ABSTRACT
Colorectal cancers associated with Lynch syndrome are characterized by defective mismatch repair, microsatellite instability, high mutation rates, and a highly immunogenic environment. These features define a subset of cancer with a favorable prognosis and high likelihood to respond to treatment with anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) drugs. With the aim to define immune-evasive mechanisms and a potential impact hereof in colorectal cancers from Lynch syndrome versus hereditary cases with retained mismatch repair function, we immunohistochemically and transcriptionally profiled 270 tumors. Lynch syndrome-associated tumors showed an overrepresentation of tumor-infiltrating CD3, CD8 and CD68 positive cells, loss of beta-2-microglobulin (B2M) and up-regulation of PD-L1 on tumor cells. The gene expression signature of Lynch syndrome tumors was characterized by upregulation of genes related to antigen processing and presentation, apoptosis, natural killer cell-mediated cytotoxicity, and T cell activation. Tumors with loss of B2M and up-regulation of PD-L1 showed distinctive immunogenic profiles. In summary, our data demonstrate a complex tumor-host interplay where B2M loss and PD-L1 up-regulation influence immunological pathways and clinical outcome in Lynch syndrome tumors. Immunological classification may thus aid in the preselection of colorectal cancers relevant for treatment with anti-PD-1/PD-L1 therapies.
KEYWORDS: Hereditary non-polyposis colorectal cancer, familial colorectal cancer type X, mismatch repair, microsatellite instability, immunophenotypes
Introduction
Defective DNA mismatch-repair (MMR) gives rise to microsatellite instability (MSI), a high mutation rate, and a highly immunogenic tumor phenotype with abundant lymphocytic infiltrates.1–4 The MMR defect may be constitutional, in which it defines Lynch syndrome, or somatic caused by hypermethylation of the MLH1 gene promoter. Tumor-infiltrating lymphocytes have been associated with MSI and signify a favorable prognosis in sporadic as well as Lynch syndrome-associated colorectal cancer.4-9 Recently, MSI has also been identified as a predictor of response to immune modulatory treatment with checkpoint inhibitors. This development offers new and promising possibilities for individualized treatment in patients with MMR deficient tumors. The first studies have shown high degree of response in advanced-stage tumors, though patients with Lynch syndrome may have a somewhat lower response rate than patients with somatic MMR defects.10-12
The majority of studies have focused on sporadic MSI cancers where current data reveal an abundance of CD3 and CD8 positive T cells.5-7 MSI status and high levels of tumor-infiltrating T cells signify a favorable prognosis compared to microsatellite stable (MSS) cancers.7-9 The hypermutated tumor phenotype generates a multitude of neo-epitopes that, when presented by MHC class I receptors, may trigger a cytotoxic T cell-mediated attack.2,5 This anti-tumorigenic effect may be counter-balanced by immune-evasive mechanisms, such as loss of the MHC class I subunit, beta-2-microglobulin (B2M) and up-regulation of the T-cell inhibitor programmed death 1 ligand 1 (PD-L1).13,14 B2M loss has been associated with loss of MHC class I receptors suggesting that this subset may not elicit neoepitope-induced T cell responses.4,15 In contrast, high levels of PD-L1 have been associated with T cell exhaustion that can be reversed by inhibition of the programmed death 1 (PD-1)/PD-L1 binding.16 As a basis for preselection of patients, who may benefit from immunotherapy, we characterized the immunophenotypes and transcriptional immune profiles in Lynch syndrome versus MMR proficient colorectal cancers from the familial colorectal cancer type X (FCCTX) syndrome.
Results
Patient characteristics
In total, 169 colorectal cancers associated with Lynch syndrome and 101 cancers associated with FCCTX were collected. Key data on the two study cohorts are summarized in Table 1. The median age at onset was 54 years (range 18–88) for Lynch syndrome compared to 59 years (range 31–84) in FCCTX (P = 0.004). As expected, Lynch syndrome tumors developed significantly more often in the proximal colon (P < 0.001) and where less likely to show lymph node metastases (P < 0.001) compared to FCCTX tumors.
Table 1.
Summary of key characteristics in colorectal cancers from the two cohorts, Lynch syndrome and familial colorectal cancer type X (FCCTX).
| Lynch syndrome |
FCCTX |
||
| |
(N = 169) (%) |
(N = 101) (%) |
P |
| Families (N)/Individuals (N) | 86/141 | 53/95 | |
| Metachronous cancer (N)/Synchronous cancer (N) | 12/16 | 2/4 | |
| Sex (men N (%)) | 88 (52.1) | 54 (53.5) | 0.899 |
| Median age at onset (range) years | 54 (18–88) | 59 (31–84) | 0.004 |
| Tumor location N (%) | < 0.001 | ||
| Right colon | 108 (63.9) | 17 (16.8) | |
| Left colon and rectum | 61 (36.1) | 84 (83.2) | |
| Tumor stage N (%) | 0.386 | ||
| T1 | 5 (3.0) | 3 (3.0) | |
| T2 | 26 (15.4) | 15 (14.9) | |
| T3 | 34 (20.1) | 19 (18.8) | |
| T4 | 73 (43.2) | 34 (33.7) | |
| Unspecified | 31 (18.3) | 30 (29.7) | |
| Lymph node stage N (%) | < 0.001 | ||
| N0 | 117 (69.2) | 49 (48.5) | |
| N1 | 13 (7.7) | 9 (8.9) | |
| N2 | 28 (16.6) | 36 (35.6) | |
| Unspecified | 11 (6.5) | 7 (6.9) | |
| Distant metastases N (%) | 0.4563 | ||
| M0 | 139 (82.2) | 66 (65.3) | |
| M1 | 12 (7.1) | 9 (8.9) | |
| Unspecified | 18 (10.7) | 26 (25.7) | |
| Mucin-producing N (%) | 0.028 | ||
| Yes (> 50% mucinous component) | 19 (11.2%) | 4 (4.0%) | |
| No (< 50% mucinous component) | 150 (88,8%) | 97 (96%) |
Tumor-infiltrating immune cells
Lynch syndrome-associated colorectal cancers showed more abundant tumor-infiltrating immune cells with significantly increased numbers of CD3, CD8, and CD68 positive cells (P < 0.001, Supplementary figure 1). As expected, the level of infiltrating CD3 positive cells was significantly associated with increased levels of CD8 positive cells (Pearson’s correlation coefficient, r = 0.81, P < 0.001). Further, presence of CD3 and CD8 positive cells were associated with CD68 positive cells (P < 0.001).
