ABSTRACT
Therapeutic vaccination as a treatment option for HPV-induced cancers is actively pursued because the two HPV proteins E6 and E7 represent ideal targets for immunotherapy, as they are non-self and expressed in all tumor stages. MHC-humanized mice are valuable tools for the study of therapeutic cancer vaccines – given the availability of a suitable tumor model. Here, we present for the first time an HPV16 tumor model suitable for fully MHC-humanized A2.DR1 mice, PAP-A2 cells, which in contrast to existing HPV16 tumor models allows the exclusive study of HLA-A2- and DR1-mediated immune responses, without any interfering murine MHC-presented epitopes. We used several HPV16 epitopes that were shown to be presented on human cervical cancer cells by mass spectrometry for therapeutic anti-tumor vaccination in the new tumor model. All epitopes were immunogenic when rendered amphiphilic by incorporation into a molecule containing stearic acids. Prophylactic and therapeutic vaccination experiments with the epitope E7/11–19 demonstrated that effective immune responses could be induced with these vaccination approaches in A2.DR1 mice. Interestingly, the combination of E7/11–19 with other immunogenic HPV16 E6/E7 epitopes caused a reduction of vaccine efficacy, although all tested combinations resulted in a survival benefit. In summary, we present the first HPV16 tumor model for exclusive studies of HLA-A2-mediated anti-HPV tumor immune responses and show anti-tumor efficacy of minimal epitope vaccines.
KEYWORDS: Cancer immunotherapy, therapeutic vaccination, HLA-humanized mouse model, human papillomavirus (HPV), A2.DR1, PAP-A2
Introduction
High-risk types of human papillomavirus (HPV) are responsible for ca. 4.5% of the worldwide new cancer cases every year.1 Cervical cancer is the most prevalent among HPV-related cancer cases, but HPVs can also cause other genital, anal and oral cancers.1 The two high-risk HPV types HPV16 and HPV18 are responsible for 60% and 15% of all HPV-mediated cancer cases, respectively.2 A vaccine preventing the infection with HPV16 and HPV18 has been brought to the market in 2006, and a more recent version protecting against infection with the seven most prevalent HPV high-risk types in 2014.3 However, the available prophylactic vaccines are not effective against already established HPV infections or HPV-mediated transformed lesions.4 Therefore, people who could have been or have been exposed to HPV do not represent a target group for prophylactic vaccination and, thus, are still at risk to develop HPV-mediated cancers. Additionally, even in developed countries vaccination efforts are far from reaching the whole population e.g. only 49.5% of 13–17 year old girls (boys: 37.5%) in the USA received at least two doses of the vaccine5 and only 44.6% of 17-year old females (boys: no vaccination recommendation, very low coverage) in Germany.6 Due to roll-out challenges and the requirement of a constant cooling chain for the existing prophylactic vaccines, vaccination coverage in countries outside the developed world is still lower,7 leaving the vast majority of people worldwide at risk.
Therefore, vaccines that are effective in a therapeutic setting are being investigated (reviewed in refs. 8, 9). These vaccines can target premalignant lesions as well as carcinomas. Several clinical studies for therapeutic cervical cancer vaccines have been8,10 and are currently being conducted with a variety of vaccine constructs like RNA-based or emulsion-based vaccines (e.g. NCT03418480, NCT02865135). HPV-induced cancers or premalignant lesions represent ideal targets for therapeutic cancer vaccination since the transformation of the infected cells is caused by the expression of two viral proteins: E6 and E7. These proteins cause continuous cell divisions and hamper the induction of apoptosis.2 Since the malignant phenotype relies on the expression of E6 and E7, cancer cells cannot evade the immune system’s attack by downregulating the expression of these proteins, which are therefore expressed in all malignant cells in all tumor stages.9 However, the development of a therapeutic vaccine against HPV cancers is challenging since the virus employs several mechanisms to avoid detection by the immune system (reviewed in ref. 11). These immune evasion mechanisms for example lead to destruction of HPV epitopes so that these epitopes are presented only at very low abundance on the surface of HPV-positive cancer cells.12
Mass spectrometry (MS) nowadays allows to verify the surface presentation of major histocompatibility complex (MHC)-bound epitopes.13 To verify the presence of HPV epitopes on the cell surface, we developed a LC-MS3 approach especially tailored to detect low abundant MHC class I epitopes.14 Our study demonstrated that out of 17 monitored HPV E6/E7-derived human leukocyte antigen (HLA)-A2-binders, eleven were found to be presented on the cell surface of human cervical cancer cells. Knowing the bona fide presented HPV target epitopes on these cells allowed us to focus our therapeutic vaccine on these targets to avoid unproductive immune responses and induce effective anti-tumor responses.
Most preclinical studies for the development of a therapeutic vaccine targeting HPV16 E6/E7 were performed in C57BL/6 mice with the TC-1 tumor model (C57BL/6 lung cells transduced with HPV16 E6/E7 and a constitutively activated version of H-ras (H-ras V12) to render the cells tumorigenic).15 This model limits the choice of epitopes to murine epitopes and therefore cannot be used for testing of epitopes that are presented on HLA molecules. Nowadays, the use of HLA-transgenic mice, which were generated to study vaccination approaches with human epitopes in a small animal model, allows overcoming this problem.16 The most commonly used transgenic mouse model to date is the AAD mouse,17 which – in addition to having all murine MHCs – carries an HLA-A2 transgene. Two HPV16 tumor models suitable for AAD mice have been published: TC-1/A2, which are TC-1 cells transduced with HLA-A2,18 and HLF16 cells, which are heart fibroblasts from AAD mice that – like the TC-1 cell line – were transduced with HPV16 E6/E7 (with E7 lacking the immunodominant murine epitope in this case) and H-ras V12.19,20 The immunodominant H-2Db-restricted epitope E7/49–57 was deleted in HLF16 cells since it has been shown that epitopes restricted to murine MHCs are preferred over HLA-A2-restricted epitopes in humanized mice.18 Since no additional H-2Db-restricted HPV16 epitopes are known, H-2Db-restricted anti-HPV16 immune responses can be virtually excluded by this approach. However, as H-2Kb-restricted immune responses are not excluded,21 anti-tumor responses observed in this model are still not necessarily HLA-A2-restricted. Furthermore, H-2Db- and H-2Kb-restricted responses against neoepitopes derived from mutations can be induced by antigen spreading and the AAD model does not allow for the study of human MHC class II epitopes.
In contrast, the A2.DR1 mice used in this study22–24 were shown to mount functional CD4+ and CD8+ T cell responses against multiple epitopes restricted by HLA-A2 and HLA-DR1.22 At the same time, they are completely devoid of murine MHCs and therefore allow the exclusive examination of HLA-A2 and HLA-DR1-restricted immune responses. Here, we present the first HPV16 E6+/E7+ tumor model for a MHC-humanized mouse strain that is completely devoid of murine MHC molecules. We used this new model to examine vaccinations with several HLA-A2-restricted HPV16 epitopes that are presented on human cervical cancer cells for their anti-tumor effects.
