ABSTRACT
The cancer stem cell (CSC) paradigm posits that specific cells within a tumor, so-called CSC-like cells, have differing levels of tumorigenicity and chemoresistance. Original studies of CSCs identified them in human cancers and utilized mouse xenograft models to define the cancer initiating properties of these cells, thereby hampering the understanding of how immunity could affect CSCs. Indeed, few studies have characterized CSCs in the context of cancer immunoediting, and it is currently not clear how immunity could impact on the levels or stem-like behavior of CSCs. Using the well-studied 3′methylcholanthrene (MCA) model of primary sarcoma formation, we have defined a CSC-like population within MCA-induced sarcomas as expressing high levels of stem cell antigen-1 (Sca-1) and low levels of CD90. These Sca-1+CD90− CSC-like cells had higher tumor initiating ability, could spontaneously give rise to Sca-1-negative cells, and formed more sarcospheres than corresponding non-CSC-like cells. Moreover, when examining MCA-induced sarcomas that were in the equilibrium phase of cancer growth, higher levels of CSC-like cells were found compared to MCA-induced sarcomas in the escape phase of cancer progression. Notably, CSC-like cells also emerged during escape from anti-PD-1 or anti-CTLA4 therapy, thus suggesting that CSC-like cells could evade immune therapy. Finally, we demonstrate that paradoxically, interferon (IFN)-γ produced in vivo by immune cells could promote the emergence of CSC-like cells. Our findings define the existence of a Sca1+CD90− CSC-like population in the MCA-sarcoma model capable of differentiation, tumorsphere formation, and increased tumor initiation in vivo. These cells may also act as mediators of immune resistance during cancer immunoediting and immune therapy.
KEYWORDS: cancer stem cells, cancer immune surveillance, cancer immunoediting, immune therapy, MCA Sarcoma
Introduction
Over the two last decades, the heterogeneity of cancers has been documented and dissected. From these studies, the cancer stem cell (CSC) paradigm emerged, stipulating that within tumors, a subset of cells endowed with stem-like properties initiates, maintains and propagates cancers.1–3 The requirement for CSCs in cancer formation4–6 and relapse7 has made the eradication of CSCs a paramount goal in cancer therapy.8–10 Cancer stemness may refer to both the cell of origin of tumors11–13 and cell reprogramming induced by oncogenesis.14,15 CSCs initiate and sustain tumor progression and are responsible for tumor metastasis via the expression of the transcription factor Twist5 among other genes. They resist conventional treatments such as radiation and chemotherapy and are believed to account for tumor resurgence after therapy.2,12 Importantly, many studies defining CSCs used xenograft models, and thus the interaction between CSCs and immune cells has not been well explored or defined.
Only a few reports document the interaction between CSCs and the immune system, resulting in no clear consensus on CSC immunogenicity. Some reports show that CD8+ T cells and NK cells can destroy CSCs,16,17 whereas others show their resistance to cancer immune surveillance.10,18 CSCs also secrete immunomodulatory cytokines19 and can take advantage of tumor promoting immunity.20,21 In a recent elegant study, antitumor immune responses mediated the dedifferentiation of melanoma cells towards a “stem-like” state via the cytokine tumor necrosis factor (TNF).18 In addition, type I and type II IFNs have been demonstrated to activate hematopoietic stem cells (HSCs) and CSCs in chronic myeloid leukemia.22–24 Thus, whereas multiple studies have shown that CSCs clearly promote cancer progression and resist conventional chemotherapy, whether CSCs resist immune therapy and how the immune system impacts on CSCs remains to be further defined.
Cancer immunoediting refers to the process by which tumor cells and immune cells interact with one another, leading to a sculpting of the cancer cell repertoire.25–27 Immunoediting begins on a substrate of highly immunogenic “unedited” cancer cells that can be completely eliminated by the immune system. In some instances a mixture of edited and unedited cancer cells are not completely eliminated and in fact can co-exist over long periods of time in the presence of immune pressure, representing an equilibrium phase.28,29 From this phase “edited” cancer cells can emerge, and these escaped cells can proceed to grow and become clinically significant. Edited cancer cells presumably have evaded immunity, but whether they have accumulated stem-like properties compared to unedited cells is not known.
Elimination, equilibrium, and escape phases of immunoediting27 have been modeled extensively using 3′methylcholanthrene (MCA) primary carcinogenesis rodent models.30 In this system, MCA induces sarcomas whose immunogenicity can be defined by transplanting them as tumor chunks31 into syngeneic recipients. Notably, MCA-induced sarcomas can also be easily adapted to culture to generate MCA-induced sarcoma cell lines, which can be transplanted at defined doses into syngeneic animals of varying levels of immunity, thereby allowing for more quantitative estimates of their immunogenicity.26,32,33 Since MCA sarcoma cell lines already have mesenchymal features, the CSC-like cells within MCA sarcomas have not been defined. We previously generated a panel of MCA sarcoma cell lines32 and herein define the CSC-like cell population within these cell lines. We found that high Sca-1 expression and low CD90 expression defined a CSC-like cell population that had increased cancer initiating properties and could be enriched during equilibrium or escape from immune therapy. Our studies therefore suggest that CSCs could emerge from immune pressure and imply that successful immune therapy of cancer will also need to target CSC-like cells.
