ABSTRACT
Colitis is a frequent, clinically-significant immune-related adverse event caused by anti-programmed death-1 (PD-1). The clinical features, timing, and management of colitis with anti-PD-1-based regimens are not well-characterized. Patients with advanced melanoma that received either anti-PD-1 monotherapy (“monotherapy”) or combined with ipilimumab (“combination therapy”) were screened from 8 academic medical centers, to identify those with clinically-relevant colitis (colitis requiring systemic steroids). Of 1261 patients who received anti-PD-1-based therapy, 109 experienced colitis. The incidence was 3.2% (30/937) and 24.4% (79/324) in the monotherapy and combination therapy cohorts, respectively. Patients with colitis from combination therapy had significantly earlier symptom onset (7.2 weeks vs 25.4 weeks, p < 0.0001), received higher steroid doses (median prednisone equivalent 1.5 mg/kg vs 1.0 mg/kg, p = 0.0015) and experienced longer steroid tapers (median 6.0 vs 4.0 weeks, p = 0.0065) compared to monotherapy. Infliximab use and steroid-dose escalation occurred more frequently in the combination therapy cohort compared to monotherapy. Nearly all patients had resolution of their symptoms although one patient died from complications. Anti-PD-1 associated colitis has a variable clinical presentation, and is more frequent and severe when associated with combination therapy. This variability in checkpoint-inhibitor associated colitis suggests that further optimization of treatment algorithms is needed.
KEYWORDS: Colitis, immune-related adverse events, anti-programmed-death-1, immunotherapy, melanoma
Introduction
Recent advances in immunotherapy have produced unprecedented clinical improvements for patients with melanoma. Specifically, immune checkpoint inhibitors (ICIs) targeting cytotoxic T lymphocyte associated antigen 4 (CTLA-4; ipilimumab) and programmed death receptor 1 (PD-1; nivolumab, pembrolizumab) trigger anti-tumor immunity by releasing potent tumor-infiltrating T cell responses. Five-year overall survival (OS) has more than tripled (34% vs historical 10%) for patients with metastatic melanoma in the past 10 years with single-agent anti-PD-1 therapy.1,2 However, the enthusiasm for these treatments has been somewhat tempered by uncommon life-threatening and the potential for highly-morbid immune-related adverse events (irAEs). These toxicities are caused by aberrant T-cell activation targeting normal host tissue resulting in inflammation of the colon, upper gastrointesintal tract, lung, endocrine glands, skin, liver and other organs.3,4 In most cases, irAEs are reversible with prompt corticosteroid treatment or therapy cessation, but fulminant cases leading to death may occur in up to 1% of patients.5–7 In particular, colitis, a syndrome primarily characterized by diarrhea and less often abdominal pain and hematochezia, remains one of the most frequent and morbid irAE. Ipilimumab-related colitis has been well characterized in melanoma, with a reported incidence up to 22% and median time to onset of 8 weeks.8,9 By contrast, the clinical features of colitis caused by anti-PD-1 based therapies are not well described. Detailed characterization of anti-PD-1 associated toxicities is particularly critical since these regimens are approved or being developed for numerous cancers and in earlier, adjuvant settings.10–12
To address this, we retrospectively analyzed patients with colitis from eight large academic centers. We aimed to characterize the incidence, timing, and clinical features of immune-related colitis with single agent anti-PD-1 and in combination with anti-CTLA-4 therapies in advanced melanoma. Further, we assessed the efficacy of immunosuppressive treatment and post-colitis outcomes.
Results
Incidence of colitis
From a total of 1261 patients screened from 8 academic centers, 937 patients received anti-PD-1 monotherapy and 324 received combination therapy with ipilimumab and nivolumab/pembrolizumab. In the entire cohort, 109 (8.6%) patients developed immune-related colitis. There was an incidence of 3.2% (30/937) in patients treated with monotherapy compared with 24.4% (79/324) in patients treated with combination ipilimumab and nivolumab/pembrolizumab (p < 1x107). Rates of colitis are listed by center (Supplementary Table S1).
