ABSTRACT
Controversy surrounds the role of cytomegalovirus (CMV) in glioblastoma (GBM). However, several studies have shown that CMV nucleic acids and proteins are present within GBM tumor tissue. CMV has been implicated in GBM pathogenesis by affecting tumor stem cell factors, angiogenesis and immune pathways. Anti-viral therapy has not been found to definitively improve outcomes for patients with GBM. Several studies have leveraged CMV by targeting CMV antigens using ex-vivo expanded T cells or dendritic cell vaccines. The initial results from these studies are promising and larger studies are underway.
KEYWORDS: Glioblastoma, brain tumors, immunotherapy, dendritic cells, vaccines, cytomegalovirus
Introduction
The poor clinical outcomes in patients with glioblastoma (GBM) has fueled a vigorous search for a driver of tumor growth and resistance. GBM is a high-grade glioma associated with a median survival of 15–20 months.1,2 Cytomegalovirus (CMV) has captured the interest of investigators due to the chronic inflammation found in GBM and the immunosuppressed state of patients. CMV is a double-stranded DNA virus that encodes approximately 200 genes as well as micro RNA (miRNA) and non-coding transcripts.3 CMV can infect many cell types including and, like all herpes viruses, can result in life long persistence after the initial acute infection.4 Therefore, the immunosuppression associated with GBM5,6 would be the perfect environment for CMV re-activation in tumor cells. CMV DNA and protein products have been discovered in multiple tumor types7-9 including low grade glioma and higher grade glioma (e.g. GBM) samples.10 These findings have led to inquiry into the effect of CMV on glioma growth patterns, stem cell properties of the tumor cells and therapeutic targeting.
In this review, we will discuss the role of CMV in GBM. The controversies around the detection of CMV in glioma samples, and the possible implications of CMV in the role of glioma-genesis or treatment resistance will be reviewed. We will also discuss therapeutic implications and how a small amount of CMV nuclei acids or proteins may result in large treatment effect when targeted with immunotherapy. Immunotherapeutic approaches leveraging CMV for the treatment of GBM have not been limited by the presence of the blood brain barrier.3 The preclinical and clinical studies of CMV based immunotherapies for GBM will be discussed.
CMV detection in gliomas
One of the first descriptions of CMV genomic expression in glioma was authored by the group led by Dr. Charles Cobbs. They found 27 out of 27 human glioma samples demonstrated expression of CMV genomic and protein material as measured by immunohistochemistry (IHC) and in situ hybridization (ISH).10 Other groups also described CMV viral material in glioma samples using similar methodology.11,12 Mitchell et al. found that a large percentage of GBM samples were positive for CMV immediate-early (IE)1, pp65 or glycoprotein B using IHC, ISH and polymerase chain reaction (PCR).13 However, subsequent to these initial reports, other groups published reports of no detectable CMV protein or genomic material in glioma samples.14,15 These findings were challenged due to the variability of CMV detection depending on assay sensitivity.16 More recent studies using PCR have also failed to detect CMV glycoprotein B and IE in GBM patient peripheral blood or tumor samples.16 Recently, an analysis of 94 formalin fixed, paraffin embedded and 28 snap frozen glioma samples using IHC and PCR found no CMV viral products in the samples.17
Despite these studies demonstrating no detectable CMV in glioma samples, multiple reports from several independent laboratories have confirmed the detection of CMV in glioma and other tumor samples. Scheurer et al. found that the majority of glioma samples had CMV viral genes and that the number of CMV positive cells was higher in GBM compared to low grade gliomas.11 This phenomenon has been reported by other groups as well.18,19 Studies using PCR techniques to detect 20 different regions of the CMV genome found CMV positivity was much more likely in GBM samples compared to epilepsy samples.20 They also found that CMV detection was higher in more recent samples compared to older samples. More recent studies utilizing IHC have found pp65 positivity in gliomas, medulloblastomas, CNS lymphoma and meningiomas.21 Others have found that 25% of patients with GBM had detectable levels of a CMV micro RNA (miR-UL112-3p) in their blood.22 (Table 1)
Table 1.
CMV detection methods in glioma.
