Abstract
The primary chemotherapeutic agents for epithelial ovarian cancer are platinum-based drugs, which are commonly used in combination with a taxane regimen. These treatments are generally effective at achieving remission, but the remission is often followed by a relapse and acquired resistance to chemotherapy. In order to overcome these barriers of drug resistance, it is important to understand the underlying mechanisms regulating the development of drug-resistant tumors. Tumors evolve through interactions with the surrounding microenvironment, which are comprised of a complex mixture of cells including fibroblasts and immune cells. In ovarian cancer, fibroblasts can make up a significant component of the primary tumor. While fibroblasts are known to influence the behavior of cancer cells directly through secretion of growth factors, and extracellular matrix (ECM) proteins, the interactions between fibroblasts and immune cells are less understood. In a recently published study from Cell, Wang and colleagues present intriguing work characterizing the role of fibroblast and T cells in modulating platinum resistance in ovarian cancer. Here, we briefly summarize and comment on their findings in relation to the tumor microenvironment and chemoresistance.
Keywords: Ovarian cancer, fibroblasts, effector T cell, chemo-resistance, platinum
Introduction
The primary chemotherapeutic agents for epithelial ovarian cancer are platinum-based drugs, which are commonly used in combination with a taxane regimen. These treatments are generally effective at achieving remission, but the remission is often followed by a relapse and acquired resistance to chemotherapy (1,2). Accordingly, acquired resistance is one of the main contributing factors for treatment failure and cancer-associated mortality.
Traditionally, resistance to chemotherapy involves multiple molecular mechanisms that include pharmacokinetics, tumor seclusion, cellular transport, inactivation/sequestration, and alterations in the drug target (3). Mechanisms involving cellular repair pathways, cell survival signals, and inhibition of cell death could also prevent chemotherapy effectiveness. The tumor microenvironment impacts drug sensitivity, through the actions of tumor-associated fibroblasts and ECM factors (2,4). In some patients, the stromal proportion of the ovarian carcinoma tissue can be up to 83% (5). Tumor-associated fibroblasts regulate cancer cell behavior by secreting growth factors, angiogenic factors, cytokines, and proteases, which may support a niche for cancer stem cells (6). Moreover, fibroblasts express matrix proteins including collagen IV, fibrillin-1, and amplification of cyclin E that are associated with chemotherapy resistance (2). In theory, matrix-associated factors may be conferring resistance to otherwise sensitive tumor cells, or possibly providing a niche for dormant cells or cancer stem cells to reside in the tumor.
Recent studies indicate that conventional chemotherapies may exert unwanted side effects on fibroblasts. In breast cancer, doxorubicin acts on fibroblasts to enhance drug resistance in cancer cells through high mobility group box-1 (7). Acting as a cytokine, high mobility group box-1 protein increases recruitment of bone marrow cells to promote primary tumor progression (8). Thus, in the presence of chemotherapies, fibroblasts may alter the immune landscape in favor of primary tumor growth and survival. Effector CD8+ T cells are an opposing immune cell and a favorable prognostic factor for breast and ovarian cancer, particularly in recurrence-free survival (9–11). Effector T cells infiltrate the tumor microenvironment to inhibit tumor growth and progression by targeting tumor cells for cytolytic cell death (12); these effects can be enhanced by immunotherapies (9,11,13–15).
Interplay between fibroblasts and T cells in ovarian cancer
A recent study by Wang and colleagues provide an interesting twist on the interplays between fibroblasts and effector T cells in modulating cancer cell responses to cisplatin in high-grade serous ovarian carcinoma (HGSOC) (16) (Figure 1). In initial experiments, they use primary epithelial ovarian cancer cells and fibroblasts isolated from an HGSOC patient to show that xenografts generated with tumor cells and fibroblasts are less sensitive to cisplatin compared to tumor cells grafted alone. In vitro, cisplatin induces apoptosis in cancer cells, but cisplatin-induced apoptosis is attenuated when cancer cells are supplemented with the fibroblast-conditioned medium. Interestingly, fibroblast-mediated cisplatin resistance in cancer cells is abrogated when the fibroblasts are pre-incubated with medium from activated CD8+ T cells. The medium from the effector T cells contains high levels of interferon-gamma (IFNγ). Pre-treating fibroblasts with IFNγ increase apoptosis in cultured cells and is attenuated by neutralizing IFNγ receptor antibodies. IFNγ also reduces the tumor volume in cisplatin-treated mice with cancer cells and fibroblasts. These studies indicate that IFNγ from T cells affects the ability of fibroblasts to promote platinum resistance in cancer cells.
