Abstract
Gender differences in the incidences of cancers have been found in almost all human cancers. However, the mechanisms that underlie gender disparities in most human cancer types have been under-investigated. Here, we provide a comprehensive overview of potential mechanisms underlying sexual dimorphism of each cancer regarding sex hormone signaling. Fully addressing the mechanisms of sexual dimorphism in human cancers will greatly benefit current development of precision medicine. Our discussions of potential mechanisms underlying sexual dimorphism in each cancer will be instructive for future cancer research on gender disparities.
Keywords: sexual dimorphism, cancer incidence, sex hormones, estrogen receptor, androgen receptor
Introduction
Sexual dimorphism is an important feature of human cancers but has been under-investigated and mostly neglected in clinical diagnosis and therapy. Our recent study showed that almost all human cancers showed significant differences between two genders (under paralleled review). Both genetic and environmental factors contribute to the initiation and progression of cancer in the form of germline genetic variations and defects, somatic mutations, and the inflammatory responses resulting from exposure to toxic chemicals, excessive alcohol consumption, and/or viral infection [1–3]. However, the mechanisms that underlie gender disparities in most human cancer types have been under-investigated. Sex hormones, i.e., estrogens in women and androgens in men, are the drivers of sexual dimorphism in general. Estrogen receptor-dependent estrogen signaling through three estrogen receptors, estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and G protein-coupled estrogen receptor (GPER1), and androgen receptor (AR) -dependent androgen signaling play the major roles in sexual dimorphism of human cancers. Here, we summarize previous studies and current progresses on sex hormone signaling in human cancers. The mechanisms of sexual dimorphism in human cancers have not been heavily investigated, except for some conclusive evidences in mice, e.g., sexual dimorphism of liver cancer is controlled by Foxa1/2-dependent ERα-mediated prevention and AR-mediated promotion on tumor growth in mice [4], which parallel well with the male-dominant liver cancer in humans. Here, we provide a comprehensive overview of potential regulatory mechanisms underlying sexual dimorphism of each cancer by focusing on sex hormone signaling and sex hormone receptor-dependent regulation. Our comprehensive overview about gender differences in cancer incidence and sex hormone regulation in cancer initiation and progression will provide a general guidance for the investigation of sexual dimorphism in human cancers and will be instructive and beneficial for cancer research and cancer diagnosis, prognosis, and therapy.
Summary of findings
I. The overview of gender differences in human cancers
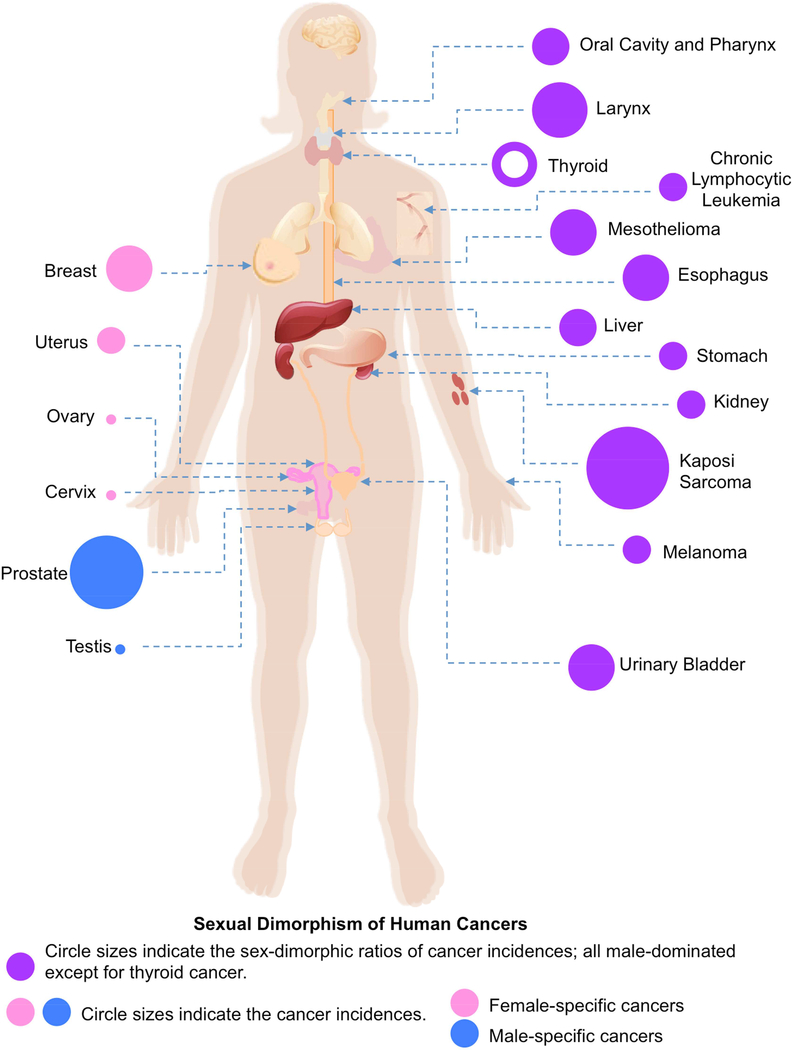
According to sex-specific organs or development, we divide all cancer types into two groups: sex-dimorphic and sex-specific (or extremely sex-dimorphic with single sex-oriented, including male-specific and female-specific) cancers. In our recent study, we calculated the average incidence ratios between men and women for all human cancer the SEER data in 1970–2014 (under paralleled review). Among the 30 types of human cancers included in the SEER data (Fig. 1), 24 of them are sex-dimorphic and all have statistically significant incidence differences between men and women, two types are men-specific (prostate cancer and testicular cancer), and four types of cancers are women-specific (breast cancer, cancer of the cervix uteri, cancer of the corpus and uterus, NOS, and ovarian cancer). Although males have a rare occurrence of breast cancer [5, 6], we considered breast cancer as female-specific because mammary glands are barely developed in men. For all 24 sex-dimorphic cancers, 23 of them are male-dominant, and interestingly, only one cancer, thyroid cancer, is female-dominant.
Figure 1.
II. Sex-dimorphic cancers
Next, we summarize the incidence data and potential mechanisms of sexual dimorphism in ten human cancer types with higher incidence ratios of at least 2-fold.
a). Kaposi Sarcoma
Kaposi sarcoma is a vascular neoplasm with nodular lesions on mucous membranes, skin, and connective tissues like bone, blood vessels, lymph nodes, and muscle with aberrant differentiation, inflammation, proliferation, and angiogenesis [7, 8]. Four types of clinical variations of Kaposi sarcoma have been identified, including classic (sporadic), endemic (African), epidemic (AIDS-associated), and iatrogenic (post-transplant) sarcoma [9, 10]. Classic Kaposi sarcoma was found in older men of European origin and caused by the Kaposi Sarcoma-associated herpesvirus (KSHV) [9, 11]. Kaposi sarcoma is rarely found in immuno-competent people, but the incidence of Kaposi sarcoma dramatically increases in homosexual and bisexual AIDS patients due to immunodeficiency, and about 15% to 20% AIDS patients develop Kaposi sarcoma [11, 12].
According to the SEER data, we found that the averaged incidence ratio of Kaposi sarcoma between men and women was 27.29 in USA during 1975–2014 (Fig. 1), which is the highest incidence ratio of sexual dimorphism in all types of human cancers. A recent study showed that the case ratio of the classic Kaposi sarcoma between men and women was 10.0 and the case ratio of the AIDS-associated Kaposi sarcoma was 3.0 among 105 Chinese patients [13]. Interestingly, in the highly HIV-infected regions, such as south and west Africa, the incidence of Kaposi sarcoma was much less sex-dimorphic compared to the SEER data [14–19].
Kaposi sarcoma is thus more common in males due to a higher incidence of AIDS in homosexual males in the western world, but apart from this, it remains a male-dominant cancer in humans worldwide. However, this sexual dimorphism has been understudied. A few studies showed that HIV-positive patients with Kaposi sarcoma had significantly higher levels of serum androgens than HIV-positive patients without Kaposi sarcoma and HIV-negative men, and proposed that excess amount of androgen might stimulate the function of suppressor T-cells [20–22].
b). Cancer of the larynx
Cancer of the larynx or laryngeal cancer arises in the throat, and cigarette smoking is one of the strongest environmental risk factors of laryngeal tumorigenesis [23–25]. This cancer includes three subtypes based on locations: glottis, supraglottis, and subglottis, with most larynx cancers being of the glottis subtype.
According to the SEER data, the average incidence ratio between men and women was about 5.18 in 1975–2014 and the second highest sexual dimorphism of all human cancers in the SEER data (Fig. 1).
