ABSTRACT
In healthy tissue, the tight junction protein Claudin 18.2 (CLDN18.2) is present only in the gastric mucosa. Upon malignant transformation of gastric epithelial tissue, perturbations in cell polarity lead to cell surface exposure of CLDN18.2 epitopes. Moreover, CLDN18.2 is aberrantly expressed in malignancies of several other organs, such as pancreatic cancer (PC). A monoclonal antibody, zolbetuximab (formerly known as IMAB362), has been generated against CLDN18.2. In a phase 2 clinical trial (FAST: NCT01630083), zolbetuximab in conjunction with chemotherapy prolonged overall and progression-free survival over chemotherapy alone and improved quality of life. In this study, the mechanism of action and antitumor activity of zolbetuximab were investigated using nonclinical PC models. Zolbetuximab bound specifically and with strong affinity to human PC cells that expressed CLDN18.2 on the cell surface. In ex vivo systems using immune effector cells and serum from healthy donors, zolbetuximab induced antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), resulting in the lysis of cultured human PC cells. The amplitude of ADCC and CDC directly correlated with cell surface CLDN18.2 levels. The chemotherapeutic agent gemcitabine upregulated CLDN18.2 expression in cultured human PC cells and enhanced zolbetuximab-induced ADCC. In mouse xenograft tumors derived from human PC cell lines, including gemcitabine-refractory ones, zolbetuximab slowed tumor growth, benefited survival, and attenuated metastases development. The results presented here validate CLDN18.2 as a targetable biomarker in PC and support extension of the clinical development of zolbetuximab to patients with CLDN18.2-expressing PC.
KEYWORDS: Claudin 18.2, zolbetuximab, IMAB362, monoclonal antibody, targeted therapy, pancreatic cancer, antibody-dependent cellular cytotoxicity, ADCC, complement-dependent cytotoxicity, immunotherapy
Introduction
Targeted therapy with monoclonal antibodies (mAbs) has become a key component in the treatment of many cancers.1 Monoclonal antibodies can exert their effects via various mechanisms, including blocking of growth factor signaling pathways in cancer cells and cytolysis by activation of immune effectors. Activation of immune effectors is mediated by the Fc-domain of the mAb and includes the processes of antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).2 These cytotoxic processes are initiated when specific mAbs bind to cellular targets, identifying the cell for immune-mediated destruction.3 For hematologic malignancies, highly effective mAbs (eg, rituximab,4 daratumumab5), which act predominantly via ADCC and/or CDC, have been developed. Although this mechanism of action has scarcely been implemented for solid tumors because of a lack of suitable cell surface targets, it may offer a promising approach for the treatment of a variety of cancers with high unmet medical needs.
An ideal therapeutic mAb would be expected to have tumor-specific action, high and sustained potency, applicability to a large population of patients, and low toxicity. For these desirable characteristics to be met, finding a suitable specific target is critical. Previous work has identified the tight junction protein Claudin-18 (CLDN18) as an attractive target candidate in several solid tumors (eg, gastric, pancreatic, lung, and bile duct cancers).6 This transmembrane protein is normally expressed as two tissue-specific splice variants: CLDN18.1, predominantly expressed in the lung, and CLDN18.2, expressed exclusively in the stomach and maintained in a substantial proportion of gastric cancers (GCs).6 We have previously reported that a chimeric monoclonal IgG1 antibody, zolbetuximab, was developed as a specifically CLDN18.2-targeting therapeutic mAb.7,27 In a phase 2 trial (FAST; NCT01630083), zolbetuximab in combination with chemotherapy significantly prolonged survival with acceptable safety and tolerability in patients with CLDN18.2-positive (CLDN18.2+) advanced/recurrent GC and gastroesophageal junction (GEJ) cancers.8 Furthermore, health-related quality of life was maintained for a longer duration in patients who received zolbetuximab plus chemotherapy compared with chemotherapy alone.9
In pancreatic cancer (PC), CLDN18.2 is aberrantly expressed with a prevalence of 60–90% reported in pancreatic ductal adenocarcinoma, the most common form of PC.10–12 Aberrantly expressed CLDN18.2 appeared in precancerous lesions, indicating it may be valuable as an early marker of malignant transformation.12,13 Depending on disease stage and patient health, systemic treatment of PC is currently based primarily on gemcitabine monotherapy, Abraxane or combinations of highly toxic fluoropyrimidine-, paclitaxel-, or oxaliplatin-based chemotherapy regimens (eg, FOLFIRINOX).14-17 There are few approved targeted therapy options for PC: the kinase inhibitor erlotinib has marginal benefit15 and the checkpoint inhibitor pembrolizumab can be used for the small fraction of patients with mismatch repair-deficient PC per a biomarker-based label approval in the United States.16 Our earlier studies showed that aberrant transcriptional induction of CLDN18.2 in pancreatic neoplasms may render a fraction of PCs targetable by zolbetuximab.12 Thus, the tumor-specific expression of CLDN18.2 in PC, as well as evidence demonstrating the beneficial effects of zolbetuximab in GC, prompted the exploration of zolbetuximab as a potential therapeutic agent for PC.
Using in vitro and in vivo nonclinical studies, we investigated the binding characteristics of zolbetuximab to CLDN18.2 in human PC cell lines, the mechanism of action of zolbetuximab, and the potential antitumor activity of zolbetuximab in mouse xenograft models of human PC cell-derived tumors. In addition, we examined the combined effect of zolbetuximab and the chemotherapeutic agent gemcitabine in these nonclinical tumor models.
Results
Zolbetuximab binds with high specificity and affinity to cldn18.2-expressing cells
Findings from our group and others have demonstrated that patients with PC frequently (60–90%) express high levels of CLDN18.2 in their tumor tissue (Figure 1a). To identify PC cell lines that endogenously express CLDN18.2, we screened 26 human PC cell lines by RT-PCR; of these screened cell lines, three were considered CLD18.2+ (Patu 8988S, DAN-G, YAPC; Figure 1b). Furthermore, protein expression was also observed in these cell lines as well as in BxPC-3 and Panc 05.04 cells (Figure 1c; Suppl Table 1). This difference of CLDN18.2 expression prevalence between tumor tissue resectates and cell lines has also been observed for gastric cancer (data not shown). An interesting observation in this context is that expression of CLDN18.2 mRNA in cell lines declines over sequential passages in cell culture (Suppl Figure 1 A and B). This implies that culturing is associated with downregulation of CLDN18.2 and may explain why cell lines with pronounced levels of endogenous CLDN18.2 are rare in the first place.