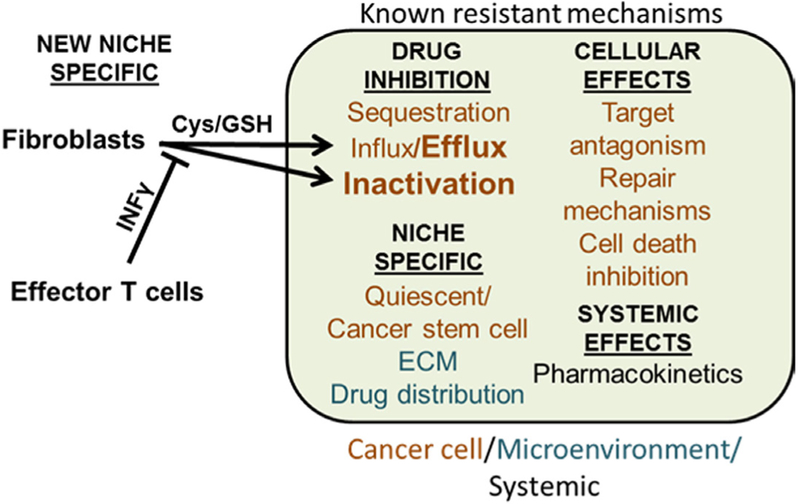
Figure 1.
Schematic diagram of known chemotherapy resistance mechanisms in solid tumors and a new mechanism proposed by Wang et al. The drug can be inhibited from reaching tumor cells by pharmacokinetics and insufficient drug distribution to the tumor. Tumor cells may be dormant or receive support from stromal fibroblasts and extracellular matrix (ECM). Within the tumor cell, pathways exist to reduce availability and inhibit the target effects through enhanced repair or decrease cell death. The pathway reported by Wang and colleagues shown in bold, now implicates the role of cysteine (Cys) and reduced glutathione (GSH) secreted by fibroblasts. These antioxidants inhibit cisplatin by binding the drug and enhancing the efflux of cisplatin through transporters. This molecular mechanism contributes to fibroblast-mediated cisplatin resistance in cancer cells. In the presence of effector T cells secreting interferon-gamma (IFNγ), IFNγ lowers the levels of GSH in tumor microenvironment by inhibiting the transport of cystine into fibroblasts. Since cystine serves as a precursor for Cys, which is required for the synthesis of GSH, decreased transport of cystine into fibroblasts resulted in decreased synthesis of GSH. Therefore, the presence of effector T cells abrogates fibroblast-mediated cisplatin resistance in cancer cells.
Wang et al. continue with the investigation into the mechanisms by which IFNγ alleviates fibroblast-associated cisplatin resistance. The intracellular content of cisplatin and DNA-cisplatin adducts in cancer cells are reduced by the addition of fibroblasts. Similar results are found in the immunocompromised mouse model, and the effect is limited by adding CD8+ T cells but not in the presence of IFNγ antibodies. The addition of fibroblasts or fibroblast-conditioned medium, which contains high levels of glutathione (GSH) and cysteine (Cys), increase the cancer cell intracellular GSH content. Intracellular GSH can chelate cisplatin and efflux the complex from the cell (17).
Treatment of A2780 cells with GSH and Cys has a similar effect as the fibroblast medium (16). Additionally, the similar effects between GSH and Cys suggest the cells can either transfer GSH or generate this from precursors such as Cys. Treatment of fibroblasts with CD8+ T cells or IFNγ reduces the GSH content of the medium. IFNγ treatment of fibroblasts also decreased Cys synthesis and expression of the cystine transporter. This effect is not observed in cancer cells. Expression of the cell membrane enzyme gamma-glutamyl-transferase, which breaks down extracellular GSH, is increased in fibroblasts after IFNγ treatment. When this enzyme is knocked down in fibroblasts, the effect of IFNγ to enhance sensitivity to cisplatin in tumor cells was partially attenuated.
GSH is particularly interesting as an antioxidant, which binds cisplatin along with other thiol-containing molecules (18), and has been shown to enhance cisplatin resistance (19). Conjugation of reduced form of GSH to cisplatin likely inactivates the drug and enhances the efflux of GSH-conjugated cisplatin via an ATP-binding cassette efflux pump (17). Cys is a critical component of GSH synthesis, and Cys can be derived from cystine. However, some cancer cells have a limited ability to uptake cystine based on low transporter expression (20). In this study, Wang et al. also demonstrates that cystine is transported into stromal cells, converted to Cys, which supports GSH synthesis and secretion to the tumor microenvironment. In co-culture studies, they further demonstrate that IFNγ derived from effector CD8+ T cells controls the production of Cys and GSH through two different mechanisms. In one mechanism, IFNγ regulates gene expression of gamma-glutamyltransferase 5, which controls the breakdown of extracellular GSH. Second, through a STAT1-dependent mechanism, IFNγ negatively regulates protein expression of cXT and SLC3A2, components of an amino acid transporter regulating the exchange of Cys and glutamate. These two different mechanisms modulated by T cells are important in preventing efflux of cisplatin from tumors, and re-sensitizing tumor cells to platinum-induced cell death.
