Abstract
Increasing evidence indicates that multiple actions of the immune system are closely intertwined with the development of depression and subsequent recovery processes. One of these interactions is substantial evidence that the TH17 subtype of CD4+ T cells promotes susceptibility to depression-like behaviors in rodents. Comparing subtypes of CD4+ T cells, we found that administration of TH17 cells, but not TH1 cells or TREGS, promoted susceptibility to learned-helplessness depressive-like behavior and accumulated in the hippocampus of learned helpless mice. Adoptively transferred TH17 cells into Rag2−/− mice that are devoid of endogenous T cells increased susceptibility to learned helplessness, demonstrating that increased peripheral TH17 cells are capable of modulating depression-like behavior. Moreover, in wild-type mice, adoptively transferred TH17 cells accumulated in the hippocampus of learned-helpless mice and induced endogenous TH17 cell differentiation. Hippocampal TH17 cells from learned-helpless mice expressed markers of pathogenic TH17 cells (CCR6, IL-23R) and of follicular cells (CXCR5, PD-1), indicating that the hippocampal cells are TFH-17-like cells. Knockout of CCR6 blocked TH17 cells from promoting learned helplessness, which was associated with increased expression of PD-1 in CCR6-deficient TH17 cells. In summary, these results reinforce the conclusion that depression-like behaviors are selectively facilitated by TH17 cells, and revealed that these cells in the hippocampus of learned helpless mice display characteristics of TFH-17-like cells, which may contribute to their pathogenic actions in promoting depression.
1. Introduction
CD4+ cells are present in the brain (Korn and Kallies, 2017), but their functions in the brain remain to be fully determined. It is thought that CD4+ cells survey the microenvironment and help to maintain homeostasis. CD4+ cells modulate mood and learning and memory, actions that mainly have been demonstrated using lymphopenic mice, such as T cell- and B cell-deficient Rag2−/− mice, that were replenished with adoptive transfer of splenocytes or lymphoid cells (Brachman et al., 2015; Clark et al., 2016; Filiano et al., 2017). Little is known about the role of CD4+ cells in modulating depressive-like behavior in non-lymphopenic mice or about which subset of CD4+ cells modulates behavior.
We previously reported that T helper (TH) CD4+ cells expressing IL-17A (TH17) cells promote susceptibility to depressive-like behaviors in non-lymphopenic mice and accumulate in the brains of mice that exhibit depressive-like behaviors (Beurel et al., 2013). The signature cytokine of TH17 cells is IL-17A (Korn et al., 2009), and the transcription factor RAR-related orphan receptor gamma (RORγ)T is required for TH17 cell differentiation (Ivanov et al., 2006). The limited information available about the role of TH17 cells in depression (Beurel and Lowell, 2017) includes the correlative association that depressed patients have elevated blood levels of TH17 cells (Chen et al., 2011), in vitro activation of CD4 cells isolated from patients with generalized anxiety disorder induces them to acquire a TH17 phenotype (Ferreira et al., 2011; Vieira et al., 2010), and patients with autoimmune diseases with elevated TH17 cells often exhibit comorbid depression (Kurd et al., 2010; Patten et al., 2017). Consistent with these findings, IL-17A was elevated in some (Chen et al., 2011; Davami et al., 2016), but not all (Kim et al., 2013; Liu et al., 2012), depressed patients, IL-17A levels predicts treatment response to certain antidepressants (Jha et al., 2017), administration of IL-17A in rodents promotes depressive-like behaviors (Nadeem et al., 2017), and anti-IL-17A therapy induces remission of depression in 40% of psoriasis patients experiencing moderately severe depression (Griffiths et al., 2017). However, the mechanisms of action of TH17 cells in depression remain unclear. One facet of this requires identification of the localization, the source, and the characteristics of TH17 cells associated with depression to determine if TH17 cells might represent a potential new biomarkers for depression.
TH17 cells are elevated in several autoimmune diseases and rodent models and are considered to be pathogenic for the central nervous system (CNS) (Cua et al., 2003). Not all TH17 cells are pathogenic though (Awasthi and Kuchroo, 2009), and the acquisition of the pathogenic phenotype of TH17 cells results from changes in the expression of proteins involved in pathology (Lee et al., 2012). Thus, it is well-accepted that the acquisition of the pathogenic phenotype occurs after expression of the IL-23 receptor (IL-23R) by TH17 cells (Ghoreschi et al., 2010; Langrish et al., 2005), which is induced after stimulation by IL-21. Although IL-23 is not required for the differentiation of TH17 cells, IL-23 is required to maintain and stabilize pathogenic TH17 cells (McGeachy et al., 2009), to suppress IL-10 production (McGeachy et al., 2007), and to promote the production of effector molecules (El-Behi et al., 2011). Besides IL-23R expression, IL-21 also promotes expression of IL-17 and RORγT, and RORγT promotes C-C chemokine receptor type 6 (CCR6) expression (Hirota et al., 2007). CCR6 is a chemokine receptor that promotes the recruitment of pathogenic T cells to inflammatory sites (Hirota et al., 2007; Kohler et al., 2003). CCR6 expression in human TH17 cells is associated with an increased memory phenotype (Acosta-Rodriguez et al., 2007; Annunziato et al., 2007). In addition, the ligand of CCR6, chemokine (C-C motif) ligand 20 (CCL20), is induced by IL-17, suggesting that TH17 cells might induce a positive loop that attracts further TH17 cells to the site of inflammation. However, the actual migratory effect of CCR6 on TH17 cells in vivo remains to be determined. It has also been proposed that upon specific antigen activation, TH17 cells may develop into subtypes, including (1) effector TH17 cells with upregulated CCR6 expression that migrate to target organs, (2) memory TH17 cells that remain in secondary lymphoid organs, or (3) an intermediate subset of TH17 cells that express high levels of IL-23R, inducible T cell costimulator (ICOS), and CXCR5, that share characteristics with T follicular helper (TFH) cells and that are present in germinal centers (Bauquet et al., 2009). TFH cells are thought to be distinct from conventional CD4 cells (Nurieva et al., 2008; Nurieva et al., 2009) and were first characterized by the expression of the chemokine receptor CXCR5, which supports their migration to germinal centers.
Here, we describe the localization and distinct characteristics of the TH17 cells found in the hippocampus of learned helpless mice. These TH17 cells were found to have the phenotype of pathogenic and follicular TH17 cells, identifying a potential new TH17 cell population implicated in brain immunity that modulates depressive-like behaviors.
2. Materials and methods
2.1. Mice
Male C57BL/6 wild-type, Rorc(γT)+/GFP, CD45.1, Rag2−/−, CCR6−/− mice were used except when indicated that female mice were used. Mice were either bred in the University of Miami animal facility or purchased from Taconic or Jackson Laboratories. 6-10 week-old mice were used to prepare T cells in vitro and 8-12 week old mice were used for behavioral assessments. For each experiment, either Jackson or Taconic mice were used. The Rorc(γT)+/GFP mice were obtained by crossing Rorc(γT)+/GFP x Rorc(γT)+/GFP, to produce 50% Rorc(γT)+/GFP, 25% wild-type, and 25% Rorc(γT)GFP/GFP mice, and behavioral experiments were carried out with littermates. Mice were housed in light and temperature controlled rooms and treated in accordance with NIH and university Institutional Animal Care and Use Committee regulations.