Immune evasion in Lynch syndrome
Immune evasive features were more frequent in Lynch syndrome tumors compared to FCCTX tumors with loss of B2M in 28% vs. 2% (P < 0.001) and presence of PD-L1 on tumor cells in 64% vs 20% (P < 0.001, Figure 1). Loss of B2M expression was associated with high levels of tumor-infiltrating CD8 positive cells irrespective of MMR status (P = 0.044). Likewise, high levels of CD8 positive cells were associated with up-regulation of PD-L1 expression on tumor cells (P < 0.001) and tumor-infiltrating immune cells (P = 0.010). Subset analyses in Lynch syndrome tumors showed significant association between high levels of PD-L1 and high levels of CD3 and CD8 (P = 0.002 and 0.010, respectively), whereas B2M expression was not correlated to CD3/CD8 levels. These data indicate that massive immune infiltration promotes clonal expansion of tumor cells expressing immune evasion mechanisms. The correlation between PD-L1 and CD3/CD8 positive cells suggest that PD-L1 upregulation is a late tumorigenic event, while B2M loss may present earlier and affect the amount of recruited T cells.
Figure 1.
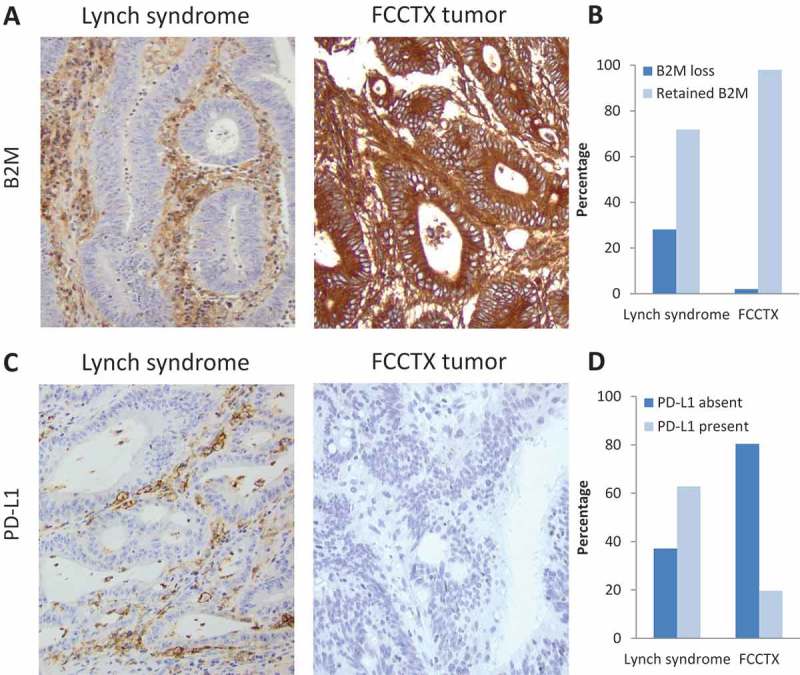
(A) Immunohistochemical stainings of B2M in a Lynch syndrome (left) and FCCTX (right) tumor. (B) Frequency of B2M loss in Lynch syndrome and FCCTX tumors, respectively. (C) Immunohistochemical stainings of PD-L1 at the core of the tumor in a Lynch syndrome (left) and FCCTX (right) tumor. (B) Frequency of PD-L1 positive staining on tumor cells in Lynch syndrome and FCCTX tumors, respectively.
Immune profiling
We investigated the expression profile of 1419 immune genes in relation to the MMR status in 76 tumors from our two cohorts. Herein, significant analyses of microarray (SAM) identified 292 significantly differentially expressed genes between Lynch syndrome and FCCTX (Supplementary Figure 2 and Supplementary Table 1). The 176 up-regulated genes in Lynch syndrome cancers included genes related to the antigen processing and presentation pathway (e.g. CIITA, CD74, and MHC class II receptors), apoptosis (e.g. CASP3, FAS, BCL2, TRAILR2), natural killer cell-mediated cytotoxicity (e.g. CD226, GZMA, GZMH, CXCL16, but also inhibitory genes like IDH1, KIR2DL1, and IL18BP), and T cell activation (e.g. NFATC1, CD86, CD247, MALT1). These data support that Lynch syndrome tumors are infiltrated with CD3 and CD8 positive T cells, but also indicate involvement of natural killer cells and immune-mediated apoptosis.
Figure 2.
