Index
The Insomnia Severity Index (ISI) is a validated instrument for the English language, translated and used to quantify the perceived severity of insomnia. It is based on the insomnia criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), evaluating recent problems to initiate and maintain sleep, early awakening, interference in daytime activities, concern with sleep problems, and sleep satisfaction. The following three questions from this questionnaire were used to identify the diurnal consequences associated with insomnia disorder classification: to what extent do you consider your sleep problem to interfere with your daily functioning?; how noticeable to others do you think your sleeping problem is in terms of impairing the quality of your life?; how worried/distressed are you about your current sleep problem?25
Beck Depression Inventory
The Beck Depression Inventory is composed of 21 items related to depressive symptoms in the past week, whose objective is to measure the severity of depressive symptoms. The questionnaire has a maximum score of 63 and the categories are: minimal depressive symptoms (0–10), mild depressive symptoms (11–19), moderate depressive symptoms (20–30), and severe depressive symptoms (31–63).26
Pre-Sleep - Previous Day and Night
The Pre-Sleep - Previous Day and Night instrument consists of 26 questions created by a panel of sleep specialists concerning aspects of the day preceding the PSG night that could influence sleep parameters. This questionnaire was used to obtain data about current medications use.27
Clinical Assessment
The measurement of weight and height was performed in the morning after the PSG. The body mass index (BMI) was obtained from the ratio between the body weight and height squared. Neck, hip, and waist circumferences were collected at night with a tape measure in a standardized way to guarantee the reliability of the data.
Systolic and diastolic blood pressure were evaluated before PSG as the volunteer sat and rested for at least 5 minutes approximately at the same time for all volunteers.
Biochemical Examinations
On the morning after PSG, after 12 hours of fasting, participants had their blood collected for biochemical examinations. The enzymatic colorimetric assay was used to assess the concentrations of glucose, triglycerides (Advia 1650/2400/Siemens Healthcare Diagnostics Inc., Hoffman Estates, Illinois, U) and high-density lipoprotein (HDL) cholesterol (Advia 1650/2400/ Kovalent, Brazil).
Comorbidities
The presence of comorbidities was diagnosed as follows:
Hypertension: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medication;
Diabetes: fasting glucose ≥ 126 mg/dL or use of antiglycemic medication28;
Dyslipidemia: presence of hypolipidemia or hypertriglyceridemia. Hypolipidemia was defined as HDL cholesterol < 40 mg/dL in men, < 50 mg/ dL in women or treatment to increase levels of HDL cholesterol29; and hypertriglyceridemia was considered positive if triglycerides ≥ 150 mg/dL or treatment to reduce its circulating levels29;
Visceral obesity: abdominal circumference ≥ 102 cm in non-Asian men, ≥ 88 cm in non-Asian women30, ≥ 90 cm in Asian men, and ≥ 80 cm in Asian women.31
Polysomnography
All participants underwent a baseline full-night PSG at the Sleep Institute, São Paulo (Brazil) using a digital system (EMBLA N7000, Embla Systems Inc., Broomfield, Colorado, United States). PSG was scored according to standardized international criteria for sleep staging.32 Physiological variables monitored during PSG included: electroencephalogram (C3-A2, C4-A1, O1-A2, O2-A1), electrooculogram (EOG-Left-A2, EOG-Right-A1), electromyogram (submentonian region, masseter region, anterior tibialis, and seventh intercostal space), electrocardiogram (derivation V1 modified), airflow detection (thermocouple and nasal pressure), respiratory effort (thorax and abdomen) via inductance plethysmography trace belts, snoring and body position by EMBLA sensors, and percutaneous oxygen saturation (SpO2) by EMBLA oximeter.
The AASM Manual for Scoring Sleep and Associated Events: Rules, Terminology and Technical Specifications32 was the reference used to score sleep-related respiratory events and arousals. Hypopneas were scored according to the alternative rule, that is, a decrease of 50% in the respiratory flow associated with an arousal or 3% oxygen desaturation. The 3% oxygen desaturation index (3% ODI) comprised the number of 3% oxygen desaturations per hour of sleep, whereas the apnea-hypopnea index (AHI) was defined as the total number of respiratory events (apnea and hypopneas) per hour of sleep.