Finally, Wang and colleagues determine the associations between stroma or T cell content in tumor specimens and clinical outcome in patients with ovarian cancer. Using platinum sensitive and platinum resistant HGSOC samples, they find that higher expression of α-smooth muscle actin positive cells and lower levels of CD8+ T cells are associated more with platinum-resistant samples and correlated with shorter survival compared to patient samples with lower stroma. In their model, IFNγ produced from the CD8+ T cells alters the expression of cystine transporters on the fibroblasts, which in turn lowers the concentrations of extracellular Cys and GSH, and provides a mechanism of drug detoxification by promoting drug conjugation and efflux from cancer cells. Overall, these studies provide a plausible answer to why patients with tumor infiltrating T cells have significantly better prognosis than those without tumor-infiltrating T cells.
Discussion
Overall, this study shows that tumor-associated fibroblasts confer cisplatin resistance to tumor cells through GSH pathways, and this can be overcome with IFNy or activated CD8+ T cells (16). They also demonstrate the clinical relevance of CD8+ and α-smooth muscle actin positive expression in platinum resistant and platinum sensitive HGSOC patient samples. IFNγ inhibition of fibroblast-mediated cisplatin resistance in ovarian cancer is very interesting, and it would be important to test if IFNy therapy can enhance cisplatin response in ovarian cancer. In addition, anti-programmed death ligand 1 (PD-L1) blockage therapies may prevent T cell exhaustion and enhance secretion of IFNγ by T cells (13,21). Peng et al. have reported that epigenetic modulators induce tumor expression of CXCL9 and CXCL10, which enhance T cell trafficking and slow tumor progression (22). These immunotherapies and epigenetic therapies may enhance T cell function and trafficking to tumor microenvironment, and counteract the effect of fibroblasts in enhancing resistance to cisplatin.
Another interesting aspect of this study is the role of GSH in cisplatin resistance. If GSH is the main mechanism by which fibroblasts mediate cisplatin resistance in ovarian cancer cells, it may be possible to disrupt this mechanism through chemical inhibition to enhance cisplatin sensitivity. Endogenous antioxidant molecules are essential for the balance of reactive oxygen species and antioxidants and affect cell signaling and viability (23,24). Most agents used to treat various cancers generate reactive oxygen species, and the endogenous antioxidant response may reduce the effectiveness of these drugs. The two major antioxidant systems in cancer cells are GSH and thioredoxin (25). Several studies have targeted antioxidant inhibitors as a method of increasing cellular reactive oxygen species in ovarian cancer; for example, APR-246, which binds to Cys in GSH and sensitizes tumor cells to cisplatin and doxorubicin (26). Thus, strategies to modulate drug metabolism in cancer cells, either through controlling the microenvironment or targeting cancer cells directly, hold promise for resensitizing cancers to chemotherapy.
Currently, immunotherapy has some success in melanomas and leukemias (27,28) and may hold promise for ovarian cancer. Ultimately, successful immunotherapy in carcinomas may depend on overcoming multiple barriers. Evidence indicates that fibroblasts regulate the immune landscape in carcinomas through secretion of cytokines. In pancreatic ductal carcinoma, fibroblasts overexpress CXCL12, which modulates expression of immunosuppressive molecules cytotoxic T-lymphocyte-associated protein 4 and PD-L1 in cancer cells, and inhibited recruitment and activity of effector T cells (29). In animal models of skin carcinogenesis, fibroblast secretion of CCL2 is important for regulating macrophage recruitment (30). In invasive breast carcinomas, the level of cytotoxic T cells inversely correlates with regulatory T cells and antigen presenting cells (10,31). These studies suggest that deregulated expression of cytokines may alter the immune landscape in favor for the tumor.