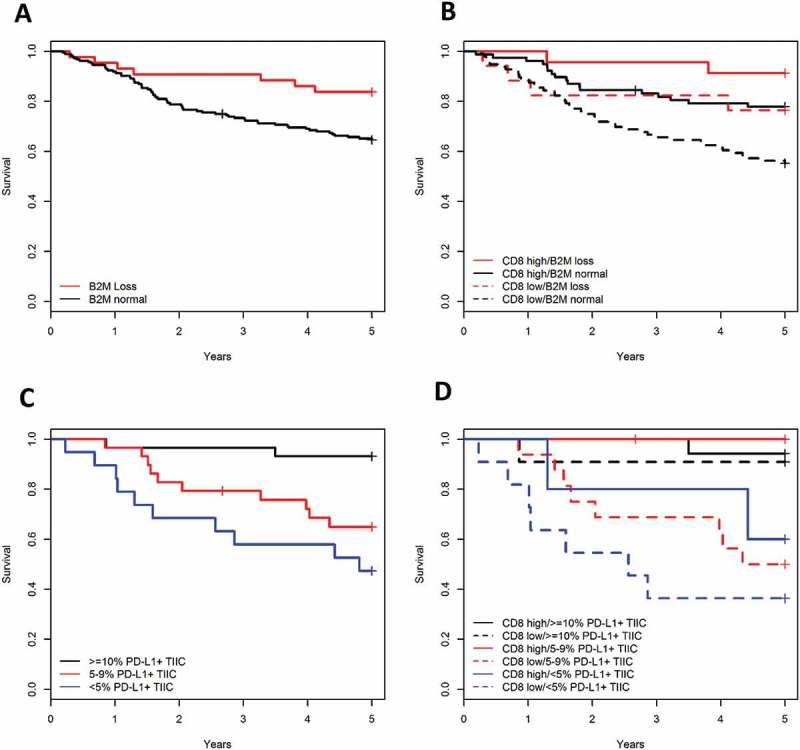
(A) Kaplan Meier plots showing the crude survival of cancer patients with tumors showing absence or presence of B2M protein expression on tumor cells. (B) B2M loss of expression increased the survival rate in tumors with high or low infiltration of CD8 positive cells. (C) Kaplan Meier plots showing the crude survival of cancer patients with different levels of PD-L1 positive tumor-infiltrating immune cells (TIIC). (D) PD-L1 expression on tumor-infiltrating immune cells increased the survival rate in cancer patients regardless of the infiltration level of CD8 positive cells.
We further investigated the association between immune evasion mechanisms, i.e. loss of B2M and up-regulation of PD-L1, and the 1419 immune-related gene expressions. B2M loss were associated with 58 differentially expressed genes including upregulation of genes involved in apoptosis (CASP3 and BNIP3L) and natural killer cell-mediated cytotoxicity (GZNA, GNZH, LCK, and KIR2DL1) and down-regulation of antigen presentation, folding, assembling and loading of MHC class I receptors (HLA-F, UBE2D2, SEC31A and ITGB5) (Supplementary table 2). These data support the immunohistochemical B2M loss observed in Lynch syndrome tumors and indicate involvement of natural killer cell-mediated apoptosis in this subset. SAM analyses identified 85 significantly differentially expressed genes with 64 genes linked to PD-L1 up-regulation including apoptosis (FAS, CASP3, BIRC3, PARP1) and T cell receptor signaling (CD3D, CD247, MALT1). These data support the association between CD8 and PD-L1 up-regulation, but also indicate that T cell-mediated apoptosis is present to some extend in this subset (Supplementary table 3).
Prognostic analyses
Increased levels of tumor-infiltrating CD3 and CD8 positive cells predicted a longer survival (log-rank P < 0.001, Supplementary figure 3). These results remained stable when adjusting for MMR status, TNM stage, sex and age at onset for CD3 with an HR of 2.5, but not for CD8 (Table 2). Sample size and lack of standardized and validated CD3/CD8 cut off values motivated analyses based on median levels, which may have been too high, since tertile and quartile sub-division of CD8 increased significance from lowest to highest subgroup (P = 0.002 and P = 0.028, respectively). High levels of CD68 positive cell infiltration were associated with a favorable prognosis with an HR of 2.1 (P = 0.043) for CD68 low compared to CD68 high (un-adjusted log-rank P = 0.003, Supplementary figure 3, Table 2). Multivariate subset analyses within the Lynch syndrome cohort increased prognostic value of CD3 (HR = 3.4, P = 0.002), CD8 (HR = 2.0, P = 0.058), and CD68 (HR = 6.5, P = 0.004) (Table 2).
Table 2.
Multivariate survival analyses of the immunological biomarkers affecting crude survival in the total cohort and Lynch syndrome subset using Cox propotional hazard model.