2.2. Learned-helplessness
Learned-helplessness was measured using a standard learned-helplessness paradigm or a modified reduced intensity inescapable shock protocol, as described previously (Beurel et al., 2013). The reduced intensity paradigm is used so wild-type C57BL/6 mice do not develop learned helplessness, allowing measurements of increased susceptibility to learned helplessness after T cells transfer.
Briefly, mice were placed in one side of a Gemini Avoidance system shuttle box (San Diego Instruments, San Diego, CA, USA or Med Associates, St Albans, VT, USA) with the gate between chambers closed. For standard learned helplessness, 180 inescapable foot shocks were delivered at an amplitude of 0.3 mA, a duration of 6-10 s per shock, and a randomized inter-shock interval of 5–45 s (Beurel et al., 2013). In a modified inescapable shock protocol, referred to as the reduced intensity learned helpless protocol, mice were given 180 foot shocks with amplitude of 0.3 mA and fixed 4 s shock duration, and a randomized inter-shock interval of 5–45 s. Twenty-four hours after inescapable foot shocks, mice were returned to the shuttle box and the escape task was tested by giving 30 escape trials with each trial using a 0.3 mA foot shock for a maximum duration of 24 s. The door of the chamber opens at the beginning of the foot shock administration to allow mice to escape. Latency to escape the shock was recorded using Gemini software, and trials in which the mouse did not escape within the 24 s time limit were counted as escape failures. Mice with greater than 15 escape failures were defined as learned helpless. When indicated, mice received i.v. TH17 cells prepared as described below.
2.3. TH17 cell preparation and transfer
CD4+ cells were isolated as described previously (Beurel et al., 2013). CD4+ cells cultured with irradiated (3,000 rads) splenic feeder cells were activated with 2.5 μg/mL of anti-CD3 (clone 145-11, Bio X Cell), and differentiated to TH17 cells by addition of IL-6 (20 ng/mL; Peprotech), TGFβ (5 ng/mL, Peprotech), anti-IL-4 (10 μg/mL; clone 11B11, Bio X Cell) and anti-IFNγ (10 μg/mL; clone XMG1.2, Bio X Cell), to Th1 cells by addition of IL-12 (10 ng/mL, R&D Systems) and anti-IL-4 (10 μg/mL; clone 11B11 Bio X Cell), or to Tregs by addition of TGFβ (5 ng/mL, Peprotech), anti-IL-4 (10 μg/mL; clone 11B11, Bio X Cell) and anti-IFN-γ (10 μg/mL; clone XMG1.2, Bio X Cell) in R10 medium (RPMI 1640 medium supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 1 × non-essential amino acids, 1 μM sodium pyruvate, 2.5 μM β-mercaptoethanol and 2 mM L-glutamine). After 4 days of differentiation toward TH17 cells, some cells were transfected with 150 pmole of scrambled siRNA or CCR6 siRNA and lipofectamine RNAiMAX according to the manufacturer’s instructions (Invitrogen) for 24 h. To inject the cells after differentiation, cells were recovered after histopaque gradient purification, and resuspended in PBS. An aliquot of cells was used to evaluate the percent of TH17 cells, Th1 cells, or Tregs, and ~5-20×106 undifferentiated CD4, TH17, TH1 or TREGS cells were injected i.v. in 200μL PBS by tail vein 24-48 h before behavioral testing. Depending on the number of mice to be injected, cells transferred were pooled from 1-2 donors.
2.4. Perfusion and Intracellular staining
Intracellular cytokine staining was carried out as described previously (Beurel et al., 2013). Immediately after behavior measurements, mice were anesthetized and spleens were recovered, and mice were perfused with PBS transcardially, and the brains were recovered. The hippocampi or other brain regions when indicated were dissected with care taken to not include meninges or choroid plexus, then passed through a 70 μm cell strainer (BD Bioscience) and the cell suspension was mixed (vol/vol) to obtain a 30% Percoll/R1 medium that was overlayed on 70% Percoll/R1 medium in a centrifuge tube, and centrifuged at 2000 rpm for 10 min without using the brake. The cells at the interface of the 30/70% Percoll gradient were recovered, washed one time, resuspended in R10 media, aliquoted into a 96 well plate, and stimulated for 4 h with phorbol myristate acetate (PMA, 50 ng/mL; Alexis) and ionomycin (750 ng/mL; Sigma) in the presence of Protein Transport Inhibitor Cocktail (eBioscience) at the recommended concentrations. Standard intracellular cytokine staining was carried out as described (Beurel et al., 2013) using the Staining Intracellular Antigens for Flow Cytometry Protocol (eBioscience). Cells were first stained extracellularly with FITC-conjugated anti-CD45 (to identify myeloid cells), PECy7-onjugated anti-CD11c (to identify DCs), PE-conjugated anti-F4/80 (to identify activated cells), eFluor450-conjugated anti-CD8, APC–conjugated anti-CD4 (eBioscience), or APC–conjugated anti-CD4, PE-Cy7-conjugated anti-CXCR5, PerCP-conjugated anti-CCR6, FITC-conjugated IL-23R, PerCP-conjugated anti-ICOS, eFluor450-conjugated anti-PD-1, or APC-eFluor 780 anti-CCR7, PerCP-conjugated anti-CD45.1, APC-conjugated anti-CD45.2 and fixed and permeabilized with permeabilization solution (eBioscience) and then were stained intracellularly with eFluor450-conjugated anti-IFN-γ (which is used in combination with CD4+ staining to identify Th1 cells), phycoerythrin-conjugated anti-IL-17A (to identify TH17 cells), or FITC-FoxP3 (to identify Tregs), APC-conjugated anti-Bcl6 (eBioscience) or GFP was analyzed. Intracellular cytokine staining was carried out as described previously (Beurel et al., 2011). All the catalog numbers of the antibodies are displayed in Supplementary Table 1.