Results
MCA sarcoma CSC-like cells could be defined by Sca-1+CD90− expression
To identify MCA sarcoma CSC-like cells, we first examined the expression of mesenchymal stem cell (MSC) markers (CD45−CD31−CD44+Sca-1+CD29+CD105+CD90+)34,35 as well as other CSC and iPS markers in several MCA sarcoma cell lines. Table 1 and Supplementary Fig. 1 show that all the tested MCA sarcoma cell lines had co-expression of the MSC markers CD44 and CD29, but these markers did not show heterogeneity. CD105, CD117 and CD133 were absent in most cell lines. iPS proteins (Oct4, Sox2, Nanog) were expressed but their expression lacked heterogeneity. CD90 and Sca-1 expression appeared to be the most heterogeneous markers and displayed an expression profile varying in the different cell lines tested (Table 1, Supplementary Fig. 1). Additionally, we analyzed the phenotype of primary MCA-induced sarcoma tumors (Supplementary Fig. 1). They displayed heterogeneity in a number of MSC and stemness markers. It was not possible to attribute this phenotype solely to the tumor cells as the tumor mass contained a variety of cells from the microenvironment which could not be excluded from the analysis. To alleviate this problem, we chose to focus our studies on MCA sarcoma cell lines, which provided a more robust and reliable model to study CSC-like cells.
Table 1.
Expression of CSC-like markers in MCA-sarcoma cell lines.
| F244 | F535 | 6730 | 9604 | 9609 | 4862 | |
|---|---|---|---|---|---|---|
| CD44 | ++a | ++ | ++ | ++ | ++ | ++ |
| CD29 | ++ | ++ | ++ | ++ | ++ | ++ |
| CD90 | − | HETb | HET | HET | HET | HET |
| Sca-1 | HET | +c | + | + | + | HET |
| CD105 | HET | −b | HET | − | − | HET |
| CD117 | − | − | − | − | − | − |
| CD133 | − | − | − | − | − | − |
| Oct4 | + | + | + | + | + | + |
| Sox2 | + | + | + | + | + | + |
| Nanog | + | + | + | + | − |
:++: Strong expression
:HET: Heterogeneous expression
:+: Intermediate expression
:−: No expression
Figure 1.
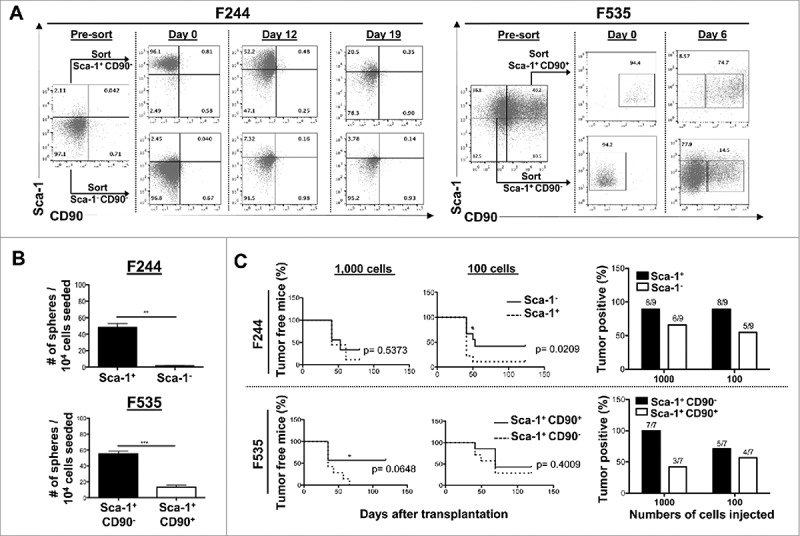
Identification of CSC-like cells in murine MCA-sarcoma cell lines. Analysis of the CSC-like potential of the various subpopulations based on Sca-1 and CD90 expression in the MCA-sarcoma cell lines F244 and F535. F244 was sorted based on Sca-1 expression into Sca-1+ and Sca-1− populations. F535 was sorted into Sca-1+CD90− and Sca-1+CD90+ populations. Each subpopulation was analyzed for their capacity to: (A) reconstitute the initial cell line heterogeneity in vitro by cultivating the sorted subpopulations and analyzing the reconstitution of the initial cell line heterogeneity over time by FACS analysis of cell surface expression of Sca-1 and CD90, (B) form sarcosphere in anchorage and serum-independent conditions, shown are the number of spheres per number of cells seeded ± S.E.M. (n = 3 in each condition), and (C) initiate tumor formation in vivo in Rag2−/− × γc−/−. Survival curves show the percentage of tumor-free mice over time after transplantation of each subpopulation. Histograms present the percent of tumor-bearing mice at the end of the experiment. The presented data are combining two independent experiments. *reflects the p values obtained in between groups at a given time point calculated by the Student t test, while the indicated p values represent the significance between the two groups in the global experiment calculated by the log-rank (Mantel-Cox) test. All the experiments were repeated at least twice.
Since the most heterogeneous phenotype in our panel of MCA-sarcomas was CD90 heterogeneity in a context where Sca-1 expression was positive, we tested these as potential markers of MCA sarcoma CSC-like cells. First, we examined the F244 cell line, which had Sca-1+ and Sca-1− populations. Figure 1A shows that sorted Sca-1+ populations are capable of giving rise to Sca-1− populations, suggesting that Sca-1 marked a CSC-like population. In the F535 MCA-sarcoma cell line, Sca-1+ cells could further be subdivided into CD90− and CD90+ populations. In this scenario, the Sca-1+CD90− cells had CSC-like features because they could give rise to the other populations but not vice versa (Fig. 1A, right panel). Isotype controls for Fig. 1A are shown in Supplementary Fig. 2A. This result was seen with other cell lines (Supplementary Fig. 2B), thus confirming that the Sca-1+CD90− population had a CSC-like repopulation capacity by reconstituting the initial tumor heterogeneity in vitro in an active process involving proliferation and differentiation. Given that Sca-1+ and Sca-1− as well as Sca-1+CD90− and Sca-1+CD90+ fractions have a similar growth rate in 2D culture (data not shown), we speculate that the regeneration of the initial tumor cell line heterogeneity is not due to the outgrowth of a contaminant Sca-1− and Sca-1+ CD90+ fraction after sort.