| Total cohort (N = 236)* |
Lynch syndrome (N = 141)** |
||||||
|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P | |
| CD3 | |||||||
| Low vs. high | 2.5 | 1.4–4.6 | 0.002 | 3.4 | 1.6–7.3 | 0.002 | |
| CD8 | |||||||
| Low vs. high | 1.6 | 0.9–2.8 | 0.122 | 2.0 | 1.0–4.2 | 0.058 | |
| CD68 | |||||||
| Intermediate vs. high | 1.9 | 0.9–4.1 | 0.065 | 3.4 | 0.9–12.3 | 0.083 | |
| Low vs. high | 2.1 | 1.0–4.4 | 0.043 | 6.5 | 1.8–23.3 | 0.004 | |
| B2M | |||||||
| Normal vs. loss | 3.4 | 1.4–8.7 | 0.009 | 3.5 | 1.2–10.1 | 0.022 | |
| Normal vs. loss*** | 3.7 | 1.3–10.1 | 0.012 | – | – | – | |
| PD-L1+ TC | |||||||
| 5–9% vs. ≥ 10% | 1.0 | 0.2–4.9 | 0.953 | – | – | – | |
| < 5% vs. ≥ 10% | 1.5 | 0.3–7.3 | 0.635 | – | – | – | |
| PD-L1+ TIIC | |||||||
| 5–9% vs. ≥ 10% | 3.5 | 0.7–17.3 | 0.117 | – | – | – | |
| < 5% vs. ≥ 10% | 4.7 | 0.9–23.7 | 0.059 | – | – | – | |
| 5–9% vs. ≥ 10%*** | 3.5 | 0.6–19.0 | 0.146 | – | – | – | |
| < 5% vs. ≥ 10%*** | 7.9 | 1.5–40-4 | 0.013 | – | – | – | |
Abbreviations: HR, hazard ratio; CI, confidence intervals; TC, tumor cells; TIIC, tumor-infiltrating immune cells; MMR, mismatch repair; TNM, tumor node metastasis.
*Analyses were adjusted for MMR status, TNM stage, sex and age.
**Subset analyses in the Lynch syndrome cohort were adjusted for TNM stage, sex and age.
***Analyses were adjusted for MMR status, TNM stage, sex, age and CD8 expression levels.
B2M loss was significantly associated with improved prognosis (log-rank P = 0.021, Figure 2A) Significance remained stable when adjusting for MMR status, TNM stage, age and sex with an HR of 3.4 in the total cohort and 3.5 in the Lynch syndrome subset (Table 2). B2M loss showed significant influence towards improved survival when adjusting for the level of CD8 positive cells with an HR of 3.7 (P = 0.012) (log-rank P = 0.001) (Figure 2B, Table 2).
PD-L1 expression in tumor-infiltrated immune cells (lowest versus highest) was associated with improved prognosis (log-rank P > 0.002, Figure 2C), but was not significant after adjustment for age, sex, MMR status and TNM stage (Table 2). When adjusting for CD8 positivity, PD-L1 expression levels increased prognostic significance (log-rank P = 0.001) with and adjusted HR of 7.9 (P = 0.013) from lowest to highest expression levels (Figure 2D, Table 2). Limited sample size prohibited subset analyses in the Lynch syndrome cohort. There was no significant prognostic correlation with increased PD-L1 expression evaluated in the tumor cells (log-rank P = 0.145 and adjusted P = 0.635) (Table 2). Together these data show that B2M and PD-L1 add prognostic value to CD8.
Discussion
The immune reactive and the immune evasion mechanisms of colorectal cancer are key players in tumorigenesis and treatment response. Immune evasive mechanisms include loss of the MHC class I subunit B2M and up-regulation of the T-cell inhibitor PD-L1.13,14 Differences in mechanisms, e.g. related to deregulation of MHC class I expression, apply between sporadic and hereditary MSI tumors, and motivate mechanistic explanation related to the variable response rates observed in sporadic and Lynch syndrome-associated MSI tumors treated with anti-PD-1 inhibitors.11,15 Defining the immunogenic environment and immune evasion mechanisms in Lynch syndrome, we hypothesize that Lynch syndrome tumors could develop resistance to anti-PD-1 therapy by bypassing the PD-L1/PD-1 pathway. In Lynch syndrome, 76% of the tumors showed up-regulation of PD-L1, indicating potential response to anti-PD-1 therapy. Loss of B2M was, however, observed in 28% and may result in loss of MHC class I presentation and confer decreased sensitivity to therapies directed at cytotoxic T cells, such as anti-PD-1 therapies.11,13,15,17
A highly immunogenic profile with high levels of CD3, CD8 and CD68 positive cells signified a favorable prognosis in sporadic as well as Lynch syndrome-associated colorectal cancer (Supplementary figure 1) which supports previous observations.4,5,8,18,19 Gene expression profiling further linked Lynch syndrome with genes involved in antigen processing and presentation, T cell activation, natural killer cell-mediated cytotoxicity, and apoptosis.8,20–22 Loss of B2M in 28% of the Lynch syndrome tumors was within previous reported range of 21–30% and are likely caused by frameshift mutations in microsatellite regions of the B2M gene (Figure 1).13,15 The tumorigenic event of B2M loss is likely occurring in the middle of the adenoma-carcinoma sequence since B2M loss has not been found in metastasizing tumors or MMR deficient adenomas.13,23 The prognostic value of B2M has been investigated in several studies with contradicting results. Studies on non-selected colon cancers have associated low expression levels with lymph node metastases, T stage, and poor survival,24,25 while studies on MMR-deficient colorectal cancers have correlated B2M loss with favorable survival.13,26,27 Our results support previous studies showing that B2M loss is significantly associated with improved prognosis in Lynch syndrome tumors and that B2M loss added prognostic value to the CD8 levels. The improved prognosis observed in B2M negative tumors has been associated with complete absence of metastases.13,26,27 With three Lynch syndrome metastatic colorectal cancers showing loss of B2M, we did not find such an association, though low levels of CD3 and CD8 positive cells was observed in all three cases.
