Abstract
Thyroid stimulating hormone and insulin-like growth factor 1 receptors (TSHRs and IGF1Rs, respectively) interact leading to additive or synergistic stimulation of cellular responses. Recent findings provide evidence that the interaction between TSHRs and IGF1Rs is similar to that described for other G protein-coupled receptors and receptor tyrosine kinases. These types of interactions occur at or proximal to the receptors and are designated “receptor cross-talk.” Herein, we describe our studies in human thyrocytes, human retro-orbital fibroblasts from Graves’ orbitopathy patients and a model cell line that support the concept of TSHR/IGF1R cross-talk. We also discuss how receptor cross-talk is involved in stimulation by a monoclonal TSHR-stimulating antibody and how targeting both receptors may lead to novel treatments of Graves’ orbitopathy.
Keywords: TSH, IGF1, receptor cross-talk
Introduction
Interactions between G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) leading to robust stimulation of cellular responses is well-established and extensively reviewed (1–3). The interaction between a GPCR and a RTK, receptor cross-talk, rests on the principle that GPCRs and RTKs share molecular components of signal transduction and that activation of both receptors leads to additive or synergistic effects. Cross-talk interactions, which are rapid and take place at or proximal to the receptor, are traditionally thought to occur through three mechanisms: 1) GPCR stimulation by an agonist leads to the production or increased availability of RTK agonists; 2) GPCR signaling results in Tyr phosphorylation of the RTK; or 3) RTK signaling appropriates molecular effectors normally associated with the GPCR pathway, e.g. G proteins or β-arrestins. However, additive and synergistic effects have been detected at times much later and at steps further downstream of agonist binding also. Pyne et al. (2) expanded these ideas of GPCR/RTK cross-talk when they introduced the concept that RTKs and GPCRs may reside in “signaling platforms” that permit bi-directional interactions. In their view, transactivation could occur both ways, i.e. RTK signaling can lead to activation, usually by phosphorylation, of a GPCR in addition to the reverse. Another possibility is that GPCR-RTK interactions could occur in these signaling platforms without a post-translational modification of either receptor by activation of one receptor causing activation-inducing conformational changes in the other receptor.
Interdependence between TSHR and IGF1R signaling has been reported in several non-human thyroid-derived cell lines (4,5). Synergistic increases in DNA synthesis or cell cycle progression was observed in rodent-derived cell lines and dog thyrocytes following simultaneous activation of TSHR and IGF1R (5–7). In sheep thyrocytes, neither TSH nor IGF1 alone could stimulate thyroid function, whereas combination treatments could (7). A common theme emerging from these data is the idea that potentiating effects necessitated binding of exogenous agonists to both receptors, but this is not correct (see below). It is noteworthy that TSHRs and IGF1Rs have been found to physically interact in primary cultures of fibroblasts from the retro-orbital spaces of Graves’ orbitopathy (GO) patients (GOFs) and in normal human thyrocytes (8) suggesting that signaling platforms containing both receptors are present in human cells naturally expressing both receptors.
We will summarize the findings from our studies that address the concept of TSHR/IGF1R cross-talk in human thyrocytes and GOFs, and in a model cell over-expressing TSHRs in culture.