We next tested the stemness of Sca-1+CD90− cells in anchorage and serum-independent culture conditions. F244 was sorted into Sca-1+ and Sca-1− cells, F535 was sorted into Sca-1+CD90− and Sca-1+CD90+ cells, and both were seeded into conditions that allowed for sarcosphere growth and quantitation. Figure 1B shows that most of the sphere-forming capacity of the cell lines was contained within the Sca-1+ fraction (for F244) with a 20-fold enrichment or the Sca-1+CD90− fraction (for F535) with a 5.5-fold enrichment compared to the Sca-1− or Sca-1+CD90+ populations, respectively.
To test the tumor initiating properties of Sca-1+CD90− cells, sorted cells were transplanted at various doses into Rag2−/− × γc−/− mice. In F244, the tumor initiating capacity appeared to be enriched in the Sca-1+ population (Fig. 1C) by 10-fold compared to the Sca-1− population. In F535, a similar trend is observed with an enrichment in the tumor-forming capacity in the Sca-1+CD90− fraction compared to Sca-1+CD90+ population (Fig. 1C). Cancer stem-cell frequencies studied largely using xenografted human cells into immune deficient mice have been shown to be highly variable depending on how the transplantation assays were performed.36–38 We speculate that our syngeneic and orthopic transplantation model gives rise to a heightened plasticity of the tumor initiating cell capacity in vivo due to the interactions of tumors cells with their original microenvironment.
In conclusion, these compiled data demonstrate the existence of a hierarchy within MCA-induced tumors, where Sca1+CD90− cells possess the ability to generate daughter Sca1−/CD90− cell compartments, an increased sarcosphere-forming capacity in vitro and an increased tumor initiation in vivo.
Primary and transplanted MCA sarcomas in equilibrium are enriched in CSC-like cells
Having defined Sca-1 and CD90 as markers of CSC-like cells in MCA sarcoma cell lines, we next examined whether immune responses in vivo could regulate the proportion of CSC-like cells. The immune response to MCA-sarcomas is divided into three phases: elimination, equilibrium and escape.39 The equilibrium phase corresponds to cancer persistence in the presence of an active immune response and could represent the “dormant” stage of cancer,29 and so we hypothesized that tumors in equilibrium could represent a CSC-mediated state. We defined tumors in equilibrium as masses < 5 mm of diameter that remained stable over a month in immunocompetent hosts. We established equilibrium in animals injected with MCA and in animals transplanted with various MCA sarcoma cell lines representing primary and transplantable MCA sarcomagenesis, respectively (Supplementary Figure 3A-B). Tumor equilibrium was an extremely rare event in transplantation experiments and occurred in less than 2% of the mice injected with MCA in the present primary MCA-induced sarcomagenesis experiment. We compared the percentage of CSC-like cells, defined by Sca-1+CD90− cells among CD45−CD44+CD29+ sarcoma cells, in MCA-sarcoma tumors in equilibrium vs non-equilibrium, which we define as “progression” tumors. We found that tumors in equilibrium had an increased percentage of CSC-like cells compared to progressively growing tumors (Fig. 2A). A representative FACS plots is shown in Fig. 2B depicting the increased percentage of Sca-1+CD90− cells among CD45−CD44+CD29+ cells.
Figure 3.

Therapeutically-induced immune responses enriched the CSC-like population of MCA-sarcoma. (A) The cell line F244 was transplanted into syngeneic WT animals treated with either anti-CTLA4 (orange line), anti-PD-1 (blue line) or corresponding isotype controls (black line). Escaping tumors, defined by a tumor progression delayed by the checkpoint blockade treatment (anti-CTLA4, n = 2; anti-PD-1, n = 3) compared to the isotype control treatment (n = 8), are highlighted on the tumor kinetic graph (Left panel). (B-C) Tumors from part (A) were harvested at endpoint, and CSC percentages were analyzed immediately ex vivo without adapting to cell culture. (B) Scatter plot and (C) representative FACS plots are shown. The experiment was repeated at least three times.
Figure 2.