Results
Generation of an A2.DR1 compatible, HPV16 E6+/E7+ tumor model
To generate an HPV16 E6+/E7+, A2.DR1 compatible tumor model, the chemically induced sarcoma cell line 2277NS,25 derived from A2.DR1 mice, was lentivirally transduced with the E6/E7 oncoproteins of HPV16 (for the vector construct, see Suppl. Figure 1). Clonal cell lines were established and one clone was selected for tumorigenicity and E6/E7 expression (data not shown). This cell line was named PAP-A2 (papilloma HLA-A2). Western blot analysis proved that the parental 2277NS cell line does not express E6 and E7. The cervical cancer-derived cell line CaSki expresses HPV16 E6/E7, whereas PAP-A2 cells express the introduced tagged versions of HPV16 E6/E7, and still do so after having grown as a tumor (Figure 1A). Interestingly, the expression of E6 is markedly lower in CaSki cells compared to PAP-A2, while the expression levels of E7 are much higher in CaSki cells than in PAP-A2 cells. Tumor growth kinetics of the new tumor model were established by injecting different numbers of PAP-A2 cells into A2.DR1 mice (Figure 1B, C). 1.5x106 cells were chosen as tumor cell injection number for all subsequent experiments since this number generated a tumor take rate of approximately 90%, while still producing tumors growing slowly enough to allow therapeutic interventions.
Figure 1.
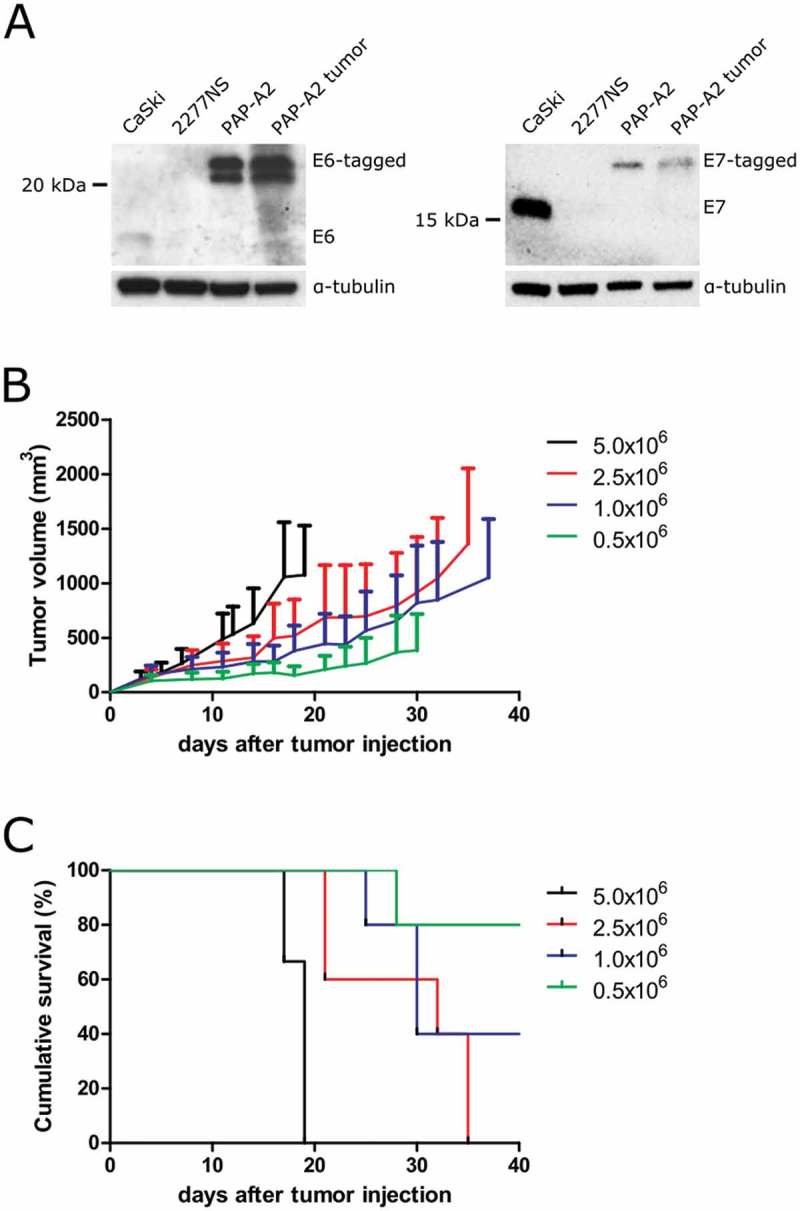
Characterization of the novel HPV16 E6/E7-expressing A2.DR1 tumor cell line, PAP-A2. (A) PAP-A2 cells were lysed and analyzed for E6 (left) and E7 (right) expression by Western blot. CaSki: HPV16+ cervical cancer cells, positive control; 2277NS: untransduced parental cell line of PAP-A2, negative control; PAP-A2: cell line lysate; PAP-A2 tumor: lysate generated from a PAP-A2 tumor having grown in an A2.DR1 mouse. The Western blots shown are representative of three Western blots. (B) Tumor growth curves of PAP-A2 cell injection number titrations in A2.DR1 mice. Mean ± SD are shown. (C) Mouse survival curves for the experiment shown in B. n = 3 (5x106) or n = 5 (other groups) mice per group.
Selected HPV16 epitopes are immunogenic in A2.DR1 mice and HPV16-epitope specific T cells specifically lyse PAP-A2 cells
Amphiphilic vaccine constructs have been shown to increase the immunogenicity of minimal epitopes as well as of synthetic long peptides.26,27 Therefore, we used this approach to generate amphiphilic versions (Figure 2A) of three HPV16 HLA-A2-restricted epitopes found on CaSki human HPV16+ cervical cancer cells: E7/7–15, E7/11–19, E7/82–90.14 We also included the epitope E6/25–33 in our studies as an example of an E6-derived epitope. These amphiphilic minimal epitope constructs (= amph-peptides) were injected s.c. with 50 µg poly I:C (pI:C) as an adjuvant (vaccination schedule: Figure 2B, upper panel). All amph-peptide vaccines induced CD8+ immune responses in A2.DR1 mice as measured by IFN-γ intracellular staining after stimulation with the respective peptide (Figure 2B, lower panel; IFN-γ mean fluorescence intensity (MFI) values: Suppl. Figure 2). Furthermore, a flow cytometry-based cytotoxicity assay (ref. 28 and Suppl. Figure 3) showed that CD8+ T cells isolated from vaccinated mice and cultured for 7 days in the presence of the respective peptide were able to specifically kill the cognate epitope-loaded parental cell line 2277NS, but not irrelevant epitope-loaded 2277NS cells (Suppl. Figure 4). Importantly, also HPV16 E6+/E7+ PAP-A2 tumor cells, but not the untransduced HPV-negative parental 2277NS cells were killed by the HPV-specific T cells (Figure 2C), proving HLA-A2 presentation of the selected epitopes on our tumor model.
