ABSTRACT
The success of chemotherapy largely depends on the anticancer immune response triggered by tumor cells that succumb to immunogenic cell death (ICD). One of the hallmarks of ICD is premortem autophagy that facilitates the release of adenosine triphosphate from dying cancer cells and acts as a chemoattractant for dendritic cell precursors. Here, we show that the immune response induced by inoculation of cancer cells undergoing ICD in response to the anthracycline mitoxantrone (MTX) can be improved by a short-term fasting regimen (48 hours of starvation) and that this effect is reversed by systemic administration of the autophagy inhibitor dimethyl α-ketoglutarate. Tumor growth reduction by MTX treatment is known to depend on autophagy induction in cancer cells as well as on an intact immune system. We compared the antitumor effects of MTX on autophagy-competent cancers implanted in wild type (WT) or partially autophagy-deficient (Becn1± or Atg4b−/-) mice. While there was no difference in the tumor growth reducing effects of MTX on tumors evolving in WT, Becn1+/- and Atg4b−/- mice, we observed an increase in the toxicity of MTX on Atg4b−/- mice. These results suggest that autophagy in cancer cells (but less so in host cells) is rate-limiting for therapeutically relevant anticancer immune responses, yet has a major role in blunting the life-threatening toxicity of chemotherapy.
KEYWORDS: Cancer, fasting, immunogenic cell death, immunotherapy, mitoxantrone
Introduction
Anticancer chemotherapies are particularly efficient if they succeed in stimulating an anticancer immune response that allows host T lymphocytes to control the growth of residual tumor cells upon discontinuation of the treatment. In mouse models, tumor growth reduction by chemotherapeutic agents often is dependent on T lymphocytes, meaning that tumors evolving in mice that lack T cells do not decrease their progression upon injection of cytotoxicants.1,2 One efficient way to stimulate such therapeutically relevant antitumor immune responses consists in the induction of immunogenic cell death (ICD). Anthracyclines (such as mitoxantrone, MTX) are able to stimulate ICD, a modality of cell death that is preceded or accompanied by the release of danger-associated molecular patterns (DAMPs), which alert innate effectors for the initiation of a cellular immune response.3–12 One of the most important DAMPs relevant to ICD is adenosine triphosphate (ATP). ATP is normally confined to the intracellular space, yet can be released from stressed and dying cells into the extracellular compartment where it interacts with purinergic receptors, in particular P2Y2 purinergic receptors to attract myeloid cells including dendritic cell (DC) precursors into the tumor bed.13,14 The release of ATP can be passive (as a result of plasma membrane permeabilization during primary or secondary necrosis) or active via lysosomal secretion.15 This latter phenomenon is linked to autophagy, which is required for optimal release of ATP from dying cancer cells.3 For this reason, autophagy-deficient cancer cells fail to release ATP in response to chemotherapy and thus are unable to recruit DCs into the tumor bed and to elicit an anti-tumor immune response.3,5
Conversely, manipulations that increase autophagy in cancer cells stimulate ATP release, enhance the recruitment of immune cells into the tumor bed and improve anticancer immunosurveillance either in baseline conditions or after chemotherapy with ICD inducers.16,17
Autophagy induction can be achieved by starvation (i.e. removal of nutrients from cultured cancer cells or from tumor-bearing mice) as well as by the administration of caloric restriction mimetics (CRMs), resulting in an enhanced anticancer immune response.18–22
Here, we investigated the question as to whether autophagy induction must also occur in the immune system to facilitate tumor growth reduction by ICD-inducing chemotherapy.
