Abstract
Intra-articular injection of hyaluronic acid (HA) as a viscosupplement is used to treat osteoarthritis (OA), yet HA is cleaved by hyaluronidase and clears out of the joint after several days, resulting in only short-term benefit. Therefore, we developed a new polymer biolubricant based on poly-oxanorbornane carboxylate to enhance joint lubrication for prolonged time. Rheological and biotribological studies of the biolubricant reveal viscoelastic properties and coefficient of friction equivalent and superior to that of healthy synovial fluid, respectively. Furthermore, in an ex vivo bovine cartilage plug model, the biolubricant exhibits superior long-term reduction of friction and wear prevention compared to saline and healthy synovial fluid. ISO 10993 biocompatibility tests demonstrate that the biolubricant polymer is non-toxic. In an in vivo rat medial meniscal tear OA model, where the performance of the leading HA viscosupplement (Synvisc-one®) is comparable to the saline control, treatment with the biolubricant affords significant chondroprotection compared to the saline control.
Keywords: Biotribology, lubricants, poly-oxanorbornane, biomechanics, chondroprotection, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a painful, chronic disease of synovial joint structure and function[1] that afflicts more than 200M individuals worldwide and has doubled in prevalence over the last half-century.[2] OA affects synovial joints by progressively breaking down hyaline cartilage, the hydrated tissue that provides a smooth, nearly frictionless surface which distributes loads applied to articulating joint surfaces.[3-5] Most of the pathologic changes observed in OA patients result from mechanical injury to the hyaline cartilage as a consequence of high body mass, joint instability, skeletal malalignment, direct trauma, and/or persistent joint incongruity. Individuals who suffer traumatic joint injuries (e.g., ligament or mensical injury[6, 7]) are at especially high risk for developing post-traumatic OA.[8-10] Clinically, OA is characterized by pain, joint deformity, and limitation of motion. Anatomically, OA is characterized by loss of cartilage volume, focal erosions, subchondral bone sclerosis, cyst formation, and marginal osteophytes.[11] Initially, patients suffering from OA are treated by a variety of non-surgical interventions[12-15] including activity modification, weight loss, brace support, exercise, and physical therapy in conjunction with anti-inflammatory drugs and/or intra-articular corticosteroid injections. Although anti-inflammatory drugs are analgesic, they do not ameliorate OA progression, and their extended use has been associated with serious side effects.[16] Surgical treatment to stabilize or re-align articular joint surfaces have moderate success in early-stage OA, but when these methods fail, the last option is total joint arthroplasty.
Healthy synovial fluid (SF) is a dialysate of blood plasma containing hyaluronic acid (HA), lubricin, and surface-active phospholipids, all of which contribute to joint lubrication.[17],[18] An important pathophysiologic change associated with OA is a decrease in the lubricity of SF owing at least in part to a decrease in both the concentration and molecular weight of HA, which adversely affect its rheological properties.[17, 19-21] Common wear patterns on osteoarthritic cartilage surfaces suggest inadequate lubrication occurs.[22] Predicated on the theory that supplementing the deficient HA will improve the rheological properties of osteoarthritic SF, viscosupplementation using gel-like HA derivatives injected intra-articularly into affected joints has been developed and commercialized.[23] However, a growing number of randomized controlled studies have challenged the efficacy of this procedure.[13, 24-27] A meta-analysis of clinical studies showed that HA viscosupplements were only 8% more effective than saline control in relieving pain.[13] [28–31] While several preclinical technologies have demonstrated protection of cartilage from wear,[32, 33] to date, no approved device or pharmacologic intervention imparts chondroprotection or reduces cartilage wear in traumatized or osteoarthritic joints. We hypothesize that supplementation of SF with a biolubricant consisting of a synthetic lubricious polymer dissolved in water designed to resist enzymatic degradation by hyaluronidase will be chondroprotective by reducing the coefficient of friction (COF) between articulating cartilage surfaces and thereby preventing wear. This is one approach to lower the COF, and other strategies investigated include the use of lubricin,[34-36] lubricin-mimetics,[32, 33, 37-39] liposomes,[40, 41] and other high-molecular-weight lubricious polymers.[42-45] These and other efforts to improve the biotribological properties of articular cartilage have been reviewed recently.[46, 47] The design criteria and rationale for the biolubricant described herein include: lack of glycosidic linkages to prevent degradation; a molecular weight greater than 1 MDa to increase joint resident time; hydrophilic repeat units within the polymer for water solubility inspired by hyaluronic acid; an overall polymer negative charge to maintain the polymer at the cartilage surface; shear thinning properties to allow for delivery using a small gauge needle; low COF to reduce wear, and preparation of the polymer via chemical synthesis giving a well defined polymer. Specifically, we synthesized poly(7-oxanorbornene-2-carboxylate) and formulated biolubricant solutions at two different concentrations, and here we report their rheological and biotribological properties, biocompatibility, and chondroprotective capabilities. The biolubricant’s in vivo efficacy to ameliorate OA is demonstrated using a rat meniscal tear OA model.
RESULTS
Synthesis
The polymer (poly(7-oxanorbornene-2-carboxylate); see Figure 1, right inset) was synthesized via ring opening metathesis polymerization (ROMP) to afford a polyanion with a Mn of 2,400,000 g/mol (PDI − 1.2) as measured via size exclusion chromatography (SEC).[48, 49] After freeze-drying, a white fibrous polymer was obtained that was subsequently dissolved in deionized water (water soluble from pH=6 to 12) at either 0.5 or 2 w/v% (wt%), pH adjusted to 7.4 with sodium hydroxide, and sterilized via filtration through a 0.2 micron filter to form the biolubricants used for the following studies.
Figure 1.