Up-regulation of PD-L1 was observed in 76% of the tumors (Figure 1). PD-L1 expression have frequently been observed in MSI tumors with strong correlation to high levels of CD3 and CD8 positive cells.12,14,28–30 In line with our results, PD-L1 expression are more frequently observed on immune cells at the invasive margin and rarely observed on tumor cells.11,14,31 PD-L1 has been detected as a soluble ligand and may potentially be secreted by MMR deficient tumor cells. At the invasive border it may bind to PD-1 receptors on CD8 positive T cells and thereby inhibit T cell-mediated apoptosis as suggested in e.g. lymphoma and malignant melanoma.32,33 This notion is supported by the fact that PD-L1 positive tumor-infiltrating immune cells, but not PD-L1 positive tumor cells, have been documented to predict prognosis, though its implication may vary according to tumor type.34,35 In contrast to other types of cancer, such as melanoma, renal cell cancer and lung cancer, PD-L1 expression have not been able to predict response to anti-PD-1 therapy in MMR deficient colorectal cancers.11 This may be explained by a large proportion of tumors developing through the B2M-driven immune evasion mechanism or differences in PD-L1 expressing cell types.14 In relation to previous studies, PD-L1 positivity could not predict survival, though it added prognostic value to the level of CD8 infiltration and has been associated with disease-free survival in MSI subgroup analyses.29
The association between tumor-infiltrating CD3 and CD8 positive cells and de-regulation of immune evasion markers likely reflect tumor-specific immune-adaptation (Figure 3). Both down-regulation of B2M and up-regulation of PD-L1 inhibitory signals can shield tumor cells from cytotoxic T cell-mediated apoptosis. Already during the early adenoma stage, the immune microenvironment may direct immune system attacks.36 In tissues highly infiltrated with immune cells, tumors may develop through an early loss of B2M heterozygosity that allows for survival in a highly immunogenic environment (Figure 3). Subgroup analyses linked B2M loss with low levels of MHC class I related genes supporting previous studies.13,15 Loss of B2M and subsequent lack of MHC class I may affect the level of CD8 cells and, in contrast, attract and activate the natural killer cell-mediated apoptosis, supported by the upregulation of Caspase 3 and the natural killer cell inhibitory ligand KIR2DL1 (Figure 3). The involvement of natural killer cells may offer an explanation to the favorable prognosis in B2M negative tumors though validation of the molecular profiles and the prognostic implications are needed.26,27 In less immunogenic baseline environments tumors may develop through a B2M proficient pathway, during which neoepitopes will recruit and activate cytotoxic T cells (Figure 3). Tumor progression will in this situation likely depend on further adaptation and immune-evasion through up-regulation of PD-L1, which is localized at the invasive margin of the tumors in contrast to complete loss of B2M. This hypothesis was supported by subgroup analyses linking PD-L1 up-regulation of tumor cells with high infiltration of CD8 cells and increased expression of genes involved in T cell receptor signaling. Up-regulation of PD-L1 has been linked to MSI tumors, and based on our data, to Lynch syndrome tumors.11,14,28,29,31
Figure 3.
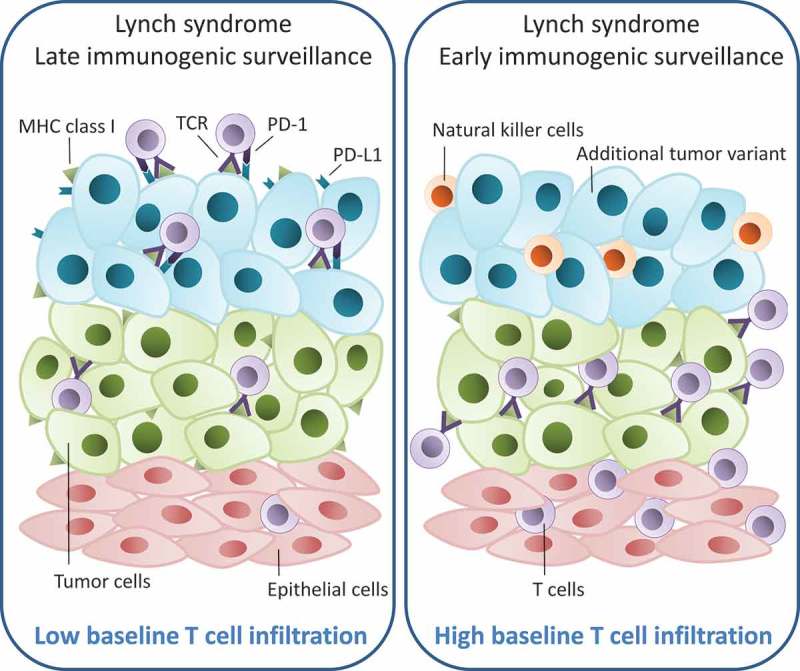
Hypothesized model showing the development of two different immune evasion mechanisms in Lynch syndrome-associated colorectal cancer. When the baseline epithelial tissue is highly infiltrated with CD3 and CD8 T cells (in purple) (panel to the right), preliminary tumor cells (in green) are forced to evade the immune system at early stages. This could be done by loss of heterozygosity of B2M and subsequent loss of MHC class I receptors generating a new tumor variant (in blue). However, if the baseline epithelial tissue has a low infiltrative level of CD3 and CD8 cells (panel to the left), there will be no selective pressure on the early tumor cells to avoid the immune system. Nevertheless, the high mutation rate seen in Lynch syndrome tumors will eventually lead to extensive presentation of neoepitopes through MHC class I receptors, which will attract and activate cytotoxic T cells. To avoid immune-mediated killing the tumor cells may adapt and shield themselves through up-regulation of PD-L1 causing T cell exhaustion. TCR = T cell receptor.