| Authors | Year | Samples | Number of samples | Detection Methods | Fraction Positive |
|---|---|---|---|---|---|
| Cobbs et al. | 2002 | Grade II-IV glioma | 27 | IHC, ISH, EM-IHC | 22/22 GBM, 1/1 AO, 4/4 LGG |
| Sabatier et al. | 2005 | Grade II-IV glioma | 97 | IHC, ISH | 10/97 GBM |
| Poltermann et al. | 2006 | Grade II-IV glioma | 40 | IHC, PCR | 0 |
| Scheurer et al. | 2008 | Grade II-IV glioma | 50 | IHC, ISH | 21/21 GBM, 9/12 AA, 14/17 LGG |
| Mitchell et al. | 2008 | Grade IV glioma | 45 | IHC, ISH, PCR | 42/45 GBM |
| Slinger et al. | 2010 | Grade IV glioma | 21 | IHC | 20/21 GBM |
| Lucas et al. | 2011 | Grade IV glioma | 49 | IHC | 25/49 pp65, 8/49 IE1 |
| Ranganathan et al. | 2012 | Grade IV glioma | 75 | PCR | yes* |
| Bhattacharjee et al. | 2012 | Grade II-IV glioma | 17 | PCR, Gene sequencing | 16/17 |
| Matlaf et al. | 2013 | Grade IV glioma | 10 | IF | 5/10 pp71 |
| Libard et al. | 2014 | Grade II-IV glioma | 469** | IHC | 197/219 HGG and LGG |
| Mohammad et al. | 2014 | Grade IV glioma | 20 | PCR of plasma | 5/20 |
| Priel et al. | 2015 | Grade IV glioma | 7 | PCR | 0 |
| Huang et al. | 2015 | Grade II-IV glioma | 60 | IHC, ISH, PCR, Western blot | yes *** |
| Garcia-Martinez et al. | 2017 | Grade II-IV glioma | 122 | PCR, IHC | 3/119 by PCR only |
* Compared GBM with epilepsy and non-malignant tumor samples. No quanitification of the number of positive glioma samples.
** Includes other tumor types such as medulloblastoma and CNS lymphoma.
*** Determined high versus low expression of various CMV markers in different grade tumors.
GBM glioblastoma; AA anaplastic astrocytoma; LGG low grade glioma; HGG high grade glioma; AO anaplastic oligodendroglioma; IHC immunohistochemistry; ISH in situ hybridization; PCR polymerase chain reaction; IF immunofluorescence; IE1 immediate early 1
Due to the variability in techniques, a consensus statement was published summarizing the data for CMV expression in gliomas. This publication established that CMV is expressed in most human glioma samples with sensitive assays.23 Peripheral blood tests do not always correlate with CMV positivity in tumor samples. Controversy remains about the role of CMV in glioma oncogenesis. Nonetheless, CMV nucleic acids and proteins have been found to be potential immunogenic antigens that could be targeted using immune-based therapy.
CMV role in glioma pathogenesis
Beyond identification of CMV within glioma samples, several studies have investigated the effect of CMV infection in glioma pathogenesis. By infecting human glioma tumor cells with CMV, investigators discovered activation of the phosphatidylinositol-3-kinase (PI3K)-Akt pathway.24 They also found phosphorylation of focal adhesion kinase (FAK) resulting in increased tumor cell migration and invasiveness. This group also described that expression of IE1 CMV genes in human GBM cell lines resulted in increased entry into the cell cycle, DNA synthesis and cellular proliferation.25 Overexpression of CMV glycoprotein B in glioma cells also induced entry into the cell cycle and increased invasiveness.26 However, this effect was mediated through phosphorylation of PDGFRα which had been previously shown to be a critical receptor for CMV infection.27 Straat et al. infected malignant glioma cell lines with CMV and found that this resulted in activated telomerase in tumor cells.28 They hypothesized that this effect could be a link between viral infection and oncogenesis.
Findings vary based on techniques utilized to overexpress CMV genes within tumor cells. Most studies have evaluated the impact of CMV on tumor cells via infection of cell lines. However, other studies utilizing transfection of glioma cell lines to overexpress the CMV IE1 proteins found a downgregulation of tumorigenic genes such as thrombospondin-1 (TSP-1) and a reduction of tumor suppressor genes like p53.29
CMV-mediated oncogenesis has also been proposed to be modulated through expression of US28, a virally encoded chemokine receptor. US28 has been found to be expressed in 60% of human GBM samples and can bind several chemokines (CCL2, CCL5, CX3CL1). During CMV infection, US28 has constitutive activity resulting in G-protein dependent signaling. Overexpression of US28 in glioma cells promoted secretion of vascular endothelial growth factor (VEGF)30, activated signal transducer and activator of transcription (STAT)3, and resulted in increased GBM cell invasiveness.31 US28 expression also accelerates glioma growth.32 De Wit et al. found that US28 resulted in activation of hypoxia inducible factor 1alpha/pyruvate kinase M2 (HIF-1alpha/PKM2) in GBM cells which resulted in increased VEGF and lactate secretion.33 Increased proliferation of cells expressing US28 was reversed by inhibiting HIF-1alpha/PKM2. In addition to promoting tumor cellular growth, other groups have found that CMV prevents apoptosis of GBM tumor cells by overexpression of activating transcription factor 5 (ATF5).34