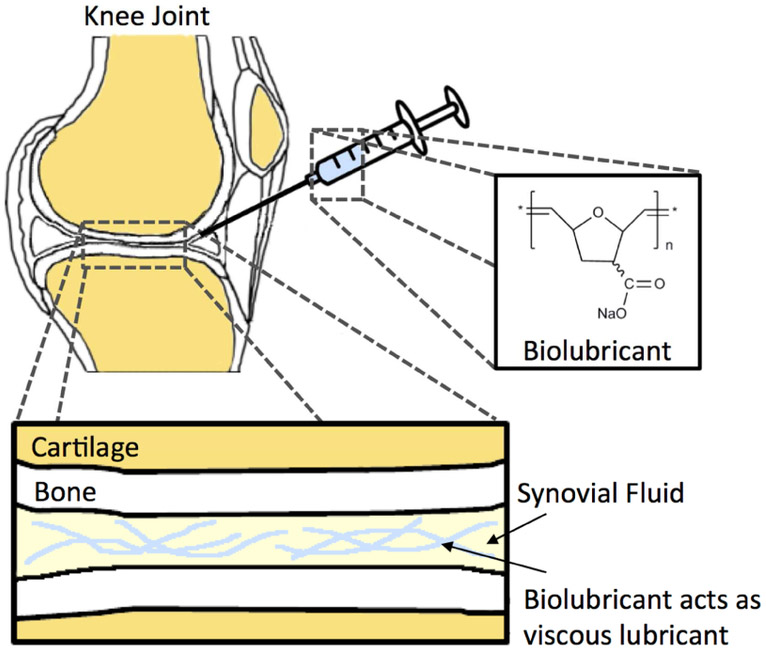
Schematic of intra-articular injection of (poly(7-oxanorbornene-2-carboxylate) of 2.4 MDa molecular weight. The polymer is dissolved in deionized water at 0.5 or 2 wt% to afford a viscous, chondroprotective biolubricant with viscoelastic properties similar to those of synovial fluid.
Cytotoxicity and inflammation
The polymer at 0.5 and 2 wt% was not cytotoxic to human chondrocytes, as the results were comparable to the untreated control (see Figure SI-1). Similar results were observed for 0.5 and 1 wt% using NIH3T3 mouse fibroblasts (see Figure SI-2). Upon exposure to macrophages for 5 or 24h, no significant inflammatory response, as measured by NO production, was observed for the polymer at 2 wt% (see Figure SI-3). NO production was similar to untreated controls and Synvisc®, and significantly different from the positive control response observed after treatment with lipopolysaccharide.
Biocompatibility
The results of the ISO 10993 biocompatibility tests, conducted at Toxikon, are summarized in Table SI-1. The biolubricant did not evoke an adverse response in the sensitization, irritation, genotoxicity, two week muscle implantation, agar diffusion, or fourteen-day subacute / subchronic toxicity tests.
Hyaluronidase degradation
The biolubricant, HA, and Synvisc® were exposed to hyaluronidase. After 24 hours, the samples were analyzed by gel electrophoresis. Both Synvisc® and HA were completely degraded by hyaluronidase, whereas the polyoxanorbornene biolubricant was not degraded by the enzyme, owing to its lack of glycosidic linkages (see SI). This result was confirmed in an independent experiment using a separate technique via GPC of the biolubricant before and after treatment with hyaluronidase (see SI). As the biolubricant lacks amide linkages it is unlikely that it will be degraded by proteases, such as matrix metalloproteinases, but this will need to be determined.
Mechanical properties of biolubricant
Healthy synovial fluid (SF) is a non-Newtonian fluid that serves both as a lubricant and shock absorber.[50, 51] At high shear loading rates such as jumping, hyaluronic acid (HA) exhibits increased elastic stiffness and reduced viscosity; at low shear loading rates such as walking, the opposite occurs. Using these properties as a reference, we evaluated the mechanical properties of the biolubricant at various concentrations.
Viscoelasticity of the biolubricant
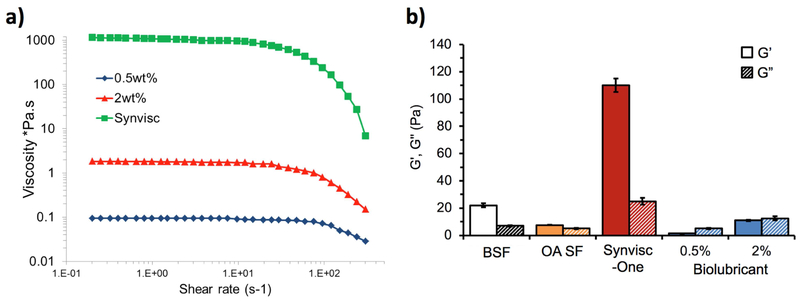
The viscoelastic properties of SF are dependent upon the size, concentration, conformation, and interactions of HA molecules within the fluid.[52, 53] The viscosity of the biolubricant at 2 wt% was similar to healthy SF[17] (ca. 1 Pa·s) with decreasing viscosity at higher shear rates (>102 s−1) (Figure 2a). At 0.5 wt%, the biolubricant exhibited viscosity an order of magnitude lower (0.1 Pa·s) due to less polymer entanglement. For reference, the viscosity of normal bovine synovial fluid (BSF) is approximately 0.05 Pa·s over the shear rate range evaluated.
Figure 2.
a) Shear thinning effects of the biolubricant at 0.5 and 2 wt% compared to Synvisc-one®. b) Storage and loss moduli at frequency of 2.5 Hz for normal bovine synovial fluid (BSF), and OA human synovial fluid, biolubricant at 0.5 and 2 wt%, and Synvisc-one® (n=3 each).
The unique rheological properties of SF are its storage modulus (G’) and loss modulus (G”), which are compared for varying concentrations of the biolubricant, Synvisc-one®, normal bovine synovial fluid (BSF, representing healthy SF) and human OA SF (Figure 2b). OA SF has lower storage and loss moduli than normal SF. Synvisc-one®, by design, has a much higher storage modulus than normal SF (110 vs. 23 Pa), thereby imparting increased elasticity compared to OA SF. The storage modulus of the 2 wt% biolubricant was comparable (12 Pa) to normal SF (23 Pa), but the loss modulus was slightly higher (16 vs 7 Pa).[48] The 0.5 wt% biolubricant exhibited relatively lower storage and loss moduli.