Mechanisms underlying sex dimorphism of laryngeal cancer has been moderately investigated. The regulations of sex hormone signaling in the proliferation of laryngeal cancer cells of squamous cell carcinoma are controversial; e.g., estrogen promoted the cell proliferation of through GPER1 or possibly ERα36 (a short isoform of ERα) but suppressed the cell proliferation through ERβ, but these differential regulations were also at a cell line-dependent manner [26–30]; although this cancer is more common in males, androgen was reported to inhibit the growth of laryngeal cancer cells [31]; thus, anti-androgen therapy had failed for patients with laryngeal tumors [32], though whether androgen receptors were expressed or involved in laryngeal tumorigenesis was still unclear [33–35].
c). Mesothelioma
Malignant mesothelioma is an aggressive neoplasm arising from mesothelial cells, and most mesothelioma locate at the pleural and peritoneal cavities, the pericardium, or the tunica vaginalis [36]. The incidence of mesothelioma has been increasing worldwide in recent decades and was peaked during the 1980s and 1990s [37]. Most (90%) malignant pleural mesothelioma might be related to prior asbestos exposure [38]. Mesothelioma was barely diagnosed before the age of 49; and mesothelioma patients died at a mean age of 70 years old [39].
The SEER data show that the average sex-dimorphic incidence ratio of mesothelioma between men and women was 4.70 (Fig. 1). Similar high sex-dimorphic incidence ratios of mesothelioma have also been reported in other countries and areas, including South Africa, Japan, France, Taiwan, Italy and Australia [40–48]. Thus, mesothelioma is a male-dominant cancer in humans.
The most commonly etiology for mesothelioma is the exposure to asbestos, which may explain the higher incidence rate in men de to male-dominant occupations, such as mining [49–52]. However, this cannot explain the consistent gender differences of mesothelioma in non-mining men and women. Investigations on the mechanism of sexual dimorphism of mesothelioma has been mainly focused on ERβ, e.g., estrogen signaling inhibited the growth of malignant mesothelioma cells through ERβ [53–56]; ERβ expression in human pleural mesothelioma cells was regulated by histone demethylase KDM6B under either normoxic or hypoxic conditions [57], indicating ERβ-mediated sexual dimorphism in mesothelioma is independent of environmental factors, such as mining; further, GPER1 activated the chemotaxis and migration of mesothelioma [58]; and ERα and AR expressed in the tumors of peritoneal mesothelioma but how they regulate the tumorigenesis were unclear [59].
d). Urinary Bladder Cancer
Urinary bladder cancer is the fifth most common malignancy in industrialized countries [60], arises from the epithelial cells of the urinary bladder, and includes two major histological types: urothelial carcinoma (> 90%) and squamous cell carcinoma (3–5%) [61]. Cigarette smoking, occupational exposures to infected waters, and chronic bladder inflammation are risk factors for urinary bladder cancer [62–64]. From the SEER data, we found that the average sex-dimorphic incidence ratio of urinary bladder cancer was 3.96 and this ratio was well maintained throughout the years in 1975–2014 (Fig. 1).
The mechanisms underlying sexual dimorphism of urinary bladder cancer have been actively investigated and are highly dependent on sex hormone signaling. Although estrogen was found to promote the growth of urothelial bladder cancer cells through both ERα and ERβ [65, 66], both estrogen therapy and anti-estrogen therapy prevented or suppressed the tumorigenesis of urothelial bladder cancer [67–69]. Also, following an antibody validation study, the expression of ERβ in the bladder or bladder cancer cells is debated [70]. Conversely, GPER1-mediated estrogen signaling inhibited the proliferation of urinary bladder cancer cells [71]. AR-mediated androgen signaling promoted the growth and metastasis of urothelial bladder cancer cells whereas androgen deprivation therapy and AR ablation or inhibition suppressed the tumorigenesis of urothelial bladder cancer [72–78]. Thus, the role of estrogen or androgen signaling, which both promote cell growth in the male-dominant sexual dimorphism in urothelial bladder cancer requires further investigation.
e). Esophageal Cancer
Cancer of the esophagus or esophageal cancer arises from the food pipe between throat and stomach. Esophageal cancer has two major subtypes: esophageal adenocarcinoma and esophageal squamous cell carcinoma [79]. Cigarette smoking, alcohol consumption, and poor oral health are risk factors for esophageal cancer [80]. Based on SEER data, the average sex-dimorphic incidence ratio of esophageal cancer was about 3.66 in 1975–2014 (Fig. 1).
Sex hormone signaling diverges between the two major subtypes of esophageal cancers. In esophageal adenocarcinoma, AR-dependent androgen signaling suppressed cell proliferation [81] whereas estrogen signaling (through both ERα and ERβ) promoted the cell growth [82, 83]. In contrast, in esophageal squamous cell carcinoma, AR-dependent androgen signaling promoted the cell growth and migration [84–86] whereas whether estrogen signaling suppressed the cell growth through ERα or ERβ was under the debate [85, 87–91]. These studies have been mainly conducted in human esophageal cancer cell lines.
f). Liver cancer
Liver cancer includes hepatocellular carcinoma (HCC) and cholangiocarcinoma. Liver cancer is closely linked to chronic liver diseases including chronic Hepatitis virus infections, exposure to aflatoxin and alcohol, and diabetes and the metabolic syndrome [92–95]. The average sex-dimorphic incidence ratio of liver cancer was 2.69 in the SEER data (Fig. 1).
Liver cancer is one of the mostly investigated cancers regarding sexual dimorphism because it is conserved in both rodents and humans [96–106]. Gender differences in the liver cancer were firstly discovered in mice in 1930s [96–98]. Later studies elucidated that ERα-mediated estrogen signaling and AR-mediated androgen signaling play the major and opposite roles in hepatic tumorigenesis, i.e., estrogen signaling prevented whereas androgen signaling promoted tumor growth in females and males, respectively [96–99, 107–112]. Although neither ERβ nor GPER1 is expressed in normal liver and liver tumors from rodents and humans, one recent study showed that global but not liver-specific ablation of Gper1 accelerated hepatocarcinogenesis [113]. Two recent studies have made great contributions on the molecular mechanisms underlying sexual dimorphism of liver cancer. Naugler et al found that MyD88-dependent IL-6 production promoted chemically induced hepatocarcinogenesis in male mice, while estrogen-mediated inhibition of IL-6 production contributed to the reduction of hepatic tumorigenesis in female mice [99]. However, employing IL-6 antagonists or estrogen and its analogs to treat HCC has brought controversial results [104, 114, 115]. Li et al found that both of the ERα-mediated protection and AR-mediated facilitation of hepatic tumorigenesis in mice depended on Foxa1/2, while deficiency of Foxa1 and Foxa2 in the mouse liver would reverse the protective role of ERα and the detrimental role of AR for HCC [4]; and genetic variants that affected the binding of FOXA2 and ERα were associated with the increased incidence of HCC in patients [4]. Recent clinical studies showed the promising evidence that estrogen supplements reduced the risk of HCC in women and increased the survival of female HCC patients [116, 117]. Thus, detailing the sex hormone signaling targets may help developing effective target therapy for HCC.
g). Cancer of oral cavity and pharynx
Cancer of the oral cavity and pharynx is mostly oral squamous cell carcinoma (OSCC), which arises from the squamous cell lining of the mouth and throat. Aging, alcohol abuse, and tobacco are risk factors for OSCC. The average sex-dimorphic incidence ratio for this group of cancers has been at a steady level around 2.59 in the SEER 1975–2014 data (Fig. 1).
It is interesting that both estrogen and androgen signaling promote the growth of OSCC cells. ERα, ERβ, GPER1, and AR were all expressed in OSCC tissues and their expression levels were possibly related to the pathological grades or malignancy of tumors [118–124]. Estrogen signaling promoted the growth of OSCC cells through ERα, ERβ, and GPER1 [122, 124–126]. AR-dependent androgen signaling promoted the growth of OSCC cells [121].
h). Stomach Cancer
Stomach cancer or gastric cancer is a malignant tumor arising from the lining of stomach. Infection by the Helicobacter pylori accounts for more than 60% of cases, and smoking, diet, and obesity are other risk factors [127–130]. About 90–95% of gastric cancer is gastric adenocarcinoma, which includes histological subtypes: diffuse and intestinal [131]. There was no significant difference in the incidence of the diffuse subtype between men and women [132–134], thus the intestinal subtype accounted for sexual dimorphism in gastric cancer. The average sex-dimorphic incidence ratio for gastric cancer was around 2.0 in the SEER data (Fig. 1).