Figure 1.
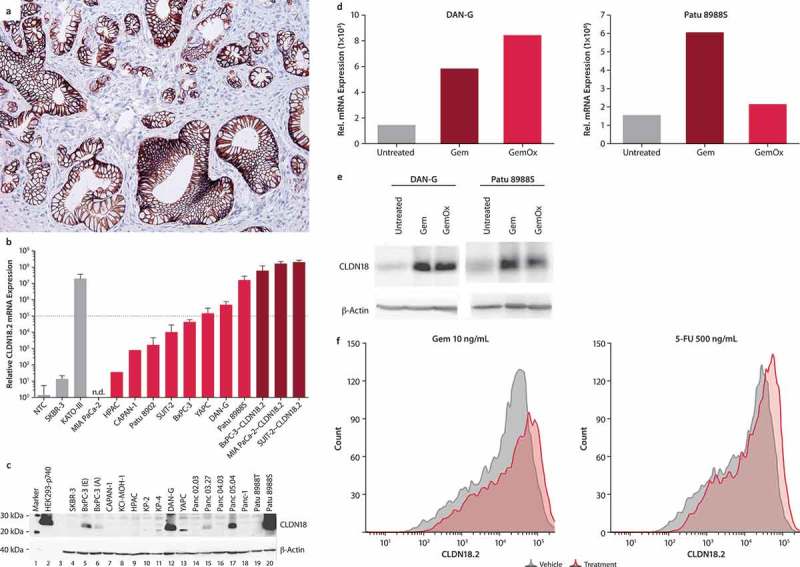
Expression of CLDN18.2 in human PC cell lines.
- (a) Typical image of CLDN18.2+ pancreatic ductal adenocarcinoma. Staining was performed with 43-14A antibody. Magnification: 200x.
- (b) Transcript (qRT-PCR) and (b) protein (western blot) levels of CLDN18.2 in PC cell lines with endogenous (light red bars) and transduced (dark red bars) CLDN18.2 expression. qRT-PCR data are mean ± SD of 1–9 independent measurements. Cell lines with a relative expression level above 1 × 105 were considered CLDN18.2+ (dotted line in a). Gray bars represent non-PC cell lines and controls.
- (c) Detection of CLDN18 protein in pancreatic cancer cell lysates. Western blot analysis was performed using a CLDN18 antibody detecting the C-terminal of CLDN18.1 and CLDN18.2 (C-term, Zymed) and a loading control antibody detecting β-actin. Lysates of SKBR-3 cells were used as negative control, whereas lysates of HEK293 cells stably transfected with CLDN18.2 (HEK293-p740) were used as positive control. BxPC-3 (e) and BxPC-3 (a) represent BxPC-3 cell lines from ECACC and ATCC, respectively.
- (d) Effect of treatment with Gem or GemOx on CLDN18.2 mRNA expression. mRNA was isolated from DAN-G cells (untreated, treated with Gem [1 ng/mL] or GemOx [Gem 1 ng/mL + Ox 10 ng/mL] for 2 days) or Patu 8988S cells (untreated or treated with Gem [10 ng/mL] or GemOx [Gem 10 ng/mL + Ox 100 ng/mL] for 3 days). RNA was reverse transcribed to cDNA and CLDN18.2 transcript levels analyzed by qRT-PCR. Expression levels are depicted relative to the housekeeping gene HPRT.
- (e) Effect of treatment with Gem or GemOx on CLDN18 protein expression. CLDN18 protein expression was analyzed in total cell lysates of untreated, Gem- (1 ng/mL) or GemOx- (Gem 10 ng/mL + Ox 100 ng/mL) treated DAN-G, or Patu 8988S cells by Western blot and detected with the Zymed C-term polyclonal antibody. Actin served as loading control.
- (f) Influence of Gem, and 5-FU on surface expression of CLDN18.2 in Patu 8988S pancreatic cancer cells. CLDN18.2 expression was detected using flow cytometry with zolbetuximab as primary antibody and anti-hu-IgG-APC as secondary antibody. Histogram shows CLDN18.2 expression on Patu 8988S cells treated for 3 days with DMSO (gray line), 10 ng/mL Gem, 500 ng/mL 5-FU, 100 ng/mL PTX, or 500 ng/mL Ox (red line).
To determine if chemotherapeutic agents commonly used in the treatment of PC may affect the expression of CLDN18.2, endogenously CLDN18.2-expressing PC cell lines (DAN-G and Patu 8988S) were treated with gemcitabine and gemcitabine plus oxaliplatin (GemOx). Gemcitabine increased both CLDN18.2 mRNA and protein expression in DAN-G and Patu 8988S cells. GemOx increased protein expression in these cells, though only a slight increase of mRNA was observed in Patu 8988S cells. (Figure 1, d and e). Furthermore, flow cytometry analysis of Patu 8988S cells revealed that single-agent gemcitabine and 5-fluorouracil (5-FU) increased CLDN18.2 on the cell surface (Figure 1 f); with paclitaxel and oxaliplatin treatment, protein expression was maintained on baseline level (data not shown).
Zolbetuximab induces ADCC and CDC in human CLDN18.2+ PC cells
Having found that endogenously CLDN18.2 expressing cell lines are scarce, and for the purposes of these studies, PC cell lines were transduced with CLDN18.2 (designated as ~ CLDN18.2) to generate models for functional studies. The transfected cell lines were found to express higher CLDN18.2 than endogenously CLDN18.2 expressing cell lines (Figure 1 b). Evaluation of the binding of zolbetuximab to PC cell lines expressing endogenous or transduced CLDN18.2 showed that zolbetuximab binds to PC cell lines that express robust levels of CLDN18.2 (Figure 2a and b). Zolbetuximab bound poorly to those that express low surface levels of CLDN18.2 (eg, Panc 05.04) and did not bind to cell lines that lacked CLDN18.2 (data not shown).