Biolubricant lubrication characteristics
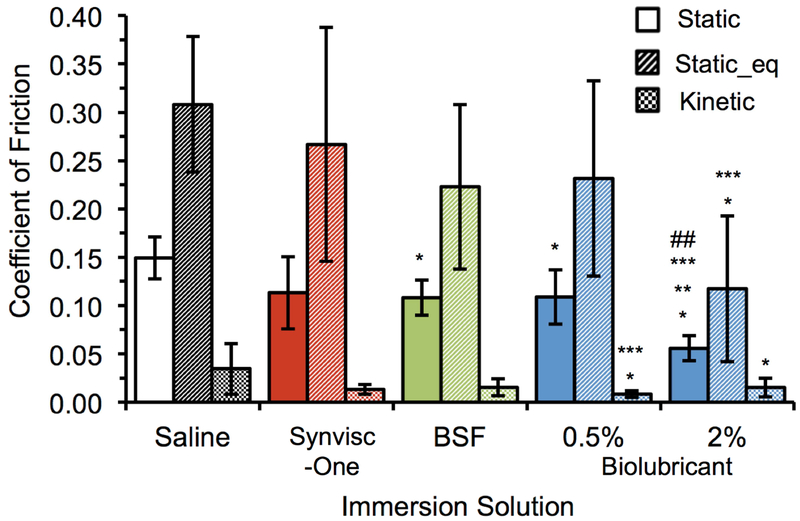
The coefficients of friction (μstatic, μstatic_eq, and μkinetic) of the biolubricant were measured using a cartilage-on-cartilage rotational friction test comprised of torsionally articulating apposed bovine osteochondral plugs excised from the patella and femoral groove surfaces. There was no statistical difference in μstatic_eq between saline and Synvisc-one®, while BSF and 0.5 and 2 wt% biolubricant solutions demonstrated statistical differences compared to Saline (Figure 3). The biolubricant (2 wt%) demonstrated significantly (p<0.05) lower μstatic_eq (0.117) compared to Saline (0.308) and Synvisc-one® (0.267). The 2 wt% biolubricant solution exhibited significantly lower μstatic (0.056) compared to all other solutions (p<0.05): saline (0.149), Synvisc-one® (0.113), BSF (0.108) and 0.5 wt% biolubricant solution (0.108). Finally, the 0.5 wt% biolubricant solution exhibited significantly (p<0.05) lower μkinetic (0.015) compared to saline (0.035).
Figure 3.
Coefficients of friction for 0.5 wt% and 2 wt% biolubricant, Synvisc-one®, Saline, and normal bovine synovial fluid, BSF, (n=6 each) obtained by torsional cartilage-on-cartilage friction testing. *= vs. Saline p<0.05, **= vs. BSF p<0.05, ***= vs. Synvisc® p<0.05, ## = vs. 0.5 wt% biolubricant p<0.05.
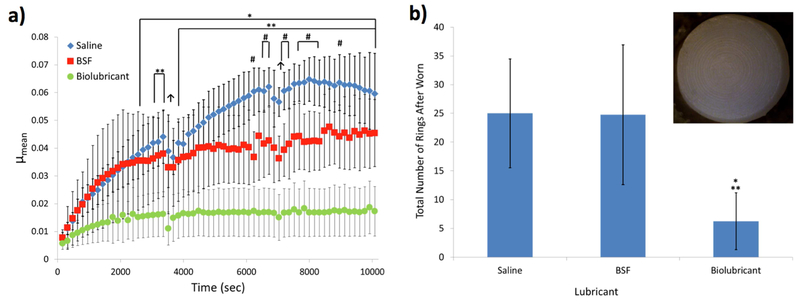
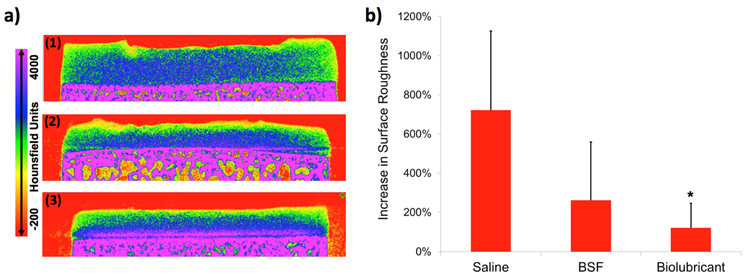
The ability of the 2 wt% biolubricant to reduce wear was evaluated using a long-duration, torsional wear test. Long-duration testing of bovine osteochondral plug pairs lubricated with the biolubricant exhibited the lowest μmean values compared to the other test solutions, being significantly lower (p<0.05) for all time points after 2560 s compared to plugs lubricated with saline, which had the greatest μmean results (Figure 4a). For all time points after 3840 s, samples lubricated with the biolubricant had significantly lower (p<0.05) μmean values compared to plug pairs lubricated with BSF. Additionally, the articulating cartilage surfaces of plug pairs lubricated with the biolubricant had 75.0% and 74.7% fewer circular wear grooves (“rings,” see Figure 4b inset) compared to samples lubricated with saline or BSF (p<0.05, Figure 4b), respectively. Following wear testing, the articular surfaces of samples lubricated with the biolubricant had noticeably less roughness than those lubricated with saline or BSF (Figure 5a). Comparing the surface roughness for the femoral groove articular specimens before and after wear testing, the percent change in surface roughness was statistically significantly lower for samples tested in the biolubricant compared to samples tested in saline (Figure 5b).
Figure 4.
a) Coefficients of friction (μmean) for bovine cartilage plug pairs (patella against femoral groove) tested in three lubricants (saline, BSF, and 2 wt% biolubricant) during the long-duration torsional friction regimen (>10,000 rotations). * = Biolubricant vs. Saline p<0.05, **= Biolubricant vs. BSF, #= BSF vs. Saline, ↑ indicates pause for lubricant extraction (plugs held apart for about 30-60 sec). b) Total number of rings on cartilage plug surfaces from each pair tested (example photo from saline test shown in inset) following long-duration torsional friction testing in three lubricants. * = vs. Saline p<0.05, ** = vs. BSF p<0.05. n>3.
Figure 5.
a) Representative CECT color maps of femoral groove plugs subjected to long-duration torsional friction testing using saline, BSF, and 2 wt% biolubricant. b) Percent increase in cartilage surface roughness following long-duration torsional friction testing in the same three lubricants. *= vs. Saline p<0.05.