Figure 2.
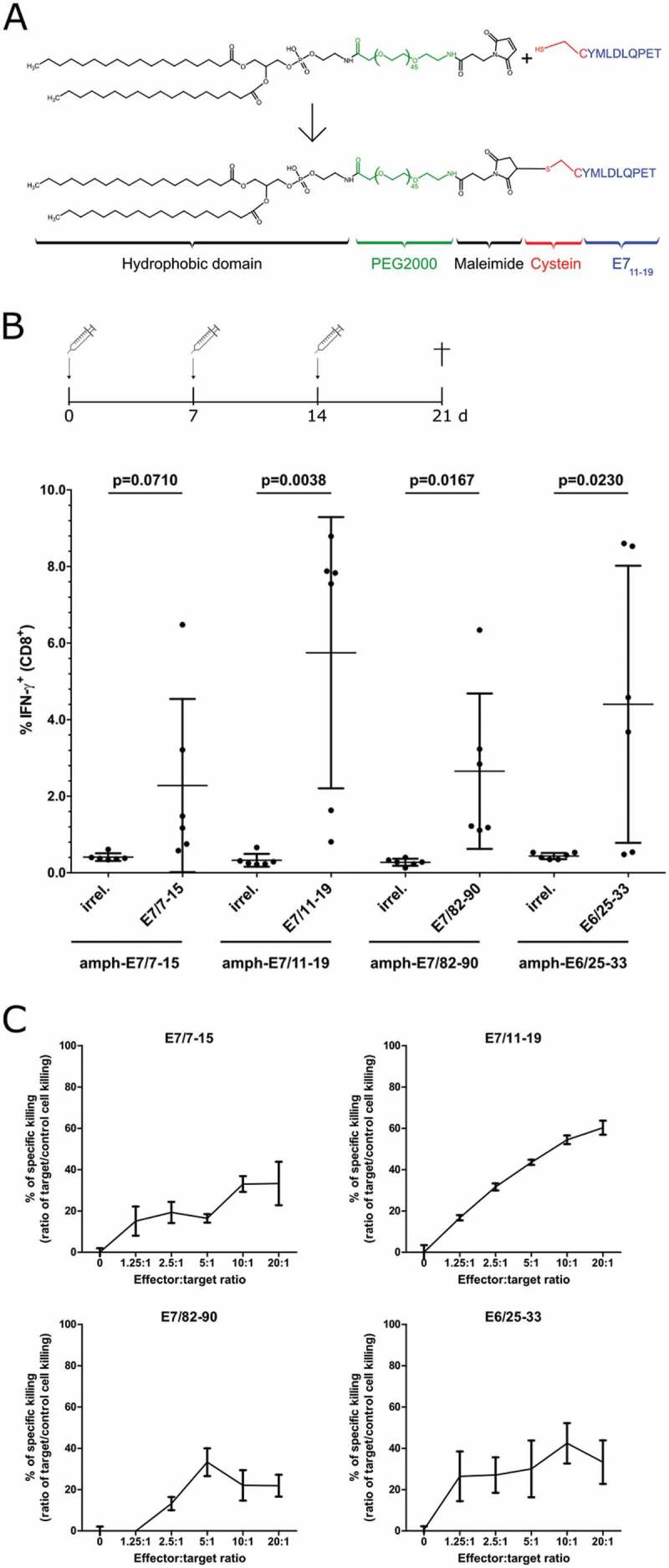
Induction of anti-HPV16 E6/E7 responses in A2.DR1 mice and specific killing of PAP-A2 tumor cells by vaccination-induced CD8+ T cells. (A) Structure and modular synthesis of amph-peptides, exemplified by amph-E7/11–19. (B) A2.DR1 mice (n = 6 per group) were injected with indicated amph-peptides + 50 µg pI:C according to the indicated schedule. Splenocytes were isolated and incubated with either an irrelevant HLA-A2-binding peptide or the cognate peptide. After 5 h of incubation, cells were stained for intracellular IFN-γ and analyzed by flow cytometry. Frequencies of IFN-γ+ cells among CD8+ T cells after incubation with the irrelevant or the cognate peptide are shown. Each dot represents one mouse, mean ± SD is indicated. Statistical analysis was performed with Student’s unpaired t test. (C) Splenocytes of amph-peptide-vaccinated A2.DR1 mice were isolated and cultured 7 days in the presence of the respective indicated cognate peptide. CD8+ T cells were isolated by untouched MACS isolation. CD8+ T cells (effector cells) were added to wells containing a 1:1 mixture of specific target cells (PAP-A2, CFSE labeled) and control target cells (parental 2277NS cells, FR labeled). 48 h after addition of CD8+ T cells, cells were analyzed via flow cytometry. “% of specific killing” was calculated from the ratio of specific to control target cell killing. The experiment was performed once in triplicates; error bars: SD.
In these experiments, vaccination of A2.DR1 mice with the epitope E7/11–19 induced the highest frequencies of IFN-γ+ CD8+ T cells and E7/11–19-specific CD8+ T cells exhibited a high capability to kill PAP-A2 cells (Figure 2B, C). Based on these results, we chose this epitope as the lead epitope for the subsequent anti-tumor vaccination experiments.
Vaccination with amph-E7/11–19 mediates anti-PAP-A2 tumor effects in prophylactic and therapeutic settings
For easier observation of any anti-tumor efficacy, we first tested a prophylactic vaccination setting. Groups of 15 mice were injected three times with amph-E7/11–19, the amphiphilic carrier moiety (vehicle control) or left untreated and challenged with PAP-A2 cells 7 days after the last vaccination (schedule: Figure 3A, right panel). Vaccination with amph-E7/11–19 significantly increased the survival compared to the control mice. The median survival of mice was more than doubled in the prophylactically vaccinated group (41 days for amph-E7/11–19-treated mice compared to 17 days for vehicle-treated mice) (Figure 3A, B).
Figure 3.