Figure 2.
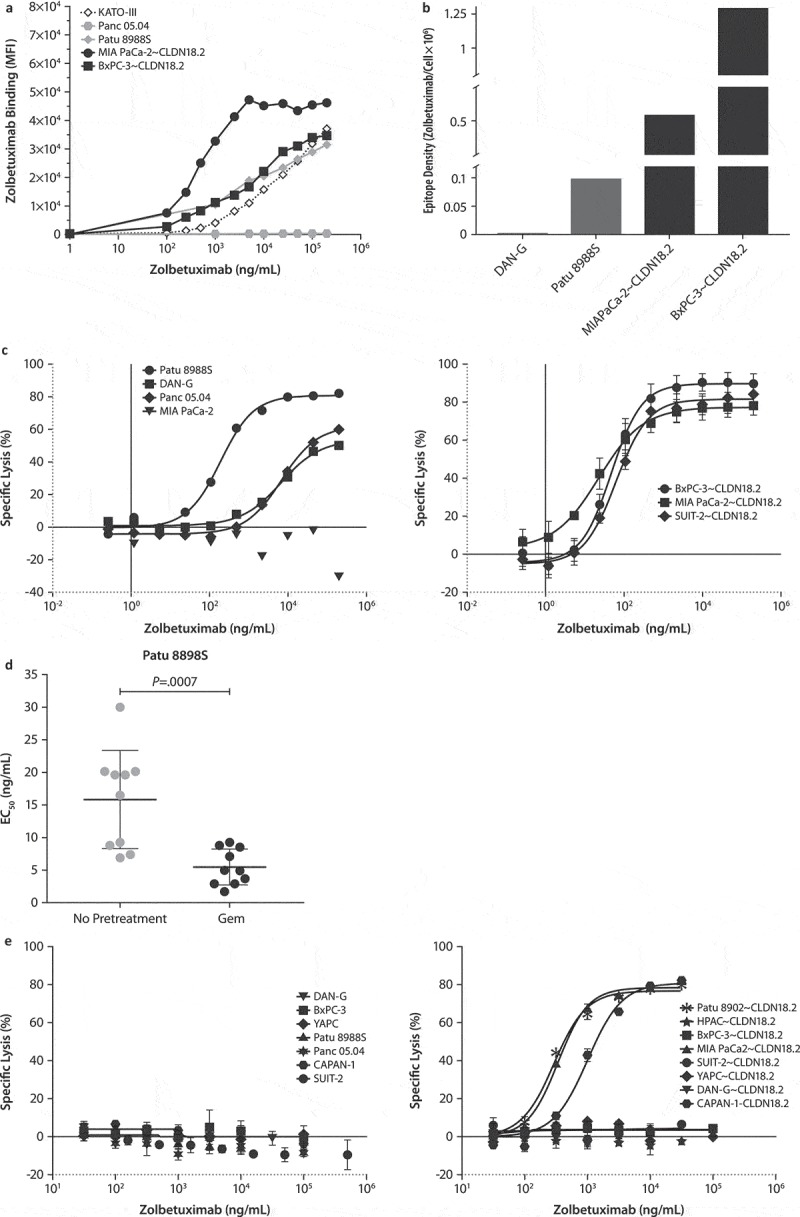
Zolbetuximab induces cytotoxicity against human PC cells.
(a) Binding dynamics of zolbetuximab (FITC-conjugated) to cell-surface CLDN18.2 on human PC cells by flow cytometry.(b) Density of the zolbetuximab epitope (zolbetuximab molecules bound per cell) on human PC cells. Data are depicted as zolbetuximab molecules bound per cell.(c) Specific lysis of CLDN18.2-expressing cell lines (left panel, endogenous; right panel, transduced) with negative control (MIA PaCa-2) by zolbetuximab-induced ADCC. Data are (a) mean of two or (b) mean ± SD of 3–5 independent donors per cell line.(d) Zolbetuximab-induced ADCC EC50 in DAN-G cells with or without 1 ng/mL Gem pretreatment. Data are mean ± SD of 10 independent donors. P value was calculated with an unpaired t-test. (e) Specific lysis of CLDN18.2-expressing cells (left panel, endogenous; right panel, transduced) by zolbetuximab-induced CDC. Healthy human serum pool served as a complement source. Data are mean ± SD of triplicate.Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; EC50, half-maximal effective concentration; SD, standard deviation.
To determine if the specific binding of zolbetuximab to CLDN18.2+ PC cells results in immune effector mediated cell killing, cells were co-incubated with human peripheral blood mononuclear cells (PBMCs) as effectors for ADCC in zolbetuximab-supplemented culture. Zolbetuximab-mediated ADCC was strictly target-dependent and highly efficient against all tested CLDN18.2-transduced PC cells (SUIT-2~ CLDN18.2, MIA PaCa-2~ CLDN18.2, and BxPC-3~ CLDN18.2) with specific lysis reaching > 78% (Figure 2c); the corresponding CLDN18.2-negative (CLDN18.2–) parental cell lines (SUIT-2, MIA PaCa-2, and BxPC-3) and other CLDN18.2 – cells were not lysed (data not shown). All three cell lines (Patu 8988S, Panc 05.04, and DAN-G) that expressed endogenous CLDN18.2 were also efficiently killed, including those with poor binding of zolbetuximab (Panc 05.04 and DAN-G; Figure 2c).