Intraarticular residence time study
Prior to performing the efficacy study, the intra-articular residence time of the 2 wt biolubricant was determined in a healthy rabbit joint. Approximately 75% of the biolubricant remained in the joint at 11 days after intra-articular injection as determined by agarose gel electrophoresis and visualization by Stains-All (see SI).
Rat meniscal tear OA model
The efficacy of the biolubricant to impart chondroprotection was evaluated using the rat knee meniscal tear OA model, which induces cartilage wear within several weeks of destabilization.[54] It is assumed that non-physiologic joint loading contributes to mechanical damage of the articular cartilage in proportion to the deviation of the applied loads and shear from baseline. This model has been previously used to successfully demonstrate efficacy of novel OA treatments.[55, 56] The right hindlimb’s medial collateral ligament and the medial meniscus were transected to destabilize the knee joint, leading to mechanical wear of the medial condyle against the tibial plateau. One week after the induction of mechanical instability, the SF of the operated knee was augmented by intra-articular injection of either 0.5 wt% or 2 wt% of the biolubricant or saline (n=20 per group) using a 26G needle. Both knee joints of the animals were histologically examined three weeks after the injection, and a number of pathological criteria relevant to OA progression were histologically evaluated – total joint score, substantial cartilage degradation width, and osteophyte score (Figure 6). The percent change in histological scores for joints treated with the biolubricant were standardized relative to the saline controls. Significant chondroprotection was observed in the destabilized knee treated with the biolubricant solutions compared to saline. Representative changes induced in the medial femoral-tibial joint space compared to a non-operated knee are shown in Figure SI-4a and -4b, respectively.
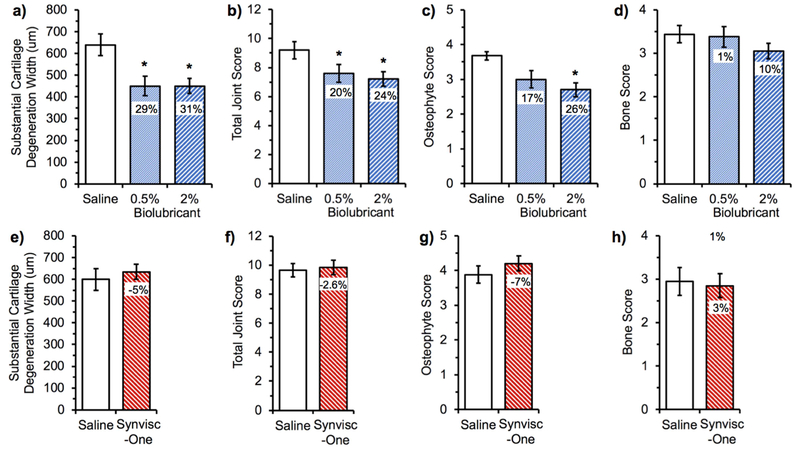
Figure 6.
Summary of histological evaluation of rat knee joints exposed to different treatments following medial meniscal tear. One week after inducing the tear, the knees were intra-articularly injected with either the biolubricant (0.5 wt% or 2 wt%), saline, or Synvisc-One® and were harvested 3 weeks later for histological analysis. Effect of the biolubricant vs. saline (a, b, c and d) at two different concentrations (0.5 and 2 wt/v%). Effect of Synvisc-One® vs. saline (e, f, g and h). The differences (in percent) were standardized against the saline control. *P<0.05 vs. saline control, n=20 per group. Baseline healthy values for an animal not receiving the medial meniscal tear nor treatment via intra-articular injection for each endpoint are as follows: Substantial cartilage degeneration width (a, e), 0 μ m; total joint score (b, f), 1; osteophyte score (c, g), 1; and bone score (d, h), 0.[34]
At the 2 wt% and 0.5 wt% doses, respectively, substantial cartilage degradation width was 31% and 29% better than saline control, and the total joint score was 24% and 20% better than the saline control (all p<0.05). Unbalanced catabolic-anabolic activities contribute to osteophyte formation in arthritic joints, and osteophyte formation was decreased by 26% for destabilized knees treated with the 2 wt% biolubricant.
In an independent animal study, Synvisc-one® (Sanofi/Genzyme) was evaluated for its chondroprotective efficacy compared to saline control (n=20 per group). Using the same histologic criteria to evaluate chondroprotective efficacy as the present study, there was no statistical difference between Synvisc-one® and the saline control (Figure 6e-h).
DISCUSSION
Most current therapies for OA focus on pain control and improved function, but to date, no non-surgical therapy has demonstrated chondroprotection. Viscosupplementation, by the intra-articular injection of HA (or its derivatives) to restore the lubricating and load dissipating characteristics of synovial fluid (SF), has only been shown to provide analgesic effect equivalent to NSAIDs. The clinical efficacy of HA viscosupplementation has been challenged[57] due to its poor lubricity and short in vivo half-life of a few days.[52, 53] Healthy SF is a non-Newtonian liquid that possesses nonlinear, shear-rate-dependent, elastic, and shear thinning effects. SF exhibits viscosities of 1-40 Pa·s, which decrease significantly at high shear rates (>102 s−1). We hypothesized that a SF supplement that imparted the rheological and tribological properties of healthy SF over an extended time period will reduce the symptoms associated with OA and protect the cartilage from continued degradation. We successfully synthesized a biolubricant that resists enzymatic degradation by hyaluronidase and that replicates the non-Newtonian fluid characteristics of SF, including thixotropy (shear-thinning behavior). These unique rheological properties allow the biolubricant to be injected through a 26-gauge needle to minimize patient discomfort compared to injection of highly viscous HA products, which require larger 18-20g needles for intra-articular administration. The biolubricant also demonstrates superior tribological and wear-preventive properties compared to healthy BSF. Using mated, apposing chondral surfaces, the biolubricant exhibits lower coefficients of friction than BSF in both short-term and long-term torsional friction tests. As a result of the reduced friction, the biolubricant prevents cartilage wear significantly better than BSF and saline (negative control), as evidenced by both the lesser number of wear rings and preservation of surface smoothness. These data provide the first direct, ex vivo evidence that the synthetic biolubricant prevents cartilage wear.