Lack of treatment data represents a major limitation to our study, though immunotherapy has not been used in any of these cases because of lack of availability at the time of treatment. Further, there is a lack of standardized scoring principles for CD3 and CD8 estimates, which led us to use the median as cut-off. Though the levels are comparable, direct comparison should not be performed.5,8,26,37
In conclusion, we demonstrate significant difference in the immunoprofiles of hereditary colorectal cancers. The Lynch syndrome subtypes show frequent infiltration by CD3, CD8 and CD68 positive cells, loss of B2M, and up-regulation of PD-L1 in the latter subtype. Expression of CD3, CD8, CD68, and B2M signified a favorable prognosis. The Lynch syndrome immunophenotypes were linked to deranged antigen processing and presentation, apoptosis, natural killer cell activation and lymphocytic markers. One out of three Lynch syndrome tumors showed loss of B2M expression, suggesting that a subset of the tumors may be less responsive to anti-PD-1 mediated reactivation of CD8 cells. Our findings reveal a complex interplay related to B2M loss and PD-L1 expression and suggest that immunoprofiling is relevant for individualized treatment decisions in Lynch syndrome.
Patients and methods
Patient selection
In total, 270 formalin-fixed, paraffin-embedded colorectal cancers from 236 individuals with Lynch syndrome and FCCTX were identified through the national Danish hereditary non-polyposis colorectal cancer (HNPCC) register. FCCTX was used for comparison as these families share phenotypic features with Lynch syndrome, e.g. fulfillment of the Amsterdam I/II criteria and a familial or hereditary risk of colorectal cancer, and yet differ as no MMR gene mutations or defects have been identified.38-40 Ethical approval for the study was obtained by the Scientific and Ethics Committee of the Capital Region of Copenhagen, Denmark (HD-2007–0032 and H-17001916) and reviewed by the Data Protection Agency (AHH-2017–071). In rectal cancer, neoadjuvant radiotherapy may induce inflammation and immune cell infiltration.41 We exclude a major impact here from since we could verify direct surgery without prior radiotherapy in 42 cases and use of neoadjuvant radiotherapy in only one case.
Immunohistochemical stainings
Immunohistochemical staining was performed using the Envision™ FLEX (DAKO, Glostrup, Denmark) and a Dako Autostainer Plus (DAKO) according to manufacturer’s instructions. Antibodies used on whole slides included anti-MLH1 (G168-15, dilution 1:100), anti-PMS2 (A16-4, 1:500), and anti-MSH6 (clone 44, 1:200) from BD Bioscience (San Jose, CA, USA), anti-MSH2 (25D12, 1:200) from Novocastra (Newcastle, UK), and PD-L1 (clone Sp142, diluted 1:100, Spring Bioscience, Ventana), while tissue microarray (TMA) (containing 2 × 1 mm biopsies from each case) immunostaining was performed using antibodies against CD3 (A0452, diluted 1:100, DAKO), CD8 (clone C8/144B, diluted 1:100, DAKO), Beta-2-Microglobulin (A0072, No pretreatment, dilution 1:600, DAKO), and CD68 (clone PG-M1 dilution 1:100). Control tissues such as brain, normal colon tissue and tonsils were used where appropriate. A pilot study of 46 tumors investigating the correlation between whole slide and tissue microarray scorings found a high correlation between the two different methods (Pearson correlation ranged from 0.75 to 0.82).
Lymphocyte quantification
Stained TMA slides were scanned using a digital scanner (AxioScan Z1, Zeiss) and evaluated using the digital pathology platform PathXL (PathXL Ltd, Belfast, UK). For CD3 (T cells) and CD8 (cytotoxic T cells) scoring, each TMA core was evaluated for infiltrating T cells at low magnification and tumor areas with most abundant infiltration were selected for scoring. According to previous studies the number of lymphocytes infiltrating the cancer nest areas was counted at high magnification in an area corresponding to 0.135 mm.6-8 The mean number of infiltrating lymphocytes in the TMA cores was used. For clinical statistics and correlation analyses the exact cell count was used. Increasing levels of CD3 and CD8 were correlated to improved survival, but where for simplicity divided into high/low expression in relation to the median level (133 cells/mm2 for CD3 and 59 cells/mm2 for CD8) in combination with B2M and PD-L1. CD68 positive macrophages were scored semi-quantitatively in the entire TMA core (area of 0.78 mm2) and the percentage (10% increments) of positive cells was reported in relation to the overall cellularity in line with previous studies.4 CD68 macrophage scores were divided into tertiles for the survival analyses since no combined survival analyses were made: CD68 low (0–9.5% CD68 positive area), CD68 intermediate (9.5–15%), and CD68 high (15–37.7%).
Immunohistochemical staining of B2M was predominantly located in the tumor cell membrane with occasional diffuse staining in the cytoplasm. Samples were classified either as complete loss (0%) or any positive staining (> 0%) and independently evaluated in the cytoplasm and in cell membrane. For whole slide PD-L1 protein staining, tumor cells and tumor infiltrating immune cells were scored at the infiltrative margins according to Horn et al. using 4 levels in each cell type: < 1%, 1–4%, 5–9% or ≥ 10% positive cells.31,42 For survival analyses the scores < 1% and 1-4% were pooled into a subset of cases with < 5% PD-L1 positive cells to avoid small sample sizes.
Three independent observers (C.T., J.W. and M.N.) evaluated the stainings with kappa values between 0.83 and 0.96. Consensus was reached in the few discordant cases.
