Abstract
Aim
Protein kinase Cα (PKCα) is a critical regulator of multiple cell signaling pathways including gene transcription, posttranslation modifications and activation/ inhibition of many signaling kinases. In regards to the control of blood pressure, PKCα causes increased vascular smooth muscle contractility, while reducing cardiac contractility. In addition, PKCα has been shown to modulate nephron ion transport. However, the role of PKCα in modulating mean arterial pressure (MAP) has not been investigated. In this study, we used a whole animal PKCα knock out (PKC KO) to test the hypothesis that global PKCα deficiency would reduce MAP, by a reduction in vascular contractility.
Methods
Radiotelemetry measurements of ambulatory blood pressure (day/night) were obtained for 18 h/day during both normal chow and high-salt (4%) diet feedings. PKCα mice had a reduced MAP, as compared with control, which was not normalized with high-salt diet (14 days). Metabolic cage studies were performed to determine urinary sodium excretion.
Results
PKC KO mice had a significantly lower diastolic, systolic and MAP as compared with control. No significant differences in urinary sodium excretion were observed between the PKC KO and control mice, whether fed normal chow or high-salt diet. Western blot analysis showed a compensatory increase in renal sodium chloride cotransporter expression. Both aorta and mesenteric vessels were removed for vascular reactivity studies. Aorta and mesenteric arteries from PKC KO mice had a reduced receptor-independent relaxation response, as compared with vessels from control. Vessels from PKC KO mice exhibited a decrease in maximal contraction, compared with controls.
Conclusion
Together, these data suggest that global deletion of PKCα results in reduced MAP due to decreased vascular contractility.
Keywords: blood pressure, hypotension, protein kinase Ca, vascular contractility, vascular reactivity
Introduction
The protein kinase C (PKC) family contains multiple isozymes, all of which play critical roles in signal transduction pathways. PKC isozymes are classified by how they are activated, either through increases in intracellular calcium (Ca2+) and/or lipid signaling molecules [1–4]. PKCα is a ‘conventional’ member of the PKC family. Previously, PKCα has been shown to be a vital part of the regulation of cardiac and vascular smooth muscle (VSM) [4] and has a role in the regulation of renal ion transporters. In the proximal convoluted tubule (PCT), PKC can inhibit the sodium (Na+)-hydrogen exchanger (NHE3), yet angiotensin II-activation of PKC increases bicarbonate and water reabsorption in the PCT [5,6]. In the PCT, PKC may also be an important mediator in fructose-stimulated NHE activity [7]. However, experiments using isolated, split-open cortical collecting ducts in PKCα-knock out (PKC KO) mice revealed increased epithelial Na+ channel (ENaC) activity [8]. These studies reveal a complicated role for PKC in the regulation of renal transporters.
PKCα has a crucial role in cardiomyocyte hypertrophic growth, via an extracellular signal-related kinase 1/2-mediated pathway [9]. In addition, PKCα has been demonstrated to play a critical role in the regulation of cardiac contractility by increasing protein phosphatase-1 and subsequent phospholambam dephosphorylation. This inhibits the sarco/endoplasmic reticulum Ca2+-ATPase-2 pump and reduces store-operated Ca2+ release [10]. This response reduces cardiac contractility. Accordingly, PKC KO mice were shown to have an increased cardiac performance and were resistant to developing heart failure in experimental animal models [11,12].
Significantly, PKCα has opposing effects in VSM, specifically in agonist-mediated contractility. PKCα phosphorylates PKC-potentiated phosphatase inhibitor protein-17 (CPI-17) and inhibits myosin light chain phosphatase, reducing myosin light chain dephosphorylation resulting in increased contraction [13]. Given the complicated, and seemingly opposing, roles of PKCα in cardiac and VSM regulation, the purpose of our study was to determine whether loss of PKCα would lead to a decrease in mean arterial pressure (MAP) through overall decrease in systemic vascular contractility, even with increased cardiac contractility.
Materials and Methods
Chemicals
Unless otherwise specified, all drugs and chemicals were purchased from Sigma Aldrich (St. Louis, Missouri, USA).
Animal studies
All experiments were performed under the guidance and approval of the Emory University Institutional Animal Care and Use Committee (IACUC protocol no. DAR-2002607-012417BN). Mice (male, 8–12 weeks) were kept in cages with autoclaved bedding and received free access to water and a standard diet [Diet 5001; Purina (St Louis Missouri, USA), 0.4% Na+], low salt (Teklad, <0.05% Na+)orhighsalt (4% Teklad). PKCα–/– (PKC KO) mice (SV129 background) were originally obtained from Dr Jeffery Molkentin (Cincinnati Children's Hospital Medical Center) and control mice (SV129; Jackson Laboratories, Bar Harbor, Maine, USA) were backcrossed with PKC KO mice (10 generations) to establish littermate controls (ctrl) for the PKC KO mice. All mice were then bred in-house at Emory University; animals were periodically genotyped to assure no genetic drift.
Radiotelemetry studies
All animal surgeries were performed in accordance with Emory University Protocol for Aseptic Technique. Mice were anesthetized using isofluorane and radiotelemetry devices (PAC-10; Data Sciences International, St. Paul, Minnesota, USA) were implanted, as described previously [14]. Animals were allowed to recover for 3–5 days; SBP/DBP, heart rate (HR) and activity were collected for 18 h per 24-h period. Data were analyzed using Power Lab (AD Instruments, Colorado Springs, Colorado, USA).
Metabolic cage studies
Mice were acclimatized to metabolic cages (Tecniplast, West Chester, Pennsylvania, USA) for 24 h, then 24-h urines were collected over the next 2 days. Urine was centrifuged (15000 rpm, 15 min) to separate any soluble substance before analysis. Urine osmolality was measured by a vapor pressure osmometer (Wescor, Logan, Utah, USA), and urea content was determined by colorimetric assay using the Infinity Urea Kit (Thermo Scientific, Waltham, Massachusetts, USA). Urinary Na+ was measured using a Na+-specific electrode (Cole Palmer, Vernon Hills, Illinois, USA).