Baseline demographics
Of the patients with anti-PD-1 monotherapy-associated colitis, 53.3% were male with a median age of 61.5 (range 33 – 88) (Table 1). Most patients (73.3%) received prior therapies; 53.3% were treated with prior ipilimumab and 11.1% with BRAF/MEK inhibitors. In comparison, for patients with combination therapy associated colitis, 62.0% were male with a median age of 59 (range 25 – 78). Moreover, most patients (59.5%) did not receive prior therapy with only 6.3% receiving prior ipilimumab and 15.4% receiving BRAF and/or MEK inhibitors. Both cohorts had excellent anti-tumor responses with objective response rates of 76.9% and 56.1% for patients treated with monotherapy and combination therapy, respectively. Median follow-up after colitis in this study was 9.7 months.
Table 1.
Baseline demographics*.
| Characteristic | Monotherapy (n = 30) | Combination therapy (n = 79) |
|---|---|---|
| Median age, range | 61.5 (33 – 88) | 59 (25 – 78) |
| Male Gender (%) | 16 (53.3%) | 49 (62.0%) |
| Median BMI, range | 27 (21 – 34) | 28 (17 – 47) |
| Primary types (%) | ||
| Cutaneous | 19 (82.6%) | 54 (72.0%) |
| Acral | 1 (4.3%) | 1 (1.3%) |
| Mucosal | 0 (0%) | 5 (6.7%) |
| Uveal | 0 (0%) | 4 (5.3%) |
| Unknown primary | 3 (13.0%) | 7 (9.3%) |
| BRAF WT, (%) | 20 (71.4%) | 49 (64.5%) |
| Prior ipilimumab (%) | 16 (53.3%) | 5 (6.3%) |
| Prior BRAF/MEK inhibitor (%) | 3 (11.1%) | 12 (15.4%) |
| Objective response rate (%) | 76.9% | 56.1% |
* % based off evaluable patients
Onset and diagnosis of colitis
Patients receiving monotherapy developed colitis at a median of 25.4 weeks (range 0.6 – 119.9 weeks), while patients on combination therapy experienced colitis significantly earlier in their treatment course, at a median of 7.2 weeks (range 0.7 – 51 weeks; p < 0.0001) (Figure 1). In the combination cohort, patients treated with sequential therapy (n = 8) had a later median onset (18 weeks) than in the concurrent combination cohort (6.1 weeks). The diagnosis of colitis was confirmed by colonoscopy and/or radiographic imaging in the majority of cases (71.9%). In both the monotherapy and combination therapy cohorts, patients were more likely to have an endoscopic evaluation (monotherapy: 52.2%, combination: 60.3%) than radiographic imaging (monotherapy: 34.8%, combination: 31.5%; all with abdominal CT or MRI scans). In patients that received colonoscopy, visual findings of inflammation were seen in 66.7% and 70.5% of patients treated with monotherapy and combination therapy, respectively. Ulcerations have been previously linked to steroid-refractory disease, and were seen in 16.7% of monotherapy and 18% combination therapy treated patient.13 Monotherapy patients that had ulcerations on colonscopy had a lower rate of infliximab use (0% vs 50%, p = 0.5), while combination therapy patients with ulcerations on colonscopy had similar rate of infliximab use (62.5% vs 52.7%, p = 0.7). Biopsies from those colonoscopies revealed pathologic findings of inflammation (active colitis, cryptitis, inflammatory cell infiltrates) in 90.9% and 90.7% of monotherapy and combination therapy patients, respectively (Figure 2). Among 17 patients that had negative visual findings on colonoscopy (i.e. normal appearing mucosa), biopsy revealed inflammatory changes in 100% (4/4) and 84.6% (11/13) of patients treated with monotherapy and combination therapy, respectively. There was no notable difference in colitis treatment outcomes for those patients that had normal biopsies or negative visual findings compared with those with confirmatory results (data not shown). In cases where radiographic imaging was performed, there were more frequent findings of colitis (wall thickening, enhancement, adenopathy) in patients treated with combination therapy than those who received monotherapy (69.6% vs 37.5%) (Figure 3).
Figure 1.