Pharmacology of TSHR and IGF1R signaling in primary cultures of human thyrocytes and GOFs
Because of IGF1’s role in growth and cancer, early reports of TSHR/IGF1R synergy in thyroid-derived cells lines focused on proliferative effects. However, whether TSH stimulates proliferation of human thyroid cells in vitro remains controversial (9), but it appears that the different findings depend on the culture conditions used in the experiments (10). Under the in vitro conditions used in our recent report, we (11) were unable to find increased proliferative activity stimulated by TSH or IGF1 in primary cultures of human thyrocytes. We found instead that TSH signaling led to thyrocyte differentiation and that IGF1 enhanced these effects (12). Specifically, IGF1 had an additive effect with TSH in the up-regulation of several thyroid-specific genes including thyroglobulin (TG), thyroid peroxidase (TPO) and deiodinase type 2 (DIO2) whereas IGF1 was synergistic with TSH in up-regulating sodium-iodide symporter (NIS) expression. More revealing were our observations of the effects of an IGF1R inhibitor, linsitinib, on stimulation by TSH alone. Linsitinib did not decrease the basal (or control) levels of TG or NIS nor the levels of TG stimulated by TSH. In contrast, linsitinib completely inhibited the up-regulation of NIS expression stimulated by TSH even in the absence of IGF1. These findings show that IGF1R participates in the TSH-induced increase in NIS expression, but not that of TG, in the absence of added IGF1. The finding that linsitinib did not lower control NIS expression levels suggests that no IGF1 was present in the culture medium from an exogenous source or from cellular secretion. These data show that IGF1Rs participate in NIS up-regulation by TSH in the absence of IGF1 activation of IGF1R and support the idea that TSHR/IGF1R cross-talk occurs after specific activation of TSHR.
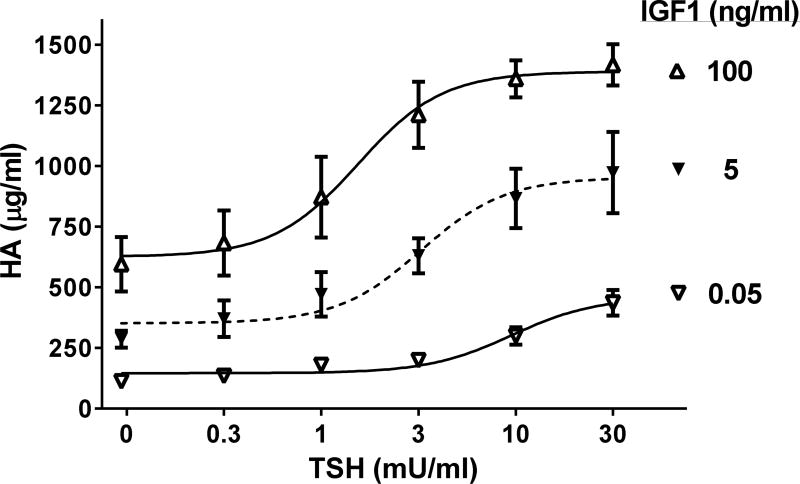
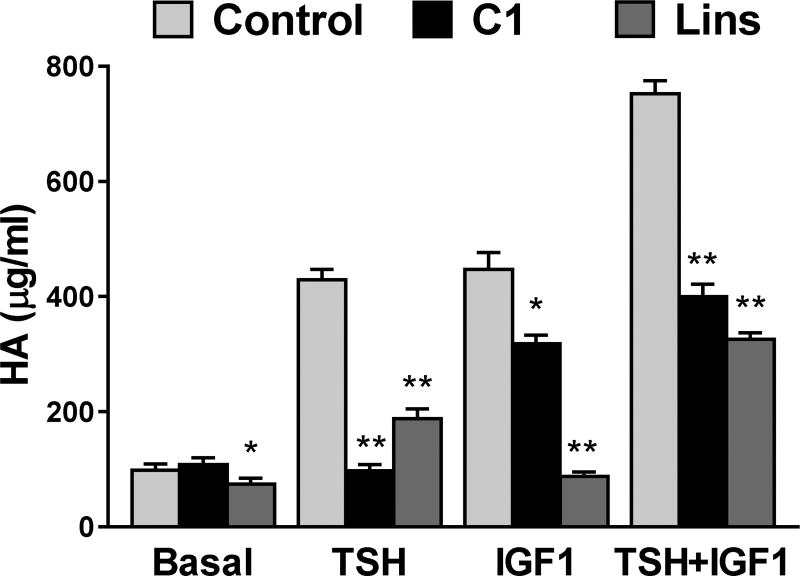
Synergistic effects of TSH and IGF1 have been found in primary cultures of GOFs also. We use stimulation of hyaluronan (hyaluronic acid, HA) production/secretion as a marker of activated GOFs as HA is a major component of the increased extracellular matrix found in patients with GO. TSHR activation by TSH results in a monophasic concentration-dependent increase in HA secretion (13). IGF1 alone increases HA secretion also. Most interestingly, we found a marked shift in TSH potency when both TSH and IGF1 receptors were simultaneously activated by their cognate ligands (Fig. 1), and the magnitude of the shift was dependent on IGF1 concentration (13). In contrast, TSH did not affect the potency of IGF1 to stimulate HA secretion. Such a shift in TSH potency by IGF1 is consistent with the idea that the activity of TSHR can be modulated by activation of IGF1R at or