The regulation of estrogen signaling in the growth of gastric cancer cells is still confusing due to limited evidence, i.e., ERα-mediated estrogen signaling promoted cell growth whereas. For example, estrogen signaling, through ERα or a splicing variant of ERα, promoted the growth of gastric cancer cells [135, 136] whereas ERβ-mediated estrogen signaling inhibited the growth of gastric cancer cells [137] and genetic variants in the ESR2 gene were highly associated with survival in patients with locally advanced gastric cancer [138]. AR-mediated androgen signaling promoted the growth and metastasis of gastric cancer cells [139].
i). Cancer of the Kidney and Renal Pelvis
Kidney or renal cancer originates from kidney and the lining of renal pelvis, and includes four major subtypes: renal cell carcinoma (about 85%), transitional cell carcinoma, Wilms tumors, and renal sarcoma [140]. Smoking, obesity and hypertension have been estimated to account for nearly half of cases [141, 142]. The average sex-dimorphic incidence ratio was stable around 2 in the SEER data (Fig. 1). Similar male dominance of kidney cancer was also observed in Denmark (1.67), Europe (2.0), and Korea (2.5) [143–146].
The mechanisms underlying sexual dimorphism of kidney cancer have been less studied. The evidence of estrogen signaling in the growth of kidney cancer cells is limited, e.g., ESR1 polymorphism was associated with the risk of kidney cancer [147]; GPER1 promoted the growth and metastasis of kidney cancer cells [148]; and AR-mediated androgen signaling promoted the growth of kidney cancer cells [149, 150].
j). Thyroid Cancer
Thyroid cancer is the most common and major lethal endocrine malignancy [151], and includes two major subtypes: papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) [152, 153]. Thyroid cancer is the only female-dominant sex-dimorphic cancer in humans with an average sex-dimorphic incidence ratio of −2.56 in the SEER data (Fig. 1).
Sexual dimorphism of thyroid cancer has been heavily investigated. ERα, ERβ, GPER1, and AR were expressed at various levels in thyroid tumor tissues [70, 154–160]. Interestingly, both estrogen signaling and androgen signaling promoted the growth of thyroid cancer cells [155, 161, 162]. Estrogen signaling through all three estrogen receptors, ERα, ERβ, and GPER1, promoted the growth and metastasis of thyroid cancer cells [155, 163–167] and ERα expression was increased in thyroid tumors compared to controls [154, 168, 169]. Recently, estrogen was shown to promote the growth of thyroid cancer stem cells in vitro and in mouse xenografts provided an additional explanation for the cause of female-dominant sexual dimorphism in thyroid cancer [170].
Discussions
1. Sex hormone signaling in sexual dimorphism of human cancers
Sexual dimorphism in human cancer susceptibility has been observed for almost a century. However, the mechanism underlying the incidence divergence between the two genders is still elusive for most cancer types. Accumulated evidences demonstrated that sex hormone receptor-mediated signaling plays the essential roles in the sexual dimorphism of human cancer incidence during the initiation, progression, metastasis, and prognosis of human cancers. Generally, three types of estrogen receptors, ERα, ERβ, and GPER1, and AR are involved in sex dimorphic regulation of human cancers at different manners. We summarize all studies that addressed sexual dimorphism of human cancers regarding sex hormone signaling and the involvement of sex hormone receptors in Table 1. However, only a few cancers have been carefully investigated for this regard, such as liver cancer. For most of other cancers, sexual dimorphism has been barely or incompletely addressed. Interestingly, sex hormone signaling or sex hormone receptor-mediated signaling does not always regulate the progression of human cancers in the same direction or plays opposite roles in two types of cancers or even in the same type of cancer; e.g., ERα-mediated estrogen signaling promotes the tumor growth of breast cancer, cancer of the cervix uteri, cancer of the corpus and uterus, and ovarian cancer, but it prevents the tumor growth of liver cancer; estrogen signaling through ERα and ERβ plays opposite roles in the same cancer, such as stomach cancer and ovarian cancer. Estrogen receptor-mediated estrogen signaling shows great tissue or cancer specificity, such as ERα in liver cancer, ERβ in mesothelioma cancer, and GPER1 in kidney cancer. Additionally, other co-regulators or epigenetic factors may be involved in the process of these identical or opposite regulations of sex hormone receptors. Some cancers have more complicated sex hormone signaling, e.g., all sex hormone receptors, ERα, ERβ, and GPER1, and AR have shown to regulate the tumorigenesis of urinary bladder cancer, oral cancer, and thyroid cancer (Table 1). Another interesting aspect in sexual dimorphism of human cancers was not discussed in this article but deserves more attention, i.e., men dominated in the cancer incidence but female cancer patients had more advanced diseases and worse survival than men. Thus, we are far away from complete understanding the mechanisms underlying sexual dimorphism of human cancers and fully elucidating the contributions of sex hormone signaling and sex hormone receptors to sexual dimorphism in each cancer type will require tremendous efforts and investigations.
TABLE 1.
Summary of Sex Hormone Signaling Studies in Human Cancers
| Cancer Type | Estrogen | ERα | ERβ | GPER1 | AR | Androgen | |
|---|---|---|---|---|---|---|---|
| 1 | Kaposi Sarcoma | − | − | − | − | − | ↑? |
| 2 | Cancer of the Larynx | ↑↓? | ↑? | ↓ | ↑ | ↑? | ↓ |
| 3 | Mesothelioma | ↑↓? | ? | ↓ | ↑? | ? | ? |
| 4 | Urinary Bladder Cancer | ↑↓ | ↑ | ↑ | ↓ | ↑ | ↑ |
| 5 | Esophageal Cancer | ↑↓ | ↑↓ | ↑↓ | − | ↑↓ | ↑↓ |
| 6 | Liver Cancer | ↓ | ↓ | − | − | ↑ | ↑ |
| 7 | Oral Cavity and Pharynx | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 8 | Stomach Cancer | ↑↓ | ↑ | ↓ | − | ↑ | ↑ |
| 9 | Cancer of the Kidney and Renal Pelvis | − | − | − | ↑ | ↑ | ↑ |
| 10 | Thyroid Cancer | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 11 | Breast Cancer | ↑ | ↑ | ↓? | ↑? | ↑ | ↑ |
| 12 | Cancer of the Cervix Uteri | ↑↓? | ↑? | − | ↓ | − | ↑ |
| 13 | Cancer of the Corpus and Uterus, NOS | ↑↓? | ↑ | ↓? | ↑ | ↓ | ↓ |
| 14 | Ovarian Cancer | ↑↓ | ↑ | ↓ | ↑↓? | ↑ | ↑? |
| 15 | Prostate Cancer | ↑↓? | ↑? | ↓? | ↓ | ↑ | ↑ |
| 16 | Testis Cancer | ↑↓? | ? | ↓ | ↑ | ↓ | ↓ |
↑, Sex hormone signaling promotes tumorigenesis in human cancers.
↓, Sex hormone signaling suppresses tumorigenesis in human cancers.
↑↓, Controversial functions of sex hormone signaling in human cancers.
−, Sex hormone signaling in human cancers has not been investigated.
?, uncertain results
2. Key challenges and future perspectives
A key challenge in deciphering the mechanisms of sexual dimorphism in human cancers is the various expression levels of sex hormone receptors in different stages of tumorigenesis (mostly silenced in advanced stages of tumors), such as liver cancer and breast cancer, which may result from sexual dimorphism in organogenesis as we observed recently in the liver [171]. Additionally, antibodies used for measuring sex hormone receptors varied in quality and some of them led to errors, such as recent evidence for anti-ERβ antibodies [70] and previous evidence of upper non-specific bands from anti-ERα antibodies (HC-20 from Santa Cruz Bio). To improve the quality for the studies of sexual dimorphism in human cancers, genetic assays using tissue-specific knockout mouse models of sex hormone receptors and using human cancer cells with CRISPR/Cas9-mediated manipulations of sex hormone receptor expression will provide more solid and direct answers for this topic. Moreover, whether or how risk factors could or could not affect sexual dimorphism of human cancer is still unclear, i.e., non-mining men still had higher incidence of mesothelioma than non-mining women [49–52], smoking men had higher incidence of lung cancer than smoking women whereas non-smoking women had higher incidence of lung cancer than non-smoking men [172], and male-dominant HIV infection did not show similar degrees of male-dominant incidence in Kaposi Sarcoma [14–19]. More importantly, there are many big questions that remain to be answered in the sexual dimorphism of human cancers; e.g., why up to three estrogen receptors are required for regulation in a single cancer? Why males dominate almost all sex-dimorphic cancers? How the only female-dominant thyroid cancer is so different from other cancers? How aging control sex hormone levels or regulate sex hormone signaling in sex-dimorphic or sex-specific cancers? Is there a general principle or mechanism of sexual dimorphism in human cancers? Last but not the least, what are the roles of X and Y chromosomes play in sexual dimorphism of human cancers? Sex is one of the most obvious features or variables in human beings or mammals. Fully understanding the mechanisms underlying sexual dimorphism in normal humans would facilitate better understanding of sexual dimorphism in human cancers; and this would be essential for developing gender-specific biomarkers or treatment for cancer patients, which would be a critical first step of gender-specific precision medicine towards genuine personalized precision medicine.