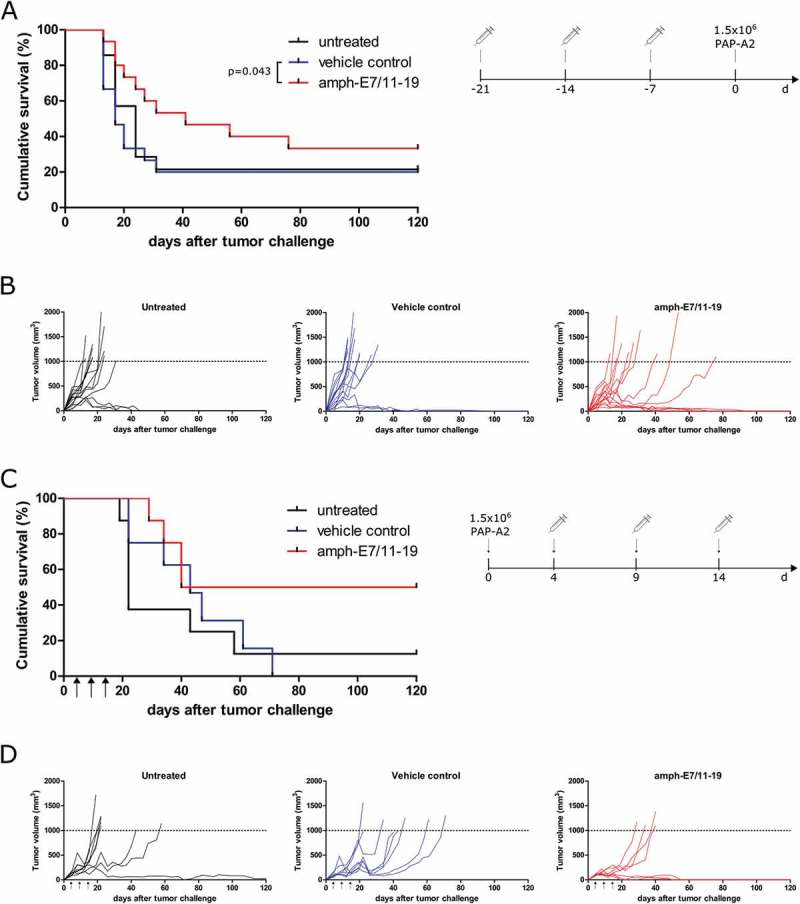
Prophylactic and therapeutic vaccination with amph-E7/11–19 reduces the growth of PAP-A2 tumors. (A, B) A2.DR1 mice (n = 15 per group for amph-E7/11–19 and vehicle, n = 14 for untreated) were treated with three injections of amph-E7/11–19 or controls and were challenged 7 days after the last vaccination with 1.5x106 PAP-A2 cells. A: Cumulative survival curves of groups, B: Tumor growth curves of individual mice. (C, D) A2.DR1 mice (n = 8 per group) were injected with 1.5x106 PAP-A2 cells and were vaccinated as indicated with amph-E7/11–19, vehicle control or left untreated. C: Cumulative survival curves of groups, D: Tumor growth curves of individual mice. Statistical analysis for differences in survival was performed with the Gehan-Breslow-Wilcoxon test.
To assess the clinically more relevant therapeutic setting, three vaccinations with amph-E7/11–19 starting 4 days after tumor injection were performed (schedule: Figure 3C, right panel). This treatment increased the survival of tumor-bearing mice and led to complete tumor rejections in 50% of the animals (Figure 3C, D). However, the separation of curves only became apparent after approximately 50 days, thus the difference in survival between HPV-vaccinated and control mice is not significant. In addition, also some animals in the untreated and vehicle control-treated groups rejected their tumor. This could be explained by the fact that we only observed a tumor take of approximately 90% with the chosen number of injected PAP-A2 cells for tumor injections (see “Generation of tumor model”, Figure 1).
Vaccination with other amphiphilic HPV16 epitopes shows less therapeutic efficacy than vaccination with amph-E7/11–19
After the experiments conducted with the lead epitope E7/11–19, we also tested the other epitopes shown to be immunogenic in A2.DR1 mice (Figure 2B) for anti-tumor efficacy. All tested vaccine formulations resulted in a survival benefit when compared to the vehicle control group in a therapeutic set-up (Figure 4), however, all observed effects were less pronounced than the effect observed for the amph-E7/11–19 vaccine (Figure 3C, D). No significant differences in survival between the groups could be observed.
Figure 4.
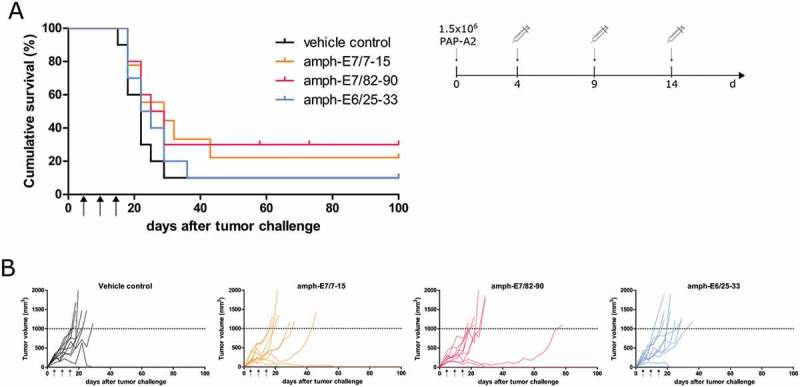
Therapeutic vaccination with the amphiphilic HPV16 E6/E7 epitopes E7/7–15, E7/82–90 and E6/25–33 only weakly reduces the growth of PAP-A2 tumors. (A, B) A2.DR1 mice (n = 10 per group) were injected with 1.5x106 PAP-A2 cells and were vaccinated as indicated with amph-peptides or the vehicle control. A: Cumulative survival curves of groups, B: Tumor growth curves of individual mice. Statistical analysis for differences in median survival was performed with the Gehan-Breslow-Wilcoxon test. All differences were found to be non-significant.
Vaccination with combinations of amph-HPV16 E6/E7 epitopes leads to reduced E7/11–19 immune responses and reduced anti-tumor effects
To assess if the combination of different amph-HPV16 E6/E7 epitopes could induce higher overall frequencies of HPV16 E6/E7-specific CD8+ T cells and thus lead to increased anti-tumor effects, we injected mice with various combinations of the before established amph-peptides. We observed that upon combining amph-E7/11–19 with other amph-peptides, CD8+ T cells of most mice still responded to restimulation with E7/11–19 with the production of IFN-γ (Suppl. Figure 5), but the frequencies of E7/11–19-specific CD8+ T cells were highly reduced (Figure 5) compared to single amph-E7/11–19 vaccination (Figure 2B). Furthermore, also the median frequencies of E7/7–15 and E6/25–33-specific CD8+ T cells were reduced in the case of combination vaccination (Figure 5). Interestingly, the response against E7/82–90 was not influenced as strongly by combination vaccination but was similar in the combination vaccinations to the response in single E7/82–90 vaccination (Figure 5). We tested if the reduced T cell frequencies would translate into worse survival in therapeutic vaccination experiments and found that only the combination E7/11–19 with E7/82–90 had a significant effect on survival (Figure 6). However, not even this treatment increased the rate of complete tumor rejections compared to vehicle control-treated animals.
Figure 5.