proximal to TSHR. There was also a synergistic increase in the maximal stimulation of HA secretion when the highest concentrations of TSH and IGF1 were administered simultaneously. We used antagonists of TSHR and IGF1R signaling to provide additional evidence of interactions between TSHR and IGF1R (Fig. 2). We found that a TSHR antagonist fully inhibited HA secretion caused by TSH and partially inhibited HA secretion stimulated by IGF1. Conversely, an IGF1R antagonist linsitinib fully inhibited HA secretion caused by IGF1 and partially inhibited secretion stimulated by TSH. These data are consistent with the idea that in GOFs both TSHR activated by TSH and IGF1R activated by IGF1 cause interactions that lead to modulation of the other receptor but that both receptors when activated separately stimulate HA secretion by other pathways also.
Figure 1. Effects of IGF1 on TSH-stimulated hyaluronan secretion by GOFs.
Adapted from (13). TSH/IGF1 synergy in hyaluronan (HA) secretion was demonstrated by the effects of IGF1 to concentration-dependently (a) increase the potency of TSH and (b) cause a more than additive increase in efficacy of maximal doses of TSH.
Figure 2. Inhibition of TSH- and IGF1-stimulated HA secretion by TSHR antagonist C1 and IGF-1R antagonist linsitinib.
Reprinted from (13). The TSHR inhibitor C1 fully inhibited TSH and partially inhibited IGF1 and TSH+IGF1 stimulation of HA secretion. The IGF1R inhibitor fully inhibited IGF1 and partially inhibited TSH and TSH+IGF1 stimulation. *P<0.03, **P<0.001
Interactions between TSHR and IGF1R constitute receptor cross-talk
Despite evidence of TSHR/IGF1R functional interactions, the studies described above in human thyrocytes and GOFs did not delineate a mechanism for synergy caused by TSH and IGF1. (We define synergy as causing a more than additive effect when TSH and IGF1 are administered simultaneously or as a shift to higher TSH potency when IGF1 is present.) Indeed, the synergistic effects were detected by measuring cellular responses at times days after agonist binding to and activation of the receptors. These responses are many steps downstream of the activated receptors. As described above, cross-talk, which is rapid and takes place at or proximal to the receptor, may occur by the following mechanisms: 1) TSHR activation leads to the production IGF1 or IGF1R stimulates production of a TSHR agonist (unlikely) that then acts in an autocrine manner to activate the receptor; 2) TSHR signaling causes Tyr phosphorylation of IGF1R or IGF1R activation causes phosphorylation (or another post-translational modification) of TSHR and this modification modulates the activity of the receptor; or 3) IGF1R signaling appropriates molecular effectors normally associated with TSHR, e.g. G proteins, or vice versa and thereby activates signaling by the other receptor’s pathway.