Sleep Disturbances
Self-reported sleep duration was obtained from the fourth question of the PSQI. This question asks: during the past month, how many hours of actual sleep did you get at night?21 Individuals who reported fewer than 6 hours of sleep were categorized as short sleepers, those between 6 and 8 hours were considered as average sleepers, and individuals with more than 8 hours as long sleepers.
OSA was defined according to AHI as mild (5 to < 15 events/h) and moderate-severe (≥ 15 events/h). Individuals who had AHI < 5 events/h were considered as non-OSA.33
The criteria for the diagnosis of self-reported insomnia were based on the DSM-IV.34 Individuals reporting regular insomnia symptoms such as difficulties to initiate and/or maintain sleep and/or early morning awakenings, occurring in the past month with diurnal consequences, were considered as having insomnia disorder. Those with the symptoms but without diurnal consequences were classified as having insomnia symptoms. Finally, individuals without any regular symptom of insomnia were classified as good sleeper34.
Last, insomnia disorder criterion was combined with the total sleep time (TST) derived from PSG for a more objective phenotype of insomnia. We used a cutoff of 360 minutes. Therefore, all individuals were additionally classified into six groups:
Good sleeper TST ≥ 360 minutes: control (non-insomnia) with TST equal or greater than 360 minutes;
Good sleeper TST < 360 minutes: control (non-insomnia) with TST lower than 360 minutes;
Insomnia symptoms TST ≥ 360 minutes: individuals with insomnia symptoms and TST equal or greater than 360 minutes;
Insomnia symptoms TST < 360 minutes: individuals with insomnia symptoms and TST lower than 360 minutes;
Insomnia disorder TST ≥ 360 minutes: individuals diagnosed with insomnia disorder and TST equal or greater than 360 minutes;
Insomnia disorder TST < 360 minutes: individuals diagnosed with insomnia disorder and TST lower than 360 minutes.
Statistical Analysis
Normality of the distribution of quantitative data was determined by Shapiro-Wilk test. Chi-square and Mann-Whitney U tests were used to verify possible associations of the groups (short telomere and non-short telomere) between categorical and continuous variables, respectively. Univariate logistic regression analyses, using the enter method, were applied to verify the association between short telomere and sleep-related variables adjusted for sex, BMI (continuous), and categorical age (20 to 39, 40 to 59, and 60 to 80 years). Statistical analyses were performed using PASW Statistics 18.0 (Chicago, Illinois, United States) and the significance level was set at 5%. Intra-assay and inter-assay coefficient of variation was calculated trough the formula: coefficient of variation = 100 × (standard deviation / mean).
RESULTS
Participant Characteristics
From the 925 individuals with DNA available (mean age 48.1 years ± 19.8, 55.4% women), 88.5% (n = 819) were included in the non-short telomere group and 11.5% (n = 106) in the short telomere group. The values of coefficient of variation were 3.72% (intra-assay) and 4.02% (inter-assay).
