ABSTRACT
We have recently demonstrated that intratumoral CpG-B vaccination enhances anti-tumor immunity and tumor regression in mice. We further show that the local delivery of TLR9 agonists converts the tolerogenic tumor microenvironment into an immunopermissive one, which may benefit current immunotherapeutic anticancer strategies by enhancing innate and adaptive tumor-associated immune cell responses.
Keywords: tumor microenvironment, local immunotherapy, CpG-B
Treatments for malignancies, including immunotherapies, are generally provided systemically, therefore frequently associated with severe toxic effects. Locally administered immunotherapy – topical, intradermal, intranodal or intratumoral – is nowadays considered as a promising approach. It may actually solve several aspects limiting immunotherapeutic strategies: local treatments necessitate lower doses to reach the required concentration, leading to reduced systemic drug levels, therefore limiting the risks of autoimmune adverse effects while enhancing the robustness of the anti-tumor immune response.1
The aim of immunotherapy is to induce a vigorous immune response against tumor antigens. The tumor microenvironment (TME) however negatively impacts the immune system through three main mechanisms, namely immunosuppression, immunoevasion and immunoediting.2 These mechanisms involve cancer and mesenchymal cells, as well as immune cells, such as myeloid-derived suppressor cells, regulatory T cells (Tregs), tumor-associated (TA-) macrophages and TA-neutrophils, which produce anti-inflammatory cytokines.2 In this regard, local immunotherapy aims at overcoming the inhibitory properties of the TME in order to “warm up” the tumor, enhance its immunogenicity and generate effector immune cells.
One conceivable local immunotherapeutic approach is the administration of TLR ligand agonists, in order to reactivate immune cells at the injection site. In many types of human cancers, the recruitment of plasmacytoid dendritic cells (pDCs) to the TME contributes to the induction of immune tolerance. Indeed, pDCs infiltrating tumors are characterized by reduced expression of costimulatory molecules, a decreased ability to produce type I IFN (IFN-I), as well as an enhanced ability to induce Tregs.3 We have previously shown that, following contralateral vaccination of mice with CpG oligonucleotides-B (CpG-B), a TLR9 agonist, together with a tumor antigen, pDCs can be activated at a site distal from the tumor and used as immunogenic antigen-presenting cells (APCs) that promote tumor-specific Th17 cells. Immune cells are further recruited to the tumor, and cytotoxic T cells (CTL) induce tumor cell death.4 Therefore, pDCs represent good candidates to boost the antitumor immune response. However, whether the tolerogenic phenotype of TA-pDCs can be reverted by intratumoral administration of TLR9 ligands, and how the TME is impacted by this approach, is still unclear.
We recently explored how intratumoral delivery of CpG-B alone or together with a tumor antigen affects tumor-infiltrating leukocytes in mice having developed solid tumors.5 Using melanoma or thymoma tumors, we observed a significant reduction of the tumor growth following intratumoral CpG-B administration. However, we did not observe any tolerogenic-to-immunogenic conversion of TA-pDC phenotype after treatment, and the genetic depletion of pDCs did not affect the efficacy of the treatment. Therefore, in our model, TA-pDCs are refractory to TLR9 stimulation and are not involved in the mechanism of action leading to tumor regression. In contrast, upon intratumoral CpG-B delivery, some changes occur in the TME, inducing an increased production of neutrophil attractants, by either tumor cells or tumor-infiltrating cells. As a consequence, neutrophils are rapidly recruited into the tumor following local CpG-B injection, and promote the activation of conventional DCs (cDCs) (Figure 1). The role of tumor-associated neutrophils is debated in the literature; these cells can have either pro- or anti-tumoral properties depending on different parameters, such as the type or the stage of the tumor.6
Figure 1.
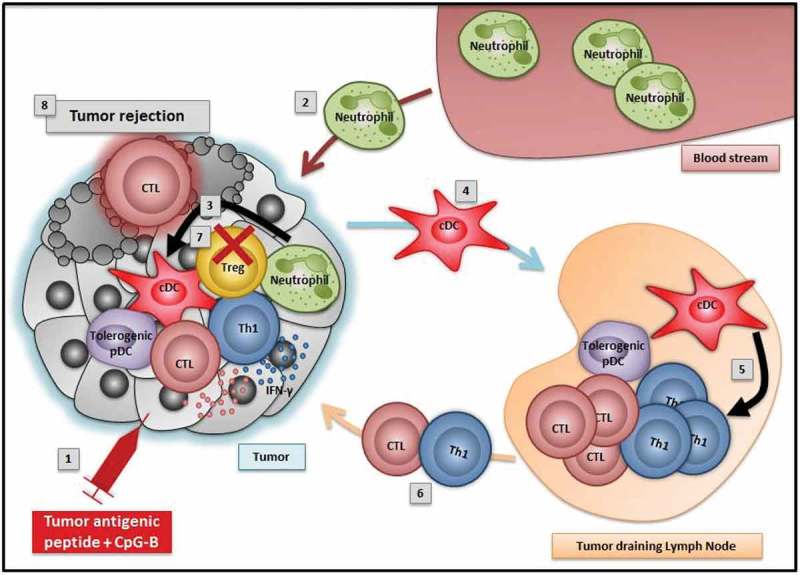
Intratumoral delivery of tumor antigenic peptide and CpG-B induces tumor rejection: working model.