Definitive chondroprotection of the biolubricant is demonstrated in vivo using the destabilized rat knee meniscal tear model of OA. Compared to saline control, the 2 wt% biolubricant reduces substantial cartilage degradation width by 31%, decreases the total joint score by 24%, and decreases the osteophyte score by 26% via semi-quantitative histological analyses (Figure 6, all p<0.05). Synvisc-One®, the most commonly injected viscosupplement in the United States, performed equivalent to saline in this model. Using this same rat model, the chondroprotective efficacy of intra-articularly administered lubricin and an MMP-13 inhibitor has been studied.[55, 56] The biolubricant performance is comparable to the chondroprotective effect and superior to the total joint score for the lubricin study. Importantly, the one intra-articular injection of our biolubricant may be more convenient than the weekly administration of lubricin or daily administration of MMP-13 inhibitor required to impart chondroprotection.
The results from the in vitro, ex vivo, and in vivo experiments are encouraging, however there are several limitations with this study. First, we used healthy bovine cartilage and normal bovine synovial fluid for the ex vivo cartilage-on-cartilage plug study to evaluate COF and wear. Additional studies using human OA cartilage and healthy human synovial fluid are warranted. When assessing wear of the cartilage plugs, the difference in the findings between the ring counting and CECT surface roughness for the saline and BSF samples indicates that the ring counting technique does not fully capture the extent of surface wear. Nevertheless, gross surface assessment using ring counting is still a useful indicator of surface wear without the need for sophisticated equipment. With regards to OA in vivo models, we selected the well-established destabilized rat knee meniscal tear model of OA as it affords OA in several weeks as a result of trauma. It is not a spontaneous model that develops OA over time, but rather a trauma-induced model reflecting more post-traumatic OA. At this time, studies in a large OA animal model (e.g., horse or goat) are not justified. Third, if the biolubricant reduces friction too greatly, joint laxity may be an issue and this will need be to determined in future studies. Finally, the treatment involved a single intra-articular injection of the biolubricant and the performance outcome from repeated treatments of the biolubricant must be investigated as this mirrors the current treatment with hyaluronic acid viscosupplement products.
Clinical viscosupplementation is frequently reviewed and subjected to meta-analysis, resulting in both recommendations for and against its use.[28, 58-60] Despite the uncertainty over HA’s effectiveness, with some randomized controlled trials demonstrating significant benefit versus saline placebo while others report that HA is indistinguishable from saline placebo, it is generally understood that the clinical mechanism of HA viscosupplementation is not directly related to lubrication; several viscosupplement pivotal clinical trials report 26 weeks of pain relief yet the intraarticular residence time half-life of these products is only several days. Thus, a long-lasting biolubricant that resists enzymatic degradation and provides tribosupplementary protection of cartilage, such as that reported herein, is a potentially improved treatment option for those with OA. While it is encouraging that the biolubricant reduces cartilage wear in an in vivo animal model of OA, OA is a complex disease that affects the entire synovial joint. The mechanical wear of cartilage may initiate the cascade of biological processes contributing to joint destruction, and further study is required to demonstrate that intra-articular injection of this biolubricant will delay or ameliorate the clinical consequences of OA in humans. Nevertheless, the data herein document this synthetic biolubricant provides longer lasting and more effective lubrication than a current viscosupplement ex vivo. In an in vivo OA model, where the performance of the leading HA viscosupplement (Synvisc-one®) is comparable to the saline control, treatment with the biolubricant affords significant chondroprotection compared to the saline control. Given the clinical need for OA treatments that address the underlying pathology to preserve cartilage tissue and halt disease progression, therapeutic concepts such as this one are in high demand to restore patient function and delay the need for surgical intervention.
MATERIALS AND METHODS
Polymer synthesis
The poly(7-oxanorbornene-2-carboxylate) was synthesized using ring opening metathesis polymerization (ROMP).[49] The resulting polymer was dissolved in aqueous solution, adjusted to pH 7.4, and sterilized via filtration through a 0.2-micron filter to form the biolubricant (see SI).
Cytotoxicity and inflammation tests
The polymer was dissolved in media and then incubated separately with NIH3T3 mouse fibroblast and human chondrocyte cells for 24 hours at 37 °C. A standard colorimetric MTS assay was used to determine the reduction in the number of metabolically active cells as a measure of cytotoxicity (see SI).
The production of NO by macrophages was measured using the fluorescent DAF-FM diacetate reagent after exposure to the polymer as a measure of inflammatory response compared to lipopolysaccharide positive control (see SI).
Biocompatibility tests
A series of biocompatibility tests were conducted by the contract research organization Toxikon Corporation (Bedford, MA) according to standard ISO 10993 and FDA G95-1 guidelines (see SI).
Measurement of Viscoelastic Properties
A TA Instrument RA 1000 controlled-strain rheometer equipped with a peltier temperature controlled plate and a 40-mm diameter aluminum plate with a 2° angle was used to measure viscosity of the biolubricant under nitrogen gas. An oscillatory strain sweep (strain amplitude from 0.01 to 10%) at fixed frequency (1 Hz) was applied to the sample to determine the Pseudo-Linear Viscoelastic Region (LVR). Steady state flow (shear stress amplitude from 0.01 to 60 Pa) at 25 °C was applied to the samples to determine their dynamic viscosity.
Preparation of osteochondral plugs for ex vivo friction studies
Osteochondral plugs were excised from the femoral groove and patellar surfaces of bovine cadaver knees. Twenty-four femoral groove plug pairs (Group 1) were used for the short-term friction experiment, while fourteen mated, patellar-groove pairs (Group 2) were used for the long-term friction and wear experiment. All the cartilage surfaces were photographed (PL-B681CU, PixeLINK, Ottawa, ON) to ensure they were comparably smooth and consistent articular surfaces. The plugs from Group 2 were then immersed in a cationic, iodinated contrast agent (CA4+) at 12 milligrams of iodine per milliliter (mgl/mL) for 24 hrs. The samples from both groups were imaged on a μCT scanner (μCT40, Scanco Medical AG, Brüttisellen, Switzerland).[61-63] The μCT data were post-processed in Analyze™ (Analyze Direct, Overland Park, KS) using previously validated protocols (see SI). The thickness of the segmented cartilage was then measured at 5 points across the width of the tissue for all coronal slices. Following imaging, CA4+ was washed from the Group 2 samples in saline for 24 hrs.