Results and discussion
Immunostimulatory effect of fasting
Starvation of mice for 48h causes a ~ 20% weight loss associated with a massive autophagic response in multiple distinct nucleated cell types.16,23 We tested the effect of fasting on the capacity of mice to mount a protective immune response against cancer cells succumbing to ICD induced by mitoxantrone (MTX) in vitro. For this, C57Bl/6 mice were injected subcutaneously (s.c.) with MTX-treated MCA205 fibrosarcoma cells into the left flank and optionally subjected to starvation (or left on standard chow as a control) for 2 days followed by rechallenge with live MCA205 cells into the opposite flank 7 days later (Figure 1A). Mice that had not been vaccinated (PBS controls) all developed tumors upon injection of live MCA205 cells. In contrast, 44% (8 out of 18) of the mice that were vaccinated with dying MCA205 cells and nourished in an uninterrupted fashion mounted a protective immune response against such cells, meaning that they did not develop tumors. This percentage raised to 77% (14 out of 18) when the mice were subjected to starvation regimen. Importantly, when starvation was combined with injection of dimethyl α-ketoglutarate (DMKG), which inhibits starvation-induced autophagy,24,25 this percentage dropped to 22% (4 out of 18), indicating a significant immunosuppressive effect of DMKG (Figure 1B). These results confirm that starvation can mediate immunostimulatory effects.9,19,20 However, they do not resolve the question whether autophagy and its modulation by DMKG impact the cancer cells or the immune system of the host.
Figure 1.
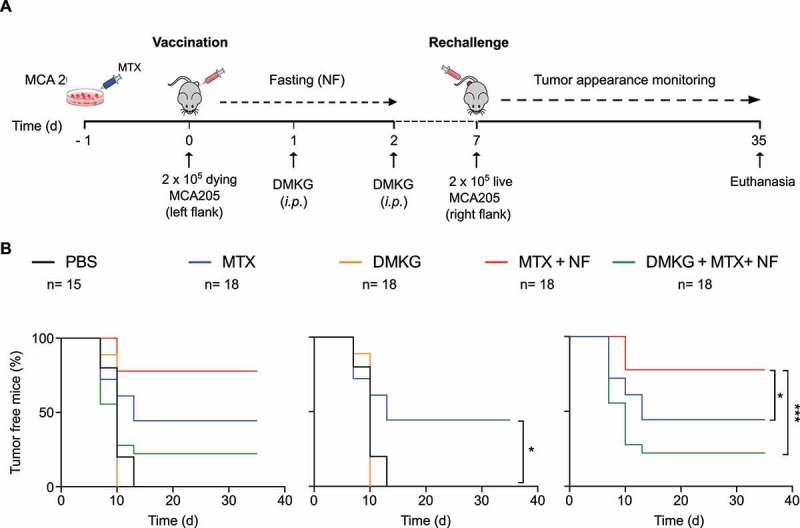
Nutrient deprivation improves tumor vaccination efficacy in an autophagy dependent manner. (A). Schematic outline of the tumor vaccination experiment used in this study. MCA205 fibrosarcoma cells, cultured for 16 hours with mitoxantrone (MTX) to trigger immunogenic cell death, were inoculated into the left flank of 6 weeks-old female C57Bl6/mice. After injection, mice were starved for 48 hours and left untreated or administered (intraperitoneally, i.p.) with the autophagy inhibitor dimethyl-2-oxoglutarate (DMKG). At day 7, mice were rechallenged by injection of live MCA205 cells in the right flank and tumor appearance was monitored over time. (B). Representative analysis of the experiment depicted in (A). Data represent the pool of two different experiments. Statistical significance was calculated by means of the likelihood ratio test. * p < 0.05; *** p < 0.001.
Normal anticancer immune responses in hosts with genetically determined autophagy defects
Autophagy-deficient MCA205 fibrosarcomas do not respond to ICD-inducing chemotherapies because they fail to recruit myeloid immune cells into the tumor bed, precluding a subsequent anticancer immune response.3,19 However, the possible contribution of autophagy in immune effectors has not been studied in this kind of system. We therefore implanted autophagy-competent MCA205 cells into autophagy-competent syngeneic wild type (WT) C57Bl/6 (Figure 2A) or autophagy-deficient hosts such as autophagy related gene 4b (Atg4b−/-) (Figure 2B) or Beclin 1 (Becn1+/-) mice (Figure 2C). The natural tumor growth (without treatment) was not influenced by the autophagy competence of the host. Moreover, a single intraperitoneal injection of MTX, that was administered when the tumor reached a surface of 25 mm2, was able to reduce tumor growth indistinguishably in WT, Atg4b−/- and Becn1+/- mice (Figure 2A-C). In conclusion, it appears that the autophagy competence of the host does not impact the efficacy of ICD-inducing chemotherapy.