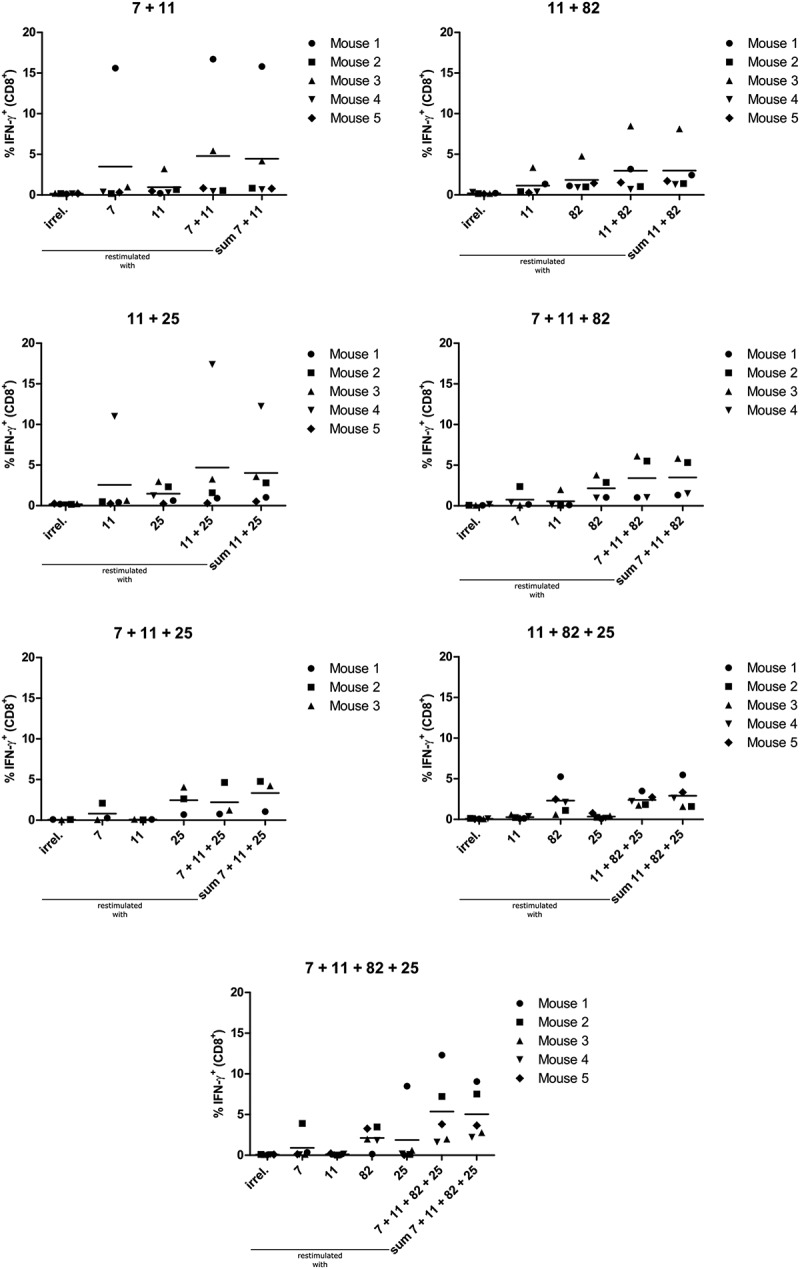
Vaccination with combinations of amphiphilic HPV16 E6/E7 epitopes leads to reduced frequencies of E7/7–15, E7/11–19 and E6/25–33-specific CD8+ cells compared to single vaccination. A2.DR1 mice (n = 5 per group) were vaccinated as indicated in Figure 2B with the combinations of amph-peptides shown in the respective graph’s title. Splenocytes were isolated and incubated with either an irrelevant HLA-A2-binding peptide, the cognate peptide or a combination of all cognate peptides. After 5 h of incubation, cells were stained for intracellular IFN-γ and analyzed by flow cytometry. Frequencies of IFN-γ+ cells among CD8+ T cells after incubation with the indicated peptide are shown. The last group in each graph shows the mathematical sum of the frequencies of all HPV16 E6/E7 epitopes used in this treatment group. Each dot represents one mouse, mean ± SD is indicated. Vaccination with amph-peptides is abbreviated as follows: 11 = amph-E7/11–19, 7 = amph-E7/7–15, 82 = amph-E7/82–90, 25 = amph-E6/25–33.
Figure 6.
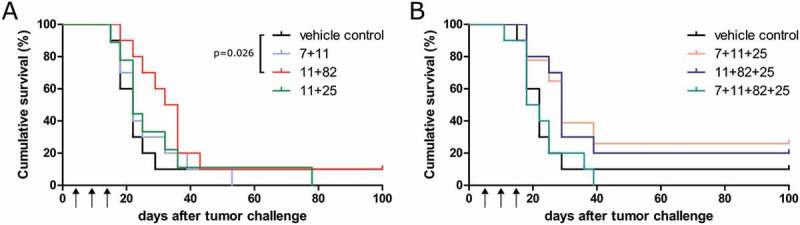
Therapeutic vaccination with combinations of amphiphilic HPV16 E6/E7 epitopes leads to reduced anti-tumor effects compared to amph-E7/11–19 single vaccination. A2.DR1 mice (n = 10 per group) were injected with 1.5x106 PAP-A2 cells and were vaccinated as indicated in the group’s title and in Figures 3C and 4A with amph-peptides or the vehicle control. Cumulative survival curves of groups are shown. Vaccination with amph-peptides is abbreviated as follows: 11 = amph-E7/11–19, 7 = amph-E7/7–15, 82 = amph-E7/82–90, 25 = amph-E6/25–33. Statistical analysis for differences in survival was performed with the Gehan-Breslow-Wilcoxon test.
Discussion
Many therapeutic anti-HPV16 vaccination approaches have yielded promising results in preclinical studies. Translational success, however, has been limited so far. To improve therapeutic efficacy and translatability into a clinical setting, we consider two aspects to be of special importance: First, the use of vaccines incorporating epitopes that are bona fide presented on HPV-transformed tumor cells and second, the use of a mouse model that allows the exclusive assessment of human epitopes. All previously established HLA-humanized mouse models still permit immune responses involving murine H-2-restricted epitopes.
In this study, we used A2.DR1 mice to find a vaccination approach eliciting robust immune responses against minimal HPV16-derived, HLA-A2-restricted peptides. Using amph-peptides as vaccination constructs, we tested the immunogenicity of three HPV16 E7 epitopes found to be presented on human cervical cancer cells.14 To this selection, we added one example of an E6 epitope. The modular synthesis of the amph-peptides allows the use of virtually every minimal epitope or also synthetic long peptides.27 We also chose this vaccine formulation because it has been shown that vaccines based on commonly used oil-emulsions like incomplete Freund’s adjuvant can retain effector T cells at the site of vaccine injection and render them dysfunctional.29,30 Therefore, it is important to use non-persistent vaccine formulations like the amph-peptides used in this study to achieve maximal anti-tumor effects. These molecules attach to albumin and are thus transported to the lymph nodes where they are taken up by antigen presenting cells, thus efficiently priming immune responses.27 Indeed, we could show that all four tested HPV16 E6/E7-derived HLA-A2-restricted epitopes are immunogenic in A2.DR1 mice when rendered amphiphilic. Two of these epitopes (E7/11–19, E6/25–33) have never been tested for their immunogenicity in humanized mice before. All E7 epitopes are also known to be immunogenic in humans since CD8+ T cells against these epitopes could be found in peripheral blood of healthy donors.14 Taken together, these findings strengthen our concept that A2.DR1 mice are a suitable model to assess HLA-A2-restricted immune responses in vivo.