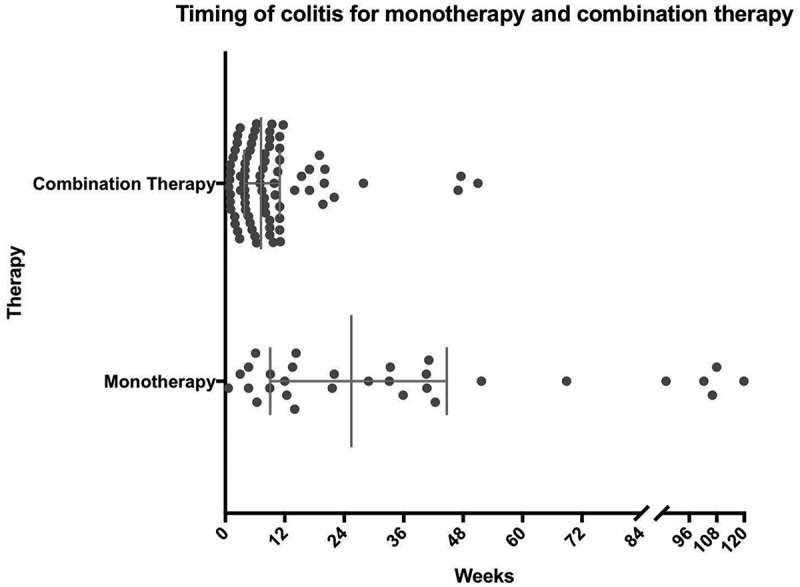
Timing of colitis: Represents time from first dose of therapy to date of colitis with interquartile range and median.
Figure 2.
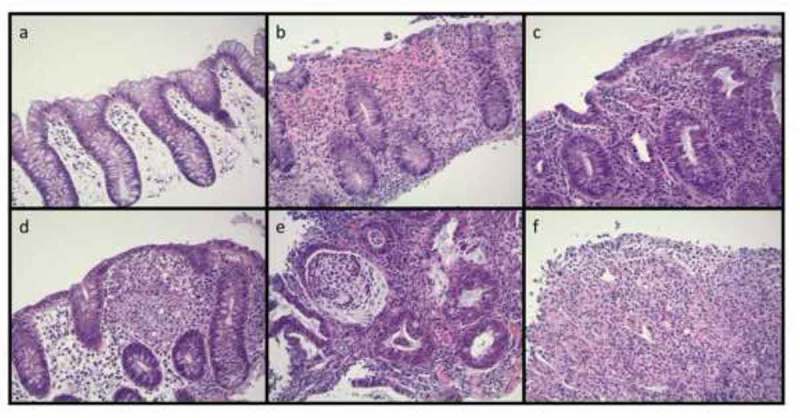
Histologic findings in checkpoint inhibitor colitis may include: a) Minimal epithelial changes with lamina propria edema; b) increased epithelial apoptotic figures; c) epithelial neutrophilic infiltrate; d) histiocytic aggregates within the lamina propria; e) neutrophilic crypt abscesses; f) ulceration with neutrophilic debris (H&E, 200x magnification).
Figure 3.
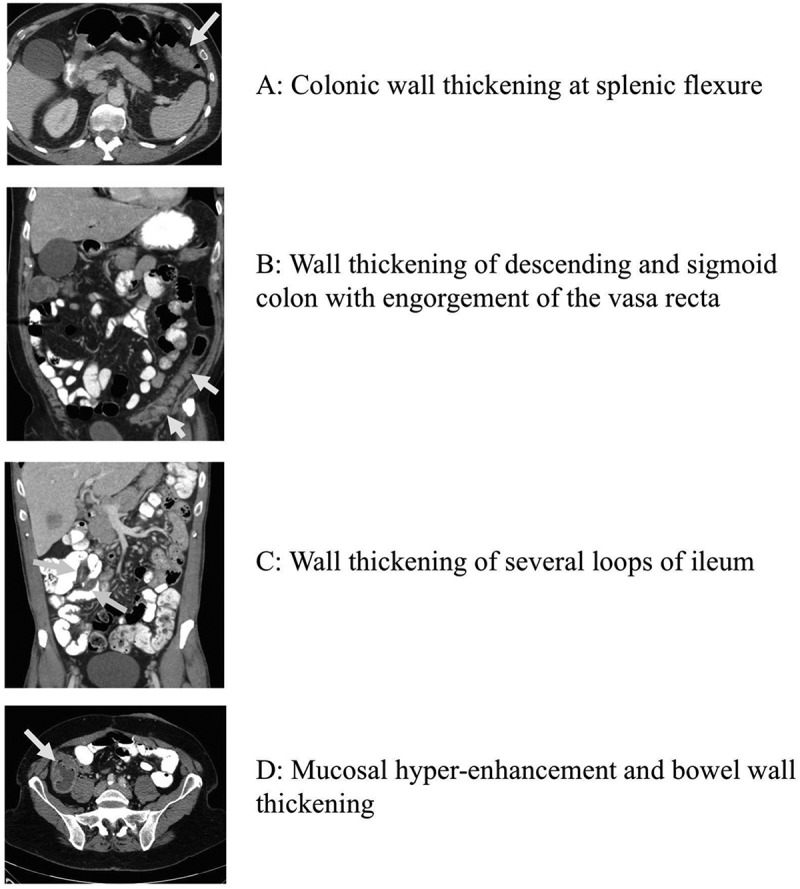
Radiographic features of colitis associated with anti-PD-1 therapy: a) colonic wall thickening at splenic flexure; b) wall thickening of descending and sigmoid colon with engorgement of the vasa recta; c) wall thickening of several loops of ileum; d) mucosal hyper-enhancement and bowel wall thickening.
Both cohorts frequently experienced grade 3 or higher colitis (monotherapy: 73.3%, combination therapy: 73.4%)(Supplementary Table S2). There was one grade 5 event in a 76 year old patient treated with combination therapy. The patient developed colitis after 2 doses of ipilimumab and nivolumab. Despite intravenous corticosteroids and infliximab, he developed ischemic colitis and died after subsequent complications.
Many patients experienced additional irAEs during their treatment course with no statistically significant difference in incidence between the combination therapy and monotherapy cohorts (60.6% vs. 45.5%, p = 0.21, respectively). The most common additional irAEs in monotherapy patients were related