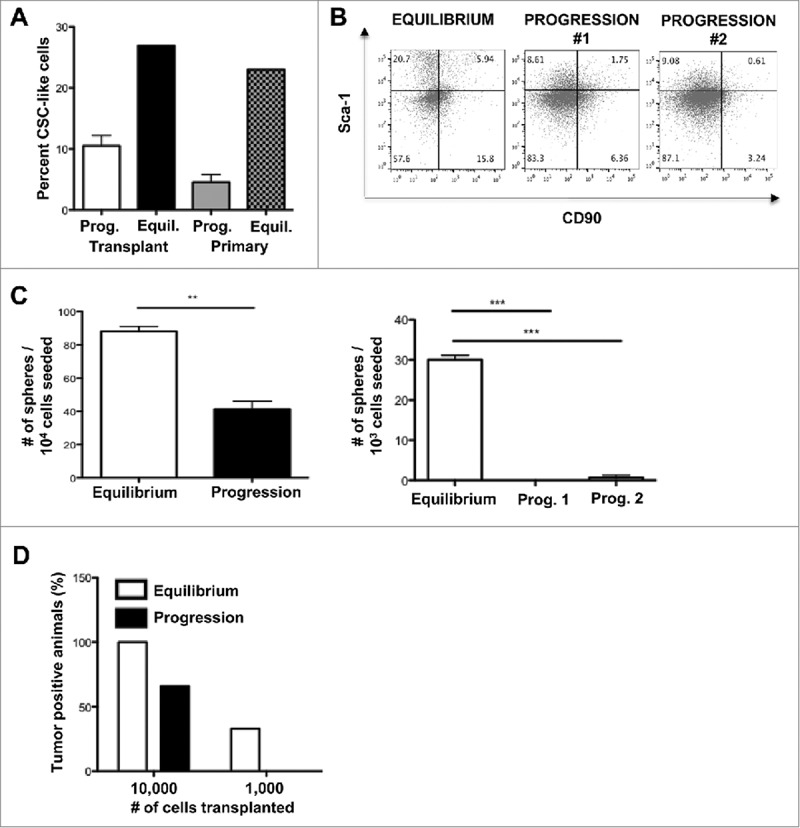
Primary and transplanted MCA sarcomas in equilibrium are enriched in CSC-like cells. (A) Transplanted F244 or 4862 MCA sarcoma cell lines or primary MCA-sarcoma tumors undergoing equilibrium or progressive growth were harvested and the percentage of CSC-like cells (Sca-1+CD90−) as a percentage of total tumor cells (CD45−CD44+CD29+) was assessed by FACS ex vivo and shown as a bar graph or (B) representative dot plots. (C) Sarcosphere-forming capacity of cell lines originating from equilibrium or representative progressive tumor transplants. Mean sarcosphere number ± S.E.M. is shown. Left panel shows the cell line 4862 and right panel shows the cell line F244. The experiment was repeated at least twice. (D) Cancer initiating property of cell lines derived from equilibrium tumors is greater than that of cell lines derived from progressing tumors. Cell lines were transplanted into in Rag2−/− × γc−/− mice at the indicated number of cells and the percentage of mice developing tumors at endpoint is shown.
To confirm that these tumors had greater CSC-like activity, cell lines were established from MCA-sarcomas undergoing either equilibrium or progression, and their sphere-forming capacity was tested (Fig. 2C). The sphere-forming capacity of cell lines generated from tumors in equilibrium was significantly higher (p<0.001) compared to the cell lines generated from progressing tumors in the two sarcoma cell lines tested. In addition, tumor initiation in severely immune-deficient hosts was increased when mice were transplanted with cell lines generated from tumors in equilibrium (Fig. 2D).
Cancers that have escaped immune therapy are enriched in CSC-like cells
To explore whether CSC-like cells resist immune therapy and may account for the relapse post-immune therapy, we set up a therapeutic protocol in vivo where tumor-bearing mice were treated with anti-PD-1, anti-CTLA4 or corresponding isotypes. In our model system, mono-therapy with checkpoint inhibitors showed three types of response (Fig. 3A): 1) no response where tumor kinetics of treated mice matched the tumor kinetic of mice treated with isotype controls (anti-CTLA4: 22% (2/9), anti-PD-1: 67% (6/9)); 2) complete response where tumors were rejected by the therapy (anti-CTLA4: 55% (5/9), anti-PD-1: 0% (0/9)); and 3) escape where the tumor initially responded to the treatment but escaped over time (anti-CTLA4: 22% (2/9), anti-PD-1: 34% (3/9)). Analysis of the tumor mass by flow cytometry in non-responsive and escaping tumors at tumor endpoint showed a significant increase in CSC-like cell percentages in escaping tumors (Fig. 3B-C, Supplementary Fig. 4). For example, tumors which escaped anti-CTLA4 treatment, showed a significantly higher percentage (p<0.001) of CSC-like cells (55.7%) compared to non-responsive tumors (13%) (Fig. 3B-C). Due to the low number of tumor bearing mice escaping CTLA4 therapy, additional experiments showing a similar trend between unresponsive and escaping tumors are shown in Supplementary Figure 4. These results suggest that CSC-like cells could mediate tumor resistance to checkpoint immune therapy.
CSC-like cells were induced by the immune system through IFN-γ
Since immune therapy is known to increase the production of IFN-γ, we next examined whether IFN-γ produced by immune cells in vivo could promote CSC-like cells. We used the well-studied F244 MCA-sarcoma cell line, as this cell line is known to induce IFN-γ production in vivo,40 while remaining a progressively growing tumor.26 F244 cells were transplanted into WT or Rag1−/− mice, which are deficient in adaptive immune cells capable of producing IFN-γ. At tumor endpoint, the proportion of CSC-like cells (CD45−CD44+CD29+Sca-1+CD90−) was investigated. Tumors transplanted into WT mice had a significantly (p<0.01) higher percentage of CSC-like cells than tumors transplanted in Rag1−/− hosts (Fig. 4A). The increase in CSC-like cells was due to endogenous production of IFN-γ since when mice were treated with blocking antibody to IFN-γ, the percentage of CSC-like cells was significantly decreased (p < 0.001) compared to treatment with control IgG (Fig. 4B). We confirmed that endogenous IFN-γ acted on cancer cells by examining MHC class I expression and PD-L1, two known targets of IFN-γ. Supplementary Figure 4 shows that in vivo IFN-γ blockade also decreased the levels of MHC class I expression on cancer cells analyzed ex vivo. In addition, the functionality of CSC-like cells was examined by sarcosphere assay, which showed an increase in the sphere-forming capacity of cell lines derived from tumors transplanted in WT compared to Rag1−/− host (Fig. 4C). These data show an endogenous induction of CSC-like cells by the adaptive immune system and IFN-γ. These results are further reinforced by the treatment of F244 with 100U/ml of IFN-γ in vitro. In a time-dependent manner, IFN-γ induced the expression of Sca-1 as early as 6 h post-treatment to reach a maximum at 24 h (Fig. 4D). This IFN-γ-induced expression of Sca-1 was shown to be reversible (Fig. 4E). These data suggest a regulation of CSC-like cells by IFN-γ in vitro and in vivo.