Measurement of short-term friction properties
An Enduratec 3230 (BOSE, Eden Prairie, MN, USA) was used to measure the torsional COF of each plug pair from Group 1 (see SI).[64] During COF testing, the cartilage surfaces were completely immersed in test solution. Three established COFs representative of the performance of articular cartilage[64] (μstatic, μstatic_eq, and μkinetic) were calculated to evaluate the efficacy of the lubricants.
Measurement of long-term friction and wear properties
Each plug pair from Group 2 was subjected to the same short-term torsional friction test described above to ensure comparable baseline performance across groups. The plug pairs were then randomly immersed in the biolubricant (n=5), BSF (n=5), or saline (n=4) for >4 hrs prior to long-duration testing. For the long-duration torsional test, the plugs from the femoral groove were rotated against the plugs from the patella at 360°/s for 10,080 alternating rotations (Enduratec 3230, see SI). The plugs were allowed to recover for >16 hrs in saline, after which, each cartilage surface was photographed again and immersed in CA4+ at 12 mgI/mL for 24 hrs for contrast-enhanced CT (CECT) analysis using μCT.
After the long-duration torsional tests, circular wear grooves (rings) developed on some of the samples, indicating surface wear. Two blinded observers counted the number of rings on the cartilage surface photographs of the groove plugs at baseline (no rings present) and after wear testing. Additionally, five sequential coronal and five sequential sagittal CECT images pre- and post-long-duration testing were evaluated to measure the change in cartilage surface roughness for each plug[65] using Analyze (see SI).
Rat meniscal tear OA model
The study design and animals used were approved by the Institutional Animal Care and Use Committee at Boulder BioPATH (Boulder, CO). Male Lewis rats weighing 275 to 300 grams (n=20 per group) were randomized to treatment group based on body weight after ≥2 days acclimation. The medial collateral ligament was transected and a full thickness cut was made through the anterior horn of the medial meniscus to simulate a complete tear and induce mechanical instability to produce traumatic OA. One week post surgery, the biolubricant (0.5 wt% or 2 wt%), saline, or Synvisc-One® (evaluated in an independent study with its own saline control group) was injected intra-articularly through an insulin syringe (40μL; n=20). The animals were euthanized three weeks later for histological evaluation of the destabilized and contra-lateral knee joints (see SI).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Coulter Foundation, FlexBiomedical, Boston University, and the NIH (R01AR066621).
COMPETING FINANCIAL INTERESTS STATEMENT
M.W.G. and B.D.S. have grant funding from the NIH and Coulter Foundation for this project. A start-up company was formed based on this technology and M.W.G. and B.D.S. had an equity stake in the company.
Footnotes
DATA AVAILABILITY STATEMENT
The raw data required to reproduce these findings are available to download from. The processed data required to reproduce these findings are available to download from.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REREFENCES
- [1].Lohmander LS, What can we do about osteoarthritis?, Arthritis research 2(2) (2000) 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE, Knee osteoarthritis has doubled in prevalence since the mid-20th century, Proceedings of the National Academy of Sciences of the United States of America 114(35) (2017) 9332–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lohmander LS, Englund PM, Dahl LL, Roos EM, The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis, Am. J. Sports Med 35(10) (2007) 1756–1769. [DOI] [PubMed] [Google Scholar]
- [4].Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM, Lifetime risk of symptomatic knee osteoarthritis, Arthritis Care & Research 29 (2008) 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cooper C, Inskip H, Croft P, Campbell L, Smith G, Mclearn M, Coggon D, Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity, Am. J. Epidemiol 147 (1998) 516–522. [DOI] [PubMed] [Google Scholar]
- [6].Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL, Incidence of acute traumatic patellar dislocation among active-duty United States military service members, Am. J. Sports Med 38 (2010) 1997–2004. [DOI] [PubMed] [Google Scholar]
- [7].Owens BD, Dawson L, Burks R, Cameron KL, Incidence of shoulder dislocation in the United States military: Demographic considerations from a high-risk population, J. Orthop. Trauma 91 (2009) 791–796. [DOI] [PubMed] [Google Scholar]
- [8].Cross JD, Ficke JR, Hsu JR, Masini BD, Wenke JC, Battlefield orthopaedic injuries cause the majority of long-term disabilities, J. Am. Acad. Orthop. Surg 19(Suppl 1) (2011) S1–S7. [DOI] [PubMed] [Google Scholar]
- [9].Masini BD, Waterman SM, Wenke JC, Owens BD, Hsu JR, Ficke JR, Resource utilization and disability outcome assessment of combat causalities from operation Iraqi freedom and operation enduring freedom, J. Orthop. Trauma 23 (2009) 261–266. [DOI] [PubMed] [Google Scholar]
- [10].Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ, Incidence of physican diagnosed osteoarthritis among active duty united states military service members, Arthritis & Rheumatism 63 (2011) 2974–2982. [DOI] [PubMed] [Google Scholar]
- [11].Goldring MB, Goldring SR, Osteoarthritis, Journal of Cellular Physiology 213(3) (2007) 626–634. [DOI] [PubMed] [Google Scholar]
- [12].Rao P, Knaus EE, Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond, J. Pharm. Pharm. Sci 11(2) (2008) 81s–110s. [DOI] [PubMed] [Google Scholar]