Although CLDN18.2 was limited on the cell surface of Pan 05.04 and DAN-G cell lines, zolbetuximab was still able to induce ADCC in these cells, indicating there was sufficient epitope to activate ADCC. However, due to the sparse presence of cell-surface CLDN18.2 in these cells, the effective concentrations for half-maximal response (EC50) values were up to > 100-fold higher than some lentivirally transduced cell lines that expressed high cell-surface CLDN18.2. The EC50 values ranged from ~ 20 ng/mL for strong CLDN18.2-expressing cell lines to > 10,000 ng/mL for cell lines with poor zolbetuximab binding (Suppl Figure 2a, right panel). Maximum lysis of target cells with endogenous CLDN18.2 was only slightly lower than that of target cells with transduced CLDN18.2 expression (range 48%–68% vs 78%–93%, respectively; Suppl Figure 2a, left panel).
To examine if gemcitabine, through upregulating CLDN18.2 expression, may have an augmenting effect on zolbetuximab-induced ADCC and EC50 values, DAN-G cells were pretreated with gemcitabine before ADCC assay was performed. Zolbetuximab-induced ADCC was more efficient in the gemcitabine-pretreated cells, as reflected in significantly lower EC50 (Figure 2d and Suppl Figure 3).
Next, to investigate the sensitivity of the PC cell lines to zolbetuximab-mediated CDC, human serum as a source of complement was added to the culture in addition to zolbetuximab. Zolbetuximab induced CDC against transduced CLDN18.2-expressing cell lines only; endogenously expressing cell lines were not killed by CDC (Figure 2e), indicating that CDC activity required higher expression levels of CLDN18.2 compared with ADCC. The EC50 values for CDC ranged between 3000–7000 ng/mL (Suppl Figure 2B). The in vitro antitumor activity of zolbetuximab was strictly target-specific for both ADCC and CDC, and while it was donor dependent, it was robust across different donors (data not shown).
Zolbetuximab exhibits in vivo antitumor activity in mouse xenograft models
To study the in vivo antitumor activity of zolbetuximab, PC cell lines, including cell lines refractory to 50 mg/kg gemcitabine, were used for subcutaneous (SC) xenografts. Mice were treated with weekly repeat doses of systemic zolbetuximab alone and in combination with gemcitabine (Figure 3 and Suppl Figure 4). Treatment of two different xenograft tumor models with zolbetuximab as a single agent resulted in significant tumor growth retardation (Figure 3, a–c).
Figure 3.
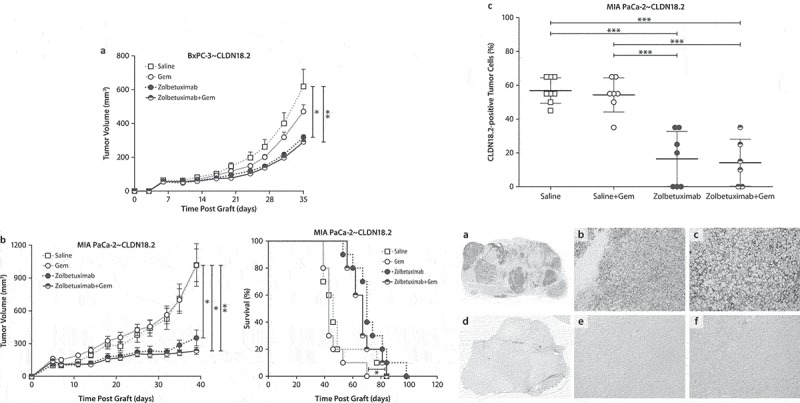
Zolbetuximab alone and in combination with gemcitabine exhibits in vivo antitumor activity in mouse xenograft models.
(a) Tumor growth kinetics of BxPC-3~ CLDN18.2 xenografts. The size of SC tumors were measured twice weekly. BxPC-3~ CLDN18.2 xenograft tumors were inoculated by SC injection of 8.5 × 106 BxPC-3~ CLDN18.2 cells into the flanks of 10 female Hsd:Athymic Nude-Foxn1nu mice per treatment group. On Day 3 after tumor cell injection, treatments were initiated with Gem (100 mg/kg IP) and were continued weekly for 6 weeks. 24 h after every injection of Gem, 200 μg zolbetuximab or saline control treatments were applied IV into the tail vein. Zolbetuximab treatment was continued semi-weekly with alternating IP and IV injections until mice were euthanized. Data are mean ± SEM. *P < .05; **P < .01 based on Tukey’s multiple comparisons test.(b) Tumor growth kinetics of MIA PaCa-2~ CLDN18.2 xenografts (left). The size of tumors was measured twice weekly. Data are mean ± SEM. Kaplan–Meier survival estimates (right). Mia PaCa2~ CLDN18.2 xenograft tumors were inoculated by SC injection of 5 × 106 MIA PaCa-2~ CLDN18.2 cells into the flank of 10 female Hsd:Athymic Nude-Foxn1nu mice per treatment group. On Day 4 after tumor cell injection, treatment was initiated with Gem (50 mg/kg IP) and was continued weekly for 6 weeks. 24 h after injection of Gem, 200 μg zolbetuximab or control treatments were applied IV into the tail vein. Zolbetuximab treatment was continued semi-weekly with alternating IP and IV injections until mice were euthanized (left). *P < .05 (zolbetuximab vs Gem, zolbetuximab+ Gem vs saline); **P < .01 (zolbetuximab+ Gem vs Gem) based on Dunn’s multiple comparisons test (right). *P < .05 (zolbetuximab+ Gem vs Gem) based on log-rank test.(c) IHC analysis of CLDN18.2+ tumor cells in MIA PaCa-2~ CLDN18.2 xenografts after treatment with zolbetuximab, Gem, or both. Percentage of CLDN18.2+ tumor cells in MIA PaCa-2~ CLDN18.2 xenografts (upper panel). Data points represent individual measurements with the horizontal line representing mean ± SD. ***P < .001 based on one-way ANOVA followed by a Tukey’s multiple comparisons test. CLDN18.2 protein expression in zolbetuximab treated and untreated MIA PaCa-2~ CLDN18.2 xenografts (lower panel). FFPE tissue sections of (a–c) isotype-treated control and (d–f) zolbetuximab-treated mice bearing MIA PaCa-2~ CLDN18.2 xenografts (a, d). 1× overview, magnification 100× (b, e) and 200× (c, f). Staining was performed with Zymed anti-CLDN18 antibody.Abbreviations: ANOVA, analysis of variance; FFPE, formalin-fixed paraffin-embedded; Gem, gemcitabine; IHC, immunohistochemistry; IP, intraperitoneal; IV, intravenous; SC, subcutaneous; SD, standard deviation; SEM, standard error of the mean.