to the skin (5), endocrine organs (3), liver (2) and joints (2), whereas liver (15), endocrine (14), skin (11), lungs (4) and joints (4) were observed in combination therapy. Patients treated with combination therapy often experienced these irAEs before (46.5%) or concurrently (30.2%) with colitis rather than after (27.9%) experiencing colitis, whereas monotherapy patients experienced irAEs more frequently before (50.0%) and after (40.0%) than concurrently (20.0%) experiencing colitis (including several patients who had > 1 additional irAE). By contrast, other severe (grade 3–4) irAEs occurred more frequently with combination patients than monotherapy patients (25.6% vs. 10.0%, respectively).
Treatment course of colitis
The course of immunosuppressive treatment for anti-PD-1 associated colitis differed based on the immunotherapy regimen. Patients treated with monotherapy received a lower initial dose of steroids (median 1.0 mg/kg vs. 1.5 mg/kg prednisone equivalent, rounded to nearest 0.5 mg/kg, p = 0.0015), and a shorter duration of steroid taper (median 4.0 vs 6.0 weeks, p = 0.0065) compared to patients treated with combination therapy (Table 2). Furthermore, monotherapy patients were less likely to require steroid dose re-escalation (23.3% vs 39.2%, p = 0.12) with their initial steroid taper, or addition of infliximab (30% vs. 44.3%, p = 0.17) although these results were not statistically significant. Most patients (74.7%) required hospitalization for the treatment of their colitis with a similar incidence in both monotherapy (78.3%) and combination therapy (73.6%).
Table 2.
Colitis treatment details*.
