ABSTRACT
Pediatric and adult patients with recurrent/refractory Burkitt lymphoma (BL) continue to have poor outcomes, emphasizing the need for newer therapeutic agents. Bruton’s tyrosine kinase (BTK) is activated following B-cell receptor stimulation and in part regulates normal B-cell development. Ibrutinib, a selective and irreversible BTK inhibitor, has been efficacious in chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), Waldenström’s macroglobulinemia, and marginal zone lymphoma. In this study, we investigated the efficacy of ibrutinib alone and in selective adjuvant combinations against BL in-vitro and in a human BL xenografted immune-deficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mouse model. Our data demonstrated that phospho-BTK level was significantly reduced in BL cells treated with ibrutinib (p < 0.001). Moreover, we observed a significant decrease in cell proliferation as well as significant decrease in IC50 of ibrutinib in combination with dexamethasone, rituximab, obinutuzumab, carfilzomib, and doxorubicin (p < 0.001). In-vivo studies demonstrated ibrutinib treated mice had a significantly prolonged survival with median survival of mice following ibrutinib treatment (32 days) (24 days) (p < 0.02). In conclusion, our findings demonstrate the significant in-vitro and preclinical in-vivo effects of ibrutinib in BL. Based on our preclinical results in this investigation, there is an on-going clinical trial comparing overall survival in children and adolescents with relapsed/refractory BL treated with chemoimmunotherapy with or without ibrutinib (NCT02703272).
KEYWORDS: Ibrutinib, Bruton’s Tyrosine Kinase, Burkitt Lymphoma, Xenografted NSG Mice, Cell Proliferation
Introduction
Burkitt lymphoma (BL) represents the most common subtype of childhood and adolescent non- Hodgkin lymphoma (NHL) (40%) but an incidence of less than 5% of adults with NHL.1–3 We and others have demonstrated that the prognosis of pediatric BL (PBL) has significantly improved over the last 40 years through the use of short and intense multi-agent chemotherapy and immunotherapy with a current 90% 5-year event-free survival (EFS).2,4–9 Specifically, we previously demonstrated 90% 5-year overall survival (OS) following treatment with a short, intensive course of chemotherapy in a cooperative international childhood B- cell NHL (B-NHL) trial (French-American-British/mature lymphoma B [FAB/LMB] 96) involving the Children’s Cancer Group (CCG), French Pediatric Oncology Group (SFOP) and United Kingdom Children’s Cancer Study Group (UKCCSG).5 However, children and adolescents with recurrent/refractory PBL have a significantly decreased and dismal prognosis and, moreover, current upfront chemotherapy protocols are associated with serious acute and long-term morbidities.5,8 It is therefore critical to investigate and to develop targeted translational therapeutic strategies in PBL with the goal of reducing acute morbidities, decreasing late effects in newly diagnosed patients, and/or providing new therapeutic options for those with chemo-immunotherapy resistant relapsed/refractory disease.
Bruton’s tyrosine kinase (BTK) is a regulator of normal B-cell development and is activated upon B-cell receptor (BCR) stimulation. Activation of the BCR signaling pathway has now emerged as a central oncogenic pathway that promotes growth and survival in both normal and malignant B-cells. Antigenic activation of the dimeric membrane immunoglobulin B-cell receptor, which induces phosphorylation of BTK and phospholipase Cγ2 (PLCγ2), results in the activation of a number of signaling pathways including mitogen-activated protein kinase (MAPK), nuclear factor (NF)-kappa B (κB) and AKT (protein kinase B).10 Chronic active BCR signaling through BTK activation and downstream pathways including the NF-kB pathways can be inhibited by the selective and covalent BTK inhibitor, ibrutinib.11,12 Ibrutinib is a selective and irreversible small molecule inhibitor of BTK and covalently binds to cysteine residue 481 on BTK, thereby inhibiting the autophosphorylation of tyrosine 223 on exon 8 resulting in irreversible inhibition of BTK enzymatic activity.13 Ibrutinib has been demonstrated to be an active agent in activated B cell-like diffuse large B-cell lymphoma (ABC-DLBCL), an NHL subtype that is characterized by constitutively activated NF-κB signaling.14
Preclinical studies of ibrutinib in chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) suggested that ibrutinib inhibits cell proliferation in-vitro in the range of 1.0 μM to 25.0 μM.15,16 Despite high ibrutinib half maximal inhibitory concentration (IC50) values in preclinical studies of CLL and MCL, ibrutinib has been highly effective in the treatment of refractory and relapsed adult patients with CLL and MCL. A recent study demonstrated that the combination of ibrutinib and B-cell lymphoma-2 (BCL2) inhibition using venetoclax was highly effective in patients with MCL.17 Ibrutinib was originally approved by the FDA in adult patients with relapsed/refractory CLL or MCL who have received at least one prior therapy (USPI)18,19 and now is approved in all lines of therapy in CLL. BL, however, is associated with tonic or possibly chronic active BCR signaling while both CLL and MCL have chronic active BCR signaling.12 Most recently, we demonstrated by genomic expression profiling a significant overexpression of BTK (9 fold) in patients with sporadic form BL treated on the Children’s Oncology Group (COG) protocol 5961.20 Bouska et al recently demonstrated that adult BL shares commonly mutated genes in the chronic BCR/BTK/NF-kB signaling pathway, which could be targeted by ibrutinib.21
Dexamethasone is often administered in conjunction with rituximab to enhance rituximab-mediated cytotoxicity.22 Carfilzomib is a second-generation proteasome inhibitor.23 It was identified as a significantly cytotoxic agent against CLL cells isolated from ibrutinib- treated patients, suggesting that carfilzomib can potentially complement ibrutinib’s anti-tumor activity.24 Idelalisib is a potent, selective small-molecule inhibitor of phosphoinositide 3-kinase delta (PI3Kδ).25 Since BTK and PI3K differentially regulate BCR signaling,26 the combination of ibrutinib and idelalisib may synergistically target BCR positive tumor cells such as CLL and MCL and other B-cell lymphomas.27 Doxorubicin has been widely used as a chemotherapeutic agent in BL to induce tumor cell death by intercalation into DNA and disruption of topoisomerase-II-mediated deoxyribonucleic acid (DNA) repair or generation of free radicals and their damage to cellular membranes, DNA and proteins.5,28 The results from an early phase 1 trial indicate that the combination of ibrutinib with the first-line therapy rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) potentially improve response rates in adults with B-NHL.29 The antitumor activity of ibrutinib alone and more importantly in combination with these regimens against BL is currently unknown. We hypothesized that ibrutinib would be an efficacious small molecule inhibitor alone and/or in selective combination with other active therapies in BL and could potentially be utilized in the future treatment of BL. Here, we investigated the in-vitro and in-vivo efficacy of ibrutinib in human BL cell xenografted immune-deficient mouse NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mouse model.