2.5. qRT-PCR
RNA from hippocampus was extracted by TRIzol using the manufacturer’s instructions (Invitrogen). RNA was retrotranscribed using ImProvII reverse transcriptase (Promega). Gene expression of the Mouse TH17 Response was analyzed by RT2 Profiler PCR array by the manufacturer’s instructions (PAMM-073ZA Qiagen) or individual gene expression of CCL20 (5’-CGACTGTTGCCTCTCGTACA-3’and 5’-CACCCAGTTCTGCTTTGGAT-3’), IL-17A (5’-CTCCAGAAGGCCCTCAGACTAC-3’ and 5’-GGGTCTTCATTGCGGTGG-3’), ICOS (5’-TGACCCACCTCCTTTTCAAG-3’ and 5’-TTAGGGTCATGCACACTGGA-3’, c-maf (5’- ACTGAACCGCAGCTGCGCGGGGTCAG-3’ and 5’-CTTCTCGTATTTCTCCTTGTAGGCGTCC-3’, IL-27 (5’-ACTTTCCAGGCATGGCATCA-3’ and 5’-GAAGGGCCGAAGTGTGGTAG-3’) and BCL-6 (5’-CACACCCGTCCATCATTGAA-3’ and 5’-TGTCCTCACGGTGCCTTTTT-3’) was assessed using SYBR green mix from qRT-PCR using a Jena Analytika instrument and the 2−ΔΔCt method was used to quantify the results.
2.6. Statistical analysis
Histograms represent mean±SEM. Statistical significance was analyzed with a one-way analysis of variance (ANOVA) for multiple comparisons with Bonferroni post-hoc test or with unpaired/paired t-test or Mann-Whitney using Prism software. p<0.05 was considered significant.
3. Results
3,1. TH17 cells but not TH1 cells promote depressive-like behaviors
We previously showed that TH17 cell administration promoted depressive-like behaviors in wild-type mice (Beurel et al., 2013). To further demonstrate that exogenous TH17 cell administration, but not proinflammatory TH1 cells, were sufficient to promote learned-helplessness, we tested if administration of TH17 or TH1 cells was sufficient to promote learned-helplessness in T cell- and B cell-deficient Rag2−/− mice. Most Rag2−/− mice given undifferentiated CD4 cells did not exhibit learned-helplessness in the reduced intensity paradigm (Fig 1A-B) that did not induce learned-helplessness in most wild-type mice (Beurel et al., 2013). TH17 or TH1 cells adoptively transfer into male Rag2−/− mice revealed that only TH17 cells, but not TH1 cells, significantly increased susceptibility to learned-helplessness (Fig 1B). These results show that TH1 cell administration does not promote susceptibility to learned-helplessness, in contrast to TH17 cells.
Figure 1:

TH17 cells promote learned-helplessness and accumulate in the hippocampus and PFC. (A) CD4+ T cells were differentiated toward either TH17, TH1 or undifferentiated CD4+ cells for 4 days in vitro and adoptively transferred to male and female Rag2−/− (B) or male wild-type (C) mice. In (B) 18% of the CD4+ cells were TH17 cells in mice receiving TH17 cells, and 39% of the CD4+ cells were TH1 cells in mice receiving TH1 cells. In mice receiving CD4+ cells, less than 1% of CD4+ cells were TH17 cells or TH1 cells. 48 h after transfer mice were subjected to the reduced paradigm of learned-helplessness. The number of escape failures was recorded. Each symbol represents an individual mouse. Mean ± SEM, one-way ANOVA, F(2,36)=4.037, Bonferroni post hoc test *p<0.05 (n=7-14). (C) Mice were subjected or not to the learned-helplessness (LH) paradigm, and were separated into 3 groups: non-shocked (NS), shocked and exhibiting >15/30 escape failures (D), or shocked and not learned-helpless (<15 escape failures, ND). (D-F) Mice were perfused, and hippocampus and PFC were processed to obtain dissociated cells. Cells were stained for CD4 and IL-17A (D), IFNγ (E) or Foxp3 (F), and gated on CD4+ cells and analyzed by flow cytometry. Data represent mean ± SEM, one-way ANOVA, F(5, 30)=10.26 (E), F(5,39)=14.64 (F), Bonferroni post hoc test *p<0.05 (n=4-11).
3.2. TH17 cells accumulate in the hippocampus and the prefrontal cortex (PFC) after induction of learned-helplessness.
Because TH17 cells increase susceptibility to depressive-like behaviors and accumulate in whole mouse brain after stress (Beurel et al., 2013), we investigated if after learned-helplessness induction, endogenous TH17 cells localize in two brain regions that are associated with depression, the PFC and hippocampus. Brain region T cells were measured in mice exhibiting learned-helplessness (D), in mice that underwent the same protocol but did not develop learned-helplessness (ND, Fig 1C and S1A), and in unshocked mice (NS). There was a significant increase of TH17 cells (Fig 1D) but not TH1 cells (Fig 1E) in the hippocampus of mice exhibiting learned-helplessness, whereas both TH17 and TH1 cells accumulated in the PFC. There were no differences in TH17 and TH1 cells in the cerebellum between the ND and D groups (Fig S1B-C). These results indicate that TH17 cells accumulate in both the hippocampus and PFC after learned-helplessness induction, whereas TH1 cells accumulate in the PFC. This was not the result of an overall influx of CD4+cells in these brain regions, as the TREGS population was unchanged by learned-helplessness in these brain regions (Fig 1F) and in the cerebellum (Fig S1D). Moreover, TREGS transfer did not change susceptibility to learned-helplessness (Fig S2). Because of the selective accumulation of TH17 cells in the hippocampus of learned-helpless mice, further experiments focused on hippocampal TH17 cells.
3.3. Peripheral CD4+cells infiltrate the hippocampus after learned-helplessness
Because in vitro TH17 differentiation requires 5 days, but brain TH17 cells are detected after the learned-helplessness paradigm which lasts 2 days, we tested if adoptively transferred undifferentiated CD4 cells or TH17 cells infiltrate the hippocampus after learned-helplessness or if host TH17 cells differentiate during learned-helplessness. To trace the fate of the cells we used the CD45.1/CD45.2 markers after adoptively transferring undifferentiated CD45.1+CD4+ or TH17-polarized CD45.1+CD4+ cells to a CD45.2 recipient mouse, and subjecting the recipient mice to the reduced-intensity learned-helplessness paradigm (Fig 2A). The mice received similar numbers of undifferentiated CD4 cells or TH17 cells, as shown by equivalent levels of CD45.1+CD4+ cells in the spleens (Fig S3A). Donor CD45.1+CD4+ cells were found in the hippocampi after learned-helplessness even though, being undifferentiated, they are not sufficient to promote learned-helplessness (Beurel et al., 2013) (Fig 2C). Furthermore, hippocampal levels of CD45.1+CD4+ cells were similar between mice that received undifferentiated CD4 cells or TH17 cells, suggesting that the infiltration of CD4 cells is independent of their differentiation status (Fig 2C). However, it is important to note that the CD4+ cells found in the hippocampus after learned-helplessness were predominantly from the host mice (CD45.2) after receiving CD45.1 TH17 cells (Fig 2A). In contrast, TH17 cells in the hippocampus after learned-helplessness originated predominantly from the donor cells in mice receiving CD45.1 TH17 cells, suggesting that adoptively transferred TH17 cells after learned-helplessness infiltrated the hippocampus (Fig 2D).