Figure 4.
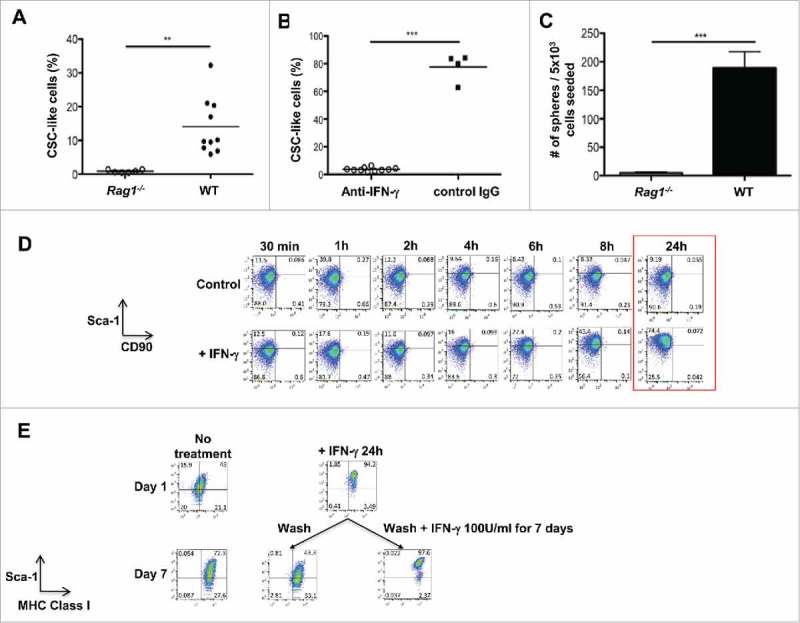
Emergence of CSC-like cells in vivo required IFN-γ production. (A) The cell line F244 was transplanted into syngeneic Rag1−/− (n = 6) or WT (n = 10) mice and CSC-like cells (Sca-1+CD90−) as a percentage of total tumor cells (CD45−CD44+CD29+) were assessed by FACS ex vivo. (B) The cell line F244 was transplanted into syngeneic WT mice treated with blocking anti-IFN-γ antibodies (n = 10) or isotype controls (n = 4). CSC-like cells were measured as in part (A). (C) Sarcosphere-forming capacity of cell lines originating from F244 tumor transplants in either Rag1−/− or WT recipients. Mean sarcosphere number ± S.E.M. is indicated (n = 3). (D) The cell line F244 was treated or not with 100U/ml of IFN-γ. Cell surface expression of Sca-1 and CD90 was analyzed at 30 minutes, 1, 2, 4, 6, 8 and 24 hours. (E) The cell line F244 was treated or not with 100U/ml of IFN-γ. Cells were washed and subsequently treated or not with 100U/ml of IFN-γ for 7 days and cell surface expression of Sca-1 and MHC Class I was analyzed All experiments in this figure were repeated at least twice.
Discussion
Although there is a general assumption that CSCs are immunoprivileged like their normal stem cell counterparts,10 most studies of CSCs make use of xenograft models involving immune deficient mice1,6,37 and therefore cannot completely address the active regulation of CSCs during cancer immunoediting. To study this process, we first identified CSC-like cells in MCA-induced primary sarcomas and sarcoma cell lines. We found that CSCs within MCA sarcomas could be identified and significantly enriched by a Sca-1+CD90− phenotype. Using this phenotypic characterization, we further showed that CSC-like cells could emerge during equilibrium, after immune therapy and after in vivo passage. In the latter process, this was due to the activity of endogenous IFN-γ.
We found that most MCA sarcoma cell lines had large populations that expressed Sca-1 but some did not, and in those cases Sca-1 marked a small CSC-like population. Other groups have similarly found that Sca-1 could serve as a potential universal stemness marker in murine cells.41–43 Interesting, Sca-1 is known to be induced by IFN-γ and is associated with stemness in the hematopoietic system.44–46 Indeed, we found that IFN-γ induced Sca-1 expression in MCA sarcoma cell lines. Notably, our results show that even in conditions without exogenous IFN-γ, Sca-1-expressing cells had better tumor initiating properties than cells lacking Sca-1.
CD90 characterizes mesenchymal stem cells (MSCs) but can also be expressed in stem-like cells in epithelial cancers.47 Surprisingly, we found it to be down-regulated on MCA sarcoma CSC-like cells. Given the broad ranges of sarcoma histologies, our results do not necessarily contradict other studies since the definition of CSC-like cells in sarcomas has not achieved consensus.48 Nevertheless, since most MCA-sarcomas expressed Sca-1, CD90 expression was the defining characteristic of non-CSC cells in our studies. Future studies will pursue whether CD90-negativity in mouse and human sarcomas could characterize a unique population of CSC-like cells that could have greater cancer initiating properties than conventional CSC-like cells that express CD90.