Since there was no HPV16 E6+/E7+ tumor model suitable for A2.DR1 mice available, we here established the first transplantable HPV16 tumor model suitable for this fully MHC-humanized mouse strain. We generated the cell line PAP-A2 to be able to study anti-tumor effects of our vaccination approach in vivo. This new model offers clear advantages over existing models18,19 because it not only allows the exclusive study of HLA-A2 mediated immune responses but also permits the use of HLA-DR1-restricted epitopes in future studies. In this first study with the new tumor model, we deliberately limited our experiments to the use of mass spectrometry (MS)-verified HLA-A2-restricted epitopes, but we plan to conduct studies with HLA-DR1-restricted HPV16 epitopes once their MS verification has been achieved. The subcutaneous injection of 1.5x106 PAP-A2 cells induced a tumor take of approximately 90%, which poses a limitation of this cell line as a tumor model since mouse numbers have to be adjusted to this fact. Inoculation with 1.5x106 PAP-A2 cells was followed by aggressive growth leading to large tumors within 2–3 weeks. We found this cell number to be the best compromise between a high tumor take-rate and tumor growth slow enough to allow for treatment intervention. Despite the aggressive growth displayed by PAP-A2 tumor cells, vaccination with the E7/11–19 epitope in a prophylactic setting was able to induce protective immunity against a challenge with PAP-A2 cells. Additionally, vaccination experiments conducted in a therapeutic setting demonstrated that vaccination with E7/11–19 led to the rejection of tumors in 50% of tumor bearing mice. In previous studies, vaccination with amph-peptides in the TC-1 model in C57BL/6 mice often resulted in higher tumor rejection rates.26,27 One factor likely limiting the CD8+ T cell-mediated anti-tumor response in A2.DR1 mice is that these mice have only one third of the CD8+ T cells that can be found in the PBMCs of a C57BL/6 WT mouse. Furthermore, TC-1 tumors present the extremely immunogenic H-2Db-restricted E7/49–57 epitope. This could represent a reason for the limited translatability of the favorable results of therapeutic vaccinations obtained in C57BL/6 mouse studies so far: HLA-A2-restricted E6/E7 epitopes are not as immunogenic as this immunodominant epitope. The direct comparison of our results to results obtained in AAD mice is also not possible, because AAD mice still allow for H-2-mediated immune responses and the results were obtained with different cell lines (TC-1/A2, HLF16), which could have different susceptibility to T cell lysis, and with different vaccine formulations and schedules.18–20,31 Interestingly, E7/11–19 was the epitope against which the highest T cell responses could be observed, both in vaccinated A2.DR1 mice in the present study, as well as in a previous study in healthy donors with CD8+ T cell responses against HPV16.14 Extrapolating from this observation, our new tumor model could give more realistic expectations about results that can be achieved in patients.
However, it has to be mentioned that none of the tested vaccination regimens could induce tumor rejection in all tested mice, although all mice used in vaccination-only experiments (Figure 2) had CD8+ T cells specific for the respective epitope. Combination of our non-persistent vaccine with checkpoint inhibitors32-34 and other immune modulators like IL-2 or anti-tumor antibodies35,36 in future experiments could improve the immune response leading to a higher proportion of tumor rejection. Also treatment regimens commencing later than four days after tumor inoculation were not successful (data not shown), most likely because the tumor mass had already grown too large and the tumor microenvironment had become too immunosuppressive. This situation of a microenvironment that is not conducive for immunotherapeutic intervention also occurs in the clinical setting37 and represents a challenge for therapeutic vaccination approaches. Therefore, we consider it best to treat low stage cancers or, with even better chances for success, precancerous lesions (e.g. CIN-I or CIN-II in the cervix) that express HPV16 E6/E7 but have not yet established a strongly immunosuppressive microenvironment. Since the potential side effects of HPV-targeted therapeutic vaccination should be very limited, this treatment option could be a true alternative to surgery. Surgery of cancer precursor lesions comes with side effects like the risk of premature deliveries after the conization of cervical precursor lesions or severe loss in quality of life after surgery to remove anal or oral HPV lesions. Treatment of HPV-positive precursor lesions or low stage cancers with immunotherapeutic approaches could also be promising for a different reason: It has been shown that with increasing cancer stage the cellular amount of the immunopeptidase ERAP1 also increases, leading to the potential destruction of immunogenic epitopes.38 According to this finding, precancerous lesions would represent better targets for therapeutic vaccinations since the presentation of immunogenic HPV epitopes should be higher than in late stage cancers.
Encouraged by the positive results of our therapeutic vaccinations with E7/11–19, we reasoned that the inclusion of several epitopes into the vaccine would give rise to higher overall numbers of potentially tumor-reactive CD8+ T cells and thus yield even better anti-tumor effects. Remarkably, the vaccination of A2.DR1 mice with combinations of amph-peptides strongly decreased the frequency of E7/11–19-specific as well as of E7/7–15 and E6/25–33-specific CD8+ T cells. This decrease was correlated with a decrease in overall survival in therapeutic tumor experiments. Since we observed that the single vaccination with E7/11–19 gave rise to substantial survival benefits, it is likely that the worse survival in the combination treatments is mainly based on the decreased frequencies of E7/11–19-specific CD8+ T cells. The general opinion that vaccines which include more than one epitope yield better anti-tumor responses39 could therefore not be justified by our results. Interestingly, the decreased frequencies of e.g. E7/11–19-specific CD8+ T cells cannot be explained by a simple immunodominance mechanism since e.g. in the case of the 7 + 11 treatment group, frequencies of both E7/7–15 and E7/11–19-specific CD8+ T cells were decreased compared to the respective single vaccinations. This finding could explain the limited success of clinical trials with therapeutic vaccines for HPV-induced malignancies since most previous studies used whole antigen, combinations of long peptides or DNA/RNA encoding for several epitopes.8,10 Our results suggest that it is important to hand-pick epitopes for therapeutic HPV vaccines for two reasons. First, as shown in this study, the inclusion of several epitopes in the vaccine can decrease the overall numbers of tumor-reactive CD8+ T cells and lead to a decrease in the magnitude of the most effective immune responses (as in our case the response against E7/11–19). Second, not all possible virus-derived epitopes are presented on the cell surface making it important to focus the immune response on the presented epitopes. The reason for this lies in the special characteristics of HPV-transformed cells, in which several immune evasion mechanisms decrease the visibility of these HPV-infected host cells to the immune system.40 Thus, if using formats other than selected minimal epitopes for vaccination, one could induce immune responses against epitopes that might be immunodominant, but are not actually presented on the target cell – und therefore not effective.
In conclusion, our new model represents an opportunity to test the efficacy of therapeutic anti-HPV16 vaccines with human epitopes in a fully MHC-humanized HPV16 tumor model, and thus could help to increase the translatability of preclinical studies into the clinical setting.