In addition to tumor proliferation and invasion, CMV expression is associated with enhancement of stem cell properties within glioma tumor cells. Soroceanu et al. found that attenuation of endogenous IE expression in glioma stem-like cells inhibited growth of the tumor cells in spheres which is a technique that enriches for stem-like cells.35 Simultaneously, non CMV-expressing cell lines that were infected with CMV had an increase in self-renewal and proliferation. The same group found that CMV pp71 was preferentially expressed in CD133+ glioma stem-like cells.36 They described that pp71 induced stem cell factor (SCF) in GBM cells which is a pro-angiogenic factor, via nuclear factor kappa light chain enhancer of activated B cells (NF-kB) signaling. Antiviral treatment resulted in less secretion of SCF from GBM cells. They also found that pp71 and NF-kB activation were augmented in mesenchymal subtypes of human GBM samples. CMV pp71 has also been implicated in diminishing the accumulation of major histocompatibility complex (MHC) class I on the cell surface of GBM cells.37
CD133 and CMV have been implicated in poorer patient outcomes. Fornara et al. reported that high expression of CD133 and CMV-IE proteins predicted poor patient survival.38 They also found that infection of GBM tumor cells led to an upregulation of stem cell markers like Notch1, Sox2, Oct4 and Nestin. CMV infection induced neurosphere formation of GBM tumor cells and this could be prevented with anti-viral treatment with ganciclovir. Recently, Ulasov et al. reported that endogenous CMV miRNA (CMV70-3P) was associated with stem cell properties in primary GBM cell lines. Inhibition of CMV70-3P reduced cell proliferation and ability to form spheres.39
Glioma stem-like cells are also associated with long-term CMV infection compared to non-stem cell glioma lines which stop expressing CMV genes 5 weeks after infection.40 Other groups have found that CMV had a tropism for CD133+ glioma stem-like cells and these cells produced CMV IL-10.41 CMV IL-10 induced immunosuppressive monocytes and expression of viral IE1. The immunosuppressive monocytes produced angiogenic VEGF, immunosuppressive transforming growth factor (TGF)-beta 1 and increased migration of glioma stem-like cells.
Others have also found that US28-CCL5 paracrine signaling may contribute to glioma progression.31 Factors associated with inflammatory macrophages has also been implicated with CMV and GBM pathogenesis. Costa et al. described an increase in arginase 2 (ARG2) in GBM cells infected with CMV that expressed the IE proteins.42 ARG2 is involved in nitric oxide metabolism. Conversely, other groups have found that CMV down-regulates ARG2 in human GBM cells with a concomitant reduction miRNA-613. These investigators found that reduced microRNA-613 was associated with worse outcomes (survival, tumor size, etc).43 Therefore, the implications of CMV in glioma growth may be partially immune mediated.
CMV impact on prognosis
Several groups have investigated the impact of CMV on outcomes in patients with GBM. In a study of 80 GBM patients, 99% of tumor samples were found to express CMV and these were graded for the number of positive cells (grade 1–4). The investigators found that patients with longer survival (> 18 months) were more likely to have tumors with low grade CMV infection.44 This group subsequently performed a retrospective study and found that GBM patients with a low grade CMV infection of their tumor sample had a median survival that was 20 months longer than those with a higher-grade infection (p 0.036).45 These studies are limited by analysis prior to routine use of temozolomide and lack of controlling for other prognostic factors such as isocitrate dehydrogenase (IDH)-1 mutational status.
The benefit of pharmacologic anti-viral treatment in GBM remains questionable. Pre-clinical studies found that treatment of human GBM cells in an orthotopic murine xenograft model with cidofovir resulted in inhibition of CMV gene expression and cellular apoptosis in both CMV infected and uninfected tumors.46 The treatment was synergistic with radiation in prolonging survival of tumor-bearing mice. In 2006, a small randomized study of adding valganciclovir to standard of care treatment for GBM found no difference in overall survival or time to progression.47 On post-hoc analysis, the investigators reported that patients who received the drug for > 6 months did have improved survival compared to those who received it for a shorter time. These results are subject to immortal time bias. Therefore, the authors re-analyzed their data to remove this bias and found similar results.48 They subsequently described a retrospective analysis of 50 GBM patients treated with valganciclovir added to standard treatment and found their medial overall survival was 25.0 months compared to 13.5 months in a comparator control group (p < 0.001).49 A small study of 13 patients who were treated with valganciclovir and bevacizumab at the time of recurrence of GBM were compared to a group of patients treated with bevacizumab alone.50 The authors reported a trend for improved overall survival (13.1 months in the combination group compared to 8.7 months in the bevacizumab alone group). These data have significant limitations due to study design. Therefore, use of anti-viral treatment in patients with GBM is not routine.