- [13].Lo GH, LaValley M, McAlindon T, Felson DT, Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis, JAMA 290(23) (2003) 3115–21. [DOI] [PubMed] [Google Scholar]
- [14].Edmonds S, Therapeutic targets for osteoarthritis, Maturitas 63(3) (2009) 191–194. [DOI] [PubMed] [Google Scholar]
- [15].Goldring SR, Needs and opportunities in the assessment and treatment of osteoarthritis of the knee and hip: The view of the rheumatologist, Journal of Bone and Joint Surgery. American Volume 91(Suppl 1) (2009) 4–6. [DOI] [PubMed] [Google Scholar]
- [16].Chikanza IC, Fernandes L, Novel strategies for the treatment of osteoarthritis, Expert Opin. Invest. Drugs 9(7) (2000) 1499–1510. [DOI] [PubMed] [Google Scholar]
- [17].Fam H, Bryant JT, Kontopoulou M, Rheological properties of synovial fluids, Biorheology 44(2) (2007) 59–74. [PubMed] [Google Scholar]
- [18].Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL, Boundary lubrication of articular cartilage: role of synovial fluid constituents, Arthritis and Rheumatism 56(3) (2007) 882–891. [DOI] [PubMed] [Google Scholar]
- [19].Bhuanantanondh P, Grecov D, Kwok E, Rheological Study of Viscosupplements and Synovial Fluid in Patients with Osteoarthritis, Journal of Medical and Biological Engineering 32(1) (2012) 12–16. [Google Scholar]
- [20].Hills BA, Monds MK, Deficiency of lubricating surfactant lining the articular surfaces of replaced hips and knees, Br. J. Rheumatol. 37(2) (1998) 143–147. [DOI] [PubMed] [Google Scholar]
- [21].Fletcher E, Jacobs JH, Markham RL, Viscosity studies on hyaluronic acid of synovial fluid in rheumatoid arthritis and osteoarthritis, Clin. Sci 14 (1955) 653–660. [PubMed] [Google Scholar]
- [22].Meachim G, Light microscopy of Indian ink preparations of fibrillated cartilage, Annals of the Rheumatic Diseases 31(6) (1972) 457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Balazs EA, Denlinger JL, Viscosupplementation: a new concept in the treatment of osteoarthritis, Journal of Rheumatology Supplement 39 (1993) 3–9. [PubMed] [Google Scholar]
- [24].Felson DT, Developments in the clinical understanding of osteoarthritis, Arthritis Research & Therapy 11(1) (2009) 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rutjes AWS, JQni P, da Costa BR, Trelle S, NQesch E, Reichenbach S, Viscosupplementation for osteoarthritis of the knee: a systematic review and meta¬analysis, Annals of internal medicine 157 (2012) 180–191. [DOI] [PubMed] [Google Scholar]
- [26].Atchia I, Kane D, Reed MR, Isaacs JD, Birrell F, Efficacy of a single ultrasound-guided injection for the treatment of hip osteoarthritis Annals of the Rheumatic Diseases 70 (2011) 110–116. [DOI] [PubMed] [Google Scholar]
- [27].Conrozier T, Vignon E, Is there evidence to support the inclusion of viscosupplementation in the treatment paradigm for patients with hip osteoarthritis?, Clinical and Experimental Rheumatology 23 (2005) 711–716. [PubMed] [Google Scholar]
- [28].Rutjes AWS, Jueni P, da Costa BR, Trelle S, Nueesch E, Reichenbach S, Viscosupplementation for Osteoarthritis of the Knee A Systematic Review and Meta-analysis, Annals of Internal Medicine 157(3) (2012) 180–191. [DOI] [PubMed] [Google Scholar]
- [29].Jevsevar D, Donnelly P, Brown GA, Cummins DS, Viscosupplementation for Osteoarthritis of the Knee A Systematic Review of the Evidence, Journal of Bone and Joint Surgery-American Volume 97A(24) (2015) 2047–2060. [DOI] [PubMed] [Google Scholar]
- [30].Divine JG, Zazulak BT, Hewett TE, Viscosupplementation for knee osteoarthritis: a systematic review, Clin Orthop Relat Res 455 (2007) 113–22. [DOI] [PubMed] [Google Scholar]
- [31].Strand V, McIntyre LF, Beach WR, Miller LE, Block JE, Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials, J Pain Res 8 (2015) 217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Samaroo KJ, Tan M, Putnam D, Bonassar LJ, Binding and lubrication of biomimetic boundary lubricants on articular cartilage, Journal of Orthopaedic Research (2016) doi: 10.1002/jor.23370 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [33].Samaroo KJ, Tan M, Andresen Eguiluz RC, Gourdon D, Putnam D, Bonassar LJ, Tunable Lubricin-mimetics for Boundary Lubrication of Cartilage, Biotribology 9(Supplement C) (2017) 18–23. [Google Scholar]
- [34].Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, Morris EA, Glasson SS, Prevention of Cartilage Degeneration in a Rat Model of Osteoarthritis by Intraarticular Treatment With Recombinant Lubricin, Arthritis and Rheumatism 60(3) (2009) 840–847. [DOI] [PubMed] [Google Scholar]
- [35].Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, Teeple E, Waller KA, Elsaid KA, Prevention of Cartilage Degeneration and Restoration of Chondroprotection by Lubricin Tribosupplementation in the Rat Following Anterior Cruciate Ligament Transection, Arthritis and Rheumatism 62(8) (2010) 2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Waller KA, Chin KE, Jay GD, Zhang LX, Teeple E, McAllister S, Badger GJ, Schmidt TA, Fleming BC, Intra-articular Recombinant Human Proteoglycan 4 Mitigates Cartilage Damage After Destabilization of the Medial Meniscus in the Yucatan Minipig, American Journal of Sports Medicine 45(7) (2017) 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lawrence A, Xu X, Bible MD, Calve S, Neu CP, Panitch A, Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface, Biomaterials 73 (2015) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Banquy X, Burdynska J, Lee DW, Matyjaszewski K, Israelachvili J, Bioinspired Bottle-Brush Polymer Exhibits Low Friction and Amontons-like Behavior, Journal of the American Chemical Society 136(17) (2014) 6199–6202. [DOI] [PubMed] [Google Scholar]