Functional studies
After euthanasia, the thoracic aorta and mesentery were rapidly excised and bathed in ice-cold physiological salt solution (NaCl 120 mmol/l, KCl 4.7 mmol/l, KH2PO4 1.18 mmol/l, NaHCO3 14.9 mmol/l, dextrose 5.6, 2.5 mmol/l CaCl2 2H2O, 0.06 mmol/l EDTA). Both vascular beds were carefully cleaned of all associated perivascular adipose tissue and cut into segments (2 mm). First-order mesenteric resistance arteries and aortic segments were mounted on Danish myo technology wire and pin myographs (Danish MyoTech, Aarhus, Denmark), respectively. Mesenteric resistance arteries were normalized to their optimal lumen diameter for active tension development, as described previously [15]. Aortic segments were set to a passive force of 5 mN. Vessels were maintained at 37 °C and continuously aerated with 95% O2, 5% CO2 and allowed to stabilize for at least 45 min. After stabilization, tissues were contracted with KCl (120 mmol/l) solution. To determine the viability of the endothelium, contraction was stimulated via phenylephrine (Phe; 10 μmol/l) followed by relaxation induced by acetylcholine (ACh; 10 μmol/l). Vessels were then washed, as described previously, before performing concentration response curves (CRCs) and after each CRC. CRCs to Phe, ACh or sodium nitroprusside (SNP) were performed in the presence of vehicle or the following inhibitors: N-nitroarginine methyl ester (L-NAME, nitric oxide synthase inhibitor; 100 μmol/l) or indomethacin [Indo, cyclooxygenase (COX) inhibitor; 10 μmol/l]. Force measurements were collected using LabChart v7 Software (ADI Instruments, Colorado Springs, Colorado, USA) for PowerLab data acquisition systems (ADI Instruments).
Tissue homogenization
Following dissection, cortex was homogenized in 1 ml of isolation buffer (10 mmol/l triethanolamine, 250 mmol/l sucrose, 1 μg/ml leupeptin and 0.1mg/ml phenylmethyl-sulfonyl fluoride, pH 7.4). Concentrated SDS was added to 1%; samples sheared by passage through a 28-ga needle and centrifuged for 10 min at 10000 × g, and protein was determined (DC protein assay kit; Bio-Rad, Hercules, California, USA).
Immunoblotting
Total cortex homogenates were electrophoresed on 7.5% gels (Bio-Rad) and transferred electrophoretically to polyvinylidene fluoride membranes. After blocking with 3% BSA, the membranes were probed with corresponding primary antibodies; antisodium chloride cotransporter (NCC) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell signaling, 1:1000) overnight at 4°C. The NCC antibody (1: 4000–1: 8000) was developed and validated in the laboratory of Robert Hoover [16]. The blots were washed in TBST (tris-buffered saline, tween 0.5%) and secondary antibodies were horseradish peroxidase-linked (Amersham, 1:5000). Supersignal West Pico was used for chemiluminescence (Thermo Scientific). Chemiluminescence was detected with G:Box (Gelbox, Frederick, Maryland, USA) and analysis by Genetools software (Syngene, Frederick, Maryland, USA).
Statistical analysis
Agonist concentration–response curves were fitted using a nonlinear interactive fitting program (GraphPad Prism 5.0 or 6.0; Graph Pad Software Inc., San Diego, California, USA), and values expressed as percentage of maximal relaxation graphed against increasing molar concentrations of agonist. Agonist potencies and maximum response are expressed as negative logarithm of the molar concentration of agonist producing 50% of the maximum response (EC50) and maximum effect elicited by the agonist (Rmax), respectively. Nonlinear regression analysis was used to determine EC50 values, in which Rmax was normalized to 100% for calculations. Data are expressed as mean ± SEM (n), in which n is the number of experiments performed and correlates with one animal. Statistical analysis of the concentration–response curves was performed by using the F test for comparisons of best-fit data between groups (EC50 and Rmax), as previously described [14,15].
For statistical comparison between groups of animals and/or conditions (hypertensive vs. normotensive and drug incubations), all vessels were grouped together for statistical analysis and comparison. Blood pressure (BP) studies were analyzed by Student's t test.
Western blot data were analyzed using the average target protein expression normalized to GAPDH. Values were analyzed for statistical significance using Student's t test for comparison between the two groups.
Urine electrolyte values were normalized as a function of body weight (100 g); one-way analysis of variance was then performed to determine statistical significance.
All data are represented as ±SEM. Values of P less than 0.05 were considered a statistically significant difference.