Gene expression profiles
Immunohistochemical data was merged with gene expression data, including 39 Lynch syndrome-associated colorectal cancers and 37 FCCTX-associated tumors, available at NCBI Gene Expression Omnibus (GEO) (Accession number GSE36335).43 An a priori gene list of immune-related genes was extracted from the AmiGO2 Gene Ontology website (http://amigo.geneontology.org/amigo) and identified 1419 immune-related gene probes in the data set. The data set was loaded into MeV software® (Version 4.9.0), log2 transformed and median centered across genes. A paired significance analysis of microarrays (SAM) was used for identification of significantly differentially expressed genes in selected subset with a false discovery rate (FDR) 5%. Based on the SAM analyses, hierarchical clustering was performed using average linkage clustering with Pearson correlation as similarity metrics. Significant gene signatures were extracted and uploaded into GSEA (http://software.broadinstitute.org/gsea/index.jsp) and Enrichr (http://amp.pharm.mssm.edu/Enrichr/) for gene ontology mapping following manufacturer’s instructions.
Data processing and statistical analyses
Clinical, genetic and histologic data were extracted from the Danish HNPCC register on January 30, 2017. Crude survival was determined as the time from date of surgical resection of colorectal cancer to date of death or exit date (January 30, 2017), whichever came first.
Clinical, immunohistochemical and gene expression data were transferred into R version 3.3.1 (R: A Language and Environment for Statistical Computing, 2011, R Foundation for Statistical Computing, Vienna, Austria). Clinical and histological differences between the Lynch syndrome and FCCTX cohorts were calculated using Fisher’s exact test or Pearson’s Chi-squared test for association of categorical variables and Welch two sample t-test (for numerical variables with assumed normal distribution) or Mann-Whitney U test (for variables with no assumption of normal distribution). Kruskal-Wallis tests were used for associations between categorical and numeric variables with the assumption of non-normal distributions.
Prognostic relevance of immunophenotypes was assessed by survival analysis. To exclude none-cancer associated deaths, survival analyses was restricted to 5 years and patients, who died due to surgical complications within the first month (n = 5), were excluded. Univariate survival analyses were performed using log rank test and the Kaplan Meier estimator, while independent associations were assessed using the multivariate Cox proportional hazard model analysis adjusting for any confounding factors, i.e. sex, age, TNM stage, and MMR gene mutation status. The proportional hazard model was evaluated by the scaled Schoenfeld residuals. The prognostic effect from B2M and PD-L1 were further adjusted for the level of CD8 positive cells using the Cox model. All P values were two sided and significance was considered at P < 0.05. Bonferroni correction was used where multiple testing occurred.
Funding Statement
This work was supported by the Danish Cancer Society Research Fund under Grant number R90-A6150; and the Swedish Cancer Society under Grant number 2014/442.
Acknowledgments
We would like to acknowledge Patrick Joost, Aleris, Medilab, for excellent advice and guidance regarding lymphocytic quantification. We would like to acknowledge all the Danish pathology departments for lending us all the tumor tissues and all the Danish clinical genetic departments for identifying and reporting the families to the Danish HNPCC register. Financial support was granted from the Danish Cancer Society and from the Swedish Cancer Society.
Disclosure of interest
The authors have declared no conflicts of interest.
Supplementary data
Supplementary data can be accessed here.
References
- 1.Klarskov L, Holck S, Bernstein I, Nilbert M.. Hereditary colorectal cancer diagnostics: morphological features of familial colorectal cancer type X versus Lynch syndrome. JClinPathol. 2012;65(4):352–356. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, Drescher KM. de la Chapelle A. Immunology and the Lynch Syndrome. Gastroenterology. 2008;134(4):1246–1249. doi: 10.1053/j.gastro.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, Von Knebel DM. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. CancerImmun. 2004;4:14. [PubMed] [Google Scholar]
- 4.De Miranda NF, Goudkade D, Jordanova ES, Tops CM, Hes FJ, Vasen HF, Van WT, Morreau H. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res. 2012;18(5):1237–1245. doi: 10.1158/1078-0432.CCR-11-1997. [DOI] [PubMed] [Google Scholar]
- 5.Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, Angell HK, Fredriksen T, Elie N, Fauquembergue E, et al. Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. CancerRes. 2015;75(17): 3446–3455. doi: 10.1158/0008-5472.CAN-14-30519. [DOI] [PubMed] [Google Scholar]
- 6.De Smedt L, Lemahieu J, Palmans S, Govaere O, Tousseyn T, Van Cutsem E, Prenen H, Tejpar S, Spaepen M, Matthijs G, et al. Microsatellite instable vs stable colon carcinomas: analysis of tumour heterogeneity, inflammation and angiogenesis. Br J Cancer. 2015;113:500–509. doi: 10.1038/bjc.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschoolmeester V, Baay M, Van ME, Weyler J, Vermeulen P, Lardon F, Vermorken JB. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44(3): 698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795): 1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22(4):813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. NEnglJMed. 2015;372(26): 2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349): 409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloor M, Michel S, Buckowitz B, Ruschoff J, Buttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121(2): 454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 14.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. CancerDiscov. 2015;5(1): 43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dierssen JW, De Miranda NF, Ferrone S, Van Puijenbroek M, Cornelisse CJ, Fleuren GJ, Van Wezel T, Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Ahn E, Ht Kissick, Ahmed R. Reinvigorating Exhausted T Cells by Blockade of the PD-1 Pathway. For Immunopathol Dis Therap. 