Would targeting fibroblasts enhance the recruitment and activity of cytotoxic T cells to the primary tumor? There are mixed results with targeting fibroblast activation protein (FAP) positive fibroblasts. Pharmacologic inhibition of FAP in immunocompetent mouse models of colon or lung cancer decreases tumor growth (32). Yet, phase II clinical trials for FAP inhibitors have failed to show significant changes in survival for metastatic colorectal patients (33). Furthermore, immune targeting of FAP has yielded limited anti-tumor effects in other mouse models of melanoma, colorectal, pancreatic, and breast carcinomas, and has resulted in cachexia and bone toxicity (34,35). One explanation for these failures may lie in studies indicating that bone marrow cells are an important source of fibroblasts and elimination of FAP-expressing cells may also eliminate bone marrow cells as well (34). In ovarian cancer, Wang et al. characterize the fibroblasts as cells that express platelet-derived growth factor receptor-α and α-smooth muscle actin. Studies have indicated that fibroblasts are a heterogeneous population of cells, characterized by expression of multiple and distinct overlapping markers including a-smooth muscle actin and fibroblast-specific protein 1 in carcinomas (36). Thus, fibroblast subpopulations may play distinct roles during tumor progression. In summary, it would be necessary to clearly understand the role of fibroblasts in tumor progression and their interactions with immune cells to improve the effectiveness of immunotherapy and chemotherapy combined.
Acknowledgements
Funding: The work is funded by the University of Kansas Endowment Association, the University of Kansas Cancer Center Support Grant (P30-CA168524), the American Cancer Society Research Scholar (125618-RSG-14-067-01-TBE), and the Department of Defense Ovarian Cancer Research Program under award number (W81XWH-10-1-0386) to J Chien, and American Cancer Society Research Scholar (RSG-13-182-01-CSM) and National Institutes of Health/National Cancer Institute (CA127357) to N Cheng.
Footnote
Provenance: This is a Guest Perspective commissioned by the Section Editor Zheng Li [Department of Gynecologic Oncology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China].
Conflicts of Interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: Disclaimer: Views and opinions of, and endorsements by the authors do not reflect those of the US Army or the Department of Defense.
References
- 1.Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Semin Oncol 2006;33:S12–6. [DOI] [PubMed] [Google Scholar]
- 2.Chien J, Kuang R, Landen C, et al. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol 2013;3:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 2003;3:502–16. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene 2012;31:1869–83. [DOI] [PubMed] [Google Scholar]
- 5.Labiche A, Heutte N, Herlin P, et al. Stromal compartment as a survival prognostic factor in advanced ovarian carcinoma. Int J Gynecol Cancer 2010;20:28–33. [DOI] [PubMed] [Google Scholar]
- 6.Li XY, Hu SQ, Xiao L. The cancer-associated fibroblasts and drug resistance. Eur Rev Med Pharmacol Sci 2015;19:2112–9. [PubMed] [Google Scholar]
- 7.Amornsupak K, Insawang T, Thuwajit P, et al. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer 2014;14:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191–5. [DOI] [PubMed] [Google Scholar]
- 9.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple negative breast cancer: a retrospective multicenter study. Breast Cancer Res 2015;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203–13. [DOI] [PubMed] [Google Scholar]
- 12.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 2015;33:445–74. [DOI] [PubMed] [Google Scholar]
- 13.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013;73:6900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562–7. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W Kryczek I, Dostal L, et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016;169:1092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HH, Kuo MT. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met Based Drugs 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307–20. [DOI] [PubMed] [Google Scholar]
- 19.Godwin AK, Meister A, O’Dwyer PJ, et al. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A 1992;89:3070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Trachootham D, Liu J, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 2012;14:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015;36:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oien DB, Moskovitz J. Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Biol 2008;80:93–133. [DOI] [PubMed] [Google Scholar]
- 24.Oien DB, Shinogle HE, Moore DS, et al. Clearance and phosphorylation of alpha-synuclein are inhibited in methionine sulfoxide reductase a null yeast cells. J Mol Neurosci 2009;39:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotblat B, Grunewald TG, Leprivier G, et al. Anti-oxidative stress response genes: bioinformatic analysis of their expression and relevance in multiple cancers. Oncotarget 2013;4:2577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohell N, Alfredsson J, Fransson A, et al. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis 2015;6:e1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccin Immunother 2016:1–11. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman CL, Gribben JG. Immunotherapy in Chronic Lymphocytic Leukaemia (CLL). Curr Hematol Malig Rep 2016;11:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Chen L, Xiao M, et al. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 2011;178:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funada Y, Noguchi T, Kikuchi R, et al. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep 2003;10:309–13. [PubMed] [Google Scholar]
- 32.Santos AM, Jung J, Aziz N, et al. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 2009;119:3613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narra K, Mullins SR, Lee HO, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther 2007;6:1691–9. [DOI] [PubMed] [Google Scholar]
- 34.Tran E, Chinnasamy D, Yu Z, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med 2013;210:1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts EW, Deonarine A, Jones JO, et al. Depletion of stromal cells expressing fibroblast activation protein-a from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 2013;210:1137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto H, Mundel TM, Kieran MW, et al. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 2006;5:1640–6. [DOI] [PubMed] [Google Scholar]