Results
Protein kinase Cα-deficiency causes hypotension
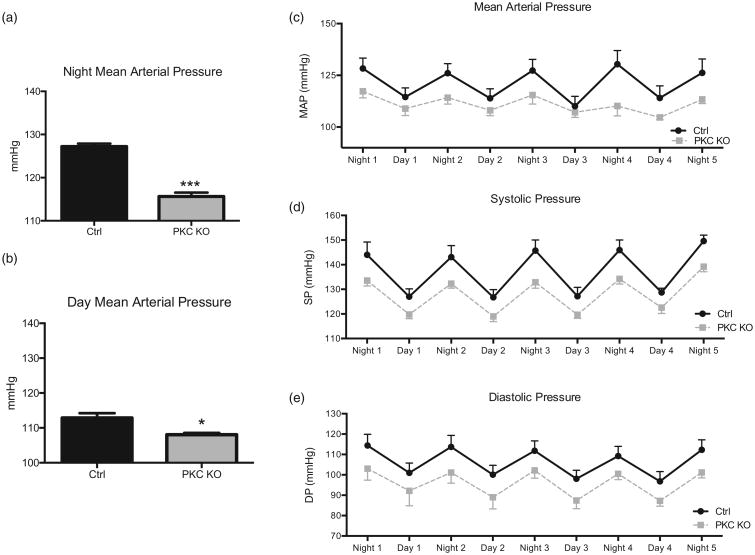
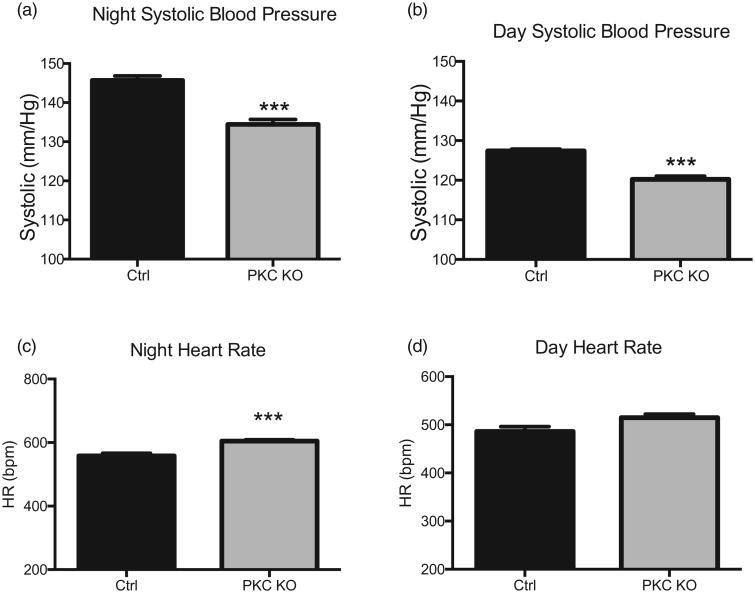
Here we report for the first time that PKC KO mice have a decreased MAP, as shown by radiotelemetry (Fig. 1a–c) during both active (night, 115.6 ± 1.5 mmHg PKC KO vs. 127.2±0.6 mmHg control, n = 4–12, ***P < 0.001) and sleep (day, 108.0 ±0.9 mmHg PKC KO vs. 112.8 ± 2.4 mmHg control, n = 4–12, *P < 0.05) periods. PKC KO mice had similarly reduced SBP, as well as DBP (Fig. 1d and e) during entire control period. Averaged data for SBP showed that PKC KO mice had a reduced active (night, 134.4 ± 1.2 mmHg PKC KO vs. 145.7 ± 1.2 mmHg control, n = 6–7, ***P < 0.001) and sleep (day, 120.2 ± 0.8 mmHg PKC KO vs. 127.4 ± 0.5 mmHg control, n = 4, ***P < 0.001) periods (Fig. 2a and b). As expected, PKC KO mice had an increased HR, during the active (night, 604.9 ± 3.6 bpm PKC KO vs. 558.1 ± 8.0 bpm control, n = 5, ***P < 0.001) period (Fig. 2c and d).
Figure 1.
Protein kinase Cα knock out mice have decreased mean arterial pressure. Radiotelemetry recordings were taken from protein kinase C knock out and control mice, following a recovery period for both night (a) and day (b) periods. Averaged blood pressure responses were compared for both, showing decreased mean arterial pressure (c). Daily SBP (d) and DBP (e) data are shown. Data shown as mean ± SEM, n = 4–12, *P < 0.05, ***P < 0.001.
Figure 2.
Protein kinase Cα knock out mice have reduced SBP and DBP. Data from radiotelemetry recordings were analyzed; night (awake) (a) and day (sleep) (b) averages for SBP are shown. Heart rate data were averaged during night (awake) (c) and day (sleep) (d) periods and are presented as beats per minute. Data shown as mean ± SEM, n = 4–12, ***P < 0.001.
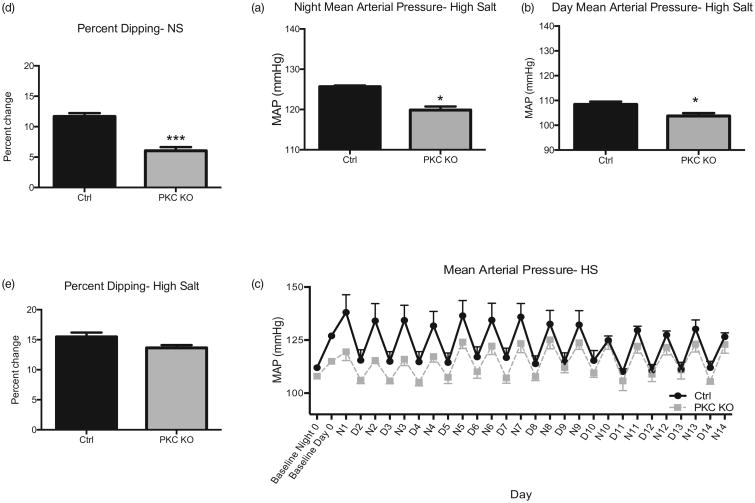
Significantly, this pressure difference was maintained following administration of a high-salt (4%) diet (Fig. 3a–c; night, 119.9 ± 0.8 mmHg PKC KO vs. 125.7 ± 0.3 mmHg control, *P less than 0.05 and day, 103.7 ± 1.2 mmHg PKC KO vs. 108.4 ± 1.1 mmHg control, *P less than 0.05; n = 4–5). However, this BP difference was attenuated during the sleeping phase (≈12 mmHg day vs. 5 mmHg night) with high salt, as compared with when the animals were fed normal chow.
Figure 3.
Protein kinase Cα knock out mice have reduced resting phase dipping blood pressure response. Mice were then fed a high salt diet (4%) for 14 days. Blood pressure responses were averaged at the end of the 14 day period for both night (a) and day (b) period. Daily mean arterial pressures following high salt (4%) chow feeding are shown (c) from baseline period. Baseline night and day measurements are averaged from previous recording period. To determine resting phase dipping blood pressure, mean arterial pressure is presented as a percentage change from night (awake) mean arterial pressures during both normal chow (d) and high salt (14 days) (e) feedings. Representative traces are shown for high salt (d) periods, with average baseline night/day blood pressure. Data shown as mean±SEM, n = 4–5, ***P < 0.001
Protein kinase Cα-deficient mice have a reduced resting phase dipping response
Next we investigated the percentage of the resting phase dipping response, as compared with the active phase BP responses in the PKC KO mice; the loss of PKCα results in a reduced dipping response (6.04 ± 0.6%, n = 8) as compared with ctrl (Fig. 3d and e; 11.67 ± 0.6%, n = 8; ***P < 0.01). These results were surprising given that PKC KO mice are hypotensive as hypertension is correlated with a decreased resting phase dipping response. When fed a high salt diet (4%), the reduced dipping response was abrogated (Fig. 3e; 15.47 ± 0.72% PKC KO vs. 13.64 ± 0.46% control, n = 14). The representative graphs of BP responses, over time, demonstrate the reduced dipping (Figs. 1c), as well as the return of the dipping response with high salt diet (Fig. 3c).