1) We have found no evidence of TSH or IGF1 in the media in which our cells are incubated in the absence of exogenous addition. 2) To our knowledge, no one has attempted to assess post-translational modification of TSHR resulting from IGF1R activity. Experiments in rat thyroid FRTL-5 cells detected tyrosine phosphorylation of IGF1R, the major initiating step in IGF1R signaling, in response to TSH stimulation (14). However, we could not detect any increase in IGF1R phosphorylation caused by TSH in human thyrocytes or GOFs (12,15). 3) We recently provided evidence that TSHR/IGF1R cross-talk occurs rapidly at a site more proximal to the receptors than had previously been appreciated (16). We found that TSHR stimulation of HA secretion was contingent on activation/phosphorylation of mitogen-activated protein kinase 1/3 (ERK1/2) because inhibition by U0126 of the kinase that phosphorylates ERK1/2 inhibited HA secretion. Phosphorylation of ERK1/2 occurs rapidly at a site proximal to the receptors. Furthermore, the concentration-response curve for TSH stimulation of ERK1/2 phosphorylation exhibited a higher potency, that is, a lower concentration needed for half-maximal efficacy, when IGF1 was simultaneously administered. The shift in the concentration response curve to higher potency for TSH stimulation of ERK2 phosphorylation is similar to the shift previously shown for stimulated HA secretion (13). These findings are most consistent with the idea that TSHR/IGF1R interactions occur at or near the receptors. We then used a model system of cells overexpressing TSHRs (HEK-TSHR cells) to attempt to identify the step upstream of ERK1/2 phosphorylation in the signaling pathway. In these cells, we found that stimulation of ERK1/2 phosphorylation by TSH exhibited a monophasic concentration-response curve whereas in the presence of IGF1, TSH elicited a biphasic concentration-response with the generation of a high potency (low concentrations of TSH) phase of stimulation. This finding is similar to that which we observed in GOFs. We then showed that knockdown of Gi/Go proteins in HEK-TSHR cells inhibited the high potency phase of TSH stimulation that we observed in the presence of IGF1. Thus, IGF1R appears to use Gi/Go proteins to mediate synergy demonstrated by a shift in the concentration-response curve of ERK1/2 phosphorylation.
Based on our findings in human thyrocytes, GOFs and HEK-TSHR cells, we suggest that the interaction between TSHR and IGF1R may involve mediation by Gi/Go proteins that are upstream members of the TSHR signaling cascade. These findings are consistent with the idea that TSHR/IGF1R cross-talk occurs via a mechanism similar to that described for other instances of GPCR/RTK cross-talk in which the RTK appropriates G proteins.
Implications of cross-talk for the treatment of GO
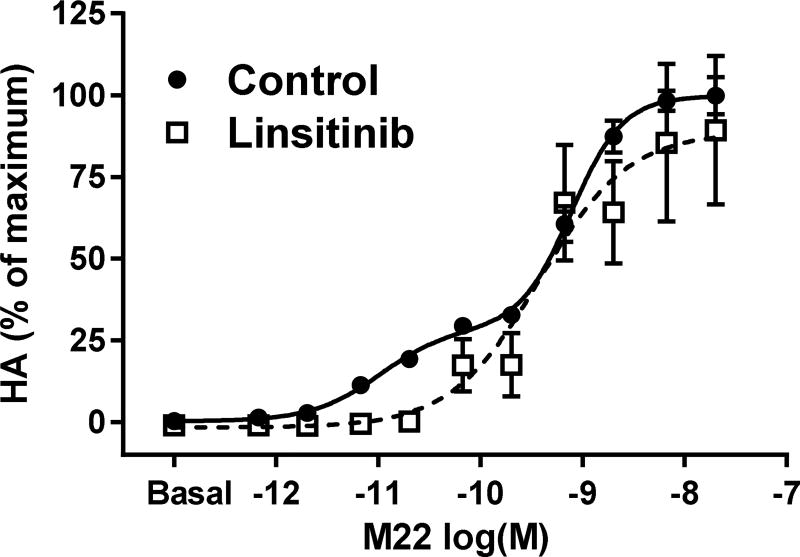
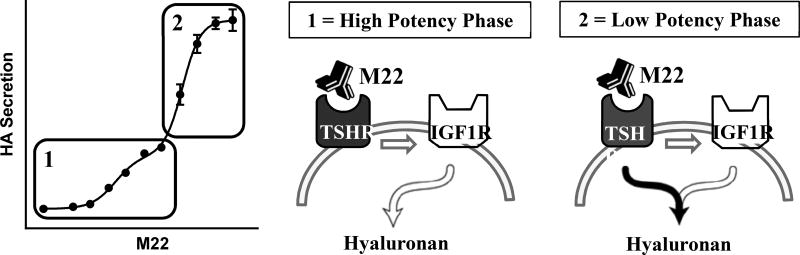