Highlights.
Sex differences in the incidences of cancers have been found in almost all human cancers.
A comprehensive overview of potential mechanisms underlying sexual dimorphism of each cancer regarding sex hormone signaling.
Fully addressing the mechanisms of sexual dimorphism in human cancers will greatly benefit current development of precision medicine.
Our discussions of potential mechanisms underlying sexual dimorphism in each cancer will be instructive for future cancer research on sexual dimorphism.
Acknowledgement
This study was partially supported by the NCI R00CA168983 to Z.L., NCI R01CA172437 (to C.W.), the Swedish Research Council (to C.W.), and the Swedish Cancer Society (to C.W.).
Abbreviations:
- (SEER)
Surveillance, Epidemiology, and End Results Program
- (ERα)
estrogen receptor alpha
- (ERβ)
estrogen receptor beta
- (GPER1)
G protein-coupled estrogen receptor
- (AR)
androgen receptor
Footnotes
Statements of Conflicts of Interest
All authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Strong LC, Genetic and environmental interactions, Cancer, 40 (1977) 1861–1866 %@ 1097–0142. [DOI] [PubMed] [Google Scholar]
- [2].Czene K, Lichtenstein P, Hemminki K, Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database, Int J Cancer, 99 (2002) 260–266. [DOI] [PubMed] [Google Scholar]
- [3].Migliore L, Coppede F, Genetic and environmental factors in cancer and neurodegenerative diseases, Mutat Res, 512 (2002) 135–153. [DOI] [PubMed] [Google Scholar]
- [4].Li Z, Tuteja G, Schug J, Kaestner KH, Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer, Cell, 148 (2012) 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vietri MT, Caliendo G, Casamassimi A, Cioffi M, De Paola ML, Napoli C, Molinari AM, A novel PALB2 truncating mutation in an Italian family with male breast cancer, Oncology reports, 33 (2015) 1243–1247. [DOI] [PubMed] [Google Scholar]
- [6].Ottini L, Male breast cancer: a rare disease that might uncover underlying pathways of breast cancer, Nature reviews. Cancer, 14 (2014) 643. [DOI] [PubMed] [Google Scholar]
- [7].Wang J, Stebbing J, Bower M, HIV-associated Kaposi sarcoma and gender, Gender medicine, 4 (2007) 266–273. [DOI] [PubMed] [Google Scholar]
- [8].Gramolelli S, Schulz TF, The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma, J Pathol, 235 (2015) 368–380. [DOI] [PubMed] [Google Scholar]
- [9].Wen KW, Damania B, Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis, Cancer Lett, 289 (2010) 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Potthoff A, Brockmeyer NH, Stucker M, Wieland U, Kreuter A, Competence Network HA, Kaposi sarcoma in a HIV uninfected man who has sex with men, European journal of medical research, 15 (2010) 79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS, Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma, Science, 266 (1994) 1865–1869. [DOI] [PubMed] [Google Scholar]
- [12].Beral V, Peterman TA, Berkelman RL, Jaffe HW, Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection?, Lancet, 335 (1990) 123–128. [DOI] [PubMed] [Google Scholar]
- [13].Wu XJ, Pu XM, Kang XJ, Halifu Y, An CX, Zhang DZ, Yakeya B, Mijit J, One hundred and five Kaposi sarcoma patients: a clinical study in Xinjiang, Northwest of China, J Eur Acad Dermatol Venereol, 28 (2014) 1545–1552. [DOI] [PubMed] [Google Scholar]
- [14].Saka B, Mouhari-Toure A, Wateba IM, Akakpo S, Kombate K, Balaka A, Sogan A, Afolabi KO, Pitche P, Tchangai-Walla K, [AIDS related Kaposi sarcoma: 103 cases in dermatology in Lome (Togo)], Medecine et sante tropicales, 23 (2013) 109–111. [DOI] [PubMed] [Google Scholar]
- [15].Khammissa RA, Pantanowitz L, Feller L, Oral HIV-Associated Kaposi Sarcoma: A Clinical Study from the Ga-Rankuwa Area, South Africa, AIDS research and treatment, 2012 (2012) 873171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Palmieri C, Dhillon T, Thirlwell C, Newsom-Davis T, Young AM, Nelson M, Gazzard BG, Bower M, Pulmonary Kaposi sarcoma in the era of highly active antiretroviral therapy, HIV medicine, 7 (2006) 291–293. [DOI] [PubMed] [Google Scholar]
- [17].Nasti G, Serraino D, Ridolfo A, Antinori A, Rizzardini G, Zeroli C, Nigro L, Tavio M, Vaccher E, Tirelli U, AIDS-associated Kaposi’s sarcoma is more aggressive in women: a study of 54 patients , Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association, 20 (1999) 337–341. [DOI] [PubMed] [Google Scholar]
- [18].Cooley TP, Hirschhorn LR, O’Keane JC, Kaposi’s sarcoma in women with AIDS, Aids, 10 (1996) 1221–1225. [DOI] [PubMed] [Google Scholar]
- [19].Phipps W, Ssewankambo F, Nguyen H, Saracino M, Wald A, Corey L, Orem J, Kambugu A, Casper C, Gender differences in clinical presentation and outcomes of epidemic Kaposi sarcoma in Uganda, PloS one, 5 (2010) e13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Christeff N, Winter C, Gharakhanian S, Thobie N, Wirbel E, Costagliola D, Nunez EA, Rozenbaum W, Differences in androgens of HIV positive patients with and without Kaposi sarcoma, J Clin Pathol, 48 (1995) 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lehmann D, Siebold K, Emmons LR, Muller H, Androgens inhibit proliferation of human peripheral blood lymphocytes in vitro, Clin Immunol Immunopathol, 46 (1988) 122–128. [DOI] [PubMed] [Google Scholar]
- [22].Araneo BA, Dowell T, Diegel M, Daynes RA, Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells, Blood, 78 (1991) 688–699. [PubMed] [Google Scholar]
- [23].Vassileiou A, Vlastarakos PV, Kandiloros D, Delicha E, Ferekidis E, Tzagaroulakis A, Nikolopoulos TP, Laryngeal cancer: smoking is not the only risk factor, B-ent, 8 (2012) 273–278. [PubMed] [Google Scholar]
- [24].Koyanagi YN, Matsuo K, Ito H, Wakai K, Nagata C, Nakayama T, Sadakane A, Tanaka K, Tamakoshi A, Sugawara Y, Mizoue T, Sawada N, Inoue M, Tsugane S, Sasazuki S, Sasazuki S, Tsugane S, Inoue M, Iwasaki M, Otani T, Sawada N, Shimazu T, Yamaji T, Tsuji I, Tsubono Y, Nishino Y, Tamakoshi A, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T, Tanaka K, Nakayama T, Sadakane A, Cigarette smoking and the risk of head and neck cancer in the Japanese population: a systematic review and meta-analysis, Japanese journal of clinical oncology, 46 (2016) 580–595. [DOI] [PubMed] [Google Scholar]
- [25].Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF, An update on larynx cancer, CA: a cancer journal for clinicians, 67 (2017) 31–50. [DOI] [PubMed] [Google Scholar]
- [26].Schwartz N, Chaudhri RA, Hadadi A, Schwartz Z, Boyan BD, 17Beta-estradiol promotes aggressive laryngeal cancer through membrane-associated estrogen receptor-alpha 36, Hormones & cancer, 5 (2014) 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schwartz N, Verma A, Muktipaty C, Bivens C, Schwartz Z, Boyan BD, Estradiol receptor profile and estrogen responsiveness in laryngeal cancer and clinical outcomes, Steroids, (2017). [DOI] [PubMed] [Google Scholar]
- [28].Li S, Wang B, Tang Q, Liu J, Yang X, Bisphenol A triggers proliferation and migration of laryngeal squamous cell carcinoma via GPER mediated upregulation of IL-6, Cell biochemistry and function, 35 (2017) 209–216. [DOI] [PubMed] [Google Scholar]
- [29].Goulioumis AK, Fuxe J, Varakis J, Repanti M, Goumas P, Papadaki H, Estrogen receptor-beta expression in human laryngeal carcinoma: correlation with the expression of epithelial-mesenchymal transition specific biomarkers, Oncology reports, 22 (2009) 1063–1068. [DOI] [PubMed] [Google Scholar]
- [30].Robbins KT, Vu TP, Diaz A, Varki NM, Growth effects of tamoxifen and estradiol on laryngeal carcinoma cell lines, Archives of otolaryngology--head & neck surgery, 120 (1994) 1261–1266. [DOI] [PubMed] [Google Scholar]