CMV as immunotherapeutic target
Several groups have explored the potential of CMV as a useful target in the immunotherapeutic treatment of GBM. Lucas et al. found that CMV pp65 and IE1 expression was mostly in the cytoplasm of GBM samples, and that CMV specific T cells were able to recognize and lyse GBM cells that were infected with CMV in the laboratory.51 The immunotherapeutic strategies for targeting CMV include adoptive T cell transfer and vaccine approaches. Adoptive T cell therapy has been mostly described using CMV-specific T cells from patients that are ex-vivo expanded. Crough et al. described CMV-specific CD8+ T cells in GBM patients who were seropositive for prior CMV infection.52 These T cells were found to be poorly functional based on cytokine production in ex vivo analyses. However, their functionality was restored in vitro with CMV peptide epitopes and IL-2 exposure. Nair et al. demonstrated that CMV antigen specific T cells could be isolated from human GBM patients.53 They described expanding these T cells ex-vivo using pp65 pulsed DCs. Expansion of these cells could represent a memory response after prior CMV infection. However, they found that these cells elicited a potent anti-tumor response in vitro in an antigen specific manner when co-cultured with autologous GBM tumor cells. These findings are significant as they demonstrated that endogenous levels of CMV antigens were sufficient to serve as targets for CMV-specific T cells in vitro. Other investigators have also been able to expand CMV specific T cells from patients with GBM.54,55 In a study of patients with recurrent GBM, these cells were given as adoptive T cell infusions in combination with chemotherapy to 11 of the patients who demonstrated an overall survival of 13.4 months. The authors found a correlation between progression-free survival and a specific gene signature of T cell activation within the ex vivo expanded, CMV-specific T cells.54
Other studies exploring vaccination strategies aimed at expanding CMV-specific immunity in vivo have demonstrated promising results in early phase studies. In murine experiments and in vitro human sample experiments, Dasari et al. demonstrated that vaccination with CMV glycoprotein B polypeptide in combination with toll like receptor agonists resulted in an influx of DC subsets.56 These cells induced activation of adaptive immunity through antigen acquisition and cross presentation. Our group has found that DCs electroporated with CMV pp65 mRNA can be delivered to GBM patients and results in a potent immunity and anti-tumor response when these cells migrate efficiently to draining lymph nodes. In a clinical trial, GBM patients were randomized to receive standard therapy with pp65 DC vaccine alone or pp65 DC vaccine combined with a tetanus diphtheria toxoid (Td) booster vaccine.57 The group that received the combination vaccination had significantly more DCs migrate to the draining lymph nodes due to an upregulation of CCL3. This increased migration correlated with significant improvement in overall survival (> 36.6 months versus 18.5 months). This publication was the first to demonstrate a link between DC migration, immunotherapy efficacy, and clinical outcomes in GBM patients.
Reap et al. subsequently found DC vaccines are important for increased functionality of adoptively transferred CMV specific T cells.58 In a pilot study, 22 patients were randomized to receive adoptive transfer of pp65 specific T cells with CMV DC vaccine or saline. The patients who received the T cells and DC vaccine had a significant increase in CMV specific CD8 T cells that were positive for IFNγ, TNFα and CCL3 compared to patients who received the T cells and saline. The increase in these polyfunctional CD8 T cells correlated with improved overall survival.
Another strategy to enhance the efficacy of CMV targeted immunotherapy, is to combine DC vaccines with dose-modified chemotherapy. In a recent study, Batich et al. described 11 GBM patients who received dose-intensified temozolomide along with at least 3 doses of the pp65 DC vaccine after completing standard temozolomide and radiation.59 After receiving 3 pp65 DC vaccines, patients had significantly increased pp65 cellular responses. Despite having an increase in peripheral immunosuppressive regulatory T cells (Tregs), the median progression-free survival was 25.3 months and overall survival was 41.1 months. Four of the eleven patients remained progression-free at 59 to 64 months. It is believed that vaccination during homeostatic recovery from the profound lymphopenia induced by dose-intensified temozolomide results in a more robust antigen-specific immune response following the period of lymphopenia.60-63
To build on these results there are ongoing studies investigating CMV based immunotherapeutic strategies. We are leading a phase 2, blinded and randomized clinical trial evaluating the efficacy of CMV pp65 RNA-pulsed DC vaccines (ATTAC II). This study (ClinicalTrials.gov, NCT02465268) is enrolling newly-diagnosed GBM patients randomized to receive either peripheral blood monocytes and saline (control group) or one of two formulations of CMV pp65 RNA conjugated to the lysosomal associated membrane protein (LAMP-1), which enhances antigen-processing and presentation. Patients receive standard concomitant radiation and temozolomide followed by adjuvant cycles of also dose-intensified temozolomide (100 mg/m2/day x 21 days).
Another group has developed an anti-CMV enveloped virus-like particle (eVLP) vaccine that is currently being tested in a Phase 1/2a study in 18 patients with recurrent GBM (ClinicalTrials.gov, NCT03382977).