Our findings most relevant to the pathogenesis of GO were obtained when we studied signaling by a monoclonal TSHR-stimulating antibody, M22 (13,17). As shown above, the concentration-response curves for HA secretion stimulated by TSH and combined TSH and IGF1 in GOFs were monophasic. In contrast, M22 stimulated HA secretion in a biphasic concentration-dependent manner (Fig. 3). The high potency (low concentrations) phase accounted for approximately 30% of the maximal response. In the presence of the IGF1R inhibitor linsitinib, the concentration response curve was monophasic and was superimposable on the low potency (high concentrations) phase in the absence of linsitinib. These findings show that the high potency phase involved interactions with IGF1R (IGF1R-dependent) whereas the low potency phase did not involve IGF1R (IGF1R-independent). In contrast to the partial inhibition caused by linsitinib, M22 stimulation was totally inhibited by a TSHR antagonist. Hence, both phases of M22 stimulation are dependent on TSHR but only the high potency effect is dependent on IGF1R. The low potency, IGF1R-independent phase is initiated by TSHR but uses a separate signaling pathway. We recently showed directly that M22 binds specifically to TSHR and does not bind to IGF1R (15). Thus, it appears that both phases of M22 stimulation are mediated by M22 binding to and directly activating only TSHR and that the high potency, IGF1R-dependent phase is mediated by TSHR/IGF1R cross-talk. Hence, there are at least two different pathways through which TSHR stimulates HA secretion. Unlike what was described for TSH plus IGF1 interactions above, the high-potency M22 phase reveals a pathway that does not depend on ligand binding to IGF1R but still involves IGF1R. A model for the cross-talk initiated by M22 is shown in Figure 4. Its general applicability and relevance to pathology are interesting questions subject to future investigations.
Figure 3. Effect of the monoclonal TSHR stimulatory antibody M22 on hyaluronan secretion in GOFs.
Adapted from (13). M22 stimulated hyaluronan secretion (HA) in a biphasic manner. The higher potency (lower concentrations of M22) phase was inhibited by the IGF1R inhibitor linsitinib whereas the lower potency (higher concentrations of M22) was not inhibited. A TSHR antagonist inhibited both phases of M22 stimulation (not shown here).
Figure 4. Proposed model for M22-initiated TSHR/IGF1R cross-talk in Graves’ orbital fibroblasts.
Adapted from [18]. The M22 concentration-response of hyaluronan (HA) secretion is shown on the left. Both phases of the M22 response are initiated by binding only to TSHR. Phase 1 is the high potency (low concentrations) phase and phase 2 is the low potency (high concentrations) phase. Within phase 1, M22 stimulation is dependent on IGF1 receptors (white arrows) whereas in phase 2 M22 stimulation is independent of IGF1R.