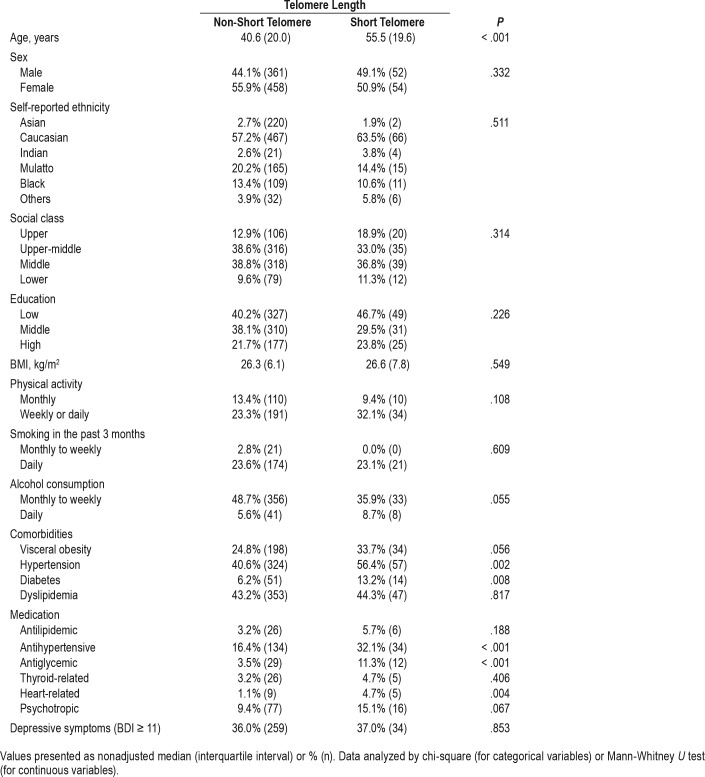
Table 1 shows the sociodemographic and clinical profile of the study participants according to the telomere length. We observed that the short telomere group was significantly older than the non-short telomere group. We did not find statistically significant differences between the groups for sex, self-reported ethnicity, social class, and education. The short telomere group presented a higher frequency of individuals with hypertension and diabetes, as well as a higher use of antihypertensive, antiglycemic, and heart-related medications compared to the non-short telomere group. We did not observe significant differences between the groups for BMI, physical activity, smoking and alcohol consumption, visceral obesity, dyslipidemia, use of antilipidemic, thyroid-related and psycho-tropic medications, and depressive symptoms.
Table 1.
Sociodemographic characteristics in the EPISONO cohort according to telomere length.
Insomnia and Self-Reported Sleep Parameters
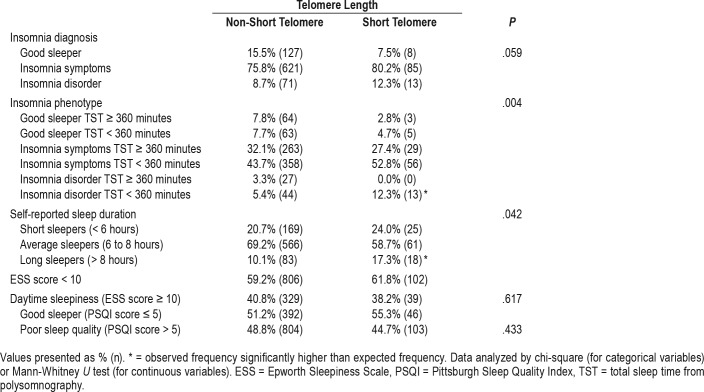
Table 2 demonstrates the distribution of insomnia and self-reported sleep parameters, the life habits, and comorbidities studied according to the telomere length. Also, we found a greater frequency of the insomnia phenotype group “insomnia disorder TST < 360 minutes” and of long sleepers in the short telomere group. Additionally, no differences were found for insomnia diagnosis, daytime sleepiness, and poor sleep quality.
Table 2.
Insomnia and self-reported sleep aspects in the EPISONO cohort according to telomere length.
OSA and Objective Sleep Parameters
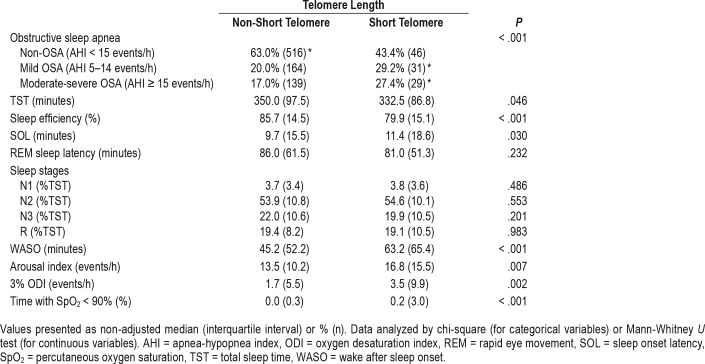
Regarding the objective variables derived from PSG and the diagnosis of OSA, Table 3 shows the results according to the telomere length. We observed a higher frequency of individuals from the mild and moderate to severe OSA groups in the short telomere group when compared to the non-OSA group. The short telomere group had significant lower TST and sleep efficiency; and greater values of sleep latency, wake after sleep onset, arousal index, 3% ODI, and percentage time with SpO2 < 90% compared to the non-short telomere group. We did not find statistically significant differences for the following variables: REM sleep latency, stage N1 sleep, stage N2 sleep, stage N3 sleep, and stage R sleep.