Methods
Mice
A2.DR1 mice, which are transgenic for HLA-A*0201 and HLA-DRB1*0101 and are engineered to lack all murine MHC molecules,22-24 were used in this study. The absence of murine MHC molecules is induced by a knockout of β2m, H-2Db and of the whole MHC class II locus. The mice were provided by the Institut Pasteur (Paris, France) and bred in-house under specific pathogen-free conditions. All national and institutional guidelines were followed and experiments were approved by governmental authorities. For experiments, female mice aged 8–16 weeks were used in age-matched groups.
Tumor cells
The cervical cancer cell line CaSki was cultured in DMEM (#D5671, Sigma) supplemented with 10% fetal bovine serum (FBS) (#10270, Gibco). 2277NS A2.DR1 sarcoma cells25 were transduced with a lentiviral pWPI vector encoding for tagged versions of full-length HPV16 E6 and E7 (E6-3xflag, E7-2xstrep) and a puromycin resistance cassette (vector map shown in Suppl. Figure 1). Clonal cell lines were established and screened for E6/E7 expression by Western blot analysis (see below). One clonal E6/E7-expressing cell line was found to be tumorigenic in in vivo experiments. The resulting tumor was reisolated, the tumor cells expanded in vitro and used as PAP-A2 cells in subsequent experiments. PAP-A2 cells were cultured in DMEM supplemented with 10% FBS, 10 mM HEPES buffer (#15630080, Gibco), 50 µM β-mercaptoethanol (#11528926, Gibco), 2 mM L-glutamine (#MT25005CI, Corning), 1 mM sodium pyruvate (#MT25000CI, Corning) and 2 µg/ml puromycin (P9620-10ML, Sigma).
Tumor inoculation
For tumor cell inoculation, PAP-A2 cells were harvested, washed several times with sterile PBS and 1.5x106 tumor cells were taken up in 50 µl sterile PBS. 50 µl of matrigel (#734–0270, Becton Dickinson (BD)) were added and the 100 µl resulting solution were injected subcutaneously in the flank of A2.DR1 mice. Tumors were measured using digital calipers two times a week and tumor volume was calculated using the formula v = 0.6 x length x width2. Mice were sacrificed when the tumor volume exceeded 1000 mm3.
Cell extracts and Western blot analysis
Cell extracts were prepared from cultured tumor cells and excised murine tumors. Cultured tumor cells were detached, washed and lysed using lysis buffer (10 mM Tris-HCl pH 7.5, 50 mM KCl, 2 mM MgCl2, 1% Triton X-100). Excised tumors were homogenized in lysis buffer. Lysates cleared of debris via centrifugation (10 min, 13,000 rpm in centrifuge #5407, Eppendorf, Hamburg, Germany) were mixed with 4x Laemmli buffer (222mM Tris pH 6.8, 3.5% SDS, 35% glycerol (#G5516-500ML, Sigma), 0.016% bromophenol blue (#A512.2, Roth), 10% β-mercaptoethanol (#4227.1, Roth)) and boiled for 5 min at 95 °C. SDS-PAGE and Western blotting was performed according to standard protocols with antibodies against E6 (clone E6-6F4, Euromedex) and E7 (clone NM2, kindly supplied by M. Müller, DKFZ) and suitable horseradish-peroxidase coupled secondary antibodies.
Peptides
Peptides derived from the HPV16 reference sequence (NC_001526) E7/7–15 (TLHEYMLDL), E7/11–19 (YMLDLQPET), E7/82–90 (LLMGTLGIV), E6/25–33 (ELQTTIHDI) were synthesized by the DKFZ peptide production facility with a purity ≥ 95% and dissolved in dimethyl sulfoxide (DMSO, #D8418, Sigma) at a concentration of 50 mM. All peptides were also synthesized with an N-terminal cysteine for subsequent coupling to (1,2-distearoyl-3-sn-phosphatidylethanolamine)-PEG-maleimide (#DSPE-PEG2000-maleimide, Laysan Bio Inc.) or for use as control peptides in in vitro assays.
Synthesis of amphiphilic peptides
For coupling to DSPE-PEG-maleimide,27 the peptides were dissolved in a 2:1 (v/v) mixture of phosphate-buffered saline (PBS) (pH 5.5) and acetonitrile. DSPE-PEG-maleimide was dissolved in a 1:1 (v/v) mixture of DMSO/PBS (pH 5.5). The DSPE-PEG-maleimide solution was mixed with the peptide solution in the stoichiometric ratio of 1:2 (DSPE-PEG-maleimide:peptide). The reaction was stirred overnight. Afterwards, the mixture was lyophilized on an Alpha 2–4 LD plus freeze dryer (Christ, Osterode, Germany). Constructs were purified by preparative high performance liquid chromatography (HPLC) and the purified product was analyzed by HPLC and HPLC-MS. Amph-peptides were dissolved in DMSO to a concentration of 25 mM.
Vaccination
Mice were injected with 50 nmol amph-peptide or 50 nmol of DSPE-PEG-maleimide without peptide as a vehicle control or left untreated with treatment regimens as displayed in the figures. In the therapeutic setting, vaccinations were given in 5 day intervals (instead of the 7 day intervals used in the prophylactic setting) to be able to vaccinate three times before tumors reached the termination criterion of 1000 mm3. 50 µg of high-molecular weight poly I:C (#vac-pic, Invivogen) per mouse were used as an adjuvant in all treated groups. The final injection volume was adjusted to 100 µl with sterile PBS and the solution was injected subcutaneously in the flank of A2.DR1 mice (contralateral flank in tumor-bearing mice). In combination vaccinations, only one amph-peptide was injected per vaccination site with the 50 µg poly I:C distributed equally to all administered amph-peptide solutions.
IFN-γ intracellular staining
Spleens of vaccinated mice were aseptically removed, minced with a scalpel and pressed through a 70 µm cell strainer. After red blood cell removal via ACK (ammonium-chloride-potassium) lysis and several washing steps, cells were incubated with peptides in the presence of GolgiStop (#554724, BD) and GolgiPlug (#555029, BD) in U-bottom 96 well plates. After 5 h of incubation at 37 °C, cells were stained with anti-mouse CD3-PE/Cy7, CD4-FITC, CD8-PE, IFN-γ-APC (BD), and Zombie Aqua (#423101, Biolegend) for dead cell exclusion. Samples were analyzed via flow cytometry on a FACS Canto II (BD, Franklin Lakes, USA). Data were analyzed with the FlowJo10 software (Treestar, Ashland, USA).
VitalFR cytotoxicity assay
Specific cytotoxicity was determined with a flow cytometry-based assay as previously described in ref. 28 and Suppl. Figure 3. In brief, splenocytes of amph-peptide-vaccinated A2.DR1 mice were isolated and cultured seven days in the presence of their cognate peptide. CD8+ T cells were isolated by untouched MACS isolation (#130–104-075, Miltenyi Biotech, Bergisch Gladbach, Germany). CD8+ T cells (effector cells) were added to wells containing a 1:1 mixture of specific target cells (carboxyfluorescein succinimidyl ester (CFSE) (#C1157, Invitrogen) -labeled) and control target cells (CellTrace Far Red (FR) (#C34564, Invitrogen) -labeled). After 48 h of incubation, cells were trypsinized, fixed and analyzed via flow cytometry on a FACS Canto II (BD). Data were analyzed with the FlowJo10 software. Specific killing was calculated with the formula 100-((% specific target cells with T cells/% control target cells with T cells)/(% specific target cells without T cells/% control target cells without T cells)x100.