Protein kinase Cα-deficient mice have increased sodium retention and sodium chloride cotransporter expression
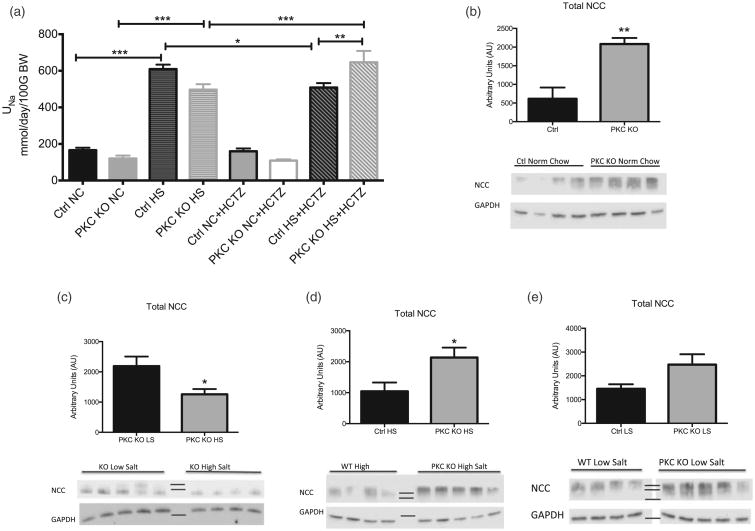
To investigate whether the mechanism for this decreased MAP response in PKCα is renal in nature, mice were housed in metabolic cages and urine collected and analyzed. We observed no differences between urine sodium excretion (UNa) in ctrl and PKC KO mice fed a normal chow diet (Fig. 4a). With an high salt diet, as expected we saw significant increases in UNa excretion (609.2 ± 24.6 mmol/day/100 g/body weight (BW) control high salt vs. 165.7 ± 13.5 mmol/day/100 g/BW control normal chow, n = 6, ***P < 0.001 and 496.6 ± 30.1 mmol/day/100 gBW PKC KO high salt vs. 120.4 ± 15.9 mmol/day/100 gBW PKC KO normal chow, n = 6, ***P < 0.001), in addition to trending toward an increase in sodium retention in PKC KO mice.
Figure 4.
Characterization of renal responses in protein kinase Cα knock out mice. (a) Protein kinase C knock out mice were placed in metabolic cages, following acclimation period; urine volumes were obtained and electrolytes analyzed during normal chow and high salt feedings. Mice were challenged with a bolus of the sodium chloride cotransporter inhibitor (hydrochlorothiazide, 12.5 mg/BW Intraperitoneal) and urine volumes obtained for 12 h. Data are shown in mmol/day, normalized to 100 g total BW of each mouse. Data shown as mean ± SEM, n = 5–6, *P < 0.05, **P < 0.01, ***P < 0.001. Total protein was used for Western blot analysis of sodium chloride cotransporter in protein kinase C knock out and control mice were normal chow and high salt (4%, 12 days) feedings. (b) Protein expression of sodium chloride cotransporter is shown in protein kinase C knock out mouse cortex homogenates as compared to control mice following normal chow. (c) Protein expression of sodium chloride cotransporter is shown in protein kinase C knock out mouse cortex homogenates during low salt (<0.05%, 12 days) and high salt (4%, 12 days) feeding. (d) Protein expression of sodium chloride cotransporter is shown in protein kinase C knock out mouse cortex homogenates as compared with control mice following high salt feeding. (e) Protein expression of sodium chloride cotransporter is shown in protein kinase C knock out mouse cortex homogenates as compared with control mice following low salt feeding. Representative blots for each group are shown, along with loading control, GAPDH. Sodium chloride cotransporter band densities were normalized to GAPDH values and are shown in arbitrary units. Data are shown as mean ± SEM, n = 4–5, *P < 0.05, **P < 0.01. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Single nucleotide polymorphisms in PKCα have also been correlated with increased sensitivity to thiazide diuretics in patient populations [17,18]. Thus, we assessed whether PKCα may be down-regulating NCC expression, leading to a decreased BP response. Using total cortex homogenates, we investigated total NCC protein expression. Here, we report significant increases in total NCC expression (Fig. 4b) in PKC KO mice (≈3.3-fold change, n = 4–5, **P < 0.001), as compared with ctrl when fed a normal chow diet. Given that high salt diet would suppress NCC [19,20], mice were then fed high salt or low salt chow for 12 days to determine, if the mice had an inability to regulate NCC expression. When comparing NCC levels in PKC KO mice fed high salt vs. low salt diet (Fig. 4c), there was an appropriate suppression of total NCC protein.
Nonetheless, NCC levels in high salt -fed PKC KO mice were still significantly higher (≈two-fold change, n = 4–5, *P < 0.05) compared with levels in high salt -fed control mice (Fig. 4d). Furthermore, when comparing PKC KO and control mouse cortical NCC expression on low salt diet, no significant differences were observed (Fig. 4e). These data suggest that NCC expression may be increased on a normal chow or high salt diet, as a compensatory response to the decreased MAP.
To assess the in-vivo activity of NCC further, metabolic cage studies were performed with a bolus of hydrochlorothiazide (HCTZ), an NCC inhibitor. As shown in Fig. 4a, although no differences showed on normal chow, when mice were fed a high salt diet, PKC KO mice had a significantly increased UNa excretion (646.3 ± 62.4 mmol/day/100 gBW PKC KO high salt + HCTZ vs. 508.3 ± 24.2 mmol/day/100 gBW control high salt + HCTZ, n = 6, **P < 0.01). This suggests increased NCC activity is present in the PKC KO mice on high salt diet, consistent with the increased protein expression.