Table 3.
Polysomnographic parameters in the EPISONO cohort according to telomere length.
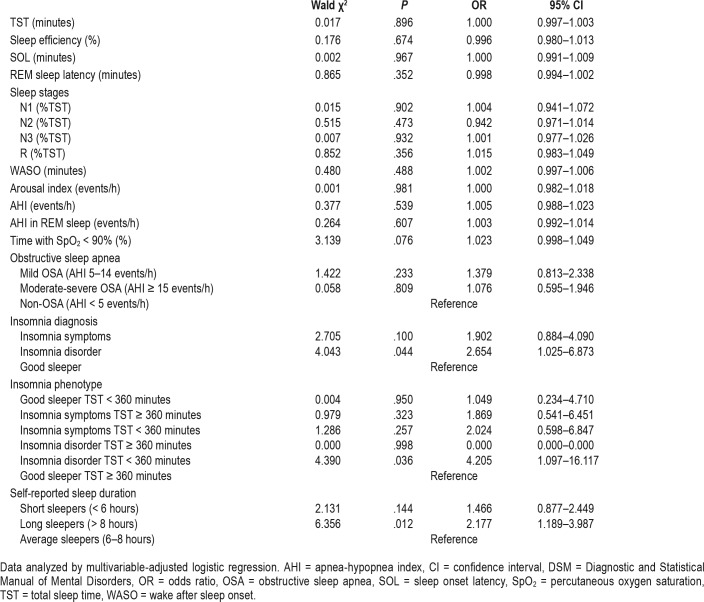
Independent Associations Between Short Telomere and Sleep
As demonstrated in Table 4, the univariate regressions adjusted for sex, BMI, and age showed an independent association between short telomere length and the following sleep-related variables: insomnia diagnosis, insomnia phenotype, and self-reported sleep duration. The presence of insomnia disorder was associated with an odds ratio (OR) of 2.654 (95% CI = 1.025– 6.873, P = .044) for short telomere in comparison with being good sleepers. Moreover, belonging to the “Insomnia disorder TST < 360 minutes” phenotype was associated with an OR of 4.205 (95% CI = 1.097–16.117, P = .036) for short telomere compared to those belonging to the “Good sleeper TST > 360 minutes” group; while being a long sleeper was associated with an OR of 2.177 (95% CI = 1.189–3.987, P = .012) for short telo-mere in relation to being an average sleeper.
Table 4.
Association between sleep and short telomere adjusted for age, sex, and body mass index in the EPISONO sample.
DISCUSSION
In the current study, we observed an independent association of short LTL with the self-reported long sleep duration (> 8 hours versus 7 to 8 hours), insomnia (disorder versus good sleeper) and insomnia phenotype (objective short-sleeper insomnia versus objective average good sleeper). To the best of our knowledge, the current study is the first to analyze the association between short telomere length and sleep in a broader sense, including both self-reported and objective parameters.
Overall, the literature suggests that shorter sleep duration is associated with shorter telomeres.7,8 In the current study, although we found lower objective TST in the short telomere group, after adjustment for age, sex, and BMI, these results were no longer significant, mostly suggesting a confounder effect of age. Regarding the self-reported sleep duration, being a long sleeper (> 8 hours) was independently associated with short telomere. Thus, there is a considerably important role of both short and long sleep duration on LTL, whose adjacent mechanisms have yet to be determined. We also have to consider the different methodologies applied because they were highly heterogeneous. Most of the studies used self-report instruments such as the PSQI to access sleep duration. Furthermore, the cutoff for short and long sleep duration was not standardized (≤ 6 hours versus ≤ 7 hours; > 7 hours versus ≥ 9 hours).