1) Intratumoral injection of tumor antigenic peptide and CpG-B2) Activated neutrophils are recruited into the tumor3) Neutrophils promote the activation of conventional dendritic cells (cDCs)4) Activated cDCs migrate to the tumor-draining lymph nodes (TdLNs)5) Activated cDCs prime cytotoxic T cells (CTLs) and Th1 cells in the TdLNs6) Effector Th1 and CTLs migrate to the tumor and secrete IFN-γ7) Treg numbers are decreased in the tumor8) Tumor cells are killed
Following intratumoral CpG-B injection, activated cDCs migrate from the tumor bed to the draining lymph nodes to activate tumor-specific cytotoxic T lymphocytes (CTLs) and Th1 cells (Figure 1). Effector cells subsequently infiltrate solid tumors, which also contain reduced numbers of Tregs. Therefore, the balance effector T cells/Treg is modulated in favour of effector T cells following local immunotherapy and results in tumor cell elimination and tumor regression (Figure 1). Importantly, all these events are dependent on the primary recruitment of neutrophils after CpG-B administration, since both cDC activation and tumor-specific T cell priming are abrogated upon neutrophil depletion.5
Interestingly, the efficacy of CpG-B immunotherapy was potentialized by the addition of a tumor antigen, suggesting that when an immunogenic tumor antigen is available for presentation, in situ TLR9 vaccination may induce the conversion of dysfunctional into immunogenic antigen-presenting cells and generate efficient antitumor T-cell responses.
Local TLR9 delivery did not induce a robust systemic antitumoral immune response in our models, with a rather modest inhibition of contralateral tumor growth. This is consistent with a recent study showing that intratumoral CpG-C does not impact the growth of distal tumors, unless it is combined with anti-OX40 agonistic antibodies.7 In human, systemic tumor-specific CD8+ T cell responses have been observed in tumor patients treated with CpG-B combined to low dose radiotherapy.8 Therefore, preclinical and early clinical studies propose that intratumoral therapies (in situ vaccination or local radiation), could convert “cold” TME into “hot” TME, thus enhancing the potential for a response after its combination with systemic immunotherapies, such as immune checkpoint inhibitors. For instance, sialic acid sugars, which promote an immunosuppressive TME, can be blocked to induce an immune-permissive TME, suitable for combination with immune-based interventions.9
Some limitations, such as tumor accessibility or the need for an optimization of the delivery methods (route, dose, volume), currently restrain the development of localized immunotherapeutic approaches. However, in situ vaccination strategies are achievable and can induce an immune response and tumor regression while reducing systemic toxic events. Recently, in melanoma patients, a single local injection of low-dose CpG-B, at the tumor excision site resulted in lymph node cDC activation and protection against relapse.10 Future work deciphering the mechanisms involved in the protection observed after intratumoral CpG-B treatment will improve anticancer therapies, for example by combining different approaches such as checkpoint inhibitors or techniques inducing the release of tumor antigens.
Funding Statement
This work was supported by the European Research Council [281365];Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung [PP00P3_152951]; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung [310030_166541].
References
- 1.Fransen MF, Ossendorp F, Arens R, Melief CJ.. Local immunomodulation for cancer therapy: providing treatment where needed. Oncoimmunology. 2013;2:e26493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Reviews Immunol. 2006;6:715–727. [DOI] [PubMed] [Google Scholar]
- 3.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629–4640. [DOI] [PubMed] [Google Scholar]
- 4.Guery L, Dubrot J, Lippens C, Brighouse D, Malinge P, Irla M, Pot C, Reith W, Waldburger J-M, Hugues S. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74:6430–6440. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Guery L, Brighouse D, Lemeille S, Hugues S. Intratumoral CpG-B promotes antitumoral neutrophil, cDC, and T-cell cooperation without reprograming tolerogenic pDC. Cancer Res. 2018;78:3280–3292. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46:15–28. [DOI] [PubMed] [Google Scholar]
- 7.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy, R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10(426). eaan4488. doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe R T, Knox SJ, Shin LK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 2010;28:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull C, Boltje TJ, Balneger N, Weischer SM, Wassink M, van Gemst JJ, Bloemendal VR, Boon L, van der Vlag J, Heise T, et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. Cancer Res. 2018;78:3574–3588. [DOI] [PubMed] [Google Scholar]
- 10.Koster BD, van den Hout M, Sluijter BJR, Molenkamp BG, Vuylsteke R, Baars A, van Leeuwen Paul A M, Scheper RJ, Petrousjka van den Tol M, van den Eertwegh Alfons JM, et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I-II Melanoma: data from two randomized phase II trials. Clin Cancer Res. 2017;23:5679–5686. [DOI] [PubMed] [Google Scholar]