Figure 2.
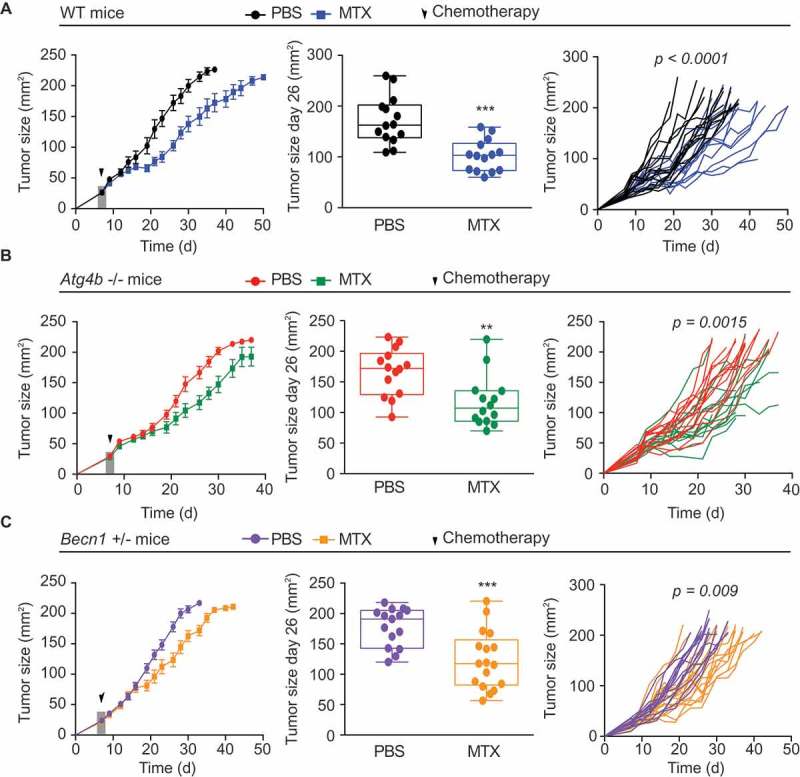
Systemic autophagy deficiency does not impact on the efficacy of anthracycline-based immunogenic chemotherapy. (A-C) Wild type (WT) autophagy competent C57BL/6 mice (A) or autophagy deficient Atg4b−/- (B) and Becn1+/- (C) mice were inoculated subcutaneously (s.c.) with murine fibrosarcoma MCA205 cells. When tumors reached a size of 25 mm2, mice received a single injection (i.p) of mitoxantrone (MTX) or an equivalent volume of PBS and tumor growth was routinely assessed. From left to right: average (± S.E.M) tumor growth curves of treated with PBS or MTX-based chemotherapy; tumor size distribution at day 26 of data; individual growth curves from mice treated with MTX or PBS. Data represent a pool of three different experiments. Statistical analysis of tumor growth curves was performed by Wald test whereas tumor size distribution at defined time points was analyzed by means of an unpaired t test. ** p < 0.01; *** p < 0.001.
Increased toxicity of mitoxantrone in atg4b−/- mice
A reduction in bodyweight is a proxy of toxicity. MTX-treated mice exhibited a significant decrease in bodyweight. This MTX effect was found for all three mice genotypes analyzed here, namely, WT (Figure 3A), Atg4b−/- (Figure 3B) and Becn1+/- (Figure 3C), when compared to their respective vehicle-only (PBS)-treated controls. However, the body weight reduction appeared stronger for MTX-treated Atg4b−/- mice than for any other treatment group (Figure 3D). These results confirm prior studies showing that the toxic effects of anthracyclines (for instance on the heart) are attenuated by autophagy induction and hence exacerbated in conditions of autophagy inhibition.26–29 Accordingly, when the cause of death in each experimental group were analyzed, deaths that could not be attributed to cancer cell proliferation (and hence must result from MTX toxicity) were more frequent among MTX-treated Atg4b−/- mice bearing MCA205 tumors than in any other treatment group including MTX-treated WT and Becn1+/- mice (Figure 3E). As a result, MTX was only able to increase the overall survival of MCA205 fibrosarcoma bearing and Becn1+/- but not Atg4b−/- mice (Figure 3F).