Results
Ibrutinib inhibits the expression of p-BTK protein in BL cells
We first demonstrated that the expression of total BTK expression was similar in Raji and Ramos BL cell lines following ibrutinib treatment with varying doses (0, 0.1, 0.2, 0.5, 1.0, 5.0, and 10 μM) for five days (Figure 1A and B), respectively. The medium was refreshed daily with ibrutinib. In both Raji and Ramos BL cell lines, p-BTK at Tyr 223 was significantly decreased following exposure to ibrutinib at all doses (Figure 1A and B) (p < 0.005, p < 0.0005, p < 0.00001), respectively.
Figure 1.
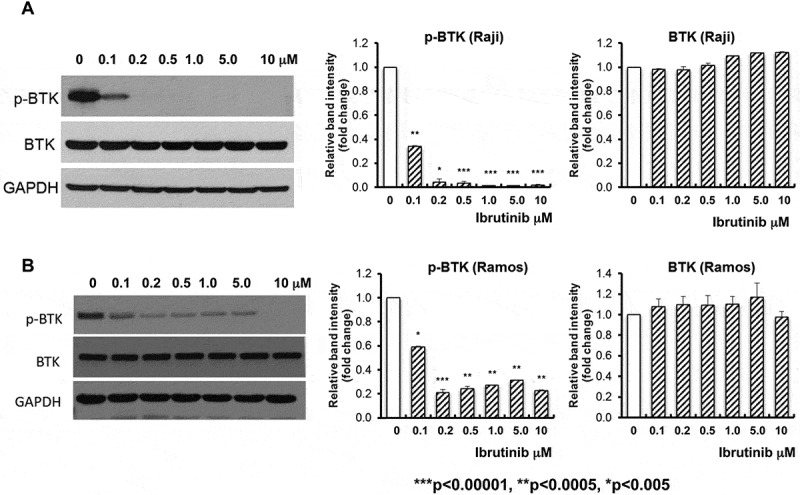
Significant inhibition of BTK phosphorylation in ibrutinib treated BL cell lines. Raji (A) and Ramos (B) BL cell lines were treated with ibrutinib at varying doses (0, 0.1, 0.2, 0.5, 1.0, 5.0, and 10 μM) for five days. Ibrutinib was dissolved in DMSO. DMSO (ibrutinib dose at 0) was used as control. The total levels of BTK protein and phosphorylated BTK protein (p-BTK) was examined by western blot analysis with specific antibody against BTK and phospho-BTK (Tyr223). GAPDH was used as loading control. Representative Western blot results are shown in the left panels of (A) and (B). Intensities of immunoreactive phospho-BTK (Tyr223) bands on Western blots shown in (A) were quantified by densitometric analysis as shown in the middle panels of (A) and (B). Intensities of immunoreactive BTK bands on Western blots shown in (A) were quantified by densitometric analysis as shown in the right panels of (A) and (B). n = 3, ***p < 0.00001, **p < 0.0005, *p < 0.005.
Ibrutinib inhibits cell proliferation in BL cells
Next, we demonstrated that in comparison to control dimethyl sulfoxide (DMSO) (1.000 ± 0.000), 5 days of ibrutinib treatment significantly inhibits proliferation of Raji cells at 5.0 μM (0.561 ± 0.170, p = 0.023) and 10 μM (0.409 ± 0.115, p = 0.044). The IC50 for inhibition of Raji cell proliferation was 5.20 μM (Figure 2A). There was a significant inhibition of cell proliferation in ibrutinib-treated Ramos vs DMSO-treated Ramos (1.000 ± 0.000) with five days of ibrutinib treatment course at 0.25 μM (0.626 ± 0.015, p = 0.0002), 0.5 μM (0.511 ± 0.131, p = 0.011), 1.0 μM (0.442 ± 0.091, p = 0.004), 5.0 μM (0.248 ± 0.056, p = 0.0009) and 10.0 μM (0.109 ± 0.018, p = 0.004). The ibrutinib IC50 for inhibition of Ramos cell proliferation was 0.868 μM (Figure 2B) (p < 0.05).
Figure 2.
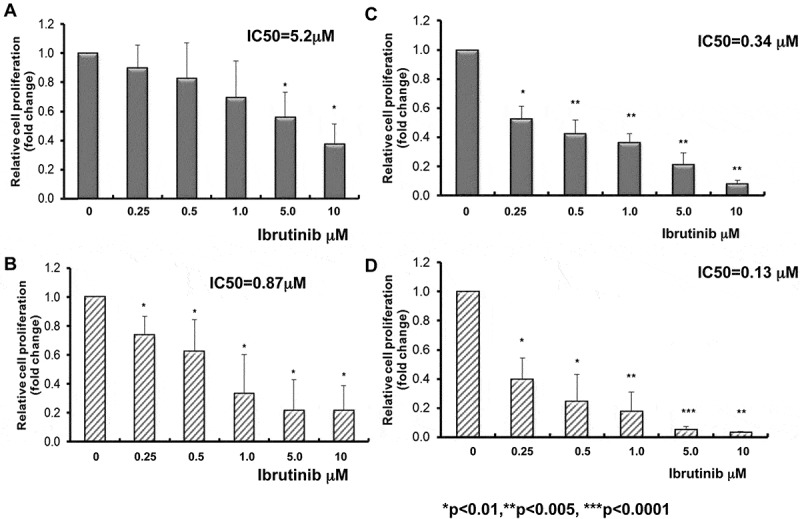
Inhibition of BL cell proliferation by ibrutinib alone or in combination with dexamethasone. Raji (A) and Ramos (B) BL cell lines were treated with ibrutinib at varying doses (0, 0.1, 0.2, 0.5, 1.0, 5.0, and 10 μM) for five days. Ibrutinib was dissolved in DMSO. DMSO (ibrutinib dose at 0) was used as control. Cell proliferation was examined by CellTiter 96® Aqueous One solution cell proliferation assays. Fold change in cell proliferation was calculated by the equation: treated A490 reading/DMSO A490 reading. DMSO A490 reading was set as 1. Significant inhibition of cell proliferation was observed in ibrutinib-treated Raji (A) and Ramos (B) BL cell lines following 5 days treatment. Raji (n = 3) IC50 = 5.2μM, Ramos (n = 3) IC50 = 0.87μM. *p < 0.05.Raji (C) and Ramos (D) BL cell lines were treated with ibrutinib at varying doses (0, 0.25, 0.5, 1.0, 5.0, and 10 μM) in combination with 1μM dexamethasone for five days. Both Ibrutinib and dexamethasone were dissolved in DMSO. DMSO (ibrutinib dose at 0 without dexamethasone) was used as control. Cell proliferation was examined by CellTiter 96® Aqueous One solution cell proliferation assays. Cell proliferation fold change was calculated by the equation: treated A490/DMSO A490. DMSO A490 was set as 1. IC50 was determined with CompuSyn software. Raji (C) and Ramos (D) cell proliferations were significantly decreased with the combination of ibrutinib and dexamethasone. IC50 of ibrutinib and dexamethasone against Raji is 0.34 μM compared to ibrutinib alone 5.2μM (n = 3, p < 0.01) and IC50 of ibrutinib and dexamethasone against Ramos is 0.13 μM compared to ibrutinib alone 0.87μM (n = 3, p < 0.01). *p < 0.01, **p < 0.005, ***p < 0.0001.