In the literature, the adverse effects of short sleep duration are already well described. The decrease in sleep duration has been consistently associated with reduced immune function,35 increased BMI,36 and cardiometabolic diseases.37 Nevertheless, the association between long sleep duration and mortality is also frequently reported. Compared to intermediary sleep duration (7 to 8 hours), those who slept 10 hours or more have shown to present 50% to 80% higher relative mortality risk.38 However, it is unclear whether the association of long sleep duration and mortality is causal or simply reflects the effect of confounding factors influencing sleep habits. Unlike short sleep, there are no convincing hypotheses about the relying mechanisms for the association between prolonged sleep and pathological conditions. In a review article, some mechanisms associating long sleep duration and illness or mortality were proposed, such as: increased sleep fragmentation, poor sleep perception, altered cytokine levels, and decreased exposure to mild stressors leading to beneficial physiological challenges.39 However, there are few experimental data from which inferences can be done.
Garland and colleagues evaluated for the first time the relationship between telomere length and insomnia in postmenopausal women who were breast cancer survivors.13 The authors found that LTL of women with more severe insomnia symptoms did not differ significantly from the LTL of age- and BMI-matched comparison group. The severity of insomnia was not associated with LTL in that study.13 Carroll and colleagues also investigated the association between insomnia and telomere length in older adults.12 The authors observed shorter LTL in those with insomnia aged 70 to 80 years compared to controls without insomnia in the same age group. However, in the group aged 60 to 69 years, this difference was no longer observed.12 In the current study, we found that insomnia defined by the DSM-IV was independently associated with short telo-mere compared to the good sleeper group. Although partially in agreement with the current literature, we have to consider the differences among the studies such as the studied samples (women who were breast cancer survivors versus elderly versus an adult population); the techniques used to measure telomeres (restriction fragment length polymorphism versus PCR); the use of telomere variable (continuous versus categorical); and the diagnostic criteria used for insomnia (International Classification of Sleep Disorders, Second Edition versus DSM-IV versus Insomnia Severity Index).
Current evidence suggests that insomnia may lead to an unbalance in inflammatory cascade and oxidative stress pathways, increasing the levels of inflammatory cytokines and pro-oxidant substances.40 Of note, insomnia associated with objective short sleep duration is considered to be one of the worst phenotypes of insomnia, which can be connected to cardiometabolic and neuropsychiatric morbidity and mortality.41 Also, this phenotype has been postulated to activate stress pathways, such as the hypothalamic-pituitary-adrenal axis and the sympatho-adrenal-medullary axis.42–44 Given the solid relationship between hypercortisolemia and morbidity, higher prevalence of negative outcomes is observed in patients with insomnia and short sleep duration compared to those with normal sleep duration.41 Thus, we have hypothesized that insomnia could negatively affect LTL mainly when associated with PSG short sleep duration. The accelerated decline in LTL is an important predictor of a greater risk of disease progression and mortality.2 Thus, cellular aging could be the mechanism through which insomnia would increase the risk for aging-related diseases.45 In fact, in this study, when PSG sleep duration was combined with insomnia diagnosis, we found an independent association between insomnia short sleepers (DSM-IV insomnia TST < 360 minutes) compared to the control group (good sleeper TST ≥ 360 minutes), corroborating the paradigm of insomnia, with short sleep duration being the most severe insomnia phenotype.
Despite the fact that we observed higher OSA frequency, sleep fragmentation, and sleep respiratory events as well as worse desaturation parameters in the short telomere group, the results did not remain significant after adjustment for age, sex, and BMI. It is postulated that OSA, through sleep fragmentation and hypoxia, is associated with decreased LTL length9–11 compared to non-OSA. However, recently longer telomeres have also been associated with OSA.14 This result may be partially explained by the fact that the telomeres can also be positively activated by environmental factors such as inflammation and oxidative stress. Thus, these factors seem to potentially be related to both shortening and elongation of telomere length. It is well described that aging is accompanied by several sleep disturbances, such as sleep fragmentation, early awakenings, sleep curtailment, and increased prevalence of OSA.46 Taking into consideration that age is also an important modulator of telomere length in the association with sleep,47 we believe that the univariate unadjusted association between OSA and short telomere in our study was mediated by older age in this group compared to the non-short telomere group. Although we have previously reported an association between OSA and reduced telomere length compared to controls,11 we must consider some aspects for the interpretation of the data. The aim of our previous study was to compare the telomere length between individuals with OSA and those in a control group using the T/S ratio as a continuous variable. In a different way, the current work aimed to determine the independent sleep predictors of short telomere based on the distribution of its length (T/S ratio) in the total sample of EPISONO.