2015;6:7–17. doi: 10.1615/ForumImmunDisTher.2015014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernal M, García-Alcalde F, Concha A, Cano C, Blanco A, Garrido F, Ruiz-Cabello F. Genome-wide differential genetic profiling characterizes colorectal cancers with genetic instability and specific routes to HLA class I loss and immune escape. Cancer ImmunolImmunother CII. 2012;61(6):803–816. doi: 10.1007/s00262-011-1147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echterdiek F, Janikovits J, Staffa L, Muller M, Lahrmann B, Fruhschutz M, Hartog B, Nelius N, Benner A, Tariverdian M, et al. Low density of FOXP3-positive T cells in normal colonic mucosa is related to the presence of beta2-microglobulin mutations in Lynch syndrome-associated colorectal cancer. Oncoimmunology. 2016;5:e1075692. doi: 10.1080/2162402X.2015.1075692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer K, Michel S, Reuschenbach M, Nelius N, Von Knebel Doeberitz M, Kloor M. Dendritic cell and macrophage infiltration in microsatellite-unstable and microsatellite-stable colorectal cancer. FamCancer. 2011;10(3):557–565. doi: 10.1007/s10689-011-9449-7. [DOI] [PubMed] [Google Scholar]
- 20.Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359–1371. doi: 10.1007/s00262-012-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjea A, Ahmed S, Hands RE, Huang F, Han X, Shaw PM, Feakins R, Bustin SA, Dorudi S. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. MolCancer. 2004;3:21. doi: 10.1186/1476-4598-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. AmJPathol. 1999;154(6):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clendenning M, Huang A, Jayasekara H, Lorans M, Preston S, O’Callaghan N, Pope BJ, Macrae FA, Winship IM, Milne RL, et al. Somatic mutations of the coding microsatellites within the beta-2-microglobulin gene in mismatch repair-deficient colorectal cancers and adenomas. Fam Cancer. 2018;17:91–100. doi: 10.1007/s10689-017-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrout J, Yousefzadeh M, Dodd A, Kirven K, Blum C, Graham A, Benjamin K, Hoda R, Krishna M, Romano M, et al. beta(2)microglobulin mRNA expression levels are prognostic for lymph node metastasis in colorectal cancer patients. Br J Cancer. 2008;98:1999–2005. doi: 10.1038/sj.bjc.6604399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum C, Graham A, Yousefzadeh M, Shrout J, Benjamin K, Krishna M, Hoda R, Hoda R, Cole DJ, Garrett-Mayer E, et al. The expression ratio of Map7/B2M is prognostic for survival in patients with stage II colon cancer. Int J Oncol. 2008;33:579–584. [PMC free article] [PubMed] [Google Scholar]
- 26.Koelzer VH, Baker K, Kassahn D, Baumhoer D, Zlobec I. Prognostic impact of beta-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. JClinPathol. 2012;65(11):996–1002. doi: 10.1136/jclinpath-2012-200742. [DOI] [PubMed] [Google Scholar]
- 27.Tikidzhieva A, Benner A, Michel S, Formentini A, Link KH, Dippold W, Von Knebel Doeberitz M, Kornmann M, Kloor M. Microsatellite instability and Beta2-Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer. 2012;106(6):1239–1245. doi: 10.1038/bjc.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lal N, Beggs AD, Willcox BE, Middleton GW. An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. Oncoimmunology. 2015;4(3):e976052. doi: 10.4161/2162402X.2014.976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104–1112. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 30.Ea Sloan, Kl Ring, Bc Willis, Modesitt SC, Mills AM. PD-L1 Expression in Mismatch Repair-deficient Endometrial Carcinomas, Including Lynch Syndrome-associated and MLH1 Promoter Hypermethylated Tumors. Am J Surg Pathol. 2017;41:326–333. doi: 10.1097/PAS.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 31.Valentini AM, Di PF, Cariola F, Guerra V, Giannelli G, Caruso ML, Pirrelli M. PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments. Oncotarget. 2018;9:8584–8596. doi: 10.18632/oncotarget.24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer ImmunolImmunother. 2017;66(7): 877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH, et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol Res. 2017;5(6): 480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PloSOne. 2015;10(6):e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao T, Li C, Wu Y, Li B, Zhang B. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PloSOne. 2017;12(4):e0176822. doi: 10.1371/journal.pone.0176822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder H, Hopp L, Schweiger MR, Hoffmann S, Juhling F, Kerick M, Timmermann B, Siebert S, Grimm C, Nersisyan L, et al. Genomic and transcriptomic heterogeneity of colorectal tumours arising in Lynch syndrome. JPathol. 2017;243(2): 242–254. doi: 10.1002/path.4948. [DOI] [PubMed] [Google Scholar]
- 37.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, Del TL, Macri E, Lanza G, Boiocchi M, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/S0016-5085(99)70510-X. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez-Valentin M, Therkildsen C, Da SS, Nilbert M. Familial colorectal cancer type X: genetic profiles and phenotypic features. ModPathol. 2015;28(1):30–36. doi: 10.1038/modpathol.2014.49. [DOI] [PubMed] [Google Scholar]
- 40.Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, Gallinger S, Bapat B, Aronson M, Hopper J, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293(16):1979–1985. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414–S421. doi: 10.1245/s10434-014-3584-y. [DOI] [PubMed] [Google Scholar]
- 42.Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 Status by Immunohistochemistry: challenges and Perspectives for Therapeutic Strategies in Lung Cancer Patients. Virchows Arch. 2016;468(5):511–525. doi: 10.1007/s00428-016-1910-4. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez-Valentin M, Therkildsen C, Veerla S, Jonsson M, Bernstein I, Borg A, Nilbert M. Distinct gene expression signatures in lynch syndrome and familial colorectal cancer type x. PLoS One. 2013;8:e71755. doi: 10.1371/journal.pone.0071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