- [31].Kleemann D, [Testosterone versus dihydrotestosterone effects on permanent squamous epithelial cancer cell lines of the larynx], Laryngo- rhino- otologie, 72 (1993) 402–405. [DOI] [PubMed] [Google Scholar]
- [32].Mattox DE, Von Hoff DD, McGuire WL, Androgen receptors and antiandrogen therapy for laryngeal carcinoma, Archives of otolaryngology (Chicago, Ill. : 1960), 110 (1984) 721–724. [DOI] [PubMed] [Google Scholar]
- [33].Scambia G, Panici PB, Battaglia F, Ferrandina G, Almadori G, Paludetti G, Maurizi M, Mancuso S, Receptors for epidermal growth factor and steroid hormones in primary laryngeal tumors, Cancer, 67 (1991) 1347–1351. [DOI] [PubMed] [Google Scholar]
- [34].Hagedorn HG, Nerlich AG, Analysis of sex-hormone-receptor expression in laryngeal carcinoma, Eur Arch Otorhinolaryngol, 259 (2002) 205–210. [DOI] [PubMed] [Google Scholar]
- [35].Goulioumis AK, Varakis J, Goumas P, Papadaki H, Androgen receptor in laryngeal carcinoma: could there be an androgen-refractory tumor?, ISRN oncology, 2011 (2011) 180518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ashrafian H, Athanasiou T, Yap J, DeSouza AC, Two-chamber intracardiac mesothelioma, Asian cardiovascular & thoracic annals, 13 (2005) 184–186. [DOI] [PubMed] [Google Scholar]
- [37].Taioli E, Wolf AS, Camacho-Rivera M, Flores RM, Women with malignant pleural mesothelioma have a threefold better survival rate than men, The Annals of thoracic surgery, 98 (2014) 1020–1024. [DOI] [PubMed] [Google Scholar]
- [38].Marinaccio A, Montanaro F, Mastrantonio M, Uccelli R, Altavista P, Nesti M, Costantini AS, Gorini G, Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models, Int J Cancer, 115 (2005) 142–147. [DOI] [PubMed] [Google Scholar]
- [39].Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T, Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008, Bulletin of the World Health Organization, 89 (2011) 716–724, 724A-724C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jennings CJ, Walsh PM, Deady S, Harvey BJ, Thomas W, Malignant pleural mesothelioma incidence and survival in the Republic of Ireland 1994–2009, Cancer Epidemiol, 38 (2014) 35–41. [DOI] [PubMed] [Google Scholar]
- [41].Gemba K, Fujimoto N, Aoe K, Kato K, Takeshima Y, Inai K, Kishimoto T, Treatment and survival analyses of malignant mesothelioma in Japan, Acta oncologica, 52 (2013) 803–808. [DOI] [PubMed] [Google Scholar]
- [42].Chamming’s S, Clin B, Brochard P, Astoul P, Ducamp S, Galateau-Salle F, Ilg AG, Goldberg M, Gramond C, Imbernon E, Rolland P, Pairon JC, Compensation of pleural mesothelioma in France: data from the French National Mesothelioma Surveillance Programme, American journal of industrial medicine, 56 (2013) 146–154. [DOI] [PubMed] [Google Scholar]
- [43].Kielkowski D, Nelson G, Bello B, Kgalamono S, Phillips JI, Trends in mesothelioma mortality rates in South Africa: 1995–2007, Occup Environ Med, 68 (2011) 547–549. [DOI] [PubMed] [Google Scholar]
- [44].Fujimoto N, Aoe K, Gemba K, Kato K, Yamazaki K, Kishimoto T, Clinical investigation of malignant mesothelioma in Japan, Journal of cancer research and clinical oncology, 136 (2010) 1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee LJ, Chang YY, Wang JD, Impact of malignant mesothelioma in Taiwan: a 27-year review of population-based cancer registry data, Lung cancer, 68 (2010) 16–19. [DOI] [PubMed] [Google Scholar]
- [46].Mensi C, Termine L, Canti Z, Rivolta G, Riboldi L, Pesatori AC, Chiappino G, [The Lombardy Mesothelioma Register, Regional Operating Centre (ROC) of National Mesothelioma Register: organizational aspects], Epidemiologia e prevenzione, 31 (2007) 283–289. [PubMed] [Google Scholar]
- [47].Gorini G, De Gregorio G, Silvestri S, Chellini E, Cupelli V, Seniori Costantini A, Survival of malignant pleural mesothelioma cases in the Tuscan Mesothelioma Register, 1988–2000: a population-based study, European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation, 14 (2005) 195–199. [DOI] [PubMed] [Google Scholar]
- [48].Hyland RA, Ware S, Johnson AR, Yates DH, Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia, Scandinavian journal of work, environment & health, 33 (2007) 286–292. [DOI] [PubMed] [Google Scholar]
- [49].Vianna NJ, Polan AK, Non-occupational exposure to asbestos and malignant mesothelioma in females, Lancet, 1 (1978) 1061–1063. [DOI] [PubMed] [Google Scholar]
- [50].Spirtas R, Heineman EF, Bernstein L, Beebe GW, Keehn RJ, Stark A, Harlow BL, Benichou J, Malignant mesothelioma: attributable risk of asbestos exposure, Occup Environ Med, 51 (1994) 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Boffetta P, Epidemiology of peritoneal mesothelioma: a review, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 18 (2007) 985–990. [DOI] [PubMed] [Google Scholar]
- [52].Lanphear BP, Buncher CR, Latent period for malignant mesothelioma of occupational origin, Journal of occupational medicine. : official publication of the Industrial Medical Association, 34 (1992) 718–721. [PubMed] [Google Scholar]
- [53].Pinton G, Thomas W, Bellini P, Manente AG, Favoni RE, Harvey BJ, Mutti L, Moro L, Estrogen receptor beta exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib, PloS one, 5 (2010) e14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Manente AG, Valenti D, Pinton G, Jithesh PV, Daga A, Rossi L, Gray SG, O’Byrne KJ, Fennell DA, Vacca RA, Nilsson S, Mutti L, Moro L, Estrogen receptor beta activation impairs mitochondrial oxidative metabolism and affects malignant mesothelioma cell growth in vitro and in vivo, Oncogenesis, 2 (2013) e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pinton G, Manente AG, Daga A, Cilli M, Rinaldi M, Nilsson S, Moro L, Agonist activation of estrogen receptor beta (ERbeta) sensitizes malignant pleural mesothelioma cells to cisplatin cytotoxicity, Mol Cancer, 13 (2014) 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nuvoli B, Sacconi A, Cortese G, Germoni S, Murer B, Galati R, Reduction of estradiol in human malignant pleural mesothelioma tissues may prevent tumour growth, as implied by in in-vivo and in-vitro models, Oncotarget, 7 (2016) 47116–47126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Manente AG, Pinton G, Zonca S, Tavian D, Habib T, Jithesh PV, Fennell D, Nilsson S, Moro L, KDM6B histone demethylase is an epigenetic regulator of estrogen receptor beta expression in human pleural mesothelioma, Epigenomics, 8 (2016) 1227–1238. [DOI] [PubMed] [Google Scholar]
- [58].Avino S, De Marco P, Cirillo F, Santolla MF, De Francesco EM, Perri MG, Rigiracciolo D, Dolce V, Belfiore A, Maggiolini M, Lappano R, Vivacqua A, Stimulatory actions of IGF-I are mediated by IGF-IR cross-talk with GPER and DDR1 in mesothelioma and lung cancer cells, Oncotarget, 7 (2016) 52710–52728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chua TC, Yao P, Akther J, Young L, Bao S, Samaraweera U, Yan TD, Morris DL, Differential expression of Ki-67 and sex steroid hormone receptors between genders in peritoneal mesothelioma, Pathology oncology research : POR, 15 (2009) 671–678. [DOI] [PubMed] [Google Scholar]