The efficacy of ibrutinib in combination with dexamethasone, rituximab and obinutuzumab against BL
We investigated the efficacy of ibrutinib in combination with dexamethasone in Raji and Ramos BL cells. Dexamethasone (1μM) was broadly used in-vitro alone or in combination with other drugs to inhibit proliferation or induce apoptosis in lymphoid malignancies.30 Different doses of ibrutinib and 1μM dexamethasone were co-cultured with Raji or Ramos for five days. The medium was refreshed daily with ibrutinib+ dexamethasone. Interestingly, we observed a significant decrease of cell proliferation with the combination of ibrutinib and 1μM dexamethasone in both Raji and Ramos cells and a significant reduction of IC50 compared to ibrutinib alone (Raji IC50: ibrutinib alone 5.2μM vs ibrutinib + dexamethasone 0.34 μM, p < 0.01 and Ramos IC50: ibrutinib alone 0.87μM vs ibrutinib + dexamethasone 0.13 μM, p < 0.01, respectively) (Figure 2C and 2D). To determine if anti-CD20 antibodies are additive to ibrutinib-mediated growth inhibition, next, we extended our investigation to determine the efficacy of ibrutinib in BL cells with anti-CD20 antibodies rituximab or obinutuzumab. We observed a significant decrease in cell proliferation following ibrutinib and rituximab (20ug/ml) or obinutuzumab (20 ug/ml) combination-treated Raji vs ibrutinib alone-treated Raji following ibrutinib at 5 days of treatment (Figure 3A). The ibrutinib IC50 values for inhibition of cell proliferation in Raji were significantly decreased from 4.55 ± 4.038 μM (ibrutinib alone) to 0.67 ± 0.364 μM with 20 ug/ml rituximab (p < 0.0005) and 0.16 ± 0.095 μM with 20 ug/ml obinutuzumab (p < 0.00005) combination following 5 days of ibrutinib treatment. In addition, we observed a significant decrease in cell proliferation following ibrutinib (0.2μM) and rituximab (20 μg/ml) or obinutuzumab (20 ug/ml) combination-treated Ramos vs ibrutinib alone-treated Ramos following ibrutinib at 5 days of treatment. The ibrutinib IC50 values for inhibition of cell proliferation in Ramos were significantly decreased from 1.41 ± 1.372 μM (ibrutinib alone) to 0.16 ± 0.174 μM with 20 μg/ml rituximab (p < 0.0001) and 0.01 ± 0.005 μM with 20 ug/ml obinutuzumab (p < 0.001) (Figure 3B). Our results suggest in part that by combining with rituximab or obinutuzumab, we may reduce the dose of ibrutinib on BL to limit the potential toxicity of ibrutinib.
Figure 3.
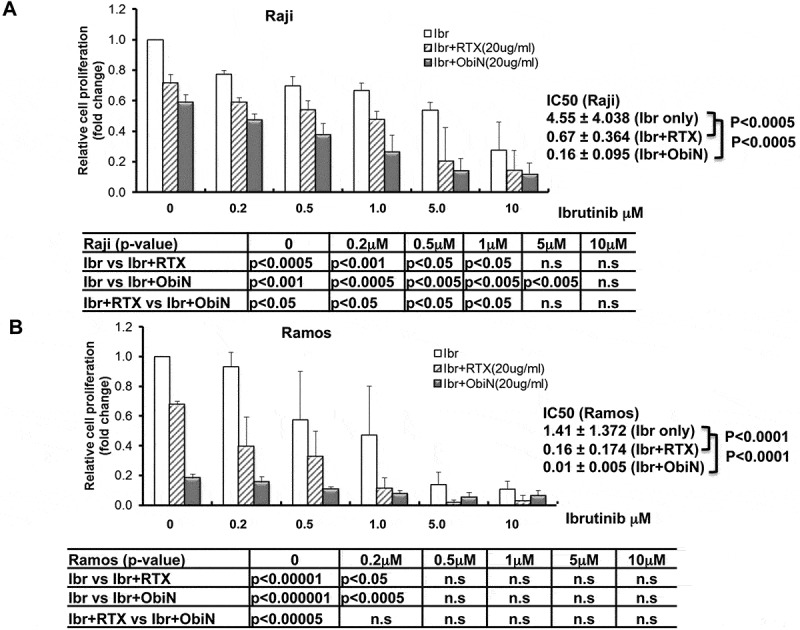
Inhibition of BL cell proliferation by ibrutinib in combination with rituximab or with obinutuzumab. Raji (A) and Ramos (B) BL cell lines were treated with ibrutinib (Ibr) with varying doses (0, 0.25, 0.5, 1.0, 5.0, and 10 μM) in combination with rituximab (RTX) (20ug/ml) or obinutuzumab (ObiN) (20ug/ml) for five days. Ibrutinib was dissolved in DMSO. DMSO (ibrutinib dose at 0 without rituximab or obinutuzumab) was used as control. Cell proliferation was examined by CellTiter 96® Aqueous One solution cell proliferation assays (MTS). Cell proliferation fold change was calculated by the equation: treated A490 reading/DMSO A490. DMSO A490 was set as 1. IC50 was determined with CompuSyn software. The second and third grey bars with ibrutinib dose at 0 represent the cells treated with 20ug/ml rituximab or 20ug/ml obinutuzumab alone. Raji (A) and Ramos (B) cell proliferations were significantly decreased with the combination of Ibr and RTX or Ibr and ObiN. IC50 of Ibr and RTX against Raji is 0.67 μM compared to Ibr alone 4.55μM (n = 3, p < 0.0005) (A) and IC50 of Ibr and RTX against Ramos is 0.16 μM compared to Ibr alone 1.41μM (n = 3, p < 0.0001) (B). IC50 of Ibr and ObiN against Raji is 0.16 μM compared to Ibr alone 4.55μM (n = 3, p < 0.00005) (A) and IC50 of Ibr and ObiN against Ramos is 0.01 μM compared to Ibr alone 1.41μM (n = 3, p < 0.001) (B).