Figure 2:

Adoptively transferred TH17 cells accumulate in the hippocampus after the learned-helplessness paradigm. (A) CD45.2 wild-type recipient mice received donor CD45.1+CD4+ T cells or CD45.1+ TH17 cells, and 48 h later were subjected to the learned-helplessness paradigm. 86% of the CD4+T cells transferred in the TH17 cells preparation were TH17 cells, whereas less than 1% TH17 cells were detected in the CD4 preparation, and 99.3% of the injected CD4 cells were CD45.1. (B) CD4+ CD45.1+ (donor cells) or CD4+CD45.2+ (recipient cells) present in the hippocampus of mice adoptively transferred with CD4 cells or TH17 cells were analyzed by flow cytometry after gating on CD4+ cells (C) CD45.1+CD4+ (donor cells) or CD45.2+IL-17A+CD4+ (recipient cells) present in the hippocampus of mice adoptively transferred with CD4 cells or TH17 cells were analyzed by flow cytometry after gating on CD45+CD4+ cells. Each symbol represents an individual mouse. Data represent mean±SEM, one-way ANOVA, F(3, 16) = 9.522, Bonferroni post-hoc test *p<0.05 (n=5). (D) CD45.1+IL-17A+CD4+ (donor cells) or CD45.2+IL-17A+CD4+ (recipient cells) present in the hippocampus of mice adoptively transferred with CD4 cells or TH17 cells were analyzed by flow cytometry after gating on CD45+IL-17A+CD4+ cells. Each symbol represents an individual mouse. Data represent mean±SEM, one-way ANOVA, F(3, 14)=9,795, Bonferroni post-hoc test *p<0.05 (n=4-5).
3.4. Learned-helplessness induces the recruitment of host TH17 cells in the hippocampus.
Because TH17 cells from the periphery infiltrate the brain during learned-helplessness, the next question was to determine if peripheral TH17 cells promote de novo TH17 cell differentiation. To determine this, we used RORγT+/GFP mice, where a GFP reporter cDNA was knocked-in at the site of initiation of RORγt translation, so CD4+cells committed toward TH17 cells express GFP. We previously reported that RORγT+/GFP mice express a very low level of IL-17A (Beurel et al., 2011) and exhibit resistance to learned-helplessness (Beurel et al., 2013). We found that administration of wild-type TH17 cells restored learned-helplessness susceptibility of RORγT+/GFP mice, as 81% of RORγT+/GFP mice receiving TH17 cells developed learned-helpless compared to 33% of RORγT+/GFP mice receiving undifferentiated CD4+cells (Fig 3A-B). To trace the fate of the TH17 cells, another cohort of RORγT+/GFP mice received wild-type TH17 cells and were subjected to learned-helplessness 2 days later. TH17 cells were analyzed in mice that developed learned-helplessness (D), in mice that were resilient (ND), and in non-shocked (NS) mice (Fig 3A). There was no difference in the percent of TH17 cells present in the hippocampus between learned-helpless and resilient mice, indicating that the migration of the donor TH17 cells to the hippocampus was induced by stress but was independent of the depressive status of the mouse (Fig 3C). Also, without foot shocks transferred TH17 cells did not accumulate in the hippocampus (Fig 3C). However, the population of hippocampal CD4+cells expressing GFP, representing the induced host TH17 cells, was 2-fold higher in the learned-helpless mice compared to resilient mice, indicating a depression-associated induction of host hippocampal TH17 cells (Fig 3D). This was not due to an induction of GFP+CD4+cells throughout the body because spleen GFP+CD4+cells were not different between learned-helpless and resilient mice (Fig 3E-F). Also, the induction of GFP+CD4+cells was restricted to mice receiving foot shocks, as CD4+cells from non-shocked mice did not express hippocampal GFP whether or not they received TH17 cells. This demonstrated that host TH17 cells were induced and accumulated in the hippocampus after TH17-associated induction of learned-helplessness.
Figure 3:
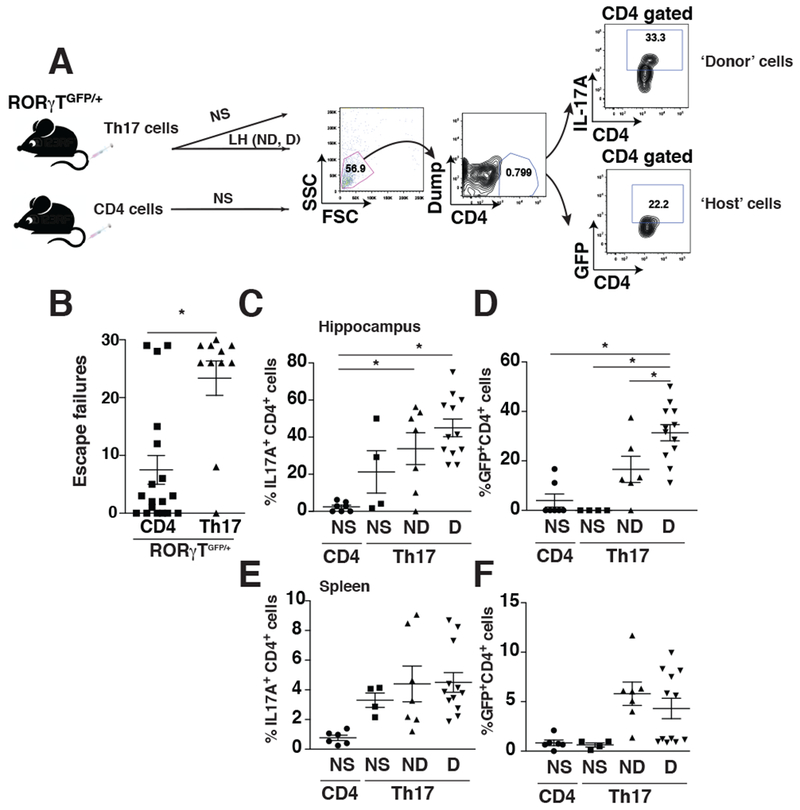
Induction of learned-helplessness increases the induction of GFP in RORγTGFP/+ mice. (A-B) RORγTGFP/+ mice adoptively transferred with undifferentiated CD4 cells or TH17 cells 48 h before being subjected to the reduced learned-helplessness paradigm, and the number of escape failures was recorded. Each symbol represents an individual mouse. Data represent mean±SEM, Mann Whitney, U=37.5, *p<0.05, (n=11-18). (C-F) Unshocked (NS) mice or mice transferred with TH17 cells (Th17), or mice exhibiting learned-helplessness (D) or non-learned-helpless mice (ND) that received TH17 cells were perfused after the last shock and hippocampal (C-D) or splenic (E-F) cells were dissociated. CD4+ T cells were stained for IL-17A, and both IL-17A+CD4+ T cells (C, E) and GFP+CD4+ T cells (D, F) were evaluated by flow cytometry after gating on CD4+ cells as described in panel (A). Each symbol represents an individual mouse. Data represent mean ±SEM, one-way ANOVA, F(3, 26)= 9,492 (B), F(3,25)=15.75 (C), Bonferroni post hoc test *p<0.05 (n=4-12).