In the MIA PaCa-2~ CLDN18.2 model, inhibition of tumor growth translated into a significant survival benefit for mice treated with zolbetuximab and gemcitabine over gemcitabine alone (Figure 3b, right panel). Further, MIA PaCa2~ CLDN18.2 tumor lesions remaining in mice that survived longer with zolbetuximab treatment had significantly lower fractions of CLDN18.2+ cells compared with tumors of control or gemcitabine-treated mice (Figure 3c), indicating eradication of target-positive cells. Visual evidence could be seen in FFPE tissue sections of isotype-treated control and zolbetuximab-treated mice bearing MIA PaCa-2~ CLDN18.2 xenografts. (Figure 3c, a-f).
A modified form of paclitaxel, nanoparticle albumin-bound paclitaxel (nab-paclitaxel), has been approved as first-line treatment for PC in combination with gemcitabine.15 We tested the antitumor activity of zolbetuximab and nab-paclitaxel in xenograft tumors, but found no additional benefit either for tumor volume reduction or for survival (Suppl Figure 5).
Zolbetuximab alone and in combination with gemcitabine prevents lung metastasis formation in intravenous mouse xenograft models
Next, the potential of zolbetuximab to prevent hematogenous dissemination of PC cells was investigated. To this aim, CLDN18.2-expressing cell lines were administered intravenously (IV) into the tail vein of mice, resulting in manifestation of lung metastases. Tumor load after treatment was assessed by two independent methods: the ratio of human to murine genomic DNA by PCR and the ratio of human MHC-I to lung cell surface by IHC. In the SUIT-2~ CLDN18.2 model, mice were treated with zolbetuximab monotherapy and control isotype antibody; in the Patu 8988S model, both zolbetuximab and the isotype were combined with gemcitabine. In both models, significantly reduced lung metastatic load was observed with zolbetuximab (Figure 4a and b).
Figure 4.
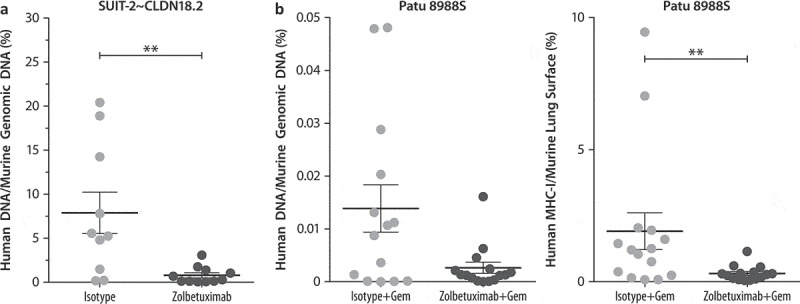
Zolbetuximab alone and in combination with gemcitabine prevents lung metastasis formation in IV mouse xenograft models.
Mice were inoculated by IV injection of 2 × 106 (a) SUIT-2~ CLDN18.2 or (b) Patu 8988S human PC cells into the tail vein of female Hsd:Athymic Nude-Foxn1nu mice. Alternating IV/IP injections with 200 µg zolbetuximab or isotype (with 100 mg/kg IP gemcitabine in combination studies) were initiated on Day 3 post-graft or 2 weeks after tumor injection and given twice per week. Mice were euthanized at different time points, or after the animals in the control group showed clear physiologic signs of metastatic disease.(a) Human DNA content with zolbetuximab (200 μg) or isotype.(b) Human DNA content (left panel) and MHC-I on lung surface (right panel) with or without zolbetuximab + Gem (zolbetuximab 200 μg + Gem 100 mg/kg semi-weekly for 4 weeks or with 200 μg isotype control antibody + 100 mg/kg Gem semi-weekly). Data are mean ± SEM. **P < .01based on Mann–Whitney U-test (two-tailed). MHC-I staining was performed with anti-human MHC-I (EPR1394Y) antibody.Abbreviations: Gem, gemcitabine; IHC, immunohistochemistry; IP, intraperitoneal; IV, intravenous; MHC, major histocompatibility complex; SEM, standard error of the mean.
Discussion
Zolbetuximab is a first-in-class mAb that specifically binds to the tight junction protein CLDN18.2 and has shown promising antitumor activity in patients with gastric and GEJ cancers.7-9,24 In these nonclinical studies, zolbetuximab effected potent PC cell lysis in vitro by inducing ADCC and CDC. ADCC was more pronounced and affected more cell lines than CDC. Consistent with this cytolytic ability, zolbetuximab demonstrated antitumor activity in human PC cell line-derived mouse xenograft tumors. In this regard, zolbetuximab is similar to mAbs such as rituximab and daratumumab, which mainly act by stimulating ADCC and/or CDC. In our experiments, the amplitude of zolbetuximab-induced cytotoxic effects correlated with the expression level of CLDN18.2 on the cell surface. An intriguing finding in our studies is that the chemotherapeutic agent gemcitabine upregulates CLDN18.2 expression in PC cell lines. This raises the possibility that the combination of zolbetuximab with certain chemotherapy may provide enhanced cytotoxic activity against CLDN18.2+ expressing cells.
Therapeutic antibodies initially developed for solid tumors acted by inhibiting tumor growth-promoting molecules (eg, growth factor receptors, angiogenic agents). A second class of mAbs for solid tumors, the immune checkpoint inhibitors that enhance T-cell-mediated cytolysis, were developed more recently and include drugs such as pembrolizumab and nivolumab. Some mAbs, such as the anti-HER2 trastuzumab, also stimulate immune effector pathways such as ADCC, although not as their primary cytolytic mechanism. A targeted, high efficacy treatment for PC remains an unmet medical need; drugs that have failed include growth factor-inhibiting mAbs and small-molecule tyrosine kinase inhibitors.18–23 In these nonclinical studies, we demonstrate that zolbetuximab, a mAb that acts through a novel target and mechanism, has antitumor activity in animal PC models utilizing human PC cell lines.