The efficacy of ibrutinib alone and in combination with carfilzomib, idelalisib, and doxorubicin on cell proliferation in Raji BL cells
Carfilzomib, idelalisib, and doxorubicin have been previously demonstrated to have significant activity against a variety of histological subtypes of B-NHL. With fixed doses of each single agent, we also investigated the efficacy of different doses of ibrutinib and in combination with fixed doses of carfilzomib, idelalisib, or doxorubicin with prolonged exposure for five days in Raji BL cells. The medium was refreshed daily with ibrutinib and in combination with carfilzomib, idelalisib, or doxorubicin. We observed a significant decrease in cell proliferation following ibrutinib and carfilzomib (2 nM and 5 nM) combination-treated Raji vs ibrutinib alone-treated Raji following 5 days of treatment. The ibrutinib IC50 values for inhibition of cell proliferation in Raji were significantly decreased from 5.83 ± 1.028 μM (ibrutinib alone) to 0.28 ± 0.085 μM (2 nM CFZ, p < 0.01) and 0.22 ± 0.04 μM (5 nM CFZ, p < 0.03) combination following 5 days of ibrutinib treatment (Figure 4A). Previously, it was reported that in-vitro exposure of BL cell lines to idelalisib in concentrations from 0.1-100µM resulted in a dose and time-dependent decrease in viable cells.31 We therefore investigated the efficacy of ibrutinib with a fixed dose of idelalisib at 10 μM. Raji cells were treated with different doses of ibrutinib with 10 μM idelalisib for 5 days. There was a significant decrease in cell proliferation following ibrutinib-treated Raji vs ibrutinib and idelalisib (10 μM) combination-treated Raji compared to DMSO-treated Raji (1.000 ± 0.000) with 0.2 μM (0.94 ± 0.036 vs 0.743 ± 0.025, p > 0.05), 0.5 μM (0.89 ± 0.019 vs 0.736 ± 0.032, p < 0.01), 1.0 μM (0.850 ± 0.013 vs 0.707 ± 0.016, p < 0.005), 5.0 μM (0.742 ± 0.015 vs 0.626 ± 0.023, p < 0.005) and 10 μM (0.468 ± 0.007 vs 0.424 ± 0.027, p < 0.05). The ibrutinib IC50 values for inhibition of cell proliferation in Raji were decreased from 13.809 ± 2.843 μM (ibrutinib alone) to 10.04 ± 1.696 μM (10 μM idelalisib) with this combination following 5 days of ibrutinib treatment, however, the differences were not statistically significant (p = 0.076) (Figure 4B).
Figure 4.
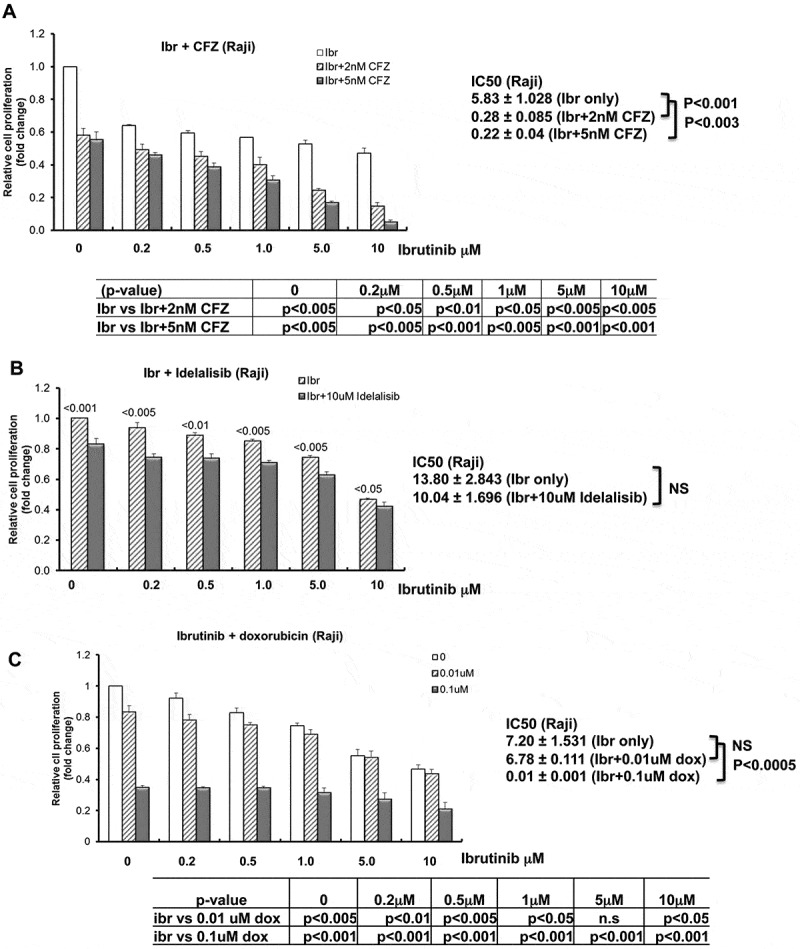
Inhibition of BL cell proliferation by ibrutinib in combination with carfilzomib, idelalisib or doxorubicin. Raji cells were treated with ibrutinib (Ibr) with varying doses (0, 0.25, 0.5, 1.0, 5.0, and 10 μM) in combination with carfilzomib (CFZ) (A), idelalisib (B), or doxorubicin (dox) (C) for five days. The doses of CFZ, idelalisib, and dox were indicated in the figures. Ibrutinib was dissolved in DMSO. DMSO (ibrutinib dose at 0 without any other drug) was used as control. The second and the third grey bars in A with ibrutinib dose at 0 represent the cells treated with carfilzomib alone. The second grey bar in B with ibrutinib dose at 0 represents the cells treated with idelalisib alone. The second and the third grey bars in C with ibrutinib dose at 0 represent the cells treated with doxorubicin alone. Cell proliferation was examined by CellTiter 96® Aqueous One solution cell proliferation assays. Cell proliferation fold change was calculated by the equation: treated A490 reading/DMSO A490 reading. DMSO A490 reading was set as 1. IC50 was determined with CompuSyn software.
Next, we also examined the efficacy (IC50) of ibrutinib alone and in combination with doxorubicin. We observed a significant decrease in cell proliferation following ibrutinib and doxorubicin (0.01 μM and 0.1 μM) combination-treated Raji vs ibrutinib alone-treated Raji following 5 days of treatment. The ibrutinib IC50 values for inhibition of cell proliferation in Raji were decreased from 7.20 ± 1.531 μM (ibrutinib alone) to 6.78 ± 1.730 μM (0.01 μM doxorubicin, p = 0.1) and significantly decreased to 0.01 ± 0.009 μM (0.1 μM doxorubicin, p < 0.005) in combination following 5 days of ibrutinib treatment (Figure 4C).