It is important to emphasize that the interpretation of the factors related to the LTL should be made carefully because the relationship found was not causal due to the cross-sectional design of the study. It is known that LTL decreases with aging and that age-related diseases and the increase of inflammatory cytokines and pro-oxidants substances may play a role in the acceleration of this process, although we have controlled the analysis by age.35 Possibly, the different characteristics of insomnia, such as exposure time, severity, and type of complaint (difficulty in initiating or maintaining sleep and early awakening) may cause different effects on LTL. In this sense, prospective studies are needed to unveil the possible association between sleep and the molecular pathways of aging, especially those related to the maintenance of telomere length.
This study has some limitations. Because the cross-sectional design of EPISONO does not allow causality, we can only infer associations between sleep parameters and short telomere length. Furthermore, there was no adaptation night for polysomnography, which can have an effect on the frequency of individuals classified as short sleepers, because the variability in TST derived from PSG is generally observed within different PSG examinations.48
Our findings demonstrate that self-reported long sleep duration and the presence of insomnia doubled the odds ratio of short telomere length, whereas the short sleep duration insomnia phenotype led to a fourfold increase in the odds ratio of short telomere length. These results suggest that longer sleepers and insomnia, especially the short sleep phenotype may play a role in the mechanisms related to biological aging.
DISCLOSURE STATEMENT
This work was performed at Universidade Federal de São Paulo. This work was supported by grants from the Associação Fundo de Incentivo à Pesquisa (AFIP), National Council for Scientific and Technological Development (CNPq) and São Paulo Research Foundation (FAPESP, #2014/15259-2 to CH and #2015/17549-0 to PFT). LB and ST are recipients of CNPq fellowship. The final version of the letter has been read and approved by all of the listed authors. There are no financial or other relationships that might lead to a conflict of interest.
ACKNOWLEDGMENTS
The authors are very grateful to Sueli Sugama, Laura Sousa, Rafael Almeida Nunes, and Diva Lima for the technical assistance in this study.
ABBREVIATIONS
- ASSIST
Alcohol, Smoking and Substance Involvement Screening Test
- BMI
body mass index
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EPISONO
São Paulo Epidemiologic Sleep Study
- LTL
leukocyte telomere length
- OSA
obstructive sleep apnea
- PCR
polymerase chain reaction
- PSQI
Pittsburgh Sleep Quality Index
- SpO2
percutaneous oxygen saturation
- TST
total sleep time
REFERENCES
- 1.Rubtsova MP, Vasilkova DP, Malyavko AN, et al. Telomere lengthening and other functions of telomerase. Acta Naturae. 2012;4(2):44–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 3.Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66(2):202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 4.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10(5):274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 5.Starkweather AR, Alhaeeri AA, Montpetit A, et al. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014;63(1):36–50. doi: 10.1097/NNR.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27(1):27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Prather AA, Puterman E, Lin J, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackowska M, Hamer M, Carvalho LA, Erusalimsky JD, Butcher L, Steptoe A. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study. PLoS One. 2012;7(10):e47292. doi: 10.1371/journal.pone.0047292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barceló A, Piérola J, López-Escribano H, et al. Telomere shortening in sleep apnea syndrome. Respir Med. 2010;104(8):1225–1229. doi: 10.1016/j.rmed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, Kwak JW, Lim SJ, Park YK, Yang HS, Kim HJ. Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Dis. 2016;7(5):604–613. doi: 10.14336/AD.2016.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempaku PF, Mazzotti DR, Hirotsu C, et al. The effect of the severity of obstructive sleep apnea syndrome on telomere length. Oncotarget. 