There are a few limitations to this study. Many of the cell lines used did not endogenously express appreciable levels of CLDN18.2, as CLDN18.2 seems to be downregulated and lost under cell culture conditions. Therefore, we lentivirally transduced cell lines, the expression levels of which may not fully reflect those naturally occurring in the pancreatic cancers of human patients. Also, while we have established ADCC and CDC as the dominant cytolytic mechanisms in ex vivo assays, we cannot discount that additional mechanisms are making a contribution, especially in in vivo environments. Another issue is that our ADCC and CDC assays were conducted with PBMC or serum pool from healthy subjects. However, in a first-in-human study, PBMC and serum from patients with GC were as capable as those from healthy donors in mediating zolbetuximab-induced cell lysis.
Our results therefore support further development of zolbetuximab as a targeted therapy for PC. In addition, these results, in conjunction with earlier findings, suggest zolbetuximab may possess a number of desirable characteristics for a therapeutic mAb: the expression of CLDN18 in a large fraction of PC (60–90% in pancreatic ductal adenocarcinoma, the most common form of pancreatic cancer) indicates that a large population of patients may be treated; the very limited expression of CLDN18.2 in normal tissue may translate into a low occurrence of adverse events; and the efficient lysis suggests it can have high potency.
Clinical data of the antitumor activity of zolbetuximab in CLDN18.2-expressing GC and GEJ cancer have been promising,7-9,24 which prompted investigations into the potential of zolbetuximab as a therapeutic agent for CLDN18.2-expressing PC as well. Our findings from this nonclinical study support the notion that zolbetuximab can be a therapeutic antibody not only for GC, but also for several other CLDN18.2-expressing solid tumors, such as PC.
Methods
Quantitative real-time PCR (qRT-PCR)
RNA was isolated with an RNeasy Mini Kit (Qiagen) and cDNA was synthesized with the Super Script III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. The CLDN18.2 DNA sequence was amplified from cDNA with primers #5054 as (5ʹ-AGAGAGCTCTGGCTTCACCGAGTG-3ʹ) and #5060 as (5ʹ-CCAGAAGTTAGTCACCAGCATGTTGG-3ʹ), which are selective for CLDN18.2 and do not cross-amplify DNA sequences for its closely related isoenzyme, CLDN18.1. The qRT-PCR reactions were prepared with SYBR Green (Qiagen) and measurements were performed using the ABI-PRISM7900 Sequence Detection System (Applied Biosystems). The relative CLDN18.2 expression was calculated using constitutive HPRT as reference gene with the ΔΔCT method.25 Samples with a relative mRNA expression of > 1 × 105 were considered CLDN18.2+.
For detection of human DNA present in murine lung genomic DNA samples, the qPCR reaction was performed with primer pair #5861 5ʹ-GGGATAATTTCAGCTGACTAAACAG-3ʹ and #5862 5ʹ-TTCCGTTTAGTTAGGTGCAGTTATC-3ʹ specifically amplifying the alpha-satellite DNA present in human chromosome 17, but not in mouse DNA. To generate a standard curve and as positive control, Patu 8988S DNA was mixed with mouse DNA and 5-fold dilutions were prepared, resulting in 100%, 20%, 4%, 0.8%, 0.16%, 0.032%, and 0.0064% human DNA in mouse DNA, respectively. The curve was used to calculate (linear regression) the amount of human metastasis-DNA present in mouse lung tissue.
Western blot
PC cells were lysed in sodium dodecyl sulfate (SDS)-buffer (34% glycine, 250 mM Tris pH 6.8, 5% β-mercaptoethanol, 8.2% SDS) and sonicated to remove genomic DNA. 75 µg total protein were electrophoresed on a 12.5% polyacrylamide gel, then blotted on a nitrocellulose membrane (90 min, 160 mA). Blots were incubated overnight at 4°C with primary antibodies (0.25 µg/mL rabbit anti-Claudin 18 [C-term, Zymed] or 0.1 µg/mL mouse anti-β-actin [Sigma] as loading control). Binding of the primary antibody was detected with labeled secondary antibodies (goat anti-rabbit IgG Fc or rabbit anti-mouse; diluted 1:1000). Protein expression was detected by the addition of 1–3 mL detection solution (Pico and Dura Detection System [Pierce]) and scanning the blots in a LAS-3000 detection box (Fujifilm).
Flow cytometry
Cells were harvested with Trypsin/EDTA (Sigma-Aldrich Co.), counted, centrifuged for 5 min (300 × g), and the pellet was resuspended in 200 μL of FACS buffer (antoMACS Running Buffer, Miltenyi Biotec). Then 100 μL of cell suspensions were plated in 96-well plates. Zolbetuximab was diluted 0.05 mg/mL in 50 μL FACS buffer and added to the cells for 30 min at 4°C as a primary antibody for detection of CLDN18.2. The plate was centrifuged (2 min, 670 × g) and then washed twice with 200 μL FACS buffer. After centrifugation, the pellet was resuspended in 100 μL of FACS buffer. Secondary goat anti-human antibody (Fc-specific, F(ab´)2 conjugated with APC, Jackson ImmunoResearch Labs, Inc.) was diluted in FACS buffer and 20 μL was added to each well. The final dilution of secondary antibody in each well was 1:100. The plate was incubated for 30 min at 4°C in the dark. After incubation, the plate was washed twice with 200 μL FACS buffer and the pellet was resuspended in 200 μL FACS buffer and filtrated through a 70 μm nylon mesh filter. Then, 100 μL FACS buffer containing 5 μL 7-AAD (BD Biosciences) was added to the cell suspension for measurement using FACSVerse (BD Biosciences). The obtained data were analyzed using FlowJo software version 10.2 (FlowJo LLC, Ashland, OR, USA). Because the experiment was conducted once, the results are therefore from single experiments (no replicates).