- [60].Reinert T, Methylation markers for urine-based detection of bladder cancer: the next generation of urinary markers for diagnosis and surveillance of bladder cancer, Advances in urology, 2012 (2012) 503271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Massaro PA, Moore J, Rahmeh T, Morse MJ, Squamous cell carcinoma of the suprapubic tract: A rare presentation in patients with chronic indwelling urinary catheters, Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 8 (2014) E510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, El-Kalawy M, Abd-Elsayed AA, Ismail K, Hablas A, Seifeldin IA, Ramadan M, Wilson ML, The changing patterns of bladder cancer in Egypt over the past 26 years, Cancer causes & control : CCC, 19 (2008) 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pinton G, Brunelli E, Murer B, Puntoni R, Puntoni M, Fennell DA, Gaudino G, Mutti L, Moro L, Estrogen receptor-beta affects the prognosis of human malignant mesothelioma, Cancer research, 69 (2009) 4598–4604. [DOI] [PubMed] [Google Scholar]
- [64].Scelo G, Brennan P, The epidemiology of bladder and kidney cancer, Nature clinical practice. Urology, 4 (2007) 205–217. [DOI] [PubMed] [Google Scholar]
- [65].Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, Sonpavde G, Ayala GE, Younes M, Lerner SP, Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue, Cancer, 106 (2006) 2610–2616. [DOI] [PubMed] [Google Scholar]
- [66].Teng J, Wang ZY, Jarrard DF, Bjorling DE, Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation, Endocrine-related cancer, 15 (2008) 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Daugherty SE, Lacey JV Jr., Pfeiffer RM, Park Y, Hoover RN, Silverman DT, Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study, International journal of cancer, 133 (2013) 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sonpavde G, Okuno N, Weiss H, Yu J, Shen SS, Younes M, Jian W, Lerner SP, Smith CL, Efficacy of selective estrogen receptor modulators in nude mice bearing human transitional cell carcinoma, Urology, 69 (2007) 1221–1226. [DOI] [PubMed] [Google Scholar]
- [69].George SK, Tovar-Sepulveda V, Shen SS, Jian W, Zhang Y, Hilsenbeck SG, Lerner SP, Smith CL, Chemoprevention of BBN-Induced Bladder Carcinogenesis by the Selective Estrogen Receptor Modulator Tamoxifen, Translational oncology, 6 (2013) 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Andersson S, Sundberg M, Pristovsek N, Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramstrom M, Soderberg O, Williams C, Asplund A, Insufficient antibody validation challenges oestrogen receptor beta research, Nat Commun, 8 (2017) 15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Huang W, Chen Y, Liu Y, Zhang Q, Yu Z, Mou L, Wu H, Zhao L, Long T, Qin D, Gui Y, Roles of ERβ and GPR30 in Proliferative Response of Human Bladder Cancer Cell to Estrogen, BioMed research international, 2015. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kawahara T, Ide H, Kashiwagi E, El-Shishtawy KA, Li Y, Reis LO, Zheng Y, Miyamoto H, Enzalutamide inhibits androgen receptor-positive bladder cancer cell growth, Urologic oncology, 34 (2016) 432, e415–423. [DOI] [PubMed] [Google Scholar]
- [73].Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T, Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats, European urology, 31 (1997) 360–364. [DOI] [PubMed] [Google Scholar]
- [74].Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C, Promotion of bladder cancer development and progression by androgen receptor signals, Journal of the National Cancer Institute, 99 (2007) 558–568. [DOI] [PubMed] [Google Scholar]
- [75].Hsu JW, Hsu I, Xu D, Miyamoto H, Liang L, Wu XR, Shyr CR, Chang C, Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor, The American journal of pathology, 182 (2013) 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Johnson DT, Hooker E, Luong R, Yu EJ, He Y, Gonzalgo ML, Sun Z, Conditional Expression of the Androgen Receptor Increases Susceptibility of Bladder Cancer in Mice, PloS one, 11 (2016) e0148851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, Chiba K, Murai T, Hirai K, Suzuki K, Fujinami K, Ueki T, Udagawa K, Kitami K, Moriyama M, Miyoshi Y, Tsuchiya F, Ikeda I, Kobayashi K, Sato M, Morita S, Noguchi K, Uemura H, Androgen deprivation therapy prevents bladder cancer recurrence, Oncotarget, 5 (2014) 12665–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jing Y, Cui D, Guo W, Jiang J, Jiang B, Lu Y, Zhao W, Wang X, Jiang Q, Han B, Xia S, Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition, Cancer letters, 348 (2014) 135–145. [DOI] [PubMed] [Google Scholar]
- [79].Enzinger PC, Mayer RJ, Esophageal cancer, The New England journal of medicine, 349 (2003) 2241–2252. [DOI] [PubMed] [Google Scholar]
- [80].Mao WM, Zheng WH, Ling ZQ, Epidemiologic risk factors for esophageal cancer development, Asian Pac J Cancer Prev, 12 (2011) 2461–2466. [PubMed] [Google Scholar]
- [81].Palethorpe HM, Drew PA, Smith E, Androgen Signaling in Esophageal Adenocarcinoma Cell Lines In Vitro, Digestive diseases and sciences, 62 (2017) 3402–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Due SL, Watson DI, Bastian I, Ding GQ, Sukocheva OA, Astill DS, Vat L, Hussey DJ, Tamoxifen enhances the cytotoxicity of conventional chemotherapy in esophageal adenocarcinoma cells, Surgical oncology, 25 (2016) 269–277. [DOI] [PubMed] [Google Scholar]
- [83].Sukocheva OA, Wee C, Ansar A, Hussey DJ, Watson DI, Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells, Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus, 26 (2013) 628–635. [DOI] [PubMed] [Google Scholar]
- [84].Dong H, Xu J, Li W, Gan J, Lin W, Ke J, Jiang J, Du L, Chen Y, Zhong X, Zhang D, Yeung SJ, Li X, Zhang H, Reciprocal androgen receptor/interleukin-6 crosstalk drives oesophageal carcinoma progression and contributes to patient prognosis, The Journal of pathology, 241 (2017) 448–462. [DOI] [PubMed] [Google Scholar]
- [85].Matsuoka H, Sugimachi K, Ueo H, Kuwano H, Nakano S, Nakayama M, Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma, Cancer research, 47 (1987) 4134–4140. [PubMed] [Google Scholar]
- [86].Zhang Y, Pan T, Zhong X, Cheng C, Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2, Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 36 (2015) 5859–5864. [DOI] [PubMed] [Google Scholar]
- [87].Zhang Z, He Q, Fu S, Zheng Z, Estrogen Receptors in Regulating Cell Proliferation of Esophageal Squamous Cell Carcinoma: Involvement of Intracellular Ca(2+) Signaling, Pathology oncology research : POR, 23 (2017) 329–334. [DOI] [PubMed] [Google Scholar]
- [88].Zuguchi M, Miki Y, Onodera Y, Fujishima F, Takeyama D, Okamoto H, Miyata G, Sato A, Satomi S, Sasano H, Estrogen receptor alpha and beta in esophageal squamous cell carcinoma, Cancer science, 103 (2012) 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Utsumi Y, Nakamura T, Nagasue N, Kubota H, Harada T, Morikawa S, Effect of 17 beta-estradiol on the growth of an estrogen receptor-positive human esophageal carcinoma cell line, Cancer, 67 (1991) 2284–2289. [DOI] [PubMed] [Google Scholar]
- [90].Ueo H, Matsuoka H, Sugimachi K, Kuwano H, Mori M, Akiyoshi T, Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line, Cancer research, 50 (1990) 7212–7215. [PubMed] [Google Scholar]
- [91].Utsumi Y, Nakamura T, Nagasue N, Kubota H, Morikawa S, Role of estrogen receptors in the growth of human esophageal carcinoma, Cancer, 64 (1989) 88–93. [DOI] [PubMed] [Google Scholar]
- [92].Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G, Hepatocellular carcinoma. [DOI] [PubMed] [Google Scholar]