| Characteristic | Monotherapy (n = 30) | Combination therapy (n = 79) |
|---|---|---|
| Grade 3 or higher (%) | 22 (73.3%) | 58 (73.4%) |
| Median onset in weeks, range | 25.4 (0.6 – 119.9) | 7.2 (0.7 – 51.0) |
| Median mg/kg (prednisone equivalent), range | 1.0 (0.5 – 3.5) | 1.5 (0.5 – 4.5) |
| Median duration of taper (weeks), range | 4.0 (0.5 – 16.0) | 6.0 (1.0 – 30.0) |
| No. requiring dose escalation (%) | 7 (23.3%) | 31 (39.2%) |
| No. treated with infliximab (%) | 9 30.0%) | 35 (44.3%) |
| No. relapse of colitis (%) | 7 (23.3%) | 15 (19.0%) |
| No. rechallenged with PD-1 inhibitor (%) | 5 (17.2%) | 38 (50.0%) |
| No. relapsed after rechallenge (%) | 1 (20%) | 5 (13.2%) |
| Hospitalizations (%) | 18 (78.3%) | 53 (73.6%) |
| Complications related to steroids (%) | 4 (18.2%) | 12 (16.9%) |
* % based off evaluable patients. Abbreviation: No. = number
Immunosuppression was generally well tolerated although 17.4% and 16.9% of patients reported complications related to steroid use or immunosuppression with monotherapy and combination therapy-related colitis, respectively. These complications included adrenal insufficiency (n = 5), hyperglycemia (n = 4), musculoskeletal issues (n = 3), volume overload (n = 2), hypertension, psychosis, insomnia, and clostridium difficile colitis.
Outcomes of colitis treatment
Nearly all patients (94.4%) had eventual resolution of colitis symptoms. While most patients experienced symptom resolution, relapses occurred in a minority of patients from both groups (monotherapy: 23.3%, combination therapy: 19.0%, p = 0.56). These relapses occurred more frequently in combination therapy treated patients when steroid tapers were shorter than the median duration (33.3% with combination vs. 14.3% with monotherapy, p = 0.64). However, monotherapy treated patients appeared more likely to relapse if treated with less than 1 mg/kg initial steroid dose when compared to those who received combination therapy (33.3% vs 14.3%, p = 0.56) although these numbers are small (Figure 4). Only 28.6% monotherapy patients required intravenous treatment (steroids and/or infliximab), while 53.3% of combination patients required intravenous treatment. The presence (vs. absence) of inflammation determined by visual analysis on colonoscopy, histopathologic evaluation, or radiographic imaging did not predict likelihood of objective response to anti-PD-1 based therapy, relapse of colitis, or use of infliximab (Supplementary Table S3).
Figure 4.
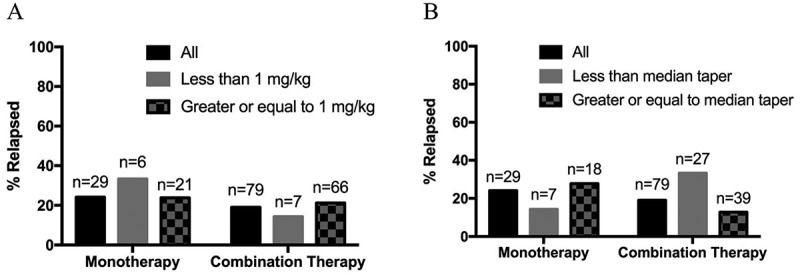
Incidence of relapse of colitis based on steroid (a) dose and (b) taper by therapy.
After resolution, a minority of monotherapy patients (16.7%) were rechallenged with anti-PD-1 therapy. Of those 5 patients rechallenged, 1 (20%) of patients developed recurrent colitis. Interestingly, half (50.0%) of the total combination therapy group were rechallenged with anti-PD-1 monotherapy, and had a low incidence of recurrent colitis after rechallenge (13.2%). One patient treated with monotherapy was rechallenged with combination therapy and had subsequent relapse of his colitis.
Discussion
Immune-related colitis is one of the most common and severe irAEs associated with anti-PD-1 based therapy. In our study, we provide a comprehensive clinical description of the incidence, clinical features, real-world diagnostic approaches, and treatment outcomes from eight large centers. Understanding the features of this irAE is increasingly important as countless patients across tumor types will receive anti-PD-1 based therapies in the coming years.
We found that patients treated with combination therapy had a higher incidence of colitis as compared to those treated with monotherapy (24.4% vs. 3.2%), similar to previous reports showing a higher incidence of colitis with combined PD-1/CTLA-4 blockade.14 While this incidence is higher than the rate of grade 3–4 colitis reported in clinical trials, it likely reflects the real-world incidence of clinically-relevant colitis seen in practice. Despite their similarities, the adverse events “colitis” and “diarrhea” are often distinguished in published clinical trials, making the true incidence of this entity more challenging to determine. Further, in clinical trials, grade 3 colitis/diarrhea (defined as severe abdominal pain and/or > 6 stools above normal) is clearly demarcated from the clinically significant grade 2 colitis (4–6 stools above baseline). Thus, we posit that “clinically-relevant colitis” defined as colitis (or diarrhea) severe enough to warrant systemic steroids, adds value to a strictly grade-based classification.