Mechanisms of action of anti-cmv based immunotherapy
The efficacy of CMV based immunotherapy observed in early phase studies in GBM has been relatively surprising since the quantitative amount of CMV genomic or protein material within glioma samples is reportedly very low. Despite this phenomenon, robust anti-CMV immune responses have been documented in a patient vaccinated with tumor-lysate pulsed DCs.64 With the caveat that early phase studies are fraught with difficulties in estimating the true efficacy of any new therapeutic modality, plausible explanations for such potential efficacy can be categorized into direct vaccine-mediated effects or indirect targeting effects on tumor cells.
Direct antigen-specific hypothesis
Strategies to generate an anti-CMV immune response result in the specific killing of CMV positive tumor cells, and the loss of these cells is what is responsible for clinical benefit. These cells have been shown to demonstrate stem cell properties35,36,38,39 and therefore may represent the tumor population driving tumor growth, invasiveness and treatment resistance. Therefore, although CMV based immunotherapy may eliminate only a portion of GBM tumor cells, these changes remain clinically significant due to the downstream impact of removing this cell population (Figure 1a).
Figure 1.
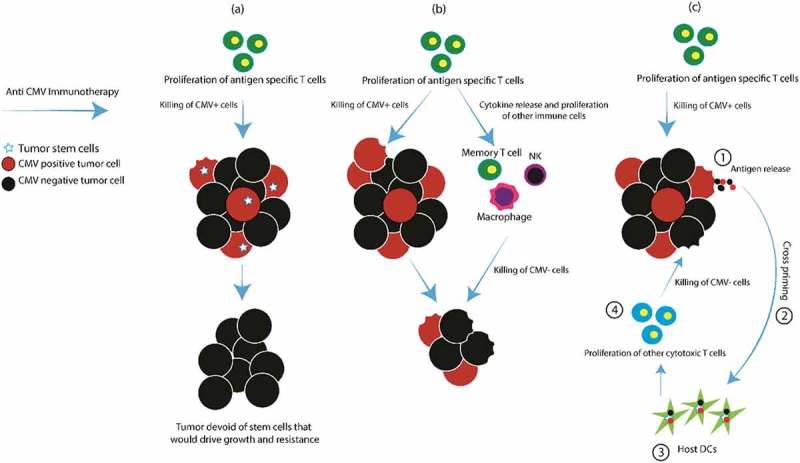
Anti-CMV immunotherapy mechanisms of action.
NK natural killer cell; DCs dendritic cells
Indirect targeting hypothesis
Due the presence of CMV antigens in most glioma samples, immunotherapy may result in a robust activation of host immunity within the tumor microenvironment. In addition to the antigen-specific immune response, the host has an increase in cytokines, NK cells, macrophages and memory T cell activation.65,66 These recruited immune effector cells can cause MHC independent killing of tumor cells and cytokines that result in a bystander T cell response67 that can also control tumor growth68 (Figure 1b). Moreover, the innate immune system can detect CMV DNA via toll-like receptor (TLR) 9 and cyclic GMP/AMP synthase (cGAS) catalyzing formation of stimulator of interferon genes (STING) followed by an upregulation of type I interferon responses69,70 that may cause indirect killing of tumor cells.
Cross-priming
CMV-specific immune killing of tumor cells results in antigen release and cross priming by dendritic cells (DCs) to other tumor-associated and tumor-specific antigens.71 These cells then magnify the anti-tumor immune response and results in killing of both CMV positive and negative tumor cells (Figure 1c).
The pre-clinical and clinical outcomes that have been reported with current CMV immunotherapeutic strategies are likely due to multiple mechanisms in play. Studies of CMV in glioma have shown that herpes viral material can be found in tumor cells. These viral proteins may serve as potent targets for immunotherapy due to their completely “foreign” nature. Despite challenges such as the blood brain barrier and heterogeneity of expression of low level CMV expression within tumor cells, initial studies of CMV targeted immunotherapy have been promising in the treatment of GBM. Further studies will be necessary to elucidate the mechanisms that result in anti-tumor efficacy and which patient populations are most likely to benefit from CMV-directed immunotherapy.