Protein kinase Cα deficient mice have reduced vascular contractile responses
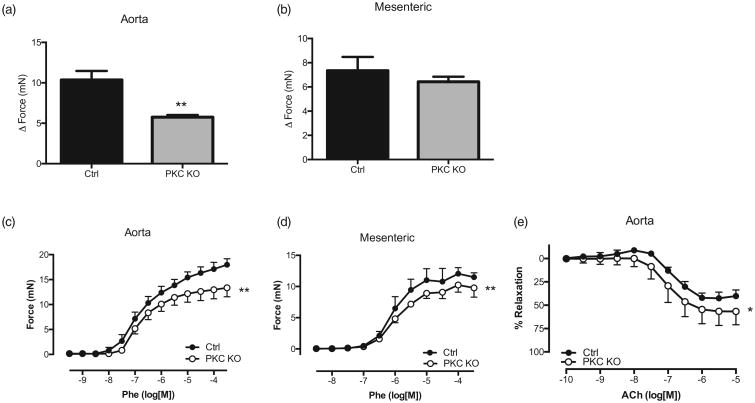
Although whole-animal PKCα deficiency results in hypotension, we found increased NCC levels, suggesting a compensatory effect and thus, not a mechanism for this decreased MAP. To determine a primary mechanism for the decreased BPs, we performed vascular contractility studies. PKCα is known to regulate smooth muscle contractility, as well as cardiac contractility [12,21–24]. For these studies, we used both aorta and first order mesenteric vessels from PKC KO and ctrl mice. In the aorta, we observed a striking reduction in receptor-independent KCl-mediated contraction (Fig. 5a; 5.8 ± 0.3 mN PKC KO vs. 10.4 ± 1.1 mN control, **P < 0.01). We did observe a trend for a decrease in the mesenteric arteries (Fig. 5b). We observed similar results when comparing receptor-mediated contractility with Phe, in both aorta and mesenteric arteries. PKC KO aorta and mesenteric arteries had decreased contractile responses to Phe, as compared with control (Fig. 5c; aorta 12.7 ± 0.5 mN Rmax vs. 16.3 ± 0.5 mN Rmax, n = 4, **P < 0.01) and mesenteric (Fig. 4d; 9.9 ± 0.3 mN Rmax vs. 11.8 ± 0.6 Rmax, n = 4, **P < 0.01). These data suggest a reduction in contractile responses in vessels from the PKC KO mice.
Figure 5.
Protein kinase Cα knock out mice have reduced vascular contractility. Relaxation responses to potassium chloride (120 mmol) were performed in aorta (a) and mesenteric arteries (b) from both protein kinase C knock out and control mice. Values shown are expressed as the change in force (mN). Data are represented as mean ± SEM; n = 4. **P < 0.01, Rmax values of protein kinase C knock out vs. Control. Concentration response curves were performed to phenylephrine (10 μmol/l) in aorta (c) and mesenteric arteries (d) from both protein kinase C knock out and control mice. Values shown are expressed as the change in force (mN). (e) Concentration response curves to acetylcholine were performed in phenylephrine-precontracted aorta. Relaxation responses were calculated relative to the contraction elicited by phenylephrine. Data are represented as mean ± SEM; n = 4. **P < 0.01, Rmax values of protein kinase C knock out vs. control.
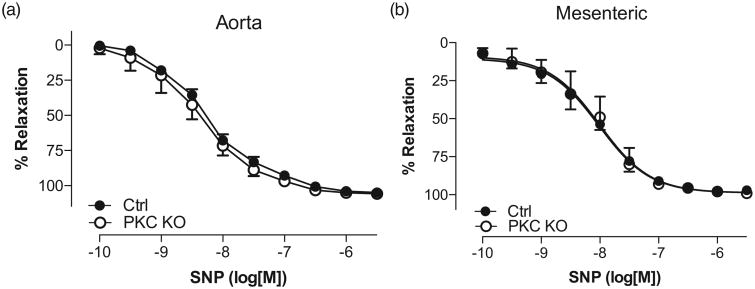
The endothelium-mediated relaxation responses to ACh were also increased in PKC KO mice, as compared with control (Fig. 5e; 59.3 ± 6.8% Rmax vs. 45.4 ± 3.2% Rmax, n = 4, *P < 0.05). These data suggest that PKCα may be negatively regulating endothelial derived relaxation factors, such as endothelial nitric oxide synthase (eNOS). When comparing direct smooth muscle relaxation using CRCs to SNP (Fig. 6a and b), no differences were observed in relaxation responses in either the aorta or mesenteric vessels from PKC KO mice, as compared with control suggesting no differences in the capacity of soluble guanylyl cyclase to cause relaxation.
Figure 6.
Vascular smooth muscle responses from protein kinase Cα knock out mice are not altered compared to control. Concentration response curves to sodium nitroprusside were performed in phenylephrine (10 μmol/l)-precontracted aorta (a) and mesenteric arteries (b). Relaxation responses were calculated relative to the contraction elicited by phenylephrine. Data are represented as mean ± SEM; n = 4, Rmax values of protein kinase C knock out vs. control
Discussion
The PKC family is composed of different isozymes that contribute to diverse intracellular signaling pathways. They have been shown to play a role in multiple cellular events, from the regulation of ion channels and exocytosis, to gene regulatory events [1,2,25–28]. In particular, PKCα is a critical regulator of cardiac myocyte and VSM cell function. Previously, PKCα has been suggested as a therapeutic target for the treatment of conditions such as ischemia-reperfusion injury and heart failure [10,12,28]. In heart failure, left ventricular hypertrophy occurs via cardiomyocyte proliferation. Using an in-vitro model with neonatal cardiomyocytes, PKCα expression was compulsory for the development of hypertrophy [9,29,30]. In addition, cardiomyocytes obtained from an aortic banding model of heart failure demonstrated that PKC levels were up-regulated in the later stages; this process led to hyper-phosphorylation of myofilaments, thus augmenting heart failure [31]. Furthermore, hearts with deletion of PKCα were found to be hypercontractile, whereas PKCα overexpressing hearts were hypocontractile [10]. Although in total, this body of work suggests that PKCα may mediate reduced cardiac output (CO), no telemetry data existed on the role of PKCα in BP, especially as it relates to sodium and water balance.