Figure 3.
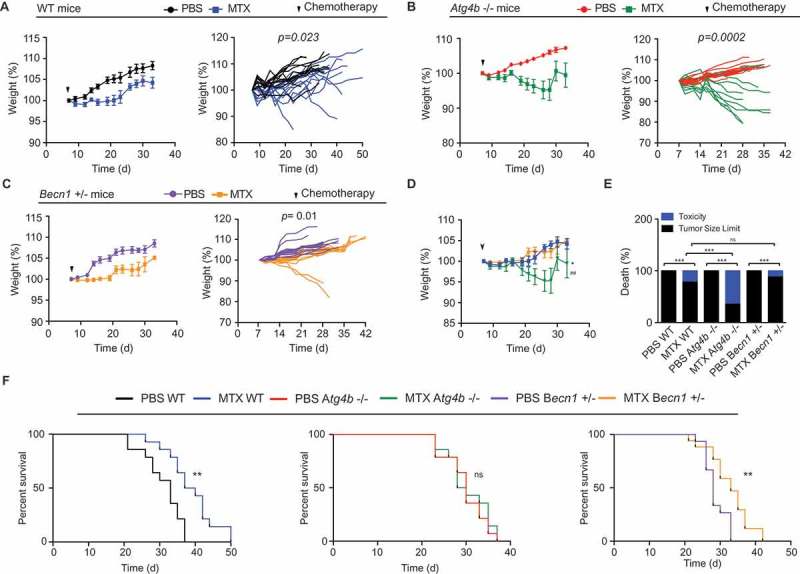
Autophagy deficient mice show increased toxicity in response to anthracyclines based chemotherapy. (A-C) Wild type (WT) autophagy competent C57BL/6 mice (A) or autophagy deficient Atg4b−/- (B) and Becn1+/- (C) mice bearing MCA205 fibrosarcoma tumors received a single injection (i.p.) of mitoxantrone (MTX) or an equivalent volume of PBS, and bodyweight was monitored over time as an indicator of chemotherapy derived toxicity. Average (± S.E.M) (left panel) and individual (right panel) bodyweight curves are shown. Data represent a pool of three different experiments. Statistical analysis of body weight curves was performed by means of linear mixed-effect modeling (over the whole time course). (D) Comparison of MTX effects on body weight between autophagy competent WT and autophagy deficient mice. * p < 0.05 (for MTX WT vs MTX Atg4b−/- comparison); ## p < 0.01 (for MTX Atg4b−/- vs MTX Becn1+/- comparison). (E) Analysis of death type from autophagy competent versus autophagy deficient mice challenged with MTX chemotherapy. ns, non significant; *** p < 0.001 (binomial test). (F) Survival curves of MCA205 tumor bearing WT, Atg4b−/- and Becn1+/- mice. Statistical significance was assessed by means of log rank test. ** p < 0.01.