We have shown that a small molecule TSHR antagonist can fully inhibit HA secretion by GOFs stimulated by M22, that is, the TSHR antagonist inhibits both phases of M22 stimulation (18). In contrast, an IGF1R antagonist, such as, the small molecule antagonist, linsitinib, or an anti-IGF1R antibody, 1H7, can only partially inhibit stimulation by M22 most likely because antagonism of the IGF1R only affects the high potency phase of stimulation. Nevertheless, studies using GOFs provided pre-clinical evidence for the use of an anti-IGF1R antibody, such as teprotumumab, in the treatment of GO (19–22). Recently, a clinical trial using teprotumumab was reported to be successful (23). We think that teprotumumab’s success likely resides in its ability to diminish TSHR/IGF1R cross-talk. However, we suggest that teprotumumab would not be as effective as TSHR antagonism and that the development of TSHR antagonists offer an attractive therapeutic approach for GO. Moreover, we have provided evidence that treatment aimed at both TSHR and IGF1R may have therapeutic advantages over treatment aimed at one of the two receptors (18). Specifically, combination therapy may be an effective strategy for dose reduction and/or to compensate for any loss of therapeutic efficacy at either receptor.
In conclusion, these findings show that TSH, IGF1 and M22 involve TSHR/IGF1R cross-talk in their mechanisms of signal transduction. In view of these observations, we suggest that both TSHR and IGF1R are targets for medical therapy and that, perhaps, targeting both receptors simultaneously may improve the therapeutic index.
Highlights.
Simultaneous activation of TSH and IGF1 receptors leads to synergistic responses
TSH and IGF1 synergism occurs proximate to the receptors (“cross-talk”)
A monoclonal TSAb activates TSHR/IGF1R cross-talk by binding only to TSHR
Abbreviations
- TSHR
Thyroid-stimulating hormone (TSH) receptor
- IGF1R
Insulin-like growth factor 1 (IGF1) receptor
- GPCR
G protein-coupled receptor
- RTK
receptor tyrosine kinase
- GO
Graves’ orbitopathy/ophthalmopathy
- TG
thyroglobulin
- TPO
thyroid peroxidase
- DIO2
deiodinase type 2
- NIS
sodium-iodide symporter
- ERK1/2
mitogen-activated protein kinase 1/3
- hyaluronic acid, HA
hyaluronan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28:602–607. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32:443–450. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology. 1986;119:940–942. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- 5.Santisteban P, Kohn LD, Di Lauro R. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J Biol Chem. 1987;262:4048–4052. [PubMed] [Google Scholar]
- 6.Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22:631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 7.Eggo MC, Bachrach LK, Burrow GN. Interaction of TSH, insulin and insulin-like growth factors in regulating thyroid growth and function. Growth Factors. 1990;2:99–109. doi: 10.3109/08977199009071497. [DOI] [PubMed] [Google Scholar]
- 8*.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181:4397–4405. doi: 10.4049/jimmunol.181.6.4397. Using co-immunoprecipitation, TSHR and IGF1R were shown to be physically associated in human fibroblasts and thyrocytes. This study was the first to show a physical association between TSHR and IGF1R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22:631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 10.Kyrilli A, Paternot S, Miot F, Corvilain B, Vassart G, Roger PP, Dumont JE. Commentary. Front Endocrinol. 2017 Aug 25;8 doi: 10.3389/fendo.2017.00214. Article 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan SJ, Neumann S, Marcus-Samuels B, Gershengorn MC. Thyrotropin Stimulates Differentiation Not Proliferation of Normal Human Thyrocytes in Culture. Front Endocrinol (Lausanne) 2016;7:168. doi: 10.3389/fendo.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Morgan SJ, Neumann S, Marcus-Samuels B, Gershengorn MC. Thyrotropin and Insulin-Like Growth Factor 1 Receptor Crosstalk Upregulates Sodium-Iodide Symporter Expression in Primary Cultures of Human Thyrocytes. Thyroid. 2016;26:1794–1803. doi: 10.1089/thy.2016.0323. TSHR/IGF1R cross-talk was shown to synergisitically up-regulate the expression of NIS in human thyrocytes in culture. This study was the first to show that TSHR/IGF1R cross-talk occurred in human thyrocytes rapidly and at a step proximate to TSHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins. The Journal of clinical endocrinology and metabolism. 