3.5. Hippocampal TH17 cells express CCR6 and IL-23R after learned-helplessness.
Because TH17 cells infiltrated the hippocampus during learned-helplessness and de novo differentiation of TH17 cells occurred, we hypothesized that endogenous TH17 cells in the hippocampus of learned helpless mice might express pathogenic markers that might control migration and/or depression-associated processes. We found that in learned-helpless mice hippocampal IL-17A mRNA levels were 1.65-fold levels of resilient or unshocked mice (Fig 4A). Using a mouse TH17 PCR Array, we found that the mRNA levels of 5 factors were increased and 2 factors were decreased in the hippocampus of learned-helpless mice compared to resilient mice using a cut-off difference of 4-fold (Fig 4B). Among these factors, the TFH cell pathway, comprising ICOS and IL-21, and the pathogenic marker CCR6, were increased (Fig 4B). We confirmed by qRT-PCR that two TFH cell markers, ICOS and c-maf, were increased in the hippocampus of learned-helpless mice compared to resilient mice or unshocked mice, and were increased in resilient mice compared to unshocked mice (Fig 4C-D). However, two other TFH cell markers, IL-27 and Bcl-6, were not different between groups (Fig 4E-F). These findings raised the possibility that hippocampal IL-17A-producing T cells in learned-helpless mice express markers of pathogenic TH17 cells and some markers that are normally characteristic of TFH cells. Consistent with this, the population of hippocampal TH17 cells expressing CCR6 was higher in learned-helpless mice than in resilient and unshocked mice (Fig 4G). Furthermore, hippocampal CCL20, the CCR6 ligand, mRNA was 5-fold higher in learned-helpless mice compared to resilient mice or unshocked mice (Fig 4H), suggesting that the CCL20/CCR6 axis is activated in hippocampal TH17 cells. Furthermore, the expression of another marker of pathogenic TH17 cells, IL-23R, was higher in IL-17A+CD4+cells in the hippocampus of learned-helpless mice than in resilient mice or unshocked mice (Fig 4I). Altogether these data revealed that pathogenic TH17 cell and TFH cell markers are elevated in the hippocampus of learned-helpless mice.
Figure 4:

Follicular and pathogenic markers are elevated in the hippocampus of learned-helpless mice. (A) Mice were subjected or not to the learned-helplessness paradigm, and mice were separated into 3 groups: learned-helpless (D), non-learned-helpless (ND) and non-shocked (NS) as described in Fig 1D. Immediately after the last foot shocks, hippocampi were collected and mRNA extracted to measure by qRT-PCR: IL-17A mRNA (A), an array of TH17 cell-related genes (B), ICOS mRNA (C), c-maf mRNA (D), IL-27 mRNA (E) and Bcl6 mRNA (F). Genes upregulated or downregulated more than 4-fold are reported in (B). Each symbol represents an individual mouse. Data represent mean±SEM, One-way ANOVA, F(2, 12)=6.212 (A), F(2, 25) = 20.21 (C), F(2,18)=57.66 (D), Bonferroni post-hoc test *p<0.05 (n=4-10). (G-I) Mice were subjected to the learned-helplessness paradigm, or not, and mice were separated into 3 groups: unshocked (NS), learned-helpless (D) and non-learned-helpless (ND) mice. Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for CD4, IL-17A, and either CCR6 (G) or IL-23R (I), and gated on IL-17A+ CD4+ cells. (H) mRNA was extracted from hippocampus and CCL20 expression was analyzed by qRT-PCR. Each symbol represents an individual mouse. Data represent mean±SEM, one-way ANOVA, F(2, 23)= 11.59 (A), F(2, 15)=16.50 (B), F(2,33)=8.034 (C), Bonferroni post-hoc test *p<0.05 (n=8-17).
3.6. TFH-17-like cells accumulate in the hippocampus of learned-helpless mice
We investigated further the follicular cell phenotype and found that TFH cells (CXCR5+CD4+) were enriched in the hippocampi of learned-helpless mice compared to resilient or unshocked mice (Fig 5A-B). TFH cells expressing IL-17A were increased in the hippocampi of learned-helpless mice (Fig 5C-D), but there was not an increase of TH17 cells not expressing CXCR5 (Fig 5E-F) or of TFH cells expressing IFNγ (TFH-1-like cells) (Fig 5G-H), compared with resilient or unshocked mice. These results indicate that the TH17 cells (IL-17A+) accumulated in the hippocampus of learned-helpless mice are actually TFH-17-like cells.
Figure 5:

TH17 cells associated with learned helplessness present a follicular phenotype. Mice were subjected to the learned-helplessness paradigm, or not, and mice were separated into 3 groups: unshocked (NS), learned-helpless (D) and non-learned-helpless (ND) as described in Fig 1D. Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for CD4, CXCR5 and gated on CD4+ cells (A-B) or cells were stained for CD4, CXCR5, IL-17A and gated (C-D) or not (E-F) on CXCR5+CD4+ cells; or cells were stained for CD4, CXCR5 and either IFNγ (G-H), or ICOS (I-J), or IL-21 (M-N), and gated on CXCR5+CD4+ cells (G-L); or cells were stained for CD4, ICOS and gated on CD4+ cells (K-L) or CD4, IL-21 and gated on CD4+ICOS+ cells (O-P). Representative plots of flow cytometry are presented in A, C, E, G, I, K, M and O, and quantifications are presented in B, D, F, H, J, L, N and P. Each symbol represents an individual mouse. Data represent mean± SEM, one-way ANOVA, F(2,32)=13.33 (B), F(2,38)= 11.20 (D), Bonferroni post-hoc test and Mann-Whitney, U=14 (J), *p<0.05 (n=4-20).
The hippocampal TFH-17-like cells associated with learned-helplessness were activated, as they express ICOS (Bauquet et al., 2009), whereas TFH-17-like cells in resilient mice do not express ICOS (Fig 5I-J). This learned-helplessness-associated increase in ICOS was specific to the TFH-17-like cells, as the overall population of CD4+cells expressing ICOS was similar between the learned-helpless and resilient mice (Fig 5K-L). There was no difference in IL-21 expression by TFH-17-like cells between learned-helpless and resilient mice (Fig 5M-N) suggesting that IL-21 was not associated with the overall TFH phenotype after learned-helplessness. However, IL-21 was elevated in activated ICOS+ TFH-17-like cells in the hippocampus of learned-helpless mice, suggesting that only activated TFH-17-like cells express more IL-21 (Fig 5O-P).
Three additional markers of follicular cells were examined, PD-1, Bcl-6 and CCR7 (Nurieva et al., 2008; Nurieva et al., 2009). There was a significant increase of the percent of TFH-17-like cells expressing PD-1 in learned-helpless mice (Fig 6A-B), whereas the percent of TFH-17-like cells expressing BCL-6 (Fig 6E-F) or CCR7 (Fig 6C-D) was similar between learned-helpless and resilient mice, suggesting that PD-1 might contribute to the hippocampal follicular cell phenotype of the TFH-17-like cells in learned-helpless mice.