Conclusion
CMV viral genes and proteins are found in most GBM tissue samples. Anti-viral treatment with drugs has not definitively been shown to improve survival in patients with GBM. However, immunotherapy has been used to target CMV antigens in GBM. Initial results of CMV-based immunotherapy are promising. The efficacy of anti-CMV immunotherapy may be due to targeting of CMV expressing cells that drive tumor growth, activation of other immune cells that cause additional killing of CMV negative cells, or cross-priming after killing of CMV positive tumor cells. Larger studies are currently underway to determine the efficacy of CMV targeted immunotherapy for the treatment of GBM.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. Jama. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 3.Lawler SE. Cytomegalovirus and glioblastoma; controversies and opportunities. Journal of Neuro-Oncology. 2015;123:465–471. doi: 10.1007/s11060-015-1734-0. [DOI] [PubMed] [Google Scholar]
- 4.Streblow DN, Nelson JA. Models of HCMV latency and reactivation. Trends in Microbiology. 2003;11:293–295. [DOI] [PubMed] [Google Scholar]
- 5.Woroniecka K, Chongsathidkiet P, Rhodin KE, Kemeny HR, Dechant CA, Farber SH, Elsamadicy AA, Cui X, Koyama S, Jackson CC, et al. T cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2018. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohme M, Schliffke S, Maire CL, Runger A, Glau L, Mende KC, Matschke J, Gehbauer C, Akyuz N, Zapf S, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2018. doi: 10.1158/1078-0432.CCR-17-2617. [DOI] [PubMed] [Google Scholar]
- 7.Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet (London, England). 2002;360:1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- 8.Huang G, Yan Q, Wang Z, Chen X, Zhang X, Guo Y, Li JJ. Human cytomegalovirus in neoplastic cells of Epstein-Barr virus negative Hodgkin’s disease. International Journal of Oncology. 2002;21:31–36. [PubMed] [Google Scholar]
- 9.Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. The Journal of Urology. 2003;170:998–1002. doi: 10.1097/01.ju.0000080263.46164.97. [DOI] [PubMed] [Google Scholar]
- 10.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Research. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 11.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathologica. 2008;116:79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, Lira SA, Soderberg-Naucler C, Smit MJ. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Science Signaling. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, Sampson JH. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology. 2008;10:10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatier J, Uro-Coste E, Pommepuy I, Labrousse F, Allart S, Tremoulet M, Delisle MB, Brousset P. Detection of human cytomegalovirus genome and gene products in central nervous system tumours. British Journal of Cancer. 2005;92:747–750. doi: 10.1038/sj.bjc.6602339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poltermann S, Schlehofer B, Steindorf K, Schnitzler P, Geletneky K, Schlehofer JR. Lack of association of herpesviruses with brain tumors. Journal of Neurovirology. 2006;12:90–99. doi: 10.1080/13550280600654573. [DOI] [PubMed] [Google Scholar]
- 16.Priel E, Wohl A, Teperberg M, Nass D, Cohen ZR. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. Journal of Clinical Neuroscience : Official Journal of the Neurosurgical Society of Australasia. 2015;22:326–330. doi: 10.1016/j.jocn.2014.06.099. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Martinez A, Alenda C, Irles E, Ochoa E, Quintanar T, Rodriguez-Lescure A, Soto JL, Barbera VM. Lack of cytomegalovirus detection in human glioma. Virology Journal. 2017;14:216. doi: 10.1186/s12985-017-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang R, Qian D, Hu M, Zhang X, Song J, Li L, Chen H, Wang B. Association between human cytomegalovirus infection and histone acetylation level in various histological types of glioma. Oncology Letters. 2015;10:2812–2820. doi: 10.3892/ol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharjee B, Renzette N, Kowalik TF. Genetic analysis of cytomegalovirus in malignant gliomas. Journal of Virology. 2012;86:6815–6824. doi: 10.1128/JVI.00015-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic loci with glioblastoma multiforme samples. Journal of Virology. 2012;86:854–864. doi: 10.1128/JVI.06097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libard S, Popova SN, Amini RM, Karja V, Pietilainen T, Hamalainen KM, Sundstrom C, Hesselager G, Bergqvist M, Ekman S, et al. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PloS one. 2014;9:e108861. doi: 10.1371/journal.pone.0108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad AA, Rahbar A, Lui WO, Davoudi B, Catrina A, Stragliotto G, Mellbin L, Hamsten A, Ryden L, Yaiw KC, et al. Detection of circulating hcmv-miR-UL112-3p in patients with glioblastoma, rheumatoid arthritis, diabetes mellitus and healthy controls. PloS one. 2014;9:e113740. doi: 10.1371/journal.pone.0113740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, Soroceanu L, Cobbs CS. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology. 2012;14:246–255. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobbs CS, Soroceanu L, Denham S, Zhang W, Britt WJ, Pieper R, Kraus MH. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. Journal of Neuro-Oncology. 2007;85:271–280. doi: 10.1007/s11060-007-9423-2. [DOI] [PubMed] [Google Scholar]