Concluding remarks
Major interventions on whole body physiology such as starvation or chemotherapy may impact on the autophagy system that – in teleological terms – aims at increasing the resilience of the organism against metabolic and toxic challenges.30 In the context of cancer, autophagy induction may have oncopreventive and improve therapeutic outcome after chemotherapy effects.18,19,31–33 In addition, starvation, which is one of the best-known inducers of autophagy, increases the resistance of mice against the toxic effects of anthracycline-based chemotherapy at the same time that it improves tumor growth reduction.20,34 This autophagy- or starvation-mediated improvement of chemotherapeutic responses has been attributed to an increase in anticancer immunity.19,20
Prior studies in which essential autophagy genes were knocked down (as shown for Atg5, Atg7, Atg10, Atg12, Bcln1, phosphatidylinositol 3-kinase catalytic subunit type 3 (Pik3c3)) or knocked out (as shown for Atg5) in malignant cells demonstrated that autophagy induction in cancer cells was a requisite for the recognition of such cells by the immune system.3,19,35 Here, we investigated whether partial effects in autophagy conferred by haploinsufficiency in Becn1 or knockout of Atg4b in the host would compromise the chemotherapy-induced anticancer immune response leading to tumor growth reduction after MTX injection. Apparently such partial autophagy defects do not cause a major immunosuppressive effect, supporting the idea that autophagy in the tumor cells (rather than autophagy in immune effectors) is relevant to the therapeutic outcome of immunogenic chemotherapy.36,37
As a caveat, we must insist on the fact that the systemic autophagy defects investigated here are only partial. Indeed, knockout of non-redundant autophagy genes (such as Atg5 or Atg7) in the hematopoietic lineage (or more specifically in myeloid or lymphoid subsets) has a strong immunosuppressive effect due to the loss of stem cell functions and an irreversible loss of cellular fitness precluding in-depth analyses of such phenotype with respect to anticancer immunosurveillance.38
Valter Longo and collaborators have evoked the possibility of using starvation regimens or hypocaloric nutrition to increase the efficacy of chemotherapy while reducing its toxicity.39,40 Based on the results shown here, we suggest that therapeutic efficacy is linked to autophagy induction in tumor cells, while toxicity reduction is tied to autophagy induction in the host.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). FP is supported by the European Molecular Biology Organization (EMBO) postdoctoral long-term fellowship (ALTF-358-2017). FC is supported by “Ligue contre le Cancer”. The authors are grateful for excellent technical assistance of the CRC Core Facilities (CEF and CGB; Centre de Recherche des Cordeliers, Paris, France).
Authors contribution
FC, FP, EV and MCM performed the experiments. FC and FP analyzed the data and performed statistical analysis. LZ, FP and GK conceived the study and wrote the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
EXPERIMENTAL PROCEDURES
Mouse Strains and Housing
Mice were maintained in specific pathogen-free conditions in a temperature controlled environment with 12-hr light/12-hr dark cycles and received food and water ad libitum (unless noted otherwise). Animal experiments were in compliance with the EU Directive 63/2010 and protocol 03981.02 was approved by the Ethical Committee of the CRC (CEEA no. 5, registered at the French Ministry of Research). Six-to 7-week-old female WT C57Bl/6 mice were obtained from Envigo France. Atg4b−/- and Becn1+/- mice were kindly provided by Drs. Carlos Lopez-Otin (Oviedo University, Spain) and Beth Levine (University of Texas Southwestern).
Mouse experiments
For tumor growth experiments, 2 × 105 MCA205 fibrosarcoma cell line were inoculated s.c. into WT or autophagy deficient Atg4b−/- and Becn1+/- C57BL/6 (H-2b) mice, and tumor surface (longest dimension × perpendicular dimension) was routinely assessed using a common caliper. When the tumor surface reached 25 mm2, mice intraperitoneally (i.p.) received a single injection of 5.17 mg/kg mitoxantrone (MTX, M6545, Sigma Aldrich) in 100μl PBS or an equivalent volume of PBS.
For tumor vaccination experiments, MCA205 fibrosarcoma cells were cultured in RPMI 1640 medium supplemented with supplemented with 10% (v/v) fetal bovine serum, 100 mg/L sodium pyruvate, 10 mM HEPES buffer, 100 IU mL-1 penicillin G sodium salt, and 100 mg/mL streptomycin sulfate under standard conditions (at 37 ºC, under 5% CO2). MCA205 cells were left untreated or treated with 5 μM MTX for 16h then s.c. inoculated (in 200 μl PBS) into the left flanks of WT mice. Mice were nourished ad libitum or underwent 48 hours fasting, combined with two i.p. injections of 300 mg/kg Dimethyl-2-Oxoglutarate (DMKG) in 200 μl of PBS or an equivalent volume of PBS. 2 × 105 live WT cells were inoculated into the opposite flank 7 days later, and tumor growth was monitored regularly for a total of 40 days. The absence of tumors was considered as an indication of efficient antitumor vaccination.
Statistical analysis
Longitudinal analysis of tumor growth and bodyweight data was performed by linear mixed effect modeling on log pre-processed tumor sizes. Type II ANOVA (Wald tests) was used to compute p values by testing jointly that both tumor growth slopes and intercepts (on a log scale) were the same between treatment groups of interest https://kroemerlab.shinyapps.io/TumGrowth/. 40 For mice euthanized before the selected sampling point, the last measure was retained.