PKCα is also integral in VSM cell contractility [21–24, 32,33]. Multiple investigators have demonstrated that activation of PKCα leads to increased contractility via ex-vivo organ bath functional and pharmacological studies [22–24, 34,35]. Earlier studies have demonstrated that in the deoxycorticosterone-salt mesenteric arteries exhibit a greater contractile response to PKC activators [36] and in genetic models of hypertension, PKC contributes to altered vascular reactivity [37,38]. Together, those data suggest a loss of PKCα would reduce preload and may reduce MAP. However, mixed results are observed with the use of PKC inhibitors, in vivo, which may be due to inhibitor specificity [39]. To determine whether whole animal PKCα deletion would impair or augment MAP, telemetry studies were performed. Significantly, we observed a significant reduction in MAP in PKC KO mice (Fig. 1a and b) during both active and resting periods. We observed corresponding decreases in both SBP and DBP (Figs. 1d and e and 2a and b) as well; however, there was an increase in HR (Fig. 2c and d). This increase in HR may be a compensatory action to restore normotension. To our knowledge, there are no data demonstrating a role for PKCα in augmenting HR or heart rhythm.
Surprisingly, PKC KO mice also exhibited a significantly reduced resting phase dipping response (≈50%, Fig. 3d and e) compared with control. Circadian rhythms are an important biological response; an aberration in this response is also a predictor for end-organ damage. This circadian rhythm translates into a reduced nighttime (or sleeping) BP, referred to as resting phase dipping, which may be mediated through changes in baroreflex activity [40]. Non-dippers have an increased propensity for cardiovascular disease and for future hypertension [41]. With an increased dietary sodium load, PKC KO mice maintained a hypotensive phenotype (Fig. 3a–c), yet the abnormal dipping response was normalized (Fig. 3e). These data suggest that although PKCα deletion may augment contractility and prevent heart failure, this does not result in an overall increase MAP. Given the hypotensive phenotype, the decreased resting phase dipping response is unlikely to be correlated to hypertension-related cardiovascular disease. However, no one has investigated the role of PKCα in other physiological mechanisms that regulate normal resting phase dipping responses, such as hormonal mediators and the baroreflex responses. Further work will be needed to determine its role in these processes.
The maintenance of BP is a function of CO and peripheral resistance that includes the regulation of sodium and water. The distal nephron is vital in this process, containing two sodium transport proteins which act to fine-tune sodium transport as well as playing a crucial role in urine concentration. Indeed, the NCC and the ENaC are two primary targets for the treatment of hypertension. The PKC KO mice have previously been shown to have a urine-concentrating defect that may be because PKCα mediates the hypertonicity-induced increases in the urea transporter (UT-A1) phosphorylation and activity in the inner medullary collecting duct [42–45]. Furthermore, PKCα has also been shown to regulate the distal sodium transporter, ENaC. By recording single ENaC channels in split open tubules from PKC KO mice, PKCα deletion was found to increase ENaC channel membrane density and open probability (NPo) [8]. In that study, BP responses using tail-cuff plethysmography were also determined. Their data showed no differences in baseline BP, but the PKCKO mice had an increase in SBP in response to high salt (8%, 14 days), as compared with control mice [8]. Although we did see a trend toward increased BP with a high salt diet, the animals remained hypotensive when compared with similarly treated control mice. This discrepancy may be due to differences in methods (telemetry vs. tail cuff), diet (4% in our study vs. 8% in the previous study) and control animal strain background [42]. We did see a normalization of the dipping response (Fig. 3e) and trending increase in MAP following 7 days of high salt feeding. However, these results did not reach significance in our experiments.
To determine how PKCα deletion affects NCC expression, whole cortex homogenates were used from PKC KO and Control mice fed NS, low salt and high salt chow. We observed an increase in total NCC expression in PKC KO mice (Fig. 4b), which was maintained with high salt feeding (Fig. 4c). The ability of the PKC KO mice to down-regulate NCC levels with high salt was also found to be intact (Fig. 4c), suggesting that the increased levels of NCC may be acting as a compensatory mechanism for the hypotensive BP seen in the PKC KO mice.
Although NS, the PKC KO mice show a similar trend for sodium retention, which correlates with the increased NCC protein expression we observed (Fig. 4a). Previously, increased ENaC activity was observed in these same mice, yet in our metabolic cage studies, we found no significant increases in sodium retention in the PKC KO mice. This may be secondary to decreased perfusion associated with hypotension. Significantly, when looking at the natriuretic response to thiazide (HCTZ) treatment, the PKC KO mice exhibited a greater natriuresis, thus responsiveness, as compared with the control mice when on a high salt diet. However, it is also possible that these differences could only be observed with the increased sodium load due to low urine volumes obtained from mice with normal chow diet.
PKC is also known to play a significant role in VSM contractile responses. PKCα is expressed ubiquitously throughout the vasculature and is activated by increased intracellular calcium levels from membrane depolarization or store-operated calcium release. Following activation in VSM, PKCα inhibits myosin light chain phosphatase by CPI-17, thus contributing to enhanced contraction [13]. In addition, PKCα can phosphorylate and inhibit calponin, a negative regulator of VSM cell contraction [46]. Pharmacological activation of PKC is known to enhanced contractility, whereas inhibition of PKC causes relaxation [22]. Significantly, the role of PKC in modulating vascular tone is not universal – PKC agonists elicit an enhanced contractility in mouse aorta, but not in the smooth muscle of the corpus cavernosum [23]. To determine how PKCα-specific deletion affects vascular reactivity, functional studies were performed in capacitance (aorta) and resistance (mesenteric) vessels. We observed significantly reduced responses to KCl-mediated and Phe-mediated (Phe) (Fig. 5) contraction in aorta from the PKC KO mice. The Phe-mediated responses were also reduced in the mesenteric arteries, suggesting a greater role in agonist-mediated responses compared with membrane depolarization (Fig. 5d).
Endothelium-mediated relaxation responses were also enhanced in the PKC KO mice (aorta, Fig. 5e). Some studies have suggested a role for PKC in modulating the enzymes that are important for endothelial-derived relaxation responses [4,47–51]. The ability of PKCα to regulate COX has been investigated, but the evidence is controversial and not well understood. Cosentino et al. [47] have demonstrated that phorbol ester-induced COX2 expression was reduced with PKC inhibition. However, during inflammatory conditions, PKC seems to increased COX levels, whereas others have shown COX2 to be a poor substrate for direct PKC phosphorylation [51].