The dose-dependent efficacy of ibrutinib alone and in combination on tumor progression and survival in Raji BL xenografted NSG mice
We first compared the efficacy of ibrutinib in a dose dependent manner on tumor progression and survival. Ibrutinib (12.5 mg/kg) treated mice had a significantly prolonged survival compared to phosphate-buffered saline (PBS) control mice (p < 0.02) with median survival of mice following ibrutinib treatment (32 days) compared to PBS control (24 days) (Figure 5A). The treatment experimental schema is shown in Figure 5B. Furthermore, we observed a significant decrease of tumor progression by measuring tumor luminescence signal intensity following ibrutinib treated Raji BL xenografted NSG mice at day 20 (p < 0.001) and at day 25 (p < 0.05) compared to control (Figure 5C and 5D).
Figure 5.
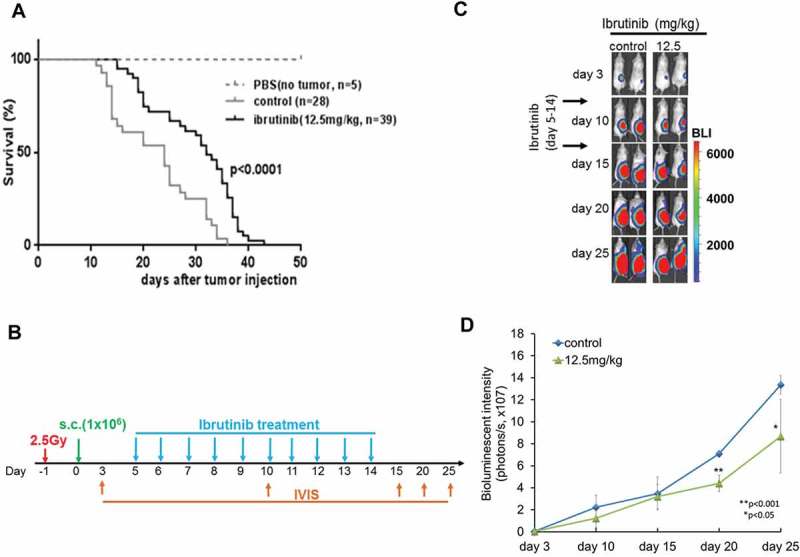
Survival of Raji BL xenografted mice after ibrutinib treatment 1 × 106 Raji-Luc cells were administered by sterile subcutaneous (s.c) injections in the lower flank of 6–8 week old female NSG mice. 5 days later after tumor xenografted. Ibrutinib (12.5 mg/kg) or PBS (control) was given to mice by oral gavage once a day for 10 continuous days. (A) Kaplan–Meier curves representing survival of mice from the beginning of tumor implantation. p < 0.0001. (B) Experimental schema showing ibrutinib treatment for different groups of irradiated BL xenografted NSG mice. (C) Bioluminescence imaging of representative mice at 3, 10, 15, 20 and 25 days post tumor cells injection are shown and mice were then monitored for quantified bioluminescence (D). (p < 0.001 at day 20, p < 0.05 at day 25, respectively).
Cyclophosphamide is an active agent in the treatment of BL and has been widely used in combination with doxorubicin, vincristine, prednisone, and rituximab to treat patients with BL.5 We investigated and compared the efficacy of ibrutinib (12.5 mg/kg) vs cyclophosphamide (CTX, 25mg/kg) vs obinutuzumab (ObiN, 30mg/kg) in Raji BL xenografted NSG mice. We observed that there was a similar median survival between the ibrutinib vs cyclophosphamide vs obinutuzumab treated Raji xenografted NSG mice (Figure 6).
Figure 6.
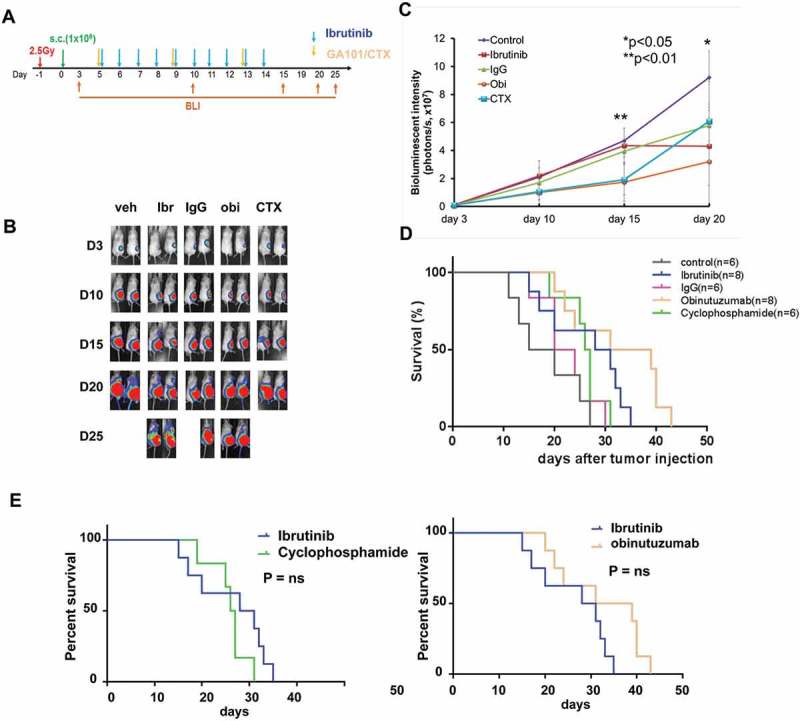
Comparison of tumor progression and survival following ibrutinib versus cyclophosphamide and obinutuzumab in BL xenografted mice. Experimental schema showing ibrutinib, cyclophosphamide or obinutuzumab treatment for different groups of irradiated BL xenografted NSG mice (A). Significantly reduced bioluminescence signal was observed in ibrutinib, cyclophosphamide (CTX) or obinutuzumab versus control treated groups at day 15 or day 20 (B and C) (p < 0.01 at day 15, p < 0.05 at day 20, respectively). Furthermore, there was equal effect of survival between ibrutinib vs CTX vs obinutuzumab. Ibrutinib significantly prolonged the survival versus control but was similar in survival when compared to single agent of CTX and obinutuzumab (D and E).
Discussion
We evaluated the in-vivo and in-vivo efficacy of ibrutinib, a FDA approved BTK inhibited, against BL. Our data demonstrated that ibrutinib significantly inhibited BL cell proliferation with concomitant decrease in phosphorylation of BTK in-vitro and secondly that ibrutinib was synergistic with either dexamethasone, rituximab, obinutuzumab, carfilzomib, or doxorubicin leading to BL growth inhibition and cell death of BL cells in-vitro. Furthermore, our data demonstrated that ibrutinib alone significantly decreased tumor progression and significantly increased the survival of BL xenografted mice and resulted in similar efficacy compared to therapeutic doses of cyclophosphamide or obinutuzumab.