2016;7(43):69216–69224. doi: 10.18632/oncotarget.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll JE, Esquivel S, Goldberg A, et al. Insomnia and telomere length in older adults. Sleep. 2016;39(3):559–564. doi: 10.5665/sleep.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland SN, Palmer C, Donelson M, Gehrman P, Johnson FB, Mao JJ. A nested case-controlled comparison of telomere length and psychological functioning in breast cancer survivors with and without insomnia symptoms. Rejuvenation Res. 2014;17(5):453–457. doi: 10.1089/rej.2014.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polonis K, Somers VK, Becari C, et al. Moderate-to-severe obstructive sleep apnea is associated with telomere lengthening. Am J Physiol Heart Circ Physiol. 2017;313(5):H1022–H1030. doi: 10.1152/ajpheart.00197.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tempaku PF, Mazzotti DR, Tufik S. Telomere length as a marker of sleep loss and sleep disturbances: a potential link between sleep and cellular senescence. Sleep Med. 2015;16(5):559–563. doi: 10.1016/j.sleep.2015.02.519. [DOI] [PubMed] [Google Scholar]
- 16.Santos-Silva R, Tufik S, Conway SG, Taddei JA, Bittencourt LR. Sao Paulo Epidemiologic Sleep Study: rationale, design, sampling, and procedures. Sleep Med. 2009;10(6):679–685. doi: 10.1016/j.sleep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Associação Brasileira de Empresas de Pesquisa. Critério de Classificação Econômica Brasil. São Paulo, Brazil: Associação Brasileira de Empresas de Pesquisa; 2003. [Google Scholar]
- 20.WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- 22.Pires ML, Benedito-Silva AA, Mello MT, Pompeia Sdel G, Tufik S. Sleep habits and complaints of adults in the city of São Paulo, Brazil, in 1987 and 1995. Braz J Med Biol Res. 2007;40(11):1505–1515. [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Menna Barreto SS, Johns MW. Portuguese-language version of the Epworth sleepiness scale: validation for use in Brazil. J Bras Pneumol. 2009;35(9):877–883. doi: 10.1590/s1806-37132009000900009. [DOI] [PubMed] [Google Scholar]
- 25.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 27.Bittencourt LR, Silva RS, Conway SG. Laboratório do Sono-Estrutura Física e Pessoal, Técnica Polissonográfica, Questionário de Sono e Banco de Dados. São Paulo, Brazil: Associação Fundo de Incentivo a Psicofarmacologia;; 2005. [Google Scholar]
- 28.American Diabetes Association. Diagnosis and classification of Diabetes Mellitus. Diabetes Care. 2010;33(Suppl1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 31.Hara K, Matsushita Y, Horikoshi M, et al. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2006;29(5):1123–1124. doi: 10.2337/diacare.2951123. [DOI] [PubMed] [Google Scholar]
- 32.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 33.The Report of an American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Wright CE, Erblich J, Valdimarsdottir HB, Bovbjerg DH. Poor sleep the night before an experimental stressor predicts reduced NK cell mobilization and slowed recovery in healthy women. Brain Behav Immun. 2007;21(3):358–363. doi: 10.1016/j.bbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Bjorvatn B, Sagen IM, Øyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16(1):66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 37.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev. 2007;11(5):337–339. doi: 10.1016/j.smrv.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31(1):15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Mendoza J, He F, Vgontzas AN, Liao D, Bixler EO. Objective short sleep duration modifies the relationship between hypertension and all-cause mortality. J Hypertens. 2017;35(4):830–836. doi: 10.1097/HJH.0000000000001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll JE, Cole SW, Seeman TE, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016;51:223–229. doi: 10.1016/j.bbi.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 47.Cribbet MR, Carlisle M, Cawthon RM, et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep. 2014;37(1):65–70. doi: 10.5665/sleep.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, Sowers M, Buysse DJ, et al. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8(1):87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]