However, it is known that PKC can phosphorylate eNOS at a site (T495) that reduces ability for cofactors to bind, thus leading to decreased eNOS activity [52,53]. Furthermore, increased phosphorylation at this site could lead to increased levels of superoxide production because of eNOS uncoupling. This imbalance of NO and superoxide may contribute to vascular dysfunction [53]. In PKC KO mice, the inhibitory effect of PKCα would be gone, perhaps increasing eNOS activity, with a secondary effect of reduced superoxide levels. Our functional data seem to corroborate the previous studies, as aorta from PKC KO mice had an enhanced ACh-mediated relaxation response. Together, these data reveal that global deletion of PKCα causes hypotension due to decreased vascular contractility and through loss of PKCα-mediated inhibition of endothelial relaxation factors.
Acknowledgments
The authors would like to thank Drs Julie A. Johnson and Rhonda Cooper-DeHoff, and the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) group for their assistance with the study.
The study was funded by the Emory University Research Committee to J.D.K., the National Institutes of Health NIDDK T32 (DK07656) to B.M.W., NHLBI (5R01HL071138-08) and (P01HL134604) to R.C.W., NIDDK (DK89828) to J.D.K., NIDDK R01 (DK-085097) to R.S.H. and NIDDK (DK056956) to A.B.C., the Department of Veteran's Affairs MERIT Award (I01BX002322–01) to R.S.H. and the American Heart Association, Predoctoral Award (13PRE14080019) to C.G.M. and Grant-in-Aid (15GRNT25700451) to R.C.W.
Abbreviations
- ACh
acetylcholine
- AngII
angiotensin II
- CRC
concentration response curve
- DCT
distal convoluted tubule
- ENaC
epithelial sodium channel
- eNOS
endothelial nitric oxide synthase
- HCTZ
hydrochlorothiazide
- MAP
Mean arterial pressure
- NCC
sodium chloride cotransporter
- NHE
sodium hydrogen exchanger
- Phe
phenylephrine
- PKC KO
PKC knock-out mouse
- PKC
protein kinase C
- SNP
sodium nitroprusside
- VSM
vascular smooth muscle
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 3.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 4.Khalil RA. Protein kinase C inhibitors as modulators of vascular function and their application in vascular disease. Pharmaceuticals (Basel) 2013;6:407–439. doi: 10.3390/ph6030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu FY, Cogan MG. Role of protein kinase C in proximal bicarbonate absorption and angiotensin signaling. Am J Physiol. 1990;258(4 Pt 2):F927–F933. doi: 10.1152/ajprenal.1990.258.4.F927. [DOI] [PubMed] [Google Scholar]
- 6.Tse CM, Levine SA, Yun CH, Brant SR, Pouyssegur J, Montrose MH, et al. Functional characteristics of a cloned epithelial Na+/H+ exchanger (NHE3): resistance to amiloride and inhibition by protein kinase C. Proc Natl Acad Sci U S A. 1993;90:9110–9114. doi: 10.1073/pnas.90.19.9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabral PD, Hong NJ, Hye Khan MA, Ortiz PA, Beierwaltes WH, Imig JD, Garvin JL. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension. 2014;63:e68–e73. doi: 10.1161/HYPERTENSIONAHA.113.02564. [DOI] [PubMed] [Google Scholar]
- 8.Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, et al. ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Calpha knockout mice. Am J Physiol Renal Physiol. 2014;306:F309–F320. doi: 10.1152/ajprenal.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braz JC, Bueno OF, De Windt LJ, Molkentin JD. PKC alpha regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2) J Cell Biol. 2002;156:905–919. doi: 10.1083/jcb.200108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Molkentin JD. Protein kinase Calpha as a heart failure therapeutic target. J Mol Cell Cardiol. 2011;51:474–478. doi: 10.1016/j.yjmcc.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, et al. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105:194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535(Pt 2):553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynne BM, Labazi H, Tostes RC, Webb RC. Aorta from angiotensin II hypertensive mice exhibit preserved nitroxyl anion mediated relaxation responses. Pharmacol Res. 2012;65:41–47. doi: 10.1016/j.phrs.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res. 2015;107:119–130. doi: 10.1093/cvr/cvv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko B, Mistry AC, Hanson L, Mallick R, Cooke LL, Hack BK, et al. A new model of the distal convoluted tubule. Am J Physiol Renal Physiol. 2012;303:F700–F710. doi: 10.1152/ajprenal.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duarte JD, Zineh I, Burkley B, Gong Y, Langaee TY, Turner ST, et al. Effects of genetic variation in H3K79 methylation regulatory genes on clinical blood pressure and blood pressure response to hydrochlorothiazide. J Transl Med. 2012;10:56. doi: 10.1186/1479-5876-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner ST, Bailey KR, Schwartz GL, Chapman AB, Chai HS, Boerwinkle E. Genomic association analysis identifies multiple loci influencing antihypertensive response to an angiotensin II receptor blocker. Hypertension. 2012;59:1204–1211. doi: 10.1161/HYP.0b013e31825b30f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon V. Regulation of the Na+–Cl− cotransporter by dietary NaCl: a role for WNKs, SPAK, OSR1, and aldosterone. Kidney Int. 2008;74:1373–1375. doi: 10.1038/ki.2008.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+–Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulopoulou S, Hannan JL, Matsumoto T, Webb RC. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am J Physiol Heart Circ Physiol. 2012;302:H2477–H2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Tostes RC, Webb RC. S-nitrosylation inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol. 2011;57:65–71. doi: 10.1097/FJC.0b013e3181fef9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin L, Teixeira CE, Webb RC, Leite R. Comparison of the involvement of protein kinase C in agonist-induced contractions in mouse aorta and corpus cavernosum. Eur J Pharmacol. 2008;590:363–368. doi: 10.1016/j.ejphar.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts SW, Chai S, Webb RC. Lead acetate-induced contraction in rabbit mesenteric artery: interaction with calcium and protein kinase C. Toxicology. 1995;99:55–65. doi: 10.1016/0300-483x(94)03003-k. [DOI] [PubMed] [Google Scholar]