Epitope density
The Quantum™ Simply Cellular® anti-human IgG kit (Bangs Laboratories, Inc.) was used according to the manufacturer’s recommendation. Cells were stained with a dilution series of FITC-conjugated zolbetuximab or FITC-conjugated isotype control antibody (rituximab). For calibration, the different beads were incubated with an excess of the respective FITC-conjugated antibodies. Mean fluorescent intensity (MFI) was measured by flow cytometry using a FACS Array Bioanalyzer (BD).
Antibody-dependent cellular cytotoxicity (ADCC) analysis
Peripheral blood mononuclear cells as ADCC effector cells (E) were isolated from human peripheral blood samples or buffy coats from healthy donors (Transfusionszentrale, Mainz) by standard Ficoll density gradient centrifugation (Ficoll-Paque Plus; VWR/GE Healthcare). Human PC cell lines as target cells (T) were prepared by transient transfection with Anti-Reverse-capped (ARCA, Ambion) luciferase RNA (provided by TRON GmbH, Mainz, Germany) to follow cell lysis. Briefly, 2.5 × 106 cells in X-Vivo medium were electroporated (Gene Pulser Xcell, Bio-Rad) in the presence of 10 µg RNA at 200–250V and 300–475 µF, depending on the cell line. 2.5 × 104 viable cells/well were seeded to confirm luciferase activity with a luminometer (Tecan Infinite200). Relative light units of > 1000 were considered as successful transfection. ADCC assays were performed with 1 × 104 target cells/well seeded in 96-well plates in triplicates in assay medium (RPMI + GlutaMax or DMEM plus 20 mM HEPES [Invitrogen]). Cells were cultured at 37°C, 5% CO2 for 4–6 h before the addition of antibodies and effector cells. Zolbetuximab and control isotype antibodies were serially diluted in PBS (Invitrogen) at concentrations ranging from 0.26 ng/mL to 200 µg/mL. PBMCs were added at an E:T ratio of 40:1 and assay plates were incubated for 24 ± 1 h at 37°C, 5% CO2. D-luciferin (BD Biosciences) was added and the reaction incubated for 80 min at room temperature in the dark. 8% Triton X-100/PBS (AppliChem) was used instead of antibody as control for maximum lysis. Assay medium without antibodies served as the negative control. Specific killing was detected and calculated as described in the supplement.
Complement-dependent cytotoxicity (CDC) analysis
CLDN18.2+ luciferase-expressing PC cells (as target cells) were generated as described for ADCC. 1.5 × 104 target cells/well were seeded in triplicates in RPMI + GlutaMax/20 mM HEPES (Invitrogen) in 96-well plates (Nunc) and cultured at 37°C, 5% CO2 for 24 h before the addition of zolbetuximab or isotype control (rituximab). Antibodies were prepared as serial dilution in PBS (Invitrogen) with assay concentrations ranging from 31.7 ng/mL to 100 µg/mL and added to target cells mixed with 20% final concentration of healthy human serum pool in assay medium as complement source. Assay medium without antibody served as negative reference. Cells were incubated for 80 min at 37°C, 5% CO2 before D-luciferin was added and the reaction was incubated for another 45 min at room temperature in the dark. Specific killing was detected and calculated as described in the supplement.
In vivo animal studies
All mouse experiments complied with the national regulations and ethical guidelines for experimental animal studies.
A total of 5 × 106 to 1 × 107 MIA PaCa-2~ CLDN18.2 or 8.5 × 106 to 1 × 107 BxPC-3~ CLDN18.2 cells, in a volume of 200 µL PBS, were injected subcutaneously into the left flank of female Hsd:Athymic Nude-Foxn1nu mice. Mice were treated twice a week with 0, 200 µg, or 400 µg zolbetuximab by alternating IV and intraperitoneal injections starting at Day 3 or Day 4 post-graft. In combination studies, low-dose gemcitabine (50 or 100 mg/kg; Hexal) was IP injected 24 h before zolbetuximab treatment for 6 weeks. Tumor size and body weight were recorded twice a week. Tumor volumes (V) were calculated with the following formula:
Mean tumor growth inhibition (TGI) was calculated as the differences in mean tumor volumes in the active treatment groups compared to the control group according to the following formula:
Day x was defined as the time point the first animal in the control group was euthanized due to a tumor volume of > 1400 mm3 or an ulcerous tumor. Tumor samples were cryoconserved or fixed in 4% formalin for subsequent analysis.
For lung metastases studies, mice were inoculated by IV injection of 2 × 106 SUIT-2~ CLDN18.2 or Patu 8988S human PC cells into the tail vein of female Hsd:Athymic Nude-Foxn1nu mice. Alternating IV/IP injections with 200 µg zolbetuximab or isotype (with 100 mg/kg IP gemcitabine in combination studies) were initiated on Day 3 post-graft (SUIT-2~ CLDN18.2) or two weeks (Patu 8988S) after tumor injection and given twice per week. Mice were euthanized at different time points, or after the animals in the control group showed clear physiologic signs of metastatic disease (eg, loss of weight, weakness, shortness of breath). All organs were macroscopically analyzed for the presence of metastasis. Patu 8988S and SUIT-2~ CLDN18.2 engraftments displayed macroscopically visible metastases in lungs and lungs/livers, respectively. The lungs were dissected into four equal pieces, two of those (lung: upper right and lower left lobe) were used for the isolation of genomic DNA. The remaining two pieces were formalin-fixed and paraffin embedded. Lungs were cryoconserved or fixed in 4% formalin for subsequent analysis.
Statistical analysis
Statistics were calculated with GraphPad Prism version 6.00 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). For correlation analysis, data were log-transformed, linear regression was plotted, and a Spearman’s rank correlation coefficient (rs) with the corresponding P value was calculated. Estimates for group differences of not-normally distributed data were calculated with a Mann–Whitney U-test (two-tailed) for comparison of two treatment groups or with a Kruskal–Wallis test followed by Dunn’s multiple comparisons test for multiple comparisons. Statistical analysis of normally distributed data sets was calculated with a Student’s t test for comparison of two unpaired treatment groups or by a one-way ANOVA followed by Tukey’s multiple comparisons test for multiple comparison. Differences in survival between groups were calculated with a log-rank (Mantel–Cox) test. All statistical tests were two-sided and differences were considered significant if P < .05.