We found that antitumor immune responses could lead to the emergence of tumors that were enriched in CSC-like cells. In these studies, increased CSC-like cells were found in the equilibrium phase of cancer immunoediting as well as when cancers emerged after checkpoint inhibition. Given that true equilibrium phase tumors are rare and difficult to model, our conclusions stem from examining three total equilibrium tumors in three different settings (two different cell lines and one primary tumor). Notably, in all three cases, the equilibrium tumors had higher CSC percentages than the control progressor / escaping tumor. Although we could establish significance based on single tumor studies, our results support the concept that equilibrium or cancer dormancy indeed is mediated by CSC-like cells.49,50 Although CSCs have been shown to mediate escape from chemoresistance, it is not clear whether immune therapy selects for pre-existing non-immunogenic CSCs or “converts” non-CSCs into CSC-like cells. We showed here that tumor escaping anti-CTLA4 blockade therapy have an increased CSC-like phenotype. It is interesting to note that tumors escaping anti-PD-1 did not seem to have as high of a percentage of CSC-like cells as tumors escaping anti-CTLA4 treatment. Although speculative, it is possible that IFN-γ could mediate this difference, through increased production of IFN-γ in conditions where mice were treated with anti-CTLA4. In fact, a recent study51 showed that PD-1 expression on macrophages could reduce responsiveness of T cells to anti-PD-1, and thus potentially reduce the impact of anti-PD-1 on IFN-γ production. Moreover, another study showed that tumors escaping a combination of anti-CTLA4 and radiation therapy,52 had a clear IFN gene signature. In this study, blocking IFN signaling led to tumor regression, suggesting that IFN-γ promoted cancer growth. The authors did not fully define the mechanism by which IFN induced cancer cell resistance to immune attack, but our results herein would suggest that IFN-γ-induction of CSC-like properties could underlie these published observations. The induction of CSC-like properties by IFN-γ could be considered a “pro-tumor” activity that contrasts with IFN's known antitumor activities.53
Notably, other groups have found that IFNs can promote stemness, typically in the hematopoietic system18,23,54 and one can infer that this new role of immune-derived cytokines in stem cell biology may be a conserved mechanism of organisms to face pathogen insult by increasing their stem cell pool.23 Future studies will focus on defining and confirming IFN-γ's pro-stem cell activities in order to limit this aspect of IFN-γ's actions while preserving its strong antitumor function.
Materials and methods
Experimental protocol
All experiments involving mice were conducted under the animal protocol approved by the University of California, San Diego Institutional Animal Care and Use Committee (IACUC protocol #S06201).
Cell lines
MCA-sarcoma cell lines were a gift from Dr. Schreiber and were generated and maintained as described previously.26,55 Cell lines tested negative on August 2016 for Mycoplasma using the Mycoplasma detection kit from Lonza.
IFN-γ treatment
Treatments were performed with 100U/ml murine IFN-γ (Biolegend).
Mice
C57 BL/6 × 129/Sv F1, C57 BL/6 WT, C57 BL/6 Rag1−/−, mice were used for tumor transplantation experiments. All mice were bred in-house and exposed to similar microbiota and were 8 to 12 weeks old at the time of the experiments. All transplants were performed on syngeneic male mice. There was no exclusion of animals in this study. Mice were randomly assigned to each experiment.
Sphere assay
Cells were seeded at 104 or 103 /well in 24-well ultra-low attachment plates (Corning) and cultured in DMEM/F12 with N2, human EGF (10ng/mL), and bFGF (10ng/mL). After 14 days, the spheres were counted under an inverted phase contrast microscope (Nikon).
MCA induction
Tumor induction by 3-methylcholanthrene (MCA) was performed as previously described.26 Cohorts of WT and Rag1−/− were injected with MCA dissolved in peanut oil at 100μg per mouse. MCA-induced and passaged sarcomas were isolated in vitro.26,40
Tumor transplantation
Tumor cell lines were harvested by trypsinization, washed thrice with PBS, and injected subcutaneously into syngeneic recipients at 1 × 106 cells.26,40 For depletion experiments, WT mice were injected intraperitoneally (i.p.) with 200µg of either hamster IgG (PIP) or anti–IFN-γ (H22/#513206/Biolegend) on days −2, 0 and every 5 days until tumor harvest. Tumor growth was measured using mean tumor diameter.
In vivo anti-PD-1 and anti-CTLA4 treatment
WT mice were transplanted with the MCA-sarcoma cell line F244. Treatments (i.p. every 3 days) with 50μg of anti-PD-1 (J43/#BE0033-2), 50μg of anti-CTLA4 (9H10/#BE0131) or 50μg of isotype controls (Bioxcell) were started when tumors reached a mean tumor diameter of 3 mm. Tumors were analyzed for CSC-like cell percentages at endpoint (15mm).
Antibodies and flow cytometry
All gates in flow cytometry are defined by isotype control staining. Tumor cell lines or tumor harvests were processed as previously described.55 The following mouse cell antibodies were used: anti-CD45 (30-F11/#103116), CD44 (IM7/#103006), CD29 (HMβ1-1/#102208), Sca-1 (D7/#108114), CD90 (53-2.1/#140312), H-2Kb/H-2Db (28-8-6/#114606), H-2Kd (SF-1.1/#116604), CD133 (315-2C11/#141207)(all from Biolegend), CD117 (2B8/#25-1171-82), Oct4 (EM92/#12-5841-82), Sox2 (Btjce/#53-9811-82), Nanog (MLC-51/#53-5761-80) and PD-L1 (MIH5/#12-5982-81) (all from eBioscience). For intracellular staining, cells were processed according to manufacturer instruction (Cytofix-Perm/Wash, BD biosciences). Cells were analyzed on a BD FACSCanto.
Cell sorting
Cells were MACS-sorted using anti-Sca-1-PE antibody in combination with anti-PE multi-sort kit (Miltenyi) or CD90 microbeads, according to the manufacturer instruction. Some cell populations were FACS-sorted on a BD FACSAria II.