- 25.Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity. Cancer Research. 2008;68:724–730. doi: 10.1158/0008-5472.CAN-07-2291. [DOI] [PubMed] [Google Scholar]
- 26.Cobbs C, Khan S, Matlaf L, McAllister S, Zider A, Yount G, Rahlin K, Harkins L, Bezrookove V, Singer E, et al. HCMV glycoprotein B is expressed in primary glioblastomas and enhances growth and invasiveness via PDGFR-alpha activation. Oncotarget. 2014;5:1091–1100. doi: 10.18632/oncotarget.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 28.Straat K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, Lou F, Liu Z, Shen J, Jia J, et al. Activation of telomerase by human cytomegalovirus. Journal of the National Cancer Institute. 2009;101:488–497. doi: 10.1093/jnci/djp031. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Jeon K, Kim JM, Kim VN, Choi DH, Kim SU, Kim S. Downregulation of GFAP, TSP-1, and p53 in human glioblastoma cell line, U373MG, by IE1 protein from human cytomegalovirus. Glia. 2005;51:1–12. doi: 10.1002/glia.20179. [DOI] [PubMed] [Google Scholar]
- 30.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America 2006; 103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, Soteropoulos P, Cobbs CS. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Research. 2011;71:6643–6653. doi: 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heukers R, Fan TS, de Wit RH, van Senten JR, De Groof TWM, Bebelman MP, Lagerweij T, Vieira J, de Munnik SM, Smits-de Vries L, et al. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene. 2018. doi: 10.1038/s41388-018-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Wit RH, Mujic-Delic A, van Senten JR, Fraile-Ramos A, Siderius M, Smit MJ. Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1alpha/PKM2 axis in glioblastoma cells. Oncotarget. 2016;7:67966–67985. doi: 10.18632/oncotarget.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Qian D, Hu M, Li L, Zhang L, Chen H, Yang R, Wang B. Human cytomegalovirus inhibits apoptosis by regulating the activating transcription factor 5 signaling pathway in human malignant glioma cells. Oncology Letters. 2014;8:1051–1057. doi: 10.3892/ol.2014.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soroceanu L, Matlaf L, Khan S, Akhavan A, Singer E, Bezrookove V, Decker S, Ghanny S, Hadaczek P, Bengtsson H, et al. Cytomegalovirus immediate-early proteins promote stemness properties in glioblastoma. Cancer Research. 2015;75:3065–3076. doi: 10.1158/0008-5472.CAN-14-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matlaf LA, Harkins LE, Bezrookove V, Cobbs CS, Soroceanu L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PloS one. 2013;8:e68176. doi: 10.1371/journal.pone.0068176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trgovcich J, Cebulla C, Zimmerman P, Sedmak DD. Human cytomegalovirus protein pp71 disrupts major histocompatibility complex class I cell surface expression. Journal of Virology. 2006;80:951–963. doi: 10.1128/JVI.80.2.951-963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornara O, Bartek J, Jr., Rahbar A, Odeberg J, Khan Z, Peredo I, Hamerlik P, Bartek J, Stragliotto G, Landazuri N, et al. Cytomegalovirus infection induces a stem cell phenotype in human primary glioblastoma cells: prognostic significance and biological impact. Cell Death and Differentiation. 2016;23:261–269. doi: 10.1038/cdd.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulasov IV, Kaverina NV, Ghosh D, Baryshnikova MA, Kadagidze ZG, Karseladze AI, Baryshnikov AY, Cobbs CS. CMV70-3P miRNA contributes to the CMV mediated glioma stemness and represents a target for glioma experimental therapy. Oncotarget. 2017;8:25989–25999. doi: 10.18632/oncotarget.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiallos E, Judkins J, Matlaf L, Prichard M, Dittmer D, Cobbs C, Soroceanu L. Human cytomegalovirus gene expression in long-term infected glioma stem cells. PloS one. 2014;9:e116178. doi: 10.1371/journal.pone.0116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dziurzynski K, Wei J, Qiao W, Hatiboglu MA, Kong LY, Wu A, Wang Y, Cahill D, Levine N, Prabhu S, et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2011;17:4642–4649. doi: 10.1158/1078-0432.CCR-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa H, Xu X, Overbeek G, Vasaikar S, Patro CP, Kostopoulou ON, Jung M, Shafi G, Ananthaseshan S, Tsipras G, et al. Human cytomegalovirus may promote tumour progression by upregulating arginase-2. Oncotarget. 2016;7:47221–47231. doi: 10.18632/oncotarget.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Zhao P, Qian D, Hu M, Zhang L, Shi H, Wang B. MicroRNA-613 is downregulated in HCMV-positive glioblastoma and inhibits tumour progression by targeting arginase-2. Tumour Biology : the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39:1010428317712512. doi: 10.1177/1010428317712512. [DOI] [PubMed] [Google Scholar]