The diagnosis of immune-related colitis is often made with symptomatic criteria although 72% of patients in our study received radiographic imaging and/or colonoscopy. These studies were usually performed to rule out infectious causes and rarely changed the course of therapy, even when negative. Interestingly, the various findings by radiographic imaging and histology underscore the heterogeneous nature of this phenomenon, which have been similarly described in immune-related pneumonitis and ipilimumab-related colitis.15–17 In particular, we noted patients that had no pathologic and radiographic findings of colitis experienced similar courses as biopsy or radiographic-proven disease. These findings reinforce the notion that immune-related colitis remains a largely symptomatic diagnosis, where adjunctive studies are helpful when ruling out competing diagnoses.
Clinical characterization of immune-related colitis revealed an impressive difference in the timing of colitis between immune-checkpoint regimens. Specifically, most patients that received combination therapy developed colitis before 8 weeks, while on average colitis occurred much later and in a wide distribution for monotherapy patients. The rapid and more predictable onset of colitis with combination treated patients may be a reflection of ipilimumab-related kinetics, which has been well described previously.8 Interestingly, the patients that received sequential treatment had a much later onset of colitis (median 18 weeks), which suggests colitis associated with concurrent combination therapy may occur even earlier. Further, colitis due to combination therapy rarely recurred when anti-PD-1 was re-introduced, suggesting that ipilimumab was the primary “driver” of these events.18 The aggressive nature of combination-induced colitis mirror the clinical patterns of other combination-induced immune-related adverse events such as myocarditis, pneumonitis, and endocrinopathies.15,19
Understanding the timing of colitis in anti-PD-1 based therapy may help to further elucidate the association of clinical benefit with immune-related adverse events. An abundance of conflicting data alternatively supports and refutes the claim that immune-related adverse events portend an objective response or clinical benefit.3,20–24 Our study illustrates the importance of timing in this interpretation. While patients in our study treated with combination therapy had a comparable response rate (56.1%) to that observed in clinical trials (~ 60%), the monotherapy cohort had a much higher response rate (76.9%) than would typically be expected (~ 45%).25 Superficially, these data suggest that immune-related colitis has prognostic value for patients treated with anti-PD-1 monotherapy. However, most monotherapy-related colitis cases arose long after their initial evaluation of response, suggesting a selection bias where patients who benefit from therapy receive much more treatment and live long enough to develop immune-related colitis. These findings need to be studied in a prospective setting and further studies are needed to elucidate any mechanisms linking toxicities with benefit.
The treatment of anti-PD-1-related colitis is largely based on the guidelines for the treatment of ipilimumab-related colitis, which recommends weight-based doses of steroids based on the severity (grade) of colitis. While this algorithm leads to the resolution of most cases of immune-related colitis, we noted that there were differences in the rate of relapse based on the initial steroid dose and length of taper based on treatment. In general, combination therapy patients appeared to relapse more frequently, although this may be mitigated by longer steroid tapers (> 6 weeks). Although these findings were based on small numbers, it warrants further prospective investigation of optimized treatment algorithms to minimize relapses and molecular characterization to understand the mechanism for steroid-resistant colitis. The importance of clearly-defined treatment algorithms is highlighted by the high incidence of hospitalization and complications from prolonged steroid tapers, which contribute to the financial toxicity of checkpoint inhibitor therapy.26
Given its retrospective nature, the study has several limitations. First, while general guidelines are available to treat immune-related colitis, there are likely differences in practice patterns at each institution which may have affected treatment outcomes (e.g. vigilance assessing symptoms, duration of tapers, timing of infliximab). However, the bias is likely minimized given the large number of patients from various institutions included. Second, only patients that received systemic steroids were included; thus, patients with sub-clinical disease that were successfully managed with other modalities (interrupting therapy, anti-motility agents) were not captured. Third, as this study was focused on the development and treatment of colitis, detailed demographic information were not collected to identify risk factors. Despite these limitations, the characterization of the patients with clinically-relevant colitis addresses pertinent gaps in our understanding of the most common morbid immune toxicity.