2015;100:1071–1077. doi: 10.1210/jc.2014-3566. Simultaneous activation by TSH and IGF1 were shown to synergistically increase HA secretion and to shift the concentration-response curve to TSH to higher potency in human Grave's orbital fibroblasts. The concetration-response curve to the monoclonal TSAb M22 was shown to be biphasic with the hgier potency phase dependent on IGF1R and the lower potency phase independent of IGF1R. This study was the first to show that a TSAb could initiate TSHR/IGF1R cross-talk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condorelli G, Formisano P, Miele C, Beguinot F. Thyrotropin regulates autophosphorylation and kinase activity in both the insulin and the insulin-like growth factor-I receptors in FRTL5 cells. Endocrinology. 1992;130:1615–1625. doi: 10.1210/endo.130.3.1311244. [DOI] [PubMed] [Google Scholar]
- 15**.Krieger CC, Neumann S, Marcus-Samuels B, Gershengorn MC. TSHR/IGF-1R Cross-Talk, Not IGF-1R Stimulating Antibodies, Mediates Graves' Ophthalmopathy Pathogenesis. Thyroid : official journal of the American Thyroid Association. 2017;27:746–747. doi: 10.1089/thy.2017.0105. This study showed that the monoclonal TSAb M22 binds to TSHR but not to IGF1R. This finding presented direct evidence that a TSAb could initiate TSHR/IGF1R cross-talk by binding only to TSHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Krieger CC, Perry JD, Morgan SJ, Kahaly GJ, Gershengorn MC. TSH/IGF-1 Receptor Cross-talk Rapidly Activates Extracellular Signal-regulated Kinases in Multiple Cell Types. Endocrinology. 2017 doi: 10.1210/en.2017-00528. https://doi.org/10.1210/en.2017-00528 . This study showed that TSHR/IGF1R cross-talk can occcur in Graves' orbital fibroblasts and human thyrocytes, and model cells made to over-express TSHRs. This allowed for the demonstration that G i /G o proteins mediate the rapid TSHR/IGF1R cross-talk responses. This was the first demonstration that the mechanism of TSHR/IGF1R cross-talk was similar to the cross-talk of other G protein-coupled receptors and receptor tyrosine kinases in that it occurred proximal to the receptors by IGF1R using a G protein originally thought to associate only with G protein-coupled receptors. [DOI] [PMC free article] [PubMed]
- 17.Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. TSH/IGF-1 Receptor Cross Talk in Graves' Ophthalmopathy Pathogenesis. The Journal of clinical endocrinology and metabolism. 2016;101:2340–2347. doi: 10.1210/jc.2016-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Place RF, Krieger CC, Neumann S, Gershengorn MC. Inhibiting thyrotropin/insulin-like growth factor 1 receptor crosstalk to treat Graves' ophthalmopathy: studies in orbital fibroblasts in vitro. Br J Pharmacol. 2017;174:328–340. doi: 10.1111/bph.13693. This study shows that stimulation of HA secretion by Graves' orbital fibroblasts in culture by the monoclonal TSAb M22 can be inhibited completely by a TSHR antagonist and partially by an IGF1R antagonist. Moreover, that treatment with both types of antagonists could lower the doses needed to achieve complete inhibition. The results of this study suggest that targeting both receptors would lead to an increase in the therapeutic index when used to treat Graves' oritopathy in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. The Journal of clinical endocrinology and metabolism. 2012;97:1681–1687. doi: 10.1210/jc.2011-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith TJ, Hegedus L, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:291–302. doi: 10.1016/j.beem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. The Journal of clinical endocrinology and metabolism. 2014;99:E1635–1640. doi: 10.1210/jc.2014-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Shan SJ, Mester T, Wei YH, Douglas RS. TSH-Mediated TNFalpha Production in Human Fibrocytes Is Inhibited by Teprotumumab, an IGF-1R Antagonist. PLoS One. 2015;10:e0130322. doi: 10.1371/journal.pone.0130322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for Thyroid-Associated Ophthalmopathy. N Engl J Med. 2017;376:1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]