- 44.Rahbar A, Stragliotto G, Orrego A, Peredo I, Taher C, Willems J, Soderberg-Naucler C. Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae. 2012;3:3. doi: 10.1186/2042-4280-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahbar A, Orrego A, Peredo I, Dzabic M, Wolmer-Solberg N, Straat K, Stragliotto G, Soderberg-Naucler C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. Journal of Clinical Virology : the Official Publication of the Pan American Society for Clinical Virology. 2013;57:36–42. doi: 10.1016/j.jcv.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Hadaczek P, Ozawa T, Soroceanu L, Yoshida Y, Matlaf L, Singer E, Fiallos E, James CD, Cobbs CS. Cidofovir: a novel antitumor agent for glioblastoma. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2013;19:6473–6483. doi: 10.1158/1078-0432.CCR-13-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stragliotto G, Rahbar A, Solberg NW, Lilja A, Taher C, Orrego A, Bjurman B, Tammik C, Skarman P, Peredo I, et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. International Journal of Cancer. 2013;133:1204–1213. doi: 10.1002/ijc.28111. [DOI] [PubMed] [Google Scholar]
- 48.Soderberg-Naucler C, Peredo I, Rahbar A, Hansson F, Nordlund A, Stragliotto G. Use of Cox regression with treatment status as a time-dependent covariate to re-analyze survival benefit excludes immortal time bias effect in patients with glioblastoma who received prolonged adjuvant treatment with valganciclovir. International Journal of Cancer. 2014;135:248–249. doi: 10.1002/ijc.28663. [DOI] [PubMed] [Google Scholar]
- 49.Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. The New England Journal of Medicine. 2013;369:985–986. doi: 10.1056/NEJMc1302145. [DOI] [PubMed] [Google Scholar]
- 50.Peng C, Wang J, Tanksley JP, Mobley BC, Ayers GD, Moots PL, Clark SW. Valganciclovir and bevacizumab for recurrent glioblastoma: A single-institution experience. Molecular and Clinical Oncology. 2016;4:154–158. doi: 10.3892/mco.2015.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas KG, Bao L, Bruggeman R, Dunham K, Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. Journal of Neuro-Oncology. 2011;103:231–238. doi: 10.1007/s11060-010-0383-6. [DOI] [PubMed] [Google Scholar]
- 52.Crough T, Beagley L, Smith C, Jones L, Walker DG, Khanna R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunology and Cell Biology. 2012;90:872–880. doi: 10.1038/icb.2012.19. [DOI] [PubMed] [Google Scholar]
- 53.Nair SK, De Leon G, Boczkowski D, Schmittling R, Xie W, Staats J, Liu R, Johnson LA, Weinhold K, Archer GE, et al. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2014. doi: 10.1158/1078-0432.CCR-13-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuessler A, Smith C, Beagley L, Boyle GM, Rehan S, Matthews K, Jones L, Crough T, Dasari V, Klein K, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Research. 2014;74:3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 55.Ghazi A, Ashoori A, Hanley PJ, Brawley VS, Shaffer DR, Kew Y, Powell SZ, Grossman R, Grada Z, Scheurer ME, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. Journal of Immunotherapy (Hagerstown, Md : 1997). 2012;35:159–168. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dasari V, Smith C, Schuessler A, Zhong J, Khanna R. Induction of innate immune signatures following polyepitope protein-glycoprotein B-TLR4&9 agonist immunization generates multifunctional CMV-specific cellular and humoral immunity. Human vaccines & immunotherapeutics. 2014;10:1064–1077. doi: 10.4161/hv.27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, Norberg PK, Herndon JE, 2nd, Healy P, Congdon KL, et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Research. 2018;78:256–264. doi: 10.1158/0008-5472.CAN-17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, Norberg PK, Herndon JE, 2nd, Healy P, Congdon KL, et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (New York, NY). 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118:3003–3012. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Perez L, Choi BD, Reap EA, Sayour EJ, Norberg P, Schmittling RJ, Archer GE, Herndon JE, 2nd, Mitchell DA, Heimberger AB, et al. BLyS levels correlate with vaccine-induced antibody titers in patients with glioblastoma lymphodepleted by therapeutic temozolomide. Cancer Immunology, Immunotherapy : CII. 2013;62:983–987. doi: 10.1007/s00262-013-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prins RM, Cloughesy TF, Liau LM. Induction of cytomegalovirus-specific anti-tumor immunity after autologous tumor lysate-pulsed dendritic cell vaccination in a patient with glioblastoma. The New England Journal of Medicine. 2008;359:539–541. doi: 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma H-L, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-γ. The Journal of Immunology. 2003;171:608–615. [DOI] [PubMed] [Google Scholar]
- 66.Dhanji S, Teh H-S. IL-2-activated CD8+CD44high cells express both adaptive and innate immune system receptors and demonstrate specificity for syngeneic tumor cells. The Journal of Immunology. 2003;171:3442–3450. [DOI] [PubMed] [Google Scholar]
- 67.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science (New York, NY). 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 68.Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paijo J, Doring M, Spanier J, Grabski E, Nooruzzaman M, Schmidt T, Witte G, Messerle M, Hornung V, Kaever V, et al. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells. PLoS Pathogens. 2016;12:e1005546. doi: 10.1371/journal.ppat.1005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pepin G, Gantier MP. cGAS-STING activation in the tumor microenvironment and its role in cancer immunity. Advances in Experimental Medicine and Biology. 2017;1024:175–194. doi: 10.1007/978-981-10-5987-2_8. [DOI] [PubMed] [Google Scholar]
- 71.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science (New York, NY). 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]