Ibrutinib’s unique biochemistry and in-vivo activities in mice and dogs paved the way for human clinical trials in adults with mature B-cell lymphomas.13 In a phase I study, ibrutinib was well tolerated in fifty evaluable adults with relapsed/refractory B-cell lymphomas including MCL, follicular lymphoma, DLBCL, marginal zone lymphoma, and CLL and was associated with an objective response rate (ORR) of 60%, including complete response (CR) 16%.32 BL is the most common subtype of pediatric NHL.3 Schmitz et al reported that 6 of 9 BL cells were clearly BCR-dependent, based on a time dependent decrease in their viability following knockdown of either CD79A or the BCR associated tyrosine kinase SYK.33 However, there is a paucity of studies investigating the efficacy of ibrutinib in BL in-vitro and in-vivo. In our preclinical studies, we demonstrated that in-vitro, ibrutinib significantly inhibited the levels of p-BTK protein in BL cells and significantly inhibited the proliferation of BL (Figure 1 and Figure 2A-B). Consistently, in Raji xenografted NSG mice, ibrutinib significantly reduced tumor burden and extended BL xenografted NSG mice survival following ibrutinib at 12.5mg/kg daily (Figure 5). Furthermore, ibrutinib resulted in significant survival compared to cyclophosphamide treated group or obinutuzumab treated group in BL xenografted NSG mice (Figure 6).
Accumulating evidence demonstrates that BTK expression and activity is crucial for the survival or proliferation of malignant B cells. Activation of BTK usually correlates with an increase in the phosphorylation of two regulatory BTK tyrosine residues: transphosphorylation at Y551 within the Src homology type 1 (SH1) domain by the Src family tyrosine kinases and autophosphorylation at Y223.34 We demonstrated that BTK was highly expressed and phosphorylated in BL cells, similarly as reported previously in CLL and MCL cells.15,35 Ibrutinib treatment resulted in diminished cell survival and proliferation and abolished BCR-stimulated AKT and extracellular signal-regulated kinase phosphorylation and BTK autophosphorylation in CLL and MCL.16,35 Consistently, our studies demonstrated that ibrutinib significantly reduced autophosphorylation in BL cells and significantly inhibited BL proliferation. Most importantly, dexamethasone significantly reduced the IC50 of ibrutinib in-vitro to less than 1 μM in BL (Figure 2C-D). Since steroids are active agents of BL chemoimmunotherapy regimens, the possibility of clinical synergy exists.4–9
In previous studies, MCL cell lines showed differential sensitivity to BTK inhibition.36 Sensitive MCL cells showed a 50% inhibitory concentration (IC50) of ibrutinib at 0.115 μM. Intermediate sensitive cells had IC50 at 0.491 μM while the least sensitive cell line with no detectable inhibition until the concentration of ibrutinib reached 16 μM.36 In our study, we demonstrated that ibrutinib significantly inhibits Raji and Ramos BL cell growth following five days of treatment. The IC50 of ibrutinib is 5.20 μM against Raji and 0.868 μM against Ramos cells (Figure 2A-B). The pharmacokinetic and pharmacodynamic studies derived from a human trial for patients with relapsed/refractory B-cell malignancies suggested that the in-vivo maximal achievable concentration of ibrutinib is 0.408 μM with a once daily dose of 560 mg.32 In the studies in BL cell lines, ibrutinib doses as low as 0.2 μM resulted in complete inhibition of BTK phosphorylation and with the addition of dexamethasone, the IC50 of ibrutinib was further reduced in-vitro to between 0.13 and 0.34 μM, a concentration achievable in-vivo.
Although ibrutinib demonstrates an impressive clinical activity in relapsed or refractory CLL, some patients achieve partial remission and the development of resistance is now well known.37 A combination of ibrutinib with other drugs such as dexamethasone targeting alternative pathways that are independent of BTK signaling could be additive or synergistic.38 In a recent in-vitro study, Manzoni et al showed that dexamethasone potentiated anti-proliferative effects of ibrutinib.38 Consistent with the previous studies, we demonstrated a significant decrease of cell proliferation with the combination of ibrutinib and dexamethasone in both Raji and Ramos with a significant reduction of IC50 compared to ibrutinib alone (Figure 2C-D). Our data indicates that the use of a dexamethasone and ibrutinib combination may be an active combination in patients with BL. Ex-vivo drug screens using targeted agents on primary CLL cells have also been performed in order to identify additional pharmacologic agents that can complement ibrutinib therapy.24 Carfilzomib (PR-171), a second-generation proteasome inhibitor, was identified as a synergistic cytotoxic agent combined with ibrutinib against primary CLL. Consistent with this report, we demonstrated that carfilzomib significantly reduced ibrutinib IC50 for inhibition of cell proliferation in Raji (Figure 4A), providing some foundation to further investigate carfilzomib-ibrutinib combination therapies in patients with relapsed/refractory BL.
Another approach to improve the activity of ibrutinib and circumvent ibrutinib resistance is to investigate the efficacy of combining ibrutinib with anti-CD20 monoclonal antibodies (mAb). While there are some reports that ibrutinib may interfere with anti-CD20 mAb activity under some experimental conditions,39 growing clinical evidence has demonstrated that the two drugs in combination can be more effective than either agent alone.40 It is notable that ibrutinib induced lymphocytosis in patients and potentially released MCL and CLL cells into the blood from tissue sites18,19 and thus anti-CD20 mAb may potentially facilitate the clearing of these tumor cells in the blood. In our in-vitro studies, we demonstrated that ibrutinib IC50 values for inhibition of cell proliferation in Raji and Ramos cells were significantly decreased with 20 ug/ml obinutuzumab to 0.16 ± 0.095 μM and 0.01 ± 0.005 μM, respectively (Figure 3), suggesting that both rituximab and obinutuzumab in fact may enhance the sensitivity of BL cells to ibrutinib.
Even though the primary target of ibrutinib is BTK and it leading to development as a treatment for B-cell malignancies, interestingly, ibrutinib is also shown as an irreversible molecular inhibitor of interleukin-2-inducible kinase (ITK). ITK is an enzyme required by Th2 T cells, allowing a shift of T-cell immune responses to a Th1 T cells and potentiating Th1-based immune responses.41 Second-generation BTK inhibitors, such as ACP-196, ONO/GS-4059, and BGB-3111 have been developed and are being investigated in clinical trials.18,19,42 These inhibitors have been suggested to have fewer off-target effects in early clinical trials. Together, with the advances in cancer immunotherapies, further investigation of the combinations of ibrutinib or second-generation BTK inhibitors with novel antibodies, bi/tri-specific antibodies, immune checkpoint inhibitors, or CAR-T/CAR-NK will be critical to improve the success of treatment in patients with B cell malignancies such as BL.43
In summary, we have demonstrated the significant in-vitro and in-vivo efficacy of ibrutinib against BL. Complete inhibition of phosphorylation of BTK occurs with doses as low as 0.2 μM and the efficacy of ibrutinib is significantly enhanced with reduced the IC50 in combination with dexamethasone. Ibrutinib showed synergistic effects in combination with other active drugs in B-NHL such as obinutuzumab, carfilzomib, or doxorubicin, and the combination significantly reduced the IC50 of ibrutinib. Most importantly, in-vivo studies show significant improvement in OS in BL xenografted NSG mice (Figure 5) and similar efficacy to cyclophosphamide or obinutuzumab (Figure 6). Based on these preliminary results, there is an on-going clinical trial comparing OS in children and adolescents with relapsed/refractory BL with chemoimmunotherapy with or without ibrutinib (NCT02703272). The result of this study will assist in the development of subsequent clinical trials to test the efficacy of ibrutinib in BL.