Funding Statement
This study was supported by Ganymed Pharmaceuticals GmbH (formerly Ganymed Pharmaceuticals AG), a wholly owned subsidiary of Astellas Pharma, Inc.
Acknowledgments
The authors would like to thank Corinna Heinz, Marie Kühnle, Marlene Knippenberg, Daniela Benkenstein, Daniela Kirsch, Martina Reis, Mario Klos and Pino Cagna (all former Ganymed), for assistance; Tim Beißert (TRON) for lentiviral transduction of human PC cells; and Stefan Jacobs for conducting the mouse experiments.
This study was funded by Ganymed Pharmaceuticals GmbH (formerly Ganymed Pharmaceuticals AG), a wholly owned subsidiary of Astellas Pharma, Inc. Financial support for this manuscript development, including medical writing under authors’ guidance and editorial assistance by Drs Amlan RayChaudhury and Regina Switzer (SuccinctChoice Medical Communications, Chicago, IL), was provided by Astellas Pharma, Inc. (Northbrook, IL, USA).
Disclosure of Potential Conflicts of Interest
Ӧzlem Türeci was a co-founder, shareholder, and former CEO of the privately owned company Ganymed Pharmaceuticals GmbH. Dr. Türeci has received consultancy fees from Astellas Pharma, Inc. and has licensed patents relevant to the work, which have been acquired by Astellas Pharma, Inc. Rita Mitnacht-Kraus and Stefan Wöll are former employees of Ganymed Pharmaceuticals GmbH and have patents relevant to the work, which have been acquired by Astellas Pharma, Inc. Ugur Sahin was a co-founder of Ganymed Pharmaceuticals GmbH and is the founder, shareholder, and CEO of the privately owned company BioNTech. Tomohiro Yamada is an employee of Astellas Pharma, Inc. Prof. Sahin has received consultancy fees from Ganymed Pharmaceuticals GmbH and has licensed patents relevant to the work, which have been acquired by Astellas Pharma, Inc
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.Scott AM, Wolchok JD, Old LJ.. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 2.Kellner C, Otte A, Cappuzzello E, Klausz K, Peipp M. Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy. Transfus Med Hemother. 2017;44(5):327–336. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud HA, Eliaou JF, Lafont V, Bonnefoy N, Gros L. Tumor antigen-targeting monoclonal antibody-based immunotherapy: orchestrating combined strategies for the development of long-term antitumor immunity. Oncoimmunology. 2014;3(9):e955684. doi: 10.4161/21624011.2014.955684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan D, Ledbetter JA, Press OW. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother. 2000;48(12):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, Oomen LA, Peipp M, Valerius T, Slootstra JW, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 6.Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624–7634. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 7.Trarbach T, Schuler M, Zvirbule Z, et al. Efficacy and safety of multiple doses of IMAB362 in patients with advanced gastro-esophageal cancer: results of a phase II study. Ann Oncol. 2014;25(suppl 4):iv218. doi: 10.1093/annonc/mdu203. [DOI] [Google Scholar]
- 8.Schuler M, Al-Batran S-E, Zvirbule Z, et al. Final results of the FAST study, an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without the anti-CLDN18.2 antibody IMAB362 as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. Ann Oncol. 2016;27(Suppl 6):vi207–vi242. doi: 10.1093/annonc/mdw141. [DOI] [Google Scholar]
- 9.Morlock R, Turnbull J, Blahut S, et al. Health-related quality-of-life results from the fast study, a phase II trial of epirubicin, oxaliplatin, and capecitabine with or without IMAB362 in patients with advanced CLDN18.2+ gastric and gastroesophageal junction adenocarcinoma. ASCO Gastrointestinal Cancers Symposium; 2018; San Francisco, CA. [Google Scholar]
- 10.Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, Schulick R, Winter J, Sharma R, Maitra A, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32(2):188–196. doi: 10.1097/PAS.0b013e31815701f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Kim KS, Kim TJ, Hong SP, Song SY, Chung JB, Park SW. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol Rep. 2011;25(4):971–978. doi: 10.3892/or.2011.1132. [DOI] [PubMed] [Google Scholar]
- 12.Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Türeci Ö. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014;134(3):731–739. doi: 10.1002/ijc.28400. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, Kokudo N, Fukayama M. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem. 2011;59(10):942–952. doi: 10.1369/0022155411420569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB, Binder E, Cardin DB, Cha C, et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(8):1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 15.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 16.FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication [accessed 2017 June 10]. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm.
- 17.Abraxane [package insert] Summit, NJ: Abraxis BioScience, LLC; 2015. [Google Scholar]
- 18.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, Ferrari D, Barni S, Aitini E, Zagonel V, et al. Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: A GISCAD randomized phase II study. Dig Liver Dis. 2014;46(2):182–186. doi: 10.1016/j.dld.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedaeinia R, Avan A, Manian M, Salehi R, Ghayour-Mobarhan M. EGFR as a potential target for the treatment of pancreatic cancer: dilemma and controversies. Curr Drug Targets. 2014;15(14):1293–1301. [DOI] [PubMed] [Google Scholar]
- 22.Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW, et al. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017;18(4):486–499. doi: 10.1016/S1470-2045(17)30084-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Rocken C, Nitsche B, Hosius C, Gschaidmeier H, Kahl S, Malfertheiner P, Ebert MPA. The tyrosine kinase inhibitor imatinib fails to inhibit pancreatic cancer progression. Cancer Lett. 2006;233(2):328–337. doi: 10.1016/j.canlet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Sahin U, Schuler M, Richly H, Bauer S, Krilova A, Dechow T, Jerling M, Utsch M, Rohde C, Dhaene K, et al. A phase 1 dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-esophageal junction cancer. Eur J Cancer. 2018;100:17–26. doi: 10.1016/j.ejca.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