- [39].Andresen Eguiluz RC, Cook SG, Tan M, Brown CN, Pacifici NJ, Samak MS, Bonassar LJ, Putnam D, Gourdon D, Synergistic Interactions of a Synthetic Lubricin- Mimetic with Fibronectin for Enhanced Wear Protection, Front Bioeng Biotechnol 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sivan S, Schroeder A, Verberne G, Merkher Y, Diminsky D, Priev A, Maroudas A, Halperin G, Nitzan D, Etsion I, Barenholz Y, Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints, Langmuir 26(2) (2010) 1107–1116. [DOI] [PubMed] [Google Scholar]
- [41].Seror J, Sorkin R, Klein J, Boundary lubrication by macromolecular layers and its relevance to synovial joints, Polymers for Advanced Technologies 25(5) (2014) 468–477. [Google Scholar]
- [42].Morgese G, Cavalli E, Rosenboom JG, Zenobi-Wong M, Benetti EM, Cyclic Polymer Grafts That Lubricate and Protect Damaged Cartilage, Angewandte Chemie- International Edition 57(6) (2018) 1621–1626. [DOI] [PubMed] [Google Scholar]
- [43].Morgese G, Ramakrishna SN, Simic R, Zenobi-Wong M, Benetti EM, Hairy and Slippery Polyoxazoline-Based Copolymers on Model and Cartilage Surfaces, Biomacromolecules 19(2) (2018) 680–690. [DOI] [PubMed] [Google Scholar]
- [44].Tnibar A, Schougaard H, Camitz L, Rasmussen J, Koene M, Jahn W, Markussen B, An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow¬up, Acta Veterinaria Scandinavica 57 (2015) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Christensen LH, Camitz L, Illigen KE, Hansen M, Sarvaa R, Conaghan PG, The effects of polyacrylamide hydrogel in normal and osteoarthritic animal joints, Osteoarthritis and Cartilage 24 (2016) S449–S450. [DOI] [PubMed] [Google Scholar]
- [46].McNary SM, Athanasiou KA, Reddi AH, Engineering Lubrication in Articular Cartilage, Tissue Engineering Part B-Reviews 18(2) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cooper BG, Bordeianu C, Nazarian A, Snyder BD, Grinstaff MW, Active agents, biomaterials, and technologies to improve biolubrication and strengthen soft tissues, (2018) DOI: 10.1016/j.biomaterials.2018.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wathier M, Lakin BA, Bansal PN, Stoddart SS, Snyder BD, Grinstaff MW, A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage, Journal of the American Chemical Society 135 (2013) 4930–4933. [DOI] [PubMed] [Google Scholar]
- [49].Wathier M, Stoddart SS, Sheehy MJ, Grinstaff MW, Acidic polysaccharide mimics via ring-opening metathesis polymerization, Journal of the American Chemical Society 132(45) (2010) 15887–9. [DOI] [PubMed] [Google Scholar]
- [50].Radin EL, Paul IL, Swann DA, Schottstaedt ES, Lubrication of synovial membrane, Annals of the Rheumatic Diseases 30(3) (1971) 322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Radin EL, Swann DA, Weisser PA, Separation of a hyaluronate-free lubricating fraction from synovial fluid, Nature 228(5269) (1970) 377–8. [DOI] [PubMed] [Google Scholar]
- [52].Laurent TC, Fraser JR, Hyaluronan SEB J. 6 (1992) 2397–2404. [PubMed] [Google Scholar]
- [53].Fraser JR, Laurent TC, Laurent UB, Hyaluronan: Its nature, distribution, functions and turnover, J. Intern. Med 242 (1997) 27–33. [DOI] [PubMed] [Google Scholar]
- [54].Bendele AM, Animal models of osteoarthritis, Journal of Musculoskeletal and Neuronal Interactions 1(4) (2001) 363–376. [PubMed] [Google Scholar]
- [55].Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, Morris EA, Glasson SS, Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin, Arthritis & Rheumatism 60(3) (2009) 840–7. [DOI] [PubMed] [Google Scholar]
- [56].Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models, Arthritis & Rheumatism 60(7) (2009) 2008–2018. [DOI] [PubMed] [Google Scholar]
- [57].Waddell DD, Viscosupplementation with hyaluronans for osteoarthritis of the knee: Clinical efficacy and economic implications, Drugs Aging 24(8) (2007) 629–642. [DOI] [PubMed] [Google Scholar]
- [58].Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G, Viscosupplementation for the treatment of osteoarthritis of the knee, Cochrane Database of Systematic Reviews (2) (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE, Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: A systematic review and meta-analysis, Seminars in Arthritis and Rheumatism 43(5) (2014) 593–599. [DOI] [PubMed] [Google Scholar]
- [60].Jevsevar DS, Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition, Journal of the American Academy of Orthopaedic Surgeons 21(9) (2013) 571–576. [DOI] [PubMed] [Google Scholar]
- [61].Lakin BA, Grasso DJ, Shah SS, Stewart RC, Bansal PN, Freedman JD, Grinstaff MW, Snyder BD, Cationic agent contrast-enhanced computed tomography imaging of cartilge correlates with the compressive modulus and coefficient of friction, Osteoarthritis and Cartilage 21(1) (2013) 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Joshi NS, Bansal PN, Stewart RC, Snyder BD, Grinstaff MW, Effect of contrast agent charge on visualization of articular cartilage using computed tomography: exploiting electrostatic interactions for improved sensitivity, Journal of the American Chemical Society 131(37) (2009) 13234–13235. [DOI] [PubMed] [Google Scholar]
- [63].Stewart RC, Bansal PN, Entezari V, Lusic H, Nazarian RM, Snyder BD, Grinstaff MW, Contrast-Enhanced CT with a High-Affinity Cationic Contrast Agent for Imaging ex Vivo Bovine, Intact ex Vivo Rabbit, and in Vivo Rabbit Cartilage, Radiology 266 (2013) 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schmidt TA, Sah RL, Effect of synovial fluid on boundary lubrication of articular cartilage, Osteoarthritis and Cartilage 15(1) (2007) 35–47. [DOI] [PubMed] [Google Scholar]
- [65].Tofte J, Elsaid K, Zhang L, Waller K, Fleming BC, Jay GD, Exercise in ACL- Transected Rats Increases Cartilage Roughness Measured by a Novel Digital Method, Orthopedic Research Society Annual Meeting, San Francisco, CA, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.