Statistical Analysis
Statistical significance between two groups at defined time points was determined by the Student t test using two-tailed analysis to obtain p-values and assuming unequal variance. For measurements where variance was observed, such as percentage of CSC-like cells, we observed a normal distribution without skewing. Variance was portrayed in some figures as standard deviation (square root of variance) or standard error of the mean (S.E.M.). The Log-Rank (Mantel-Cox) test was used to compare the survival of mice across tumor transplantation or induction conditions. Error bars are depicted using the S.E.M., mean is represented as center values and *p<0.05, **p<0.01, ***p<0.001. All experiments were done at least twice and representative data are shown. For experiments with mice, the cohort size was determined by previous experiments that we have performed with these cell lines.56
A cohort of 5 mice was considered ideal for determining survival, regression, and progression. In experiments where growth delay or kinetics were examined, depending on the extent of delay more animals were included in the cohort, or the experiment was repeated to attain statistical significance. We did not exclude any data. For mouse measurements, sphere counts, flow cytometry analysis and gating, some samples were provided to technicians who were blinded to the identity of the sample.
Table 1 summarizes cell surface expression of CD44, CD29, CD105, CD90, Sca-1, CD117 and CD133 as well as the intracellular protein expression of Oct4, Nanog and Sox2 in the murine MCA-sarcoma cell lines F244, F535, 6730, 9604, 9609 and 4862 determined by flow cytometry.
Supplementary Material
Funding Statement
J.D.B. is supported by NIH grant CA157885 and The Hartwell Foundation.
Disclosure of potential conflicts of interest
There are no conflicts of interest to disclose.
Acknowledgments
We thank Calvin Lee for technical support.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3(7):730–7. doi: 10.1038/nm0797-730. PMID:9212098 [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8(10):755–68. doi: 10.1038/nrc2499. PMID:18784658 [DOI] [PubMed] [Google Scholar]
- 3.Nassar D, Blanpain C. Cancer stem cells: Basic concepts and therapeutic implications. Annu Rev Pathol 2016;11:47–76. doi: 10.1146/annurev-pathol-012615-044438. PMID:27193450 [DOI] [PubMed] [Google Scholar]
- 4.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al.. Identification of cells initiating human melanomas. Nature 2008;451(7176):345–9. doi: 10.1038/nature06489. PMID:18202660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al.. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. PMID:18485877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. PMID:12629218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2013;2(1):3. doi: 10.1186/2001-1326-2-3. PMID:23369605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009;138(4):645–59. doi: 10.1016/j.cell.2009.06.034. PMID:19682730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013;34(6):732–40. doi: 10.1038/aps.2013.27. PMID:23685952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138(2):271–85. doi: 10.1016/j.cell.2009.05.046. PMID:19632178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature 2012;488(7412):527–30. doi: 10.1038/nature11344. PMID:22854777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012;488(7412):522–6. doi: 10.1038/nature11287. PMID:22854781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012;337(6095):730–5. doi: 10.1126/science.1224676. PMID:22855427 [DOI] [PubMed] [Google Scholar]
- 14.Munoz P, Iliou MS, Esteller M. Epigenetic alterations involved in cancer stem cell reprogramming. Molecular oncology 2012;6(6):620–36. doi: 10.1016/j.molonc.2012.10.006. PMID:23141800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al.. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Eng J Med 2004;351(7):657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 16.Liao T, Kaufmann AM, Qian X, Sangvatanakul V, Chen C, Kube T, Zhang G, Albers AE. Susceptibility to cytotoxic T cell lysis of cancer stem cells derived from cervical and head and neck tumor cell lines. J Cancer Res Clin Oncol 2013;139(1):159–70. doi: 10.1007/s00432-012-1311-2. PMID:23001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer 2011;2:443–57. doi: 10.7150/jca.2.443. PMID:21850212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M, et al.. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012;490(7420):412–6. doi: 10.1038/nature11538. PMID:23051752 [DOI] [PubMed] [Google Scholar]
- 19.Ramgolam K, Lauriol J, Lalou C, Lauden L, Michel L, de la Grange P, Khatib AM, Aoudjit F, Charron D, Alcaide-Loridan C, et al.. Melanoma spheroids grown under neural crest cell conditions are highly plastic migratory/invasive tumor cells endowed with immunomodulator function. PLoS One 2011;6(4):e18784. doi: 10.1371/journal.pone.0018784. PMID:21526207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al.. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol 2014;16(11):1105–17. doi: 10.1038/ncb3041. PMID:25266422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem cells 2013;31(2):248–58. doi: 10.1002/stem.1281. PMID:23169551 [DOI] [PubMed] [Google Scholar]
- 22.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009;458(7240):904–8. doi: 10.1038/nature07815. PMID:19212321 [DOI] [PubMed] [Google Scholar]
- 23.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 2010;465(7299):793–7. doi: 10.1038/nature09135. PMID:20535209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-gamma. J Exp Med 2013;210(3):605–21. doi: 10.1084/jem.20121229. PMID:23401488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331(6024):1565–70. doi: 10.1126/science.1203486. PMID:21436444 [DOI] [PubMed] [Google Scholar]