Statistics
Mouse experiments were conducted once with the number of mice indicated in the respective figure legend. For statistical analyses, GraphPad PRISM5® (GraphPad Software, La Jolla, USA) was used. The specific statistical parameters and tests performed are indicated in the respective figure legends.
Funding Statement
This work was supported by a grant by the German Center for Infection Research (DZIF) [grant number TTU 07.706] to ABR.
Abbreviations
HPVhuman papillomavirus
HLAhuman leukocyte antigen;
amphamphiphilic
IFN-γinterferon-γ
Acknowledgments
We thank the Institut Pasteur for providing the A2.DR1 mice, Prof. M. Müller (DKFZ) for supplying the NM2 antibody and Prof. P. Beckhove and Dr. T. Bunse (DKFZ) for supplying the 2277NS sarcoma cells. We gratefully acknowledge the DKFZ flow cytometry facility for their technical support.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Supplemental material
Supplemental data for this article can be accessed here.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S.. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffman M, Doorbar J, Wentzensen N, de Sanjose S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 3.Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2014;16:e56. doi: 10.1016/S1470-2045(14)71191-X. [DOI] [PubMed] [Google Scholar]
- 4.Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M, Sidawy M, Schiller JT, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2015;215:212.e1–212.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — united States, 2016. MMWR Morb Mortal Wkly Rep. 2016;66:874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert Koch-Institut. Epidemiologisches Bulletin. 2018;2018:8–9. [Google Scholar]
- 7.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4:e453–63. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 8.Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2017;5:46–58. doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munger K. The role of human papillomaviruses in human cancers. Front Biosci. 2002;7:d641–9. [DOI] [PubMed] [Google Scholar]
- 10.Khallouf H, Grabowska A, Riemer A. Therapeutic vaccine strategies against human papillomavirus. Vaccines (Basel). 2014;2:422–462. doi: 10.3390/vaccines2020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: an update. Int J Cancer. 2018;142:224–229. doi: 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 12.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. [DOI] [PubMed] [Google Scholar]
- 13.Di Marco M, Peper JK, Rammensee HG. Identification of immunogenic epitopes by MS/MS. Cancer J. 2017;23:102–107. doi: 10.1097/PPO.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 14.Blatnik R, Mohan N, Bonsack M, Falkenby LG, Hoppe S, Josef K, Steinbach A, Becker S, Nadler WM, Rucevic M, et al. A targeted LC-MS Strategy for low-abundant HLA class-I-presented peptide detection identifies novel human papillomavirus T-cell epitopes. Proteomics. 2018;e1700390. doi: 10.1002/pmic.v18.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 16.Pascolo S. HLA class I transgenic mice: development, utilisation and improvement. Expert Opin Biol Ther. 2005;5:919–938. doi: 10.1517/14712598.5.7.919. [DOI] [PubMed] [Google Scholar]
- 17.Newberg MH, Smith DH, Haertel SB, Vining DR, Lacy E, Engelhard VH. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]
- 18.Peng S, Trimble C, He L, Tsai Y-C, Lin C-T, Boyd D, Pardoll D, Hung C-F, Wu T-C. Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice. Gene Ther. 2006;13:67–77. doi: 10.1038/sj.gt.3302607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiben GL, Velders MP, Schreiber H, Cassetti MC, Pullen JK, Smith LR, Kast WM. Establishment of an HLA-A * 0201 human papillomavirus type 16 tumor model to determine the efficacy of vaccination strategies in HLA-A * 0201 transgenic mice. Cancer Res. 2002;62:5792–5799. [PubMed] [Google Scholar]
- 20.Daftarian PM, Mansour M, Pohajdak B, Fuentes-Ortega A, Korets-Smith E, Macdonald L, Weir G, Brown RG, Kast WM. Rejection of large HPV-16 expressing tumors in aged mice by a single immunization of VacciMax encapsulated CTL/T helper peptides. J Transl Med. 2007;5:26. doi: 10.1186/1479-5876-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng S, Ji H, Trimble C, He L, Tsai Y-C, Yeatermeyer J, Boyd DAK, Hung C-F, Wu T-C. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pajot A, Michel ML, Fazilleau N, Pancré V, Auriault C, Ojcius DM, Lemonnier FA, Lone Y-C. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1-transgenic H-2 class I-/class II-knockout mice. Eur J Immunol. 2004;34:3060–3069. doi: 10.1002/eji.200425463. [DOI] [PubMed] [Google Scholar]
- 23.Mathieu MG, Knights AJ, Pawelec G, Riley CL, Wernet D, Lemonnier F, Straten PT, Mueller L, Rees RC, McArdle SEB. HAGE, a cancer/testis antigen with potential for melanoma immunotherapy: identification of several MHC class I/II HAGE-derived immunogenic peptides. Cancer Immunol Immunother. 2007;56:1885–1895. doi: 10.1007/s00262-007-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 26.Cho H-I, Barrios K, Lee Y-R, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62:787–799. doi: 10.1007/s00262-012-1382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanke J, Hoffmann C, Erben U, von Keyserling H, Stevanovic S, Cichon G, Schneider A, Kaufmann AM. A flow cytometry-based assay to assess minute frequencies of CD8+ T cells by their cytolytic function. J Immunol Methods. 2010;360:56–65. doi: 10.1016/j.jim.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62:1149–1159. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Z, Ou R, Ni B, Tang J, Xu Y. Cytolytic activity of the human papillomavirus type 16 E711-20 epitope-specific cytotoxic T lymphocyte is enhanced by heat shock protein 110 in HLA-A*0201 transgenic mice. Clin Vaccine Immunol. 2013;20:1027–1033. doi: 10.1128/CVI.00721-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hailemichael Y, Woods A, Fu T, He Q, Nielsen MC, Hasan F, Roszik J, Xiao Z, Vianden C, Khong H, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest. 2018;128:1338–1354. doi: 10.1172/JCI93303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 34.Strauss J, Madan RA, Gulley JL. Considerations for the combination of anticancer vaccines and immune checkpoint inhibitors. Expert Opin Biol Ther. 2016;16:895–901. doi: 10.1517/14712598.2016.1170805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521:99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1:412–420. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 38.Steinbach A, Winter J, Reuschenbach M, Blatnik R, Klevenz A, Bertrand M, Hoppe S, von nebel Doeberitz M, Grabowska AK, Riemer AB. ERAP1 overexpression in HPV-induced malignancies: a possible novel immune evasion mechanism. Oncoimmunology. 2017;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Grabowska AK, Riemer AB. The invisible enemy - how human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol J. 2012;6:249–256. doi: 10.2174/1874357901206010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