Statistical significance of tumor vaccination experiment was assessed by means of the likelihood ratio test by means of TumGrowth software https://kroemerlab.shinyapps.io/TumGrowth/.40 Analysis of mouse survival curves (log rank test) and of the effect of treatment on tumor size at a selected sampling point (unpaired t test) was performed by means of Graph Pad software.
References
- 1.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, Van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 3.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 4.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 5.Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–978. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L.. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 8.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 9.Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6:e1386829. doi: 10.1080/2162402X.2017.1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, et al. Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology. 2014;3:e28473. doi: 10.4161/onci.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baracco EE, Pietrocola F, Buque A, Bloy N, Senovilla L, Zitvogel L, Vacchelli E, Kroemer G. Inhibition of formyl peptide receptor 1 reduces the efficacy of anticancer chemotherapy against carcinogen-induced breast cancer. Oncoimmunology. 2016;5:e1139275. doi: 10.1080/2162402X.2016.1139275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini J-L, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21:79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrocola F, Bravo-San Pedro JM, Galluzzi L, Kroemer G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy. 2017;13:2163–2170. doi: 10.1080/15548627.2017.1310356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14:247–258. doi: 10.1038/nrclinonc.2016.183. [DOI] [PubMed] [Google Scholar]
- 18.Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi: 10.1038/ncomms5972. [DOI] [PubMed] [Google Scholar]
- 19.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, De Cabo R, Wei M, et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell. 2016;30:136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino G, Pietrocola F, Madeo F, Kroemer G. Caloric restriction mimetics: natural/physiological pharmacological autophagy inducers. Autophagy. 2014;10:1879–1882. doi: 10.4161/auto.36413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madeo F, Pietrocola F, Eisenberg T, Kroemer G. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014;13:727–740. doi: 10.1038/nrd4391. [DOI] [PubMed] [Google Scholar]
- 23.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 24.Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014;53:710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Marino G, Pietrocola F, Kong Y, Eisenberg T, Hill JA, Madeo F, Kroemer G. Dimethyl alpha-ketoglutarate inhibits maladaptive autophagy in pressure overload-induced cardiomyopathy. Autophagy. 2014;10:930–932. doi: 10.4161/auto.28235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizarro M, Troncoso R, Martinez GJ, Chiong M, Castro PF, Lavandero S. Basal autophagy protects cardiomyocytes from doxorubicin-induced toxicity. Toxicology. 2016;370:41–48. doi: 10.1016/j.tox.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 28.An L, Hu XW, Zhang S, Hu X, Song Z, Naz A, Zi Z, Wu J, Li C, Zou Y, et al. UVRAG Deficiency Exacerbates Doxorubicin-Induced Cardiotoxicity. Sci Rep. 2017;7:43251. doi: 10.1038/srep43251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The Role of Autophagy in the Heart. Annu Rev Physiol. 2018;80:1–26. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 30.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietrocola F, Pol J, Kroemer G. Fasting improves anticancer immunosurveillance via autophagy induction in malignant cells. Cell Cycle. 2016;15:3327–3328. doi: 10.1080/15384101.2016.1224797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladoire S, Enot D, Senovilla L, Chaix M, Zitvogel L, Kroemer G. Positive impact of autophagy in human breast cancer cells on local immunosurveillance. Oncoimmunology. 2016;5:e1174801. doi: 10.1080/2162402X.2016.1174801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penault-Llorca F, Arnould L, et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy. 2016;12:864–875. doi: 10.1080/15548627.2016.1154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L, Zitvogel L, Kroemer G. Immunological Mechanisms Underneath the Efficacy of Cancer Therapy. Cancer Immunol Res. 2016;4:895–902. doi: 10.1158/2326-6066.CIR-16-0197. [DOI] [PubMed] [Google Scholar]
- 38.Shibutani ST, Saitoh T, Nowag H, Munz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 39.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enot DP, Vacchelli E, Jacquelot N, Zitvogel L, Kroemer G. TumGrowth: an open-access web tool for the statistical analysis of tumor growth curves. Oncoimmunology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