Materials and methods
Cell lines and cell culture
Raji and Ramos human BL cell lines were purchased from ATCC and cultured under RPMI1640 supplemented with 10% Fetal Bovine Serum and 2mM Glutamine in a 37°C incubator at 5% CO2 as we have previously described.44
Cell proliferation assay
Cell growth was determined by using the non-radioactive CellTiter 96® Aqueous One solution cell proliferation assay (Promega, Madison, WI) and measured by a Multilabel Counter (Perkin Elmer, Massachusetts,USA) at OD490.44
Western blotting
Western blotting was performed as we have previously described.44–46 In brief, cell lysates were prepared in ice-cold lysis buffer (50 mmol/L Tris–HCl, pH 7.4, 350 mmol/L NaCl, 1% TritonX-100, 0.5% Nonidet P-40, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L Na3O4, 1 mmol/L phenylmethylsulphonyl fluoride (PMSF) with a phosphatase inhibitor cocktail. The total lysates were then separated into SDS-PAGE and then transferred to nitrocellulose membrane (Bio-Rad) and incubated with primary antibodies according to the manufacturer’s instructions. Membrane was developed using Enhanced Chemiluminescence Reagent (GE Healthcare Bio-Sciences). Antibodies specific for phospho-BTK at Tyr 223 (cat# 5082) and total BTK (cat# 3533) were purchased from Cell Signaling Technology. GAPDH was purchased from Santa Cruz. Band intensities on SDS-PAGE gels were measured using ImageJ software program.47
Reagents and antibodies
Ibrutinib was generously provided by Janssen R & D, LLC. Carfilzomib (PR-171) was obtained from Chemi Tek (Indianapolis, IN, USA). Idelalisib (GS-1101/CAL-101) was purchased from ApexBio Technology, LLC (Boston, MA, USA) and cyclophosphamide was purchased from Sigma-Aldrich (St. Louis, MO, USA. Doxorubicin was purchased from Selleck Chemicals (Houston, TX, USA). Obinutuzumab (GA101) was generously provided by Dr. Christian Klein, PhD from Roche (Basel, Switzerland) and rituximab was purchased from Genentech Inc. (South San Francisco, CA, USA).
In-vivo xenograft models
All experimental animal procedures, animal protocols and care were approved by the Institutional Animal Care and Use Committee at New York Medical College.
Ffluc/Zeo-labeled Raji cells were generated as we previously described.48 1 × 106 genetically modified ffluc/Zeo-labeled Raji cells were administered by sterile subcutaneous (s.c) injections in the lower flank of 6–8 week-old female mice as we have previously described.48,49 Stable engraftment of the genetically engineered cell line was also verified by bioluminescence imaging as we previously described.48 The total light emissions of the tumor cells in the animal were quantified using the Living Image software (Xenogen IVIS-200, Caliper Life Science). Tumor progression was monitored at day 7 and then every five days by BLI. Survival was determined in mice xenografted with Raji and treated with ibrutinib alone and in combination. Groups of NSG mice received ibrutinib alone (12.5 mg/kg), dexamethasone (0.2 mg/kg), cyclophosphamide (CTX, 25mg/kg), obinutuzumab (ObiN, 30mg/kg), IgG(30mg/kg) or PBS (control) by oral gavage or intraperitoneal (i.p.) injection based on route of drugs. Therapy was administered daily for 10 days and in combination with other drugs. Tumor size and whole-body tumor burden were assessed and survival was measured for 60 days as we have previously described.48,49 The survival rate was performed using humane endpoints and all mice were sacrificed when tumor size was greater than 2.0 cm3 [(length × width2)/2] or with signs of ulceration, or if mice were moribund.
Statistical analysis
Average values are reported as the mean ± SD. Significant differences between two groups were determined by using one-tailed paired Student’s t-test and p-values less than 0.05 were considered significant. CompuSynTM software was utilized for the determination of IC50 value and quantitation of synergism and antagonism in drug combination.50 Kaplan-Meier method was performed for survival rate and differences were evaluated by log-rank test using the Prism Version 6.0 software.
Funding Statement
This study was supported in part by grants from the Pediatric Cancer Research Foundation (PCRF), St. Baldrick’s Foundation and Janssen Research & Development, LLC.
Authorship contributions
YC analyzed and organized the data and wrote the manuscript. SL performed the experiments, analyzed the data and wrote the manuscript. CY performed experiments, analyzed the data and wrote the manuscript. JA, EM, CV, MB, TS, RRM, PG, STG, MSL, MH, LML, LGR, SLP reviewed and approved the manuscript. MSC designed the study, analyzed the data and wrote the manuscript.
Acknowledgments
The authors would like to thank Virginia Moore, RN, for her excellent assistance with the submission of this manuscript. And the authors thank Dr. Carl Hamby (New York Medical College) for his review of the statistic analysis and Dr. Matthew Barth (State University of New York at Buffalo) for providing Raji-2R and Raji-4RH cell lines.
Financial Disclosure Statement
All authors have no financial interests to disclose.
Conflict of Interest Statement
All authors have no potential conflicts of interest to disclose.