Figure 6:

TFH-like 17 cells have increased PD-1 levels and express CCR6 and IL-23R. Mice were subjected to the learned-helplessness paradigm, and were separated into 2 groups: learned-helpless (D) and non-learned-helpless (ND). Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for CD4, CXCR5, IL-17A, and either PD-1 (A-B), or CCR7 (C-D), or BCL-6 (E-F), and gated on IL-17A+CD4+ cells (A-F). Each symbol represents an individual mouse. Data represent mean±SEM, t-test, t(10)=2.729 *p<0.05 (n=5-7). (G-N) Mice were subjected to the learned-helplessness paradigm, or not, and mice were separated into 3 groups: unshocked (NS), learned-helpless (D) and non-learned-helpless (ND) mice. Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for CD4, CXCR5, IL-17A, and either CCR6 (G-H, K-L) or IL-23R (I-J, M-N), and gated on CXCR5+IL-17A+CD4+ cells (G-J) or on CXCR5+CD4+cells (K-M). Representative plots of flow cytometry are presented in G, I, K and M, and quantifications are presented in H, J, L and N. Each symbol represents an individual mouse. Data represent mean± SEM, Mann-Whitney, U=17 (H), U=20 (J), *p<0.05 (n=8-17).
To further test if the TFH-17-like cells also exhibit pathogenic properties, we analyzed the expression of the two pathogenic markers CCR6 and IL-23R (Ghoreschi et al., 2010; Langrish et al., 2005). There was an enrichment in hippocampal TFH-17-like cells expressing CCR6 (Fig 6G-H) and IL-23R (Fig 6I-J) in learned-helpless mice compared with resilient mice. Moreover, hippocampal CD4+cells not expressing IL-17A but expressing CXCR5 and CCR6 or IL-23R were similarly present in learned-helpless and resilient mice (Fig 6K-N), showing that hippocampal TFH-17-like cells express CCR6 and IL-23R. Moreover, using an adoptive transfer approach, we found that these TFH-17-like cells arise from the host, as TFH-17-like cells in the hippocampus express mostly CD45.2 whether they received CD45.1-expressing CD4+cells or CD45.1-expressing TH17 cells (Fig 7). In the spleen, the TFH-17-like cells originated from the host after transfer of CD45.1-expressing CD4+cells, and from the donor after transfer of CD45.1-expressing TH17 cells.
Figure 7:

TFH-like 17 cells originate from the host. CD45.2 wild-type recipient mice received donor CD45.1+CD4+ T cells or CD45.1+ TH17 cells, and 48 h later were subjected to the learned-helplessness paradigm. 86% of the CD4+T cells transferred in the TH17 cells group were TH17 cells, whereas less than 1% TH17 cells were detected in the CD4 group, and 99.3% of the CD4 injected cells were CD45.1 as described in Fig 2A. (A) CD4+CD45.1+ (donor cells) or CD4+CD45.2+ (recipient cells) present in the hippocampus of mice adoptively transferred with CD4 or TH17 cells were analyzed by flow cytometry after gating on CD4+ cells. CD45.1+CXCR5+IL-17A+CD4+ (donor cells) or CD45.2+CXCR5+IL-17A+CD4+ (recipient cells) present in the hippocampus (B) or spleen (C) of mice adoptively transferred with CD4 or TH17 cells were analyzed by flow cytometry after gating on CD45+CXCR5+IL-17A+CD4+ cells as described in panel (A). Each symbol represents an individual mouse. Data represent mean±SEM, one-way ANOVA, F(2,14)=22.9 (A), F(3,16)=34.35 (B), Bonferroni post-hoc test *p<0.05 (n=5).
3.7. CCR6-deficient TH17 cells induce resilience to learned-helplessness
To determine if the pathogenic marker CCR6 or the follicular cell marker CXCR5 present on the TFH-17-like cells in learned-helpless mice are required to promote learned-helplessness, we tested the effects of knocking down CCR6 or CXCR5 in TH17 cells using either knockdown by siRNA or CCR6 or CXCR5 knockout mice. To test if CXCR5 expression in TH17 cells is necessary for the TH17 cells to promote learned-helplessness, we adoptively transferred in vitro polarized CXCR5-deficient TH17 cells into wild-type recipient mice, and subjected the mice to learned-helplessness. Mice receiving CXCR5-deficient TH17 cells were susceptible to learned-helplessness similar to mice receiving wild-type TH17 cells, suggesting that CXCR5 was not required to promote susceptibility to learned-helplessness (Fig S4).
To determine the role of CCR6 in TH17 cell-mediated learned-helplessness, we prepared CCR6-knocked down TH17 cells using first a siRNA approach. After 4 days of in vitro differentiation, TH17 cells were transfected with CCR6 siRNA, resulting in ~80% transfected cells (Fig 8A), and 24 h later cells were transferred to wild-type mice. In mice receiving CCR6-knocked down TH17 cells, only 30% exhibited learned-helplessness compared with 70% of mice receiving TH17 cells transfected with a scrambled siRNA (siSCR) (Fig 8B). There were no changes in hippocampal TH17, CD4, or Treg (Foxp3+CD4+) cells (Fig 8C-E), activated microglia (Fig 8F), macrophages (Fig 8G), or dendritic cells (Fig 8H) in mice receiving CCR6-knocked down TH17 cells compared to control mice. In mice receiving CCR6-knocked out TH17 cells, only 20% of the mice exhibited learned-helplessness compared to 80% of mice receiving TH17 cells (Fig 8I), confirming the requirement of CCR6 to promote susceptibility to learned-helplessness. The behavioral effect was independent of the accumulation of TH17 cells in the hippocampus, as similar frequencies of TH17 cells were observed in the hippocampus (Fig 8J). However, hippocampal TH17 cells express higher level of the follicular cell marker PD-1 in the mice that received CCR6-knocked out TH17 cells (Fig 8K), suggesting that PD-1 might be regulated by the pathogenic marker CCR6.