- 26.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410(6832):1107–11. doi: 10.1038/35074122. PMID:11323675 [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3(11):991–8. doi: 10.1038/ni1102-991. PMID:12407406 [DOI] [PubMed] [Google Scholar]
- 28.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol 2008;84(4):988–93. doi: 10.1189/jlb.1107774. PMID:18515327 [DOI] [PubMed] [Google Scholar]
- 29.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450(7171):903–7. doi: 10.1038/nature06309. PMID:18026089 [DOI] [PubMed] [Google Scholar]
- 30.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst 1957;18(6):769–78. PMID:13502695 [PubMed] [Google Scholar]
- 31.Prehn RT, Bartlett GL. Surveillance, latency and the two levels of MCA-induced tumor immunogenicity. Int J Cancer 1987;39(1):106–10. doi: 10.1002/ijc.2910390119. PMID:3793267 [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, et al.. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 2012;209(10):1869–82. doi: 10.1084/jem.20112738. PMID:22927549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med 2005;202(5):583–8. doi: 10.1084/jem.20050994. PMID:16129707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Ren Z, Du X, Hao M, Zhou W. The role of mesenchymal stem/progenitor cells in sarcoma: update and dispute. Stem Cell Investig 2014;1:18. PMID:27358864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarnerio J, Riccardi L, Taulli R, Maeda T, Wang G, Hobbs RM, Song MS, Sportoletti P, Bernardi R, Bronson RT, et al.. A genetic platform to model sarcomagenesis from primary adult mesenchymal stem cells. Cancer Discov 2015;5(4):396–409. doi: 10.1158/2159-8290.CD-14-1022. PMID:25614485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al.. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010;466(7302):133–7. doi: 10.1038/nature09161. PMID:20596026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008;456(7222):593–8. doi: 10.1038/nature07567. PMID:19052619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010;18(5):510–23. doi: 10.1016/j.ccr.2010.10.012. PMID:21075313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. PMID:15032581 [DOI] [PubMed] [Google Scholar]
- 40.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol 2006;176(2):905–13. doi: 10.4049/jimmunol.176.2.905. PMID:16393975 [DOI] [PubMed] [Google Scholar]
- 41.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, et al.. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports 2013;1(5):397–410. doi: 10.1016/j.stemcr.2013.09.004. PMID:24286028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houlihan DD, Mabuchi Y, Morikawa S, Niibe K, Araki D, Suzuki S, Okano H, Matsuzaki Y. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-alpha. Nat Protoc 2012;7(12):2103–11. doi: 10.1038/nprot.2012.125. PMID:23154782 [DOI] [PubMed] [Google Scholar]
- 43.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 2007;25(6):1339–47. doi: 10.1634/stemcells.2006-0644. PMID:17379763 [DOI] [PubMed] [Google Scholar]
- 44.Zhao X, Ren G, Liang L, Ai PZ, Zheng B, Tischfield JA, Shi Y, Shao C. Brief report: interferon-gamma induces expansion of Lin(−)Sca-1(+)C-Kit(+) Cells. Stem Cells 2010;28(1):122–6. doi: 10.1002/stem.252. PMID:19890981 [DOI] [PubMed] [Google Scholar]
- 45.Dumont FJ, Dijkmans R, Palfree RG, Boltz RD, Coker L. Selective up-regulation by interferon-gamma of surface molecules of the Ly-6 complex in resting T cells: the Ly-6 A/E and TAP antigens are preferentially enhanced. Eur J Immunol 1987;17(8):1183–91. doi: 10.1002/eji.1830170816. PMID:3040423 [DOI] [PubMed] [Google Scholar]
- 46.Snapper CM, Yamaguchi H, Urban JF Jr, Finkelman FD. Induction of Ly-6 A/E expression by murine lymphocytes after in vivo immunization is strictly dependent upon the action of IFN-alpha/beta and/or IFN-gamma. Int Immunol 1991;3(9):845–52. doi: 10.1093/intimm/3.9.845. PMID:1931812 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Zhang C, Zhu H, Tang J, Zhang S, Luo J, Sun X. CD90 positive cells exhibit aggressive radioresistance in esophageal squamous cell carcinoma. J Thorac Dis 2017;9(3):610–20. doi: 10.21037/jtd.2017.03.28. PMID:28449469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trucco M, Loeb D. Sarcoma stem cells: do we know what we are looking for? Sarcoma 2012;2012:291705. doi: 10.1155/2012/291705. PMID:22654552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol 2013;734:145–79. doi: 10.1007/978-1-4614-1445-2_8. PMID:23143979 [DOI] [PubMed] [Google Scholar]
- 50.Patel P, Chen EI. Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol (Lausanne) 2012;3:125. PMID:23109929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al.. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545(7655):495–9. doi: 10.1038/nature22396. PMID:28514441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint\sVictor C, Cucolo L, Lee DS, Pauken KE, Huang AC, et al.. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016;167(6):1540–54 e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, et al.. Interferon-gamma and cancer immunoediting. Immunol Res 2005;32(1–3):231–45. doi: 10.1385/IR:32:1-3:231. PMID:16106075 [DOI] [PubMed] [Google Scholar]
- 54.Jiang Q, Crews LA, Barrett CL, Chun HJ, Court AC, Isquith JM, Zipeto MA, Goff DJ, Minden M, Sadarangani A, et al.. ADAR1 promotes malignant progenitor reprogramming in chronic myeloid leukemia. Proc Natl Acad Sci U S A 2013;110(3):1041–6. doi: 10.1073/pnas.1213021110. PMID:23275297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Sullivan T, Saddawi-Konefka R, Gross ET, Washington A Jr, Bui JD. Tumor-expressed IL-17D recruits NK cells to reject tumors. Oncoimmunology 2014;3(12):e954853. doi: 10.4161/21624011.2014.954853. PMID:25964859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Sullivan T, Saddawi-Konefka R, Gross E, Tran M, Mayfield SP, Ikeda H, Bui JD. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep 2014;7(4):989–98. doi: 10.1016/j.celrep.2014.03.073. PMID:24794441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