In conclusion, we identified an incidence of approximately 3% and 24% of clinically relevant colitis in anti-PD-1 monotherapy and combination therapy patients. Diagnostic findings were diverse regardless of treatment, which emphasize that colitis remains a largely clinical diagnosis. Furthermore, the course of colitis associated with anti-PD-1 monotherapy was generally milder with a later onset, and of shorter duration requiring a lower initial dose of steroids, less common dose-escalations, and escalation to infliximab as compared to colitis due to combination therapy. Ultimately, the study highlights the need for further optimization of management for this common immune-related adverse event and understanding of the molecular mechanisms that underlie its varied presentation.
Methods
Patients
We screened all patients with melanoma who received anti-PD-1 (nivolumab or pembrolizumab) as a single-agent (“monotherapy”) or in combination with anti-CTLA-4 (ipilimumab) (“combination therapy”) at eight participating centers (n = 1261). Each site had approval from their institutional review board (IRB) in conformity with the Declaration of Helsinki prior to screening. From the screened patients, we identified 109 patients who developed clinically-relevant colitis, due to treatment with anti-PD-1 (n = 30) or combination PD-1/CTLA-4 blockade (n = 79) and requiring systemic steroids without an alternate etiology. Colitis was determined by the treating investigator and diagnosed clinically based on the symptoms defined by the Common Toxicity Criteria for Adverse Events (CTCAE) criteria of diarrhea (based on number of stools) and/or colitis (based on symptoms including abdominal pain, blood in the stool, perforation), with or without histopathologic confirmationWe defined clinically relevant colitis as CTCAE grade 3–4 events or persistent grade 2 events that were treated with corticosteroids. Combination therapy included concurrent treatment (n = 71) or sequential treatment with ipilimumab and nivolumab with planned crossover (n = 8). For sequential treatment, patients received at least one dose of ipilimumab and nivolumab. Patients treated with sequential therapy were included in the combination therapy cohort since the frequency of irAEs appears similar between sequential and concurrent nivolumab and ipilimumab.25,27 Patient data was collected from Georgetown University (n = 13), Massachusetts General Hospital (n = 29), Moffitt Cancer Center (n = 19), Northwestern University (n = 10), Roswell Park Cancer Institute (n = 4), Rutgers University (n = 4), University of Alabama at Birmingham (n = 9), and Vanderbilt University Medical Center (n = 21).
Study design
We obtained baseline demographic data including age, sex, weight, body mass index (BMI), lactate dehydrogenase (LDH), BRAF mutation status, American Joint Committee on Cancer (AJCC, 7th ed. 2010) pathologic stage, and prior treatments. Further information regarding anti-PD-1 based therapy including type of therapy, timing, and objective response based on RECIST v1.1 criteria were collected.28 For clinical characterization of colitis, we collected data on time of onset, diagnostic studies (colonoscopy, biopsy, imaging), management (dose/taper of steroids and use of infliximab or other agents), and post-colitis outcomes (resolution, relapses). Prednisone tapers were left to the clinician’s judgement based on institutional practice. Steroid dose escalation was defined as any increase in steroids (IV or PO) back to initial dosing or at least 1 mg/kg. Inflixmab dosing was standard 5 mg/kg, but the frequency of dosing was left to the discretion of the clinican. Relapse was defined as substantial clinical worsening based on symptoms per clinician’s evaluation. Resolution of symptoms was defined as improvement of diarrhea or colitis to grade 1 or less. Each case of colitis was graded by the treating investigator based on the Common Toxicity Criteria for Adverse Events V4.0 for diarrhea and/or colitis.
Statistical analysis
We compared clinical features of patients with anti-PD-1 monotherapy associated colitis vs. those with combination associated colitis using Mann Whitney U testing (continuous variables), chi-square or Fisher’s exact testing (categorical variables), and log-rank testing (time-dependent variables). Continuous and categorical variables were described using means and percentages, respectively. Analyses were performed using GraphPad Prism version 7.