References
- 1.Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS.. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 2.Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol. 2012;156:730–743. doi: 10.1111/j.1365-2141.2011.09024.x. [DOI] [PubMed] [Google Scholar]
- 3.Cairo MS, Pinkerton R. Childhood, adolescent and young adult non-Hodgkin lymphoma: state of the science. Br J Haematol. 2016;173:507–530. doi: 10.1111/bjh.14035. [DOI] [PubMed] [Google Scholar]
- 4.Cairo MS, Krailo MD, Morse M, Hutchinson RJ, Harris RE, Kjeldsberg CR, Kadin ME, Radel E, Steinherz LJ, Morris E, et al. Long-term follow-up of short intensive multiagent chemotherapy without high-dose methotrexate (‘Orange’) in children with advanced non-lymphoblastic non-Hodgkin’s lymphoma: a children’s cancer group report. Leukemia. 2002;16:594–600. [DOI] [PubMed] [Google Scholar]
- 5.Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27:1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children’s Oncology Group Report. Br J Haematol. 2014;167:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 11.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, Goldlust IS, Yasgar A, McKnight C, Boxer MB, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A. 2014;111:2349–2354. doi: 10.1073/pnas.1311846111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman SEM, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinar M, Hamedani F, Mo Z, Cinar B, Amin HM, Alkan S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk Res. 2013;37:1271–1277. doi: 10.1016/j.leukres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, Burbury K, Turner G, Di Iulio J, Bressel M, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 18.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Day NS, Miles RR, Perkins SL, Lim MS, Ayello J, Van De Ven C, Harrison L, El-Mallawany NK, Goldman S, et al. Comparative genomic expression signatures of signal transduction pathways and targets in paediatric Burkitt lymphoma: a Children’s Oncology Group report. Br J Haematol. 2017;177:601–611. doi: 10.1111/bjh.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouska A, Bi C, Lone W, Zhang W, Kedwaii A, Heavican T, Lachel CM, Yu J, Ferro R, Eldorghamy N, et al. Adult high-grade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood. 2017;130:1819–1831. doi: 10.1182/blood-2017-02-767335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, Damaj G, Gastinne T, Ribrag V, Feugier P, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250–1260. doi: 10.1056/NEJMoa1701769. [DOI] [PubMed] [Google Scholar]
- 23.Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893–897. doi: 10.1182/blood-2012-10-459883. [DOI] [PubMed] [Google Scholar]
- 24.Lamothe B, Cervantes-Gomez F, Sivina M, Wierda WG, Keating MJ, Gandhi V. Proteasome inhibitor carfilzomib complements ibrutinib’s action in chronic lymphocytic leukemia. Blood. 2015;125:407–410. doi: 10.1182/blood-2014-07-585364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lannutti BJ, Meadows SA, Herman SEM, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaargaren M, De Rooij MF, Kater AP, Eldering E. BTK inhibitors in chronic lymphocytic leukemia: a glimpse to the future. Oncogene. 2015;34:2426–2436. doi: 10.1038/onc.2014.181. [DOI] [PubMed] [Google Scholar]
- 27.De Rooij MFM, Kuil A, Kater AP, Kersten MJ, Pals ST, Spaargaren M. Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood. 2015;125:2306–2309. doi: 10.1182/blood-2014-12-619163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J, Bandyopadhyay N, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, Den Boer ML, Pieters R, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Bhatti M, Ippolito T, Mavis C, Barth M. PI3K/Akt/mTOR pathway inhibition in chemotherapy-sensitive and -resistant models of Burkitt Lymphoma. Blood. 2015;126:1558.26160184 [Google Scholar]
- 32.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J clin oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C, Scharenberg AM, Kinet JP, Witte ON. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4:515–525. [DOI] [PubMed] [Google Scholar]
- 35.Chang BY, Francesco M, De Rooij MFM, Magadala P, Steggerda SM, Huang MM, Kuil A, Herman SEM, Chang S, Pals ST, et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122:2412–2424. doi: 10.1182/blood-2013-02-482125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma J, Lu P, Guo A, Cheng S, Zong H, Martin P, Coleman M, Wang YL. Characterization of ibrutinib-sensitive and -resistant mantle lymphoma cells. Br J Haematol. 2014;166:849–861. doi: 10.1111/bjh.12974. [DOI] [PubMed] [Google Scholar]
- 37.Woyach JA, Furman RR, Liu T-M, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH-H, Steggerda SM, Versele M, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzoni D, Catallo R, Chebel A, Baseggio L, Michallet A-S, Roualdes O, Magaud J-P, Salles G, Ffrench M. The ibrutinib B-cell proliferation inhibition is potentiated in vitro by dexamethasone: application to chronic lymphocytic leukemia. Leuk Res. 2016;47:1–7. doi: 10.1016/j.leukres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Bojarczuk K, Siernicka M, Dwojak M, Bobrowicz M, Pyrzynska B, Gaj P, Karp M, Giannopoulos K, Efremov DG, Fauriat C, et al. B-cell receptor pathway inhibitors affect CD20 levels and impair antitumor activity of anti-CD20 monoclonal antibodies. Leukemia. 2014;28:1163–1167. doi: 10.1038/leu.2014.12. [DOI] [PubMed] [Google Scholar]
- 40.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, De Weerdt I, Jeyakumar G, Ferrajoli A, Cardenas-Turanzas M, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu T-M, Chang BY, Larkin KM, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam C, Grigg AP, Opat S, Ku M, Gilbertson M, Anderson MA, Seymour JF, Ritchie DS, Dicorleto C, Dimovski B, et al. The BTK Inhibitor, Bgb-3111, Is Safe, tolerable, and highly active in patients with relapsed/Refractory B-Cell Malignancies: initial report of a phase 1 first-in-human trial. Blood. 2015;126:832.26160184 [Google Scholar]
- 43.Younes A, Ansell S, Fowler N, Wilson W, De Vos S, Seymour J, Advani R, Forero A, Morschhauser F, Kersten MJ, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol. 2017;14:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu Y, Huang AY, Ayello B, Barth J, Cairo M MS. Romidepsin alone or in Combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting burkitt lymphoma in vitro and in immunodeficient mice. Oncoimmunology. 2017;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Andrieu C, Saltel F, Destaing O, Auclair J, Pouchkine V, Michelon J, Salaun B, Kobayashi R, Jurdic P, et al. IkappaB kinase beta phosphorylates Dok1 serines in response to TNF, IL-1, or gamma radiation. Proc Natl Acad Sci U S A. 2004;101:17416–17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Mallawany NK, Day N, Ayello J, Van De Ven C, Conlon K, Fermin D, Basrur V, Elenitoba-Johnson K, Lim M, Cairo MS. Differential proteomic analysis of endemic and sporadic Epstein-Barr virus-positive and negative Burkitt lymphoma. Eur J Cancer. 2015;51:92–100. [DOI] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu Y, Hochberg J, Yahr A, Ayello J, Van De Ven C, Barth M, Czuczman M, Cairo MS. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-modified expanded natural killer cells in vitro and in NSG Mice. Cancer Immunol Res. 2015;3:333–344. [DOI] [PubMed] [Google Scholar]
- 49.Ayello J, Van De Ven C, Cairo E, Hochberg J, Baxi L, Satwani P, Cairo MS. Characterization of natural killer and natural killer-like T cells derived from ex vivo expanded and activated cord blood mononuclear cells: implications for adoptive cellular immunotherapy. Exp Hematol. 2009;37:1216–1229. [DOI] [PubMed] [Google Scholar]
- 50.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. [DOI] [PubMed] [Google Scholar]