Figure 8:

CCR6 expression is required for TH17 cells to promote learned-helplessness. Male and female mice received TH17 cells transduced with either a scrambled siRNA (siSCR) or siRNA targeting CCR6 (siCCR6) and 48 h later were subjected to the reduced paradigm of learned-helplessness. (A) Before transfer, TH17 cells transduced with either a scrambled siRNA (47.4% CD4 are TH17 cells) or siRNA targeting CCR6 (43.5% CD4 are TH17 cells) were stained for CCR6, IL-17A and CD4 for analysis by flow cytometry. Mean ± SEM, Mann-Whitney, U=0, *p<0.05 (n=3-4). (B) The number of escape failures was recorded. Each symbol represents an individual mouse. Mean ± SEM, t-test, t(20)=2.132, *p<0.05 (n=11). Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for IL-17A and CD4 (C), CD4 (C), Foxp3 and CD4 (E), F4/80 and CD45 (F), F4/80 and CD45 (G), CD11c and CD45 (H) and gated on CD4+ cells (C-E). Data represent mean±SEM, (n=5). (I) Mice received TH17 cells differentiated from wild-type CD4 cells (WT, 33% CD4 are TH17 cells) or CCR6-deficient CD4 cells (CCR6 KO, 28% CD4 are TH17 cells) and 48 h later were subjected to the reduced paradigm of learned-helplessness. The number of escape failures was recorded. Each symbol represents an individual mouse. Mean ± SEM, Mann-Whitney, *p<0.05 (n=10). Immediately after the last foot shocks, mice were perfused and hippocampi were collected. Dissociated cells were stained for IL-17A and CD4, and gated on CD4+ cells (J) or stained for PD-1, IL-17A, and CD4 and gated on IL-17A+ CD4+ cells (K). Data represent mean±SEM, Mann-Whitney U=21 (I) and Wilcoxon test, W=−19 (K), *p<0.05 (n=9).
4. Discussion
Accumulating evidence indicates that TH17 cells contribute to depression and depressive-like behaviors in rodents (Beurel and Lowell, 2017), but little is known about the characteristics and mechanism of action of TH17 cells in depression. Here we report the selective modification by TH17 cells, but not TH1 cells or TREGS, of susceptibility to learned-helplessness and report the localization and characteristics of hippocampal TH17 cells in learned-helpless mice, which indicates that TFH-17-like cells are the primary pathogenic cells.
Modulation of susceptibility to learned-helplessness was limited to TH17 cells. Although there is some evidence of an association of TH1 cells and/or TH1 cell-derived cytokines with depression (Beurel et al., 2013; Myint et al., 2005), TH1 cell adoptive transfer did not promote learned-helplessness, as did TH17 cells, even though both TH17 and TH1 cells were elevated in the brains of learned-helpless mice (Beurel et al., 2013) (Fig 1). These results suggest that TH1 cells alone are not sufficient to promote learned-helplessness, although they may play a role in conjunction with other depression-inducing factors. It is also possible that TH1 cells associated with depression may have evolved from TH17 cells, since it is known that this occurs under certain conditions (Harbour et al., 2015). Similar to TH1 cells, TREGS transfer did not affect susceptibility to learned-helplessness, suggesting that TREGS suppressive immune function is not sufficient to block the development of learned-helplessness.
TH17 cells accumulate in the brains of learned-helpless mice (Beurel et al., 2013). Examination of brain regions revealed that TH17 cells accumulate in the PFC and hippocampus, two brain regions that are associated with depression (Campbell and Macqueen, 2004; Koenigs and Grafman, 2009), of mice exhibiting learned-helplessness, but not in the cerebellum. These findings raise the possibility that the hippocampus and PFC might be where TH17 cells act to promote depressive-like behaviors, although additional actions in other regions cannot be ruled out. Examining whether the accumulated TH17 cells in the hippocampus developed there or migrated, our evidence that CD45.1+ donor TH17 cells were found in the hippocampus of CD45.2 recipient mice after learned-helplessness indicates that donor TH17 cells reach the hippocampus after stress. Additionally, using RORγt-GFP reporter mice, we found that the pro-depressive capacity of donor TH17 cells also depends on the induction of endogenous TH17 cells. Thus, both peripheral and central TH17 cells contribute to promoting stress-induced depressive-like behavior, suggesting that the initial peripheral immune response may mold central immune responses. However, peripheral TH17 cells were sufficient to promote learned-helplessness in Rag2−/− mice, which are devoid of endogenous CD4+cells, indicating that a peripheral source of TH17 cells is sufficient to promote learned-helplessness, similarly to evidence that gut TH17 cells modulate neurodevelopmental processes (Kim et al., 2017).
Although the mechanisms of migration of TH17 cells to the brain during the induction of depressive-like behavior remain mainly unknown, it has been proposed in EAE that TH17 cells migrate to the CNS using VLA4 (Rothhammer et al., 2011) or chemokine receptors such as CCR6, especially in the choroid plexus (El-behi et al., 2010; Reboldi et al., 2009). Others have suggested that TH17 cells attach better to the brain endothelial cells than TH1 cells (Brucklacher-Waldert et al., 2009), and locally produce cytokines, such as IL-17A or IL-22 (Kebir et al., 2007) or other mechanisms that disrupt the blood brain barrier, facilitating the migration of TH17 cells to the brain (Huppert et al., 2010).
Characterization of hippocampal TH17 cells in learned-helpless mice revealed that they express CCR6 and IL-23R, and, more surprisingly, the follicular cell marker CXCR5. CCR6 and IL-23R have been linked to the pathogenicity of TH17 cells in autoimmune diseases (Hirota et al., 2007; Kohler et al., 2003; Langrish et al., 2005), such as multiple sclerosis, suggesting that the depression-associated hippocampal TH17 cells display CNS-directed pathogenic properties that may underlie a portion of their pro-depressive actions. This is supported by our evidence that CCR6-deficient TH17 cells did not promote learned-helplessness even though they differentiated similarly to wild-type CD4+cells, suggesting that CCR6 confers pathogenicity. CCR6 is thought to promote TH17 cell recruitment to sites of inflammation (Hirota et al., 2007). However, we did not observe any difference in the percent of TH17 cells in the hippocampus between mice receiving wild-type or CCR6-deficient TH17 cells, suggesting that the hippocampal recruitment of CCR6-expressing TH17 cells during learned-helplessness was not driving the behavior. However, we cannot exclude that in this transfer experiment, endogenous CCR6-expressing TH17 cells compensate for hippocampal CCR6-deficient TH17 cells. It is also possible that CCR6 contributes to targeting TH17 cells to sites within the hippocampus. Associated with the resilience to learned-helplessness of mice receiving CCR6-deficient TH17 cells was an increase of PD-1 expression in hippocampal TH17 cells. This reveals a potential mechanism whereby CCR6 controls pathogenicity of TH17 cells in learned-helplessness because PD-1 is known to induce anergy in TH17 cells (Luz-Crawford et al., 2012). More studies will be needed to confirm the role of PD-1 and its regulation by CCR6 in TH17 cells.
There is little known about any possible role of CCR6 in major depressive disorder (MDD). However, reduced levels of CCR6 expression were found in the blood of MDD patients, suggesting that cells expressing CCR6 might already have reached the brain or other organs (Patas et al., 2018; Powell et al., 2014).