Supplementary Material
Funding Statement
This study was supported by National Institutes of Health (NIH) grant [K23 CA204726] (Johnson); James C. Bradford Jr. Melanoma Fund (Johnson); and the Melanoma Research Foundation Young Investigator Award with support from BMS (Johnson).
Conflict of Interest and Financial Disclosures
Dr. Conry has received honoraria and participated in a speakers’ bureau for Bristol-Myers Squibb (BMS), Merck, Genentech, and Amgen. Dr. Silk has received research funding from Merck and Prometheus. Dr. Mehnert has received honoraria from Genentech and EMD Serono, consulted for Merck Sharp & Dohme and Amgen, received research funding from Merck, Sanofi, Novartis, Polynoma, Immunocore, Amgen, and AstraZeneca, and received travel, accommodations, or expenses from EMD Serono and Merck Sharp & Dohme. Dr. Puzanov has consulted for Amgen, Roche/Genentech, BMS and received travel, accommodations, or expenses from Amgen and Merck. Dr. Sosman has received honoraria from and consulted for Amgen, Merck, Array BioPharma, and BMS. Dr. Gibney has consulted for Novartis and Genentech/Roche and participated in a speaker’s bureau for Merck and Genentech. Dr. Sullivan has received honoraria from Roche/Genentech, consulted for Novartis, Biodesix, Prometheus, Amgen, Takeda, WorldCare Clinical, LLC, ACI Clinical, Merck and BiolineRx, and received research funding from Amgen, Lilly, BioMed Valley Discoveries, Merck, Deciphera, and Roche/Genentech. Dr. Johnson has consulted for BMS, Genoptix, Merck, Novartis, and Incyte and received research funding from Incyte. No other disclosures are reported.
References
- 1.Hodi FS, Kluger H, Sznol M, Carvajal RD, Lawrence D, Atkins MB, Powderly JD, Sharfman WH, Puzanov I, Smith D, et al. Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. In In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research New Orleans, LA: Philadelphia (PA): AACR; 2016. 10.1158/1538-7445.AM2016-CT001 [DOI] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ.. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Prolonged survival in stage III Melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 9.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 10.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV Melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. adjuvant pembrolizumab versus placebo in resected stage III Melanoma. N Engl J Med. 2018. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 12.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, Zobniw C, Johnson DH, Samdani R, Lum P, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 14.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. OncoImmunology. 2017;6(10):e1344805. doi: 10.1080/2162402X.2017.1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutzac C, Adam J, Soularue E, Collins M, Racine A, Mussini C, Boselli L, Kamsukom N, Mateus C, Charrier M, et al. Colon Immune-Related Adverse Events: anti-CTLA-4 and Anti-PD-1 Blockade Induce Distinct Immunopathological Entities. J Crohns Colitis. 2017;11:1238–1246. doi: 10.1093/ecco-jcc/jjx081. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Neuer M, Marmarelis ME, Jangi SR, Luke JJ, Ibrahim N, Davis M, Weinberg J, Donahue H, Bailey N, Hodi FS, et al. Diagnostic comparison of ct scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res. 2017;5:286–291. doi: 10.1158/2326-6066.CIR-16-0302. [DOI] [PubMed] [Google Scholar]
- 18.Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV, Menzies AM, Eroglu Z, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2017;28:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol. 2018;178:175–182. doi: 10.1530/EJE-17-0810. [DOI] [PubMed] [Google Scholar]
- 20.Grimaldi AM, Simeone E, Festino L, Giannarelli D, Palla M, Caraco C, Curvietto M, Esposito A, Grimaldi MC, Mozzillo N, et al. Correlation between immune-related adverse events and response to pembrolizumab in advanced melanoma patients. Cancer Immunol Immunother. 2015;3:P186. doi: 10.1186/2051-1426-3-S2-P186. [DOI] [Google Scholar]
- 21.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilir SP, Ma Q, Zhao Z, Wehler E, Munakata J, Barber B. Economic burden of toxicities associated with treating metastatic melanoma in the United States. Am Health Drug Benefits. 2016;9:203–213. [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL Jr., Lawrence DP, Logan TF, Schuchter LM, Nair S, Fecher L, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17:943–955. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