- [93].Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr., Hepatocellular carcinoma: a review, Journal of hepatocellular carcinoma, 3 (2016) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ghouri YA, Mian I, Rowe JH, Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis, Journal of carcinogenesis, 16 (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Waller LP, Deshpande V, Pyrsopoulos N, Hepatocellular carcinoma: A comprehensive review, World journal of hepatology, 7 (2015) 2648–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Andervont HB, Lorenz E, Dibenzanthracene tumors in mice. Production of subcutaneous, pulmonary and liver tumors by serum dispersions and lard solution, Public Health Reports, 52 (1937) 637–647. [Google Scholar]
- [97].Burns EL, Schenken JR, Spontaneous Primary Hepatomas in Mice of Strain C3H a Study of Incidence, Sex Distribution and Morbid Anatomy, Am J Cancer, 39 (1940) 12. [Google Scholar]
- [98].Tomita T, Studies on Experimental Production of Cancer : Hepatoma Formation and Lipoidosis, Gann, (1937) 31:255, Abst. In Am. J. Cancer 236: 308, 1939. [Google Scholar]
- [99].Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M, Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production, Science, 317 (2007) 121–124. [DOI] [PubMed] [Google Scholar]
- [100].Shimizu I, Yasuda M, Mizobuchi Y, Ma YR, Liu F, Shiba M, Horie T, Ito S, Suppressive effect of oestradiol on chemical hepatocarcinogenesis in rats, Gut, 42 (1998) 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tsutsui S, Yamamoto R, Iishi H, Tatsuta M, Tsuji M, Terada N, Promoting effect of ovariectomy on hepatocellular tumorigenesis induced in mice by 3’-methyl-4-dimethylaminoazobenzene, Virchows Arch B Cell Pathol Incl Mol Pathol, 62 (1992) 371–375. [DOI] [PubMed] [Google Scholar]
- [102].Yamamoto R, Iishi H, Tatsuta M, Tsuji M, Terada N, Roles of ovaries and testes in hepatocellular tumorigenesis induced in mice by 3’-methyl-4dimethylaminoazobenzene, Int J Cancer, 49 (1991) 83–88. [DOI] [PubMed] [Google Scholar]
- [103].Bielschowsky A, Goethe, el hombre y su obra. Refundición de J. Casán Herrera, Editorial Scientia, Barcelona,, 1944. [Google Scholar]
- [104].Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R, Role of sex steroid receptors in pathobiology of hepatocellular carcinoma, World journal of gastroenterology : WJG, 14 (2008) 5945–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ghebranious N, Sell S, Hepatitis B injury, male gender, aflatoxin, and p53 expression each contribute to hepatocarcinogenesis in transgenic mice, Hepatology, 27 (1998) 383–391. [DOI] [PubMed] [Google Scholar]
- [106].Maeda S, Kamata H, Luo JL, Leffert H, Karin M, IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis, Cell, 121 (2005) 977–990. [DOI] [PubMed] [Google Scholar]
- [107].Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM, MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits, Nature cell biology, 14 (2012) 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, Hung YC, Yeh S, Chang C, Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription, Science translational medicine, 2 (2010) 32ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bigsby RM, Caperell-Grant A, The role for estrogen receptor-alpha and prolactin receptor in sex-dependent DEN-induced liver tumorigenesis, Carcinogenesis, 32 (2011) 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ma WL, Hsu CL, Yeh CC, Wu MH, Huang CK, Jeng LB, Hung YC, Lin TY, Yeh S, Chang C, Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis, Hepatology, 56 (2012) 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lai HC, Yeh CC, Jeng LB, Huang SF, Liao PY, Lei FJ, Cheng WC, Hsu CL, Cai X, Chang C, Ma WL, Androgen receptor mitigates postoperative disease progression of hepatocellular carcinoma by suppressing CD90+ populations and cell migration and by promoting anoikis in circulating tumor cells, Oncotarget, 7 (2016) 46448–46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jiang L, Shan J, Shen J, Wang Y, Yan P, Liu L, Zhao W, Xu Y, Zhu W, Su L, Chen J, Cheng F, Yao H, Xu H, Qian C, Liang Z, Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma, Oncotarget, 7 (2016) 36814–36828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wei T, Chen W, Wen L, Zhang J, Zhang Q, Yang J, Liu H, Chen BW, Zhou Y, Feng X, Yang Q, Bai X, Liang T, G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis, Cancer Lett, 382 (2016) 195–202. [DOI] [PubMed] [Google Scholar]
- [114].Di Maio M, De Maio E, Morabito A, D’Aniello R, De Feo G, Gallo C, Perrone F, Hormonal treatment of human hepatocellular carcinoma, Ann N Y Acad Sci, 1089 (2006) 252–261. [DOI] [PubMed] [Google Scholar]
- [115].Lawrence T, Hageman T, Balkwill F, Cancer. Sex, cytokines, and cancer, Science, 317 (2007) 51–52. [DOI] [PubMed] [Google Scholar]
- [116].Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, Phan AT, Hawk E, Javle M, Lee JS, Torres HA, Rashid A, Lenzi R, Hassabo HM, Abaza Y, Shalaby AS, Lacin S, Morris J, Patt YZ, Amos CI, Khaderi SA, Goss JA, Jalal PK, Kaseb AO, Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma, Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 15 (2017) 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].McGlynn KA, Hagberg K, Chen J, Braunlin M, Graubard BI, Suneja N, Jick S, Sahasrabuddhe VV, Menopausal hormone therapy use and risk of primary liver cancer in the clinical practice research datalink, International journal of cancer, 138 (2016) 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Colella G, Izzo G, Carinci F, Campisi G, Lo Muzio L, D’Amato S, Mazzotta M, Cannavale R, Ferrara D, Minucci S, Expression of sexual hormones receptors in oral squamous cell carcinoma, International journal of immunopathology and pharmacology, 24 (2011) 129–132. [DOI] [PubMed] [Google Scholar]
- [119].Grimm M, Biegner T, Teriete P, Hoefert S, Krimmel M, Munz A, Reinert S, Estrogen and Progesterone hormone receptor expression in oral cavity cancer, Medicina oral, patologia oral y cirugia bucal, 21 (2016) e554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Doll C, Arsenic R, Lage H, Johrens K, Hartwig S, Nelson K, Raguse JD, Expression of Estrogen Receptors in OSCC in Relation to Histopathological Grade, Anticancer research, 35 (2015) 5867–5872. [PubMed] [Google Scholar]
- [121].Wu TF, Luo FJ, Chang YL, Huang CM, Chiu WJ, Weng CF, Hsu YK, Yuan TC, The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells, Oral diseases, 21 (2015) 320–327. [DOI] [PubMed] [Google Scholar]
- [122].Chang YL, Hsu YK, Wu TF, Huang CM, Liou LY, Chiu YW, Hsiao YH, Luo FJ, Yuan TC, Regulation of estrogen receptor alpha function in oral squamous cell carcinoma cells by FAK signaling, Endocrine-related cancer, 21 (2014) 555–565. [DOI] [PubMed] [Google Scholar]
- [123].Marocchio LS, Giudice F, Correa L, Pinto Junior Ddos S, de Sousa SO, Oestrogens and androgen receptors in oral squamous cell carcinoma, Acta odontologica Scandinavica, 71 (2013) 1513–1519. [DOI] [PubMed] [Google Scholar]
- [124].Bai LY, Weng JR, Hu JL, Wang D, Sargeant AM, Chiu CF, G15, a GPR30 antagonist, induces apoptosis and autophagy in human oral squamous carcinoma cells, Chem Biol Interact, 206 (2013) 375–384. [DOI] [PubMed] [Google Scholar]
- [125].Somers KD, Koenig M, Schechter GL, Growth of head and neck squamous cell carcinoma in nude mice: potentiation of laryngeal carcinoma by 17 beta-estradiol, Journal of the National Cancer Institute, 80 (1988) 688–691. [DOI] [PubMed] [Google Scholar]
- [126].Ishida H, Wada K, Masuda T, Okura M, Kohama K, Sano Y, Nakajima A, Kogo M, Kamisaki Y, Critical role of estrogen receptor on anoikis and invasion of squamous cell carcinoma, Cancer Sci, 98 (2007) 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chan AOO, Wong BC, Risk factors for gastric cancer.