- 25.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton AC, Antal CE, Steinberg SF. Protein kinase C mechanisms that contribute to cardiac remodelling. Clin Sci (Lond) 2016;130:1499–1510. doi: 10.1042/CS20160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda) 2012;27:130–139. doi: 10.1152/physiol.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkela R, Ilves M, Pikkarainen S, Tokola H, Ronkainen J, Vuolteenaho O, et al. Identification of PKCalpha isoform-specific effects in cardiac myocytes using antisense phosphorothioate oligonucleotides. Mol Pharmacol. 2002;62:1482–1491. doi: 10.1124/mol.62.6.1482. [DOI] [PubMed] [Google Scholar]
- 30.Vijayan K, Szotek EL, Martin JL, Samarel AM. Protein kinase C-alpha-induced hypertrophy of neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H2777–H2789. doi: 10.1152/ajpheart.00171.2004. [DOI] [PubMed] [Google Scholar]
- 31.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, WangH, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 32.Kanashiro CA, Khalil RA. Isoform-specific protein kinase C activity at variable Ca2+ entry during coronary artery contraction by vasoactive eicosanoids. Can J Physiol Pharmacol. 1998;76:1110–1119. doi: 10.1139/cjpp-76-12-1110. [DOI] [PubMed] [Google Scholar]
- 33.Turla MB, Webb RC. Augmented phosphoinositide metabolism in aortas from genetically hypertensive rats. Am J Physiol. 1990;258(1 Pt 2):H173–H178. doi: 10.1152/ajpheart.1990.258.1.H173. [DOI] [PubMed] [Google Scholar]
- 34.Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin ii and endothelin-1. J Am Soc Hypertens. 2009;3:84–95. doi: 10.1016/j.jash.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turla MB, Park SM, Webb RC. Vascular responsiveness to phorbol esters in coarctation-hypertensive rats. J Hypertens. 1990;8:191–196. doi: 10.1097/00004872-199002000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Turla MB, Webb RC. Vascular responsiveness to protein kinase C activators in mineralocorticoid-hypertensive rats. J Hypertens. 1991;9:209–215. doi: 10.1097/00004872-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Storm DS, Turla MB, Todd KM, Webb RC. Calcium and contractile responses to phorbol esters and the calcium channel agonist, Bay K 8644, in arteries from hypertensive rats. Am J Hypertens. 1990;3(8 Pt 2):245S–248S. doi: 10.1093/ajh/3.8.245. [DOI] [PubMed] [Google Scholar]
- 38.Turla MB, Webb RC. Enhanced vascular reactivity to protein kinase C activators in genetically hypertensive rats. Hypertension. 1987;9(6 Pt 2):III150–III154. doi: 10.1161/01.hyp.9.6_pt_2.iii150. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz RA, Dundore RL, Cumiskey WR, Harris AL, Silver PJ. Protein kinase inhibitors and blood pressure control in spontaneously hypertensive rats. Hypertension. 1991;17:91–100. doi: 10.1161/01.hyp.17.1.91. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens. 2010;19:51–58. doi: 10.1097/MNH.0b013e3283336ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin SS, O'Brien E, Staessen JA. Masked hypertension: understanding its complexity. Eur Heart J. 2017;38:1112–1118. doi: 10.1093/eurheartj/ehw502. [DOI] [PubMed] [Google Scholar]
- 42.Thai TL, Blount MA, Klein JD, Sands JM. Lack of protein kinase C-alpha leads to impaired urine concentrating ability and decreased aquaporin-2 in angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2012;303:F37–F44. doi: 10.1152/ajprenal.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Klein JD, Frohlich O, Sands JM. Role of protein kinase C-α in hypertonicity-stimulated urea permeability in mouse inner medullary collecting ducts. Am J Physiol Renal Physiol. 2013;304:F233–F238. doi: 10.1152/ajprenal.00484.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein JD, Martin CF, Kent KJ, Sands JM. Protein kinase C-alpha mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol. 2012;302:F1098–F1103. doi: 10.1152/ajprenal.00664.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Klein JD, Liedtke CM, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2010;299:F1401–F1406. doi: 10.1152/ajprenal.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998;25:974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 47.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 48.Duggan SV, Lindstrom T, Iglesias T, Bennett PR, Mann GE, Bartlett SR. Role of atypical protein kinase C isozymes and NF-kappaB in IL-1beta-induced expression of cyclooxygenase-2 in human myometrial smooth muscle cells. J Cell Physiol. 2007;210:637–643. doi: 10.1002/jcp.20901. [DOI] [PubMed] [Google Scholar]
- 49.Chen HT, Sun D, Peng YC, Kao PH, Wu YL. Novel augmentation by bufalin of protein kinase C-induced cyclooxygenase-2 and IL-8 production in human breast cancer cells. Innate Immun. 2017;23:54–66. doi: 10.1177/1753425916676347. [DOI] [PubMed] [Google Scholar]
- 50.Simeone AM, Nieves-Alicea R, McMurtry VC, Colella S, Krahe R, Tari AM. Cyclooxygenase-2 uses the protein kinase C/interleukin-8/uroki-nase-type plasminogen activator pathway to increase the invasiveness of breast cancer cells. Int J Oncol. 2007;30:785–792. [PubMed] [Google Scholar]
- 51.Vezza R, Habib A, Li H, Lawson JA, FitzGerald GA. Regulation of cyclooxygenases by protein kinase C. Evidence against the importance of direct enzyme phosphorylation. J Biol Chem. 1996;271:30028–30033. doi: 10.1074/jbc.271.47.30028. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara M, Hayashi N, Jing T, Titani K. Regulation of endothelial nitric oxide synthase by protein kinase C. J Biochem. 2003;133:773–781. doi: 10.1093/jb/mvg099. [DOI] [PubMed] [Google Scholar]
- 53.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, et al. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+ -toxins. PLoS One. 2014;9:e99823. doi: 10.1371/journal.pone.0099823. [DOI] [PMC free article] [PubMed] [Google Scholar]