TH17 cells have been proposed to exist in 3 lineages: CCR6-dependent effector cells, memory cells, or IL-23R- and CXCR5-dependent follicular TH17 cells (Awasthi and Kuchroo, 2009; Sundrud and Trivigno, 2013). We found that at least 2 lineages of TH17 cells (although we did not directly test for the presence of memory cells) are present in the hippocampus after learned-helplessness. The role of CXCR5 in TH17 cells during depression is less clear than that of CCR6 and IL-23R because there was not a change in susceptibility to learned-helplessness after transfer of CXCR5-deficient TH17 cells, which does not exclude the possibility that CXCR5 is involved in the recruitment of TH17 cells to the hippocampus in conjunction with CCR6. Indeed, a question that remains open is the potential existence of germinal centers in the brain associated with the recently discovered lymphatic system (Louveau et al., 2015), since CXCR5 is a major homing signal for germinal centers. However, as for CCR6, it is possible that CXCR5 as a chemokine receptor is attracted by the chemokine CXCL13 produced locally in the hippocampus (Michalovicz and Konat, 2014) rather than a germinal center, and that signals coming from both CCR6 and CXCR5 are required for hippocampal TH17 recruitment during depressive-like behavior, providing a possible explanation for why CCR6 deficiency is not sufficient to block the recruitment of TH17 cells to the hippocampus in learned-helpless mice receiving CCR6-deficient TH17 cells and as to why CXCR5-deficient TH17 cells are not sufficient to decrease susceptibility to learned-helplessness. Although the role of CXCR5 in promoting depressive-like behavior needs to be further explored, the fact that some follicular markers (PD-1 and CXCR5), but not all (Bcl-6, CCR7), are increased in hippocampal TH17 cells in learned-helpless mice suggests that these hippocampal TFH-17-like cells associated with learned-helplessness have unique properties compared to peripheral TFH cells. In addition, CXCR5 expressed on TH17 cells might be important to promote B cell responses and memory formation in peripheral germinal centers (Hsu et al., 2008; Mitsdoerffer et al., 2010) before recruitment to the brain, providing a potential mechanism whereby immune memory to pathogenic signals and stressful events might be generated.
5. Conclusion
Altogether this study identified the selective pathogenicity of the TH17 subtype of T cells and identified novel characteristics of hippocampal TH17 cells associated with learned-helplessness. In particular, we found that learned-helplessness is associated with the hippocampal accumulation of TFH-17-like cells, and that CCR6 is a critical mediator of pathogenicity in TH17-dependent learned-helplessness, which may provide potential new avenues for treatment.
Supplementary Material
Acknowledgements
This research was supported by grants from the NIMH (MH104656, MH110415, and MH095380).
Footnotes
Financial disclosures
The authors have no financial interests or conflicts of interest.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, and Napolitani G (2007). Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8, 639–646. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. (2007). Phenotypic and functional features of human Th17 cells. J Exp Med 204, 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, and Kuchroo VK (2009). Th17 cells: from precursors to players in inflammation and infection. Int Immunol 21, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, and Kuchroo VK (2009). The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 10, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, and Jope RS (2013). Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, and Lowell JA (2017). Th17 cells in depression. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Yeh WI, Michalek SM, Harrington LE, and Jope RS (2011). Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol 186, 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, and Herkenham M (2015). Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci 35, 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, and Tolosa E (2009). Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 132, 3329–3341. [DOI] [PubMed] [Google Scholar]
- Campbell S, and Macqueen G (2004). The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29, 417–426. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, and Sun Y (2011). Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res 188, 224–230. [DOI] [PubMed] [Google Scholar]
- Clark SM, Soroka JA, Song C, Li X, and Tonelli LH (2016). CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(−/−) mice. Stress 19, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. (2003). Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748. [DOI] [PubMed] [Google Scholar]
- Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, and Atashzar MR (2016). Elevated IL-17 and TGF-beta Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin Neurosci 7, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, and Rostami A (2011). The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12, 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-behi M, Rostami A, and Ciric B (2010). Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol 5, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TB, Kasahara TM, Barros PO, Vieira MM, Bittencourt VC, Hygino J, Andrade RM, Linhares UC, Andrade AF, and Bento CA (2011). Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J Neuroimmunol 238, 58–66. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, and Kipnis J (2017). How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci 18, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. (2010). Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, Acharya N, and Rapaport MH (2017). Impact of Ixekizumab Treatment on Depressive Symptoms and Systemic Inflammation in Patients with Moderate-to-Severe Psoriasis: An Integrated Analysis of Three Phase 3 Clinical Studies. Psychother Psychosom 86, 260–267. [DOI] [PubMed] [Google Scholar]
- Harbour SN, Maynard CL, Zindl CL, Schoeb TR, and Weaver CT (2015). Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 112, 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. (2007). Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204, 2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. (2008). Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 9, 166–175. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, et al. (2010). Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24, 1023–1034. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, and Littman DR (2006). The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, and Trivedi MH (2017). Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, and Prat A (2007). Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13, 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, Lee HJ, Ham BJ, and Lee HS (2013). Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig 10, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, et al. (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, and Grafman J (2009). The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler RE, Caon AC, Willenborg DO, Clark-Lewis I, and McColl SR (2003). A role for macrophage inflammatory protein-3 alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system. J Immunol 170, 6298–6306. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, and Kuchroo VK (2009). IL-17 and Th17 Cells. Annu Rev Immunol 27, 485–517. [DOI] [PubMed] [Google Scholar]
- Korn T, and Kallies A (2017). T cell responses in the central nervous system. Nat Rev Immunol 17, 179–194. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, and Gelfand JM (2010). The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 146, 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, and Cua DJ (2005). IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. (2012). Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ho RC, and Mak A (2012). The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis 15, 183–187. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, and Djouad F (2012). Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One 7, e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, and Cua DJ (2007). TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8, 1390–1397. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, and Cua DJ (2009). The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovicz LT, and Konat GW (2014). Peripherally restricted acute phase response to a viral mimic alters hippocampal gene expression. Metab Brain Dis 29, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, and Kuchroo VK (2010). Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107, 14292–14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, and Kim YK (2005). Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord 88, 167–173. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, and Attia SM (2017). IL-17A causes depression-like symptoms via NFkappaB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 97, 14–24. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. (2008). Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, and Dong C (2009). Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patas K, Willing A, Demiralay C, Engler JB, Lupu A, Ramien C, Schafer T, Gach C, Stumm L, Chan K, et al. (2018). T Cell Phenotype and T Cell Receptor Repertoire in Patients with Major Depressive Disorder. Front Immunol 9, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Marrie RA, and Carta MG (2017). Depression in multiple sclerosis. Int Rev Psychiatry 29, 463–472. [DOI] [PubMed] [Google Scholar]
- Powell TR, McGuffin P, D’Souza UM, Cohen-Woods S, Hosang GM, Martin C, Matthews K, Day RK, Farmer AE, Tansey KE, et al. (2014). Putative transcriptomic biomarkers in the inflammatory cytokine pathway differentiate major depressive disorder patients from control subjects and bipolar disorder patients. PLoS One 9, e91076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, and Sallusto F (2009). C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10, 514–523. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, and Korn T (2011). Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J Exp Med 208, 2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundrud MS, and Trivigno C (2013). Identity crisis of Th17 cells: many forms, many functions, many questions. Semin Immunol 25, 263–272. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, et al. (2010). Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229, 212–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