- [128].Ishaq S, Nunn L, Helicobacter pylori and gastric cancer: a state of the art review, Gastroenterology and hepatology from bed to bench, 8 (2015) S6–s14. [PMC free article] [PubMed] [Google Scholar]
- [129].Talebi Bezmin Abadi A, Helicobacter pylori and Gastric Cancer, Frontiers in medicine, 3 (2016) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zali H, Rezaei-Tavirani M, Azodi M, Gastric cancer: prevention, risk factors and treatment, Gastroenterology and hepatology from bed to bench, 4 (2011) 175–185. [PMC free article] [PubMed] [Google Scholar]
- [131].Lauren P, The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification, Acta pathologica et microbiologica Scandinavica, 64 (1965) 31–49. [DOI] [PubMed] [Google Scholar]
- [132].Wu CW, Tsay SH, Hsieh MC, Lo SS, Lui WY, P’Eng F K, Clinicopathological significance of intestinal and diffuse types of gastric carcinoma in Taiwan Chinese, Journal of gastroenterology and hepatology, 11 (1996) 1083–1088. [DOI] [PubMed] [Google Scholar]
- [133].Stemmermann GN, Brown C, A survival study of intestinal and diffuse types of gastric carcinoma, Cancer, 33 (1974) 1190–1195. [DOI] [PubMed] [Google Scholar]
- [134].Lauren PA, Nevalainen TJ, Epidemiology of intestinal and diffuse types of gastric carcinoma. A time-trend study in Finland with comparison between studies from high-and low-risk areas, Cancer, 71 (1993) 2926–2933. [DOI] [PubMed] [Google Scholar]
- [135].Wang X, Deng H, Zou F, Fu Z, Chen Y, Wang Z, Liu L, ER-alpha36-mediated gastric cancer cell proliferation via the c-Src pathway, Oncology letters, 6 (2013) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tang W, Liu R, Yan Y, Pan X, Wang M, Han X, Ren H, Zhang Z, Expression of estrogen receptors and androgen receptor and their clinical significance in gastric cancer, Oncotarget, 8 (2017) 40765–40777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kim MJ, Cho SI, Lee KO, Han HJ, Song TJ, Park SH, Effects of 17betaestradiol and estrogen receptor antagonists on the proliferation of gastric cancer cell lines, J Gastric Cancer, 13 (2013) 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sunakawa Y, Cao S, Berger MD, Matsusaka S, Yang D, Zhang W, Ning Y, Parekh A, Stremitzer S, Mendez A, Okazaki S, Wakatsuki T, Azuma M, Shimada K, Watanabe M, Koizumi W, Wu AH, Lenz HJ, Estrogen receptor-beta genetic variations and overall survival in patients with locally advanced gastric cancer, The pharmacogenomics journal, 17 (2017) 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhang BG, Du T, Zang MD, Chang Q, Fan ZY, Li JF, Yu BQ, Su LP, Li C, Yan C, Gu QL, Zhu ZG, Yan M, Liu B, Androgen receptor promotes gastric cancer cell migration and invasion via AKT-phosphorylation dependent upregulation of matrix metalloproteinase 9, Oncotarget, 5 (2014) 10584–10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Motzer RJ, Bander NH, Nanus DM, Renal-cell carcinoma, The New England journal of medicine, 335 (1996) 865–875. [DOI] [PubMed] [Google Scholar]
- [141].Chow WH, Dong LM, Devesa SS, Epidemiology and risk factors for kidney cancer, Nature reviews. Urology, 7 (2010) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Qayyum T, Oades G, Horgan P, Aitchison M, Edwards J, The epidemiology and risk factors for renal cancer, Current urology, 6 (2013) 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rampersaud EN, Klatte T, Bass G, Patard JJ, Bensaleh K, Bohm M, Allhoff EP, Cindolo L, De La Taille A, Mejean A, Soulie M, Bellec L, Christophe Bernhard J, Pfister C, Colombel M, Belldegrun AS, Pantuck AJ, George D, The effect of gender and age on kidney cancer survival: younger age is an independent prognostic factor in women with renal cell carcinoma, Urologic oncology, 32 (2014) 30 e39–13. [DOI] [PubMed] [Google Scholar]
- [144].Pelant T, Larsen EH, Lund L, Borre M, Erichsen R, Norgaard M, Jacobsen JB, Survival of patients with kidney cancer in central and northern Denmark, 1998–2009, Clinical epidemiology, 3 Suppl 1 (2011) 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ, Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993–2004, The Journal of urology, 179 (2008) 1709–1713; discussion 1713. [DOI] [PubMed] [Google Scholar]
- [146].Song W, Jeon HG, Incidence of kidney, bladder, and prostate cancers in Korea: An update, Korean J Urol, 56 (2015) 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tanaka Y, Sasaki M, Kaneuchi M, Fujimoto S, Dahiya R, Estrogen receptor alpha polymorphisms and renal cell carcinoma--a possible risk, Molecular and cellular endocrinology, 202 (2003) 109–116. [DOI] [PubMed] [Google Scholar]
- [148].Feldman RD, Ding Q, Hussain Y, Limbird LE, Pickering JG, Gros R, Aldosterone mediates metastatic spread of renal cancer via the G protein-coupled estrogen receptor (GPER), FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 30 (2016) 2086–2096. [DOI] [PubMed] [Google Scholar]
- [149].Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng J, Lin W, Huang Q, Li G, Zheng J, Chang C, LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor(AR)/miRNA-143–3p signals, Cell death and differentiation, 24 (2017) 1502–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].He D, Li L, Zhu G, Liang L, Guan Z, Chang L, Chen Y, Yeh S, Chang C, ASCJ9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2alpha/VEGF signaling pathway, Cancer research, 74 (2014) 4420–4430. [DOI] [PubMed] [Google Scholar]
- [151].Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R, Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors, J Cancer Epidemiol, 2013 (2013) 965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Passler C, Scheuba C, Prager G, Kaczirek K, Kaserer K, Zettinig G, Niederle B, Prognostic factors of papillary and follicular thyroid cancer: differences in an iodinereplete endemic goiter region, Endocrine-related cancer, 11 (2004) 131–139. [DOI] [PubMed] [Google Scholar]
- [153].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012, Int J Cancer, 136 (2015) E359–386. [DOI] [PubMed] [Google Scholar]
- [154].Magri F, Capelli V, Rotondi M, Leporati P, La Manna L, Ruggiero R, Malovini A, Bellazzi R, Villani L, Chiovato L, Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk, Endocrine-related cancer, 19 (2012) 463–471. [DOI] [PubMed] [Google Scholar]
- [155].Zhang Y, Wei F, Zhang J, Hao L, Jiang J, Dang L, Mei D, Fan S, Yu Y, Jiang L, Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway, Archives of biochemistry and biophysics, 633 (2017) 29–39. [DOI] [PubMed] [Google Scholar]
- [156].Vannucchi G, De Leo S, Perrino M, Rossi S, Tosi D, Cirello V, Colombo C, Bulfamante G, Vicentini L, Fugazzola L, Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer, Eur J Endocrinol, 173 (2015) 29–36. [DOI] [PubMed] [Google Scholar]
- [157].Magri F, Capelli V, Gaiti M, Villani L, Zerbini F, La Manna L, Rotondi M, Chiovato L, ER-alpha and ER-beta expression in differentiated thyroid cancer: relation with tumor phenotype across the TNM staging and peri-tumor inflammation, Endocrine, 49 (2015) 429–435. [DOI] [PubMed] [Google Scholar]
- [158].Liu J, Chen G, Meng XY, Liu ZH, Dong S, Serum levels of sex hormones and expression of their receptors in thyroid tissue in female patients with various types of thyroid neoplasms, Pathology, research and practice, 210 (2014) 830–835. [DOI] [PubMed] [Google Scholar]
- [159].Tang C, Yang L, Wang N, Li L, Xu M, Chen GG, Liu ZM, High expression of GPER1, EGFR and CXCR1 is associated with lymph node metastasis in papillary thyroid carcinoma, International journal of clinical and experimental pathology, 7 (2014) 3213–3223. [PMC free article] [PubMed] [Google Scholar]
- [160].Huang Y, Dong W, Li J, Zhang H, Shan Z, Teng W, Differential expression patterns and clinical significance of estrogen receptor-alpha and beta in papillary thyroid carcinoma, BMC cancer, 14 (2014) 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Stanley JA, Aruldhas MM, Chandrasekaran M, Neelamohan R, Suthagar E, Annapoorna K, Sharmila S, Jayakumar J, Jayaraman G, Srinivasan N, Banu SK, Androgen receptor expression in human thyroid cancer tissues: a potential mechanism underlying the gender bias in the incidence of thyroid cancers, The Journal of steroid biochemistry and molecular biology, 130 (2012) 105–124. [DOI] [PubMed] [Google Scholar]
- [162].Banu KS, Govindarajulu P, Aruldhas MM, Testosterone and estradiol have specific differential modulatory effect on the proliferation of human thyroid papillary and follicular carcinoma cell lines independent of TSH action, Endocr Pathol, 12 (2001) 315–327. [DOI] [PubMed] [Google Scholar]
- [163].Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG, Liu ZM, GPER/ERK&AKT/NF-kappaB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells, Mol Cell Endocrinol, 442 (2017) 68–80. [DOI] [PubMed] [Google Scholar]
- [164].Kumar A, Klinge CM, Goldstein RE, Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta, Int J Oncol, 36 (2010) 1067–1080. [DOI] [PubMed] [Google Scholar]
- [165].Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, Tiwari RK, Metastatic phenotype is regulated by estrogen in thyroid cells, Thyroid : official journal of the American Thyroid Association, 20 (2010) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chen GG, Vlantis AC, Zeng Q, van Hasselt CA, Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer, Curr Cancer Drug Targets, 8 (2008) 367–377. [DOI] [PubMed] [Google Scholar]
- [167].Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M, 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30, Molecular pharmacology, 70 (2006) 1414–1423. [DOI] [PubMed] [Google Scholar]
- [168].Rubio GA, Catanuto P, Glassberg MK, Lew JI, Elliot SJ, Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause, Surgery, 163 (2018) 143–149. [DOI] [PubMed] [Google Scholar]
- [169].Di Vito M, De Santis E, Perrone GA, Mari E, Giordano MC, De Antoni E, Coppola L, Fadda G, Tafani M, Carpi A, Russo MA, Overexpression of estrogen receptor-alpha in human papillary thyroid carcinomas studied by laser- capture microdissection and molecular biology, Cancer science, 102 (2011) 1921–1927. [DOI] [PubMed] [Google Scholar]
- [170].Zane M, Parello C, Pennelli G, Townsend DM, Merigliano S, Boscaro M, Toniato A, Baggio G, Pelizzo MR, Rubello D, Boschin IM, Estrogen and thyroid cancer is a stem affair: A preliminary study, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 85 (2017) 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zheng D, Zhao Y, Wang X, Antonson P, Gustafsson JA, Li Z, Genomics of sex hormone receptor signaling in hepatic sexual dimorphism, Molecular and cellular endocrinology, (2017) Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW, Lung cancer incidence in never smokers, Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 25 (2007) 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]