Abstract
Objectives
Despite an obvious improvement in the treatment of coronary artery disease (CAD) and survival rate of patients with CAD during recent decades, diabetes mellitus (DM) is still considered a risk factor of adverse clinical outcomes in these patients. Therefore, we sought to evaluate the clinical implications of DM in patients with CAD who underwent contemporary percutaneous coronary intervention (PCI).
Methods
Based on the National Health Insurance claims data in South Korea, patients aged 18 years or older who had undergone PCI for the diagnosis of CAD between 2011 and 2015 were analyzed. Patients were classified into the DM (n = 26,872) and non-DM (n = 54,243) groups. The primary endpoint was all-cause mortality, and it was compared between the two groups via a propensity score matching analysis.
Results
The study population was categorized as patients with angina (n = 49,228) or acute myocardial infarction (AMI, n = 31,887). The study population had a median follow-up of 2.1 years (interquartile range, 1.1–3.2). After the propensity score matching analysis, 8,157 and 4,266 pairs of patients with angina and AMI were identified, respectively. In the matched angina group, the incidence of all-cause death was significantly higher in patients with DM (adjusted hazard ratio [aHR]: 1.30; 95% confidence interval [CI]: 1.16–1.47; p<0.001) than in those without DM. Moreover, in the matched AMI group, the incidence of all-cause death was significantly higher in patients with DM (aHR: 1.35; 95% CI: 1.19–1.53; p<0.001) than in those without DM.
Conclusions
In patients undergoing contemporary PCI in Korea, the presence of DM was associated with poorer clinical outcomes.
Introduction
The prevalence of diabetes mellitus (DM) is increasing worldwide, which is driven by both population aging and an increased prevalence of overweight and obesity [1, 2]. In particular, the prevalence of DM in Asian populations has increased rapidly in the recent decades [3]. In patients with DM, the leading cause of death is coronary artery disease (CAD) [4]. Despite an obvious improvement in the treatment of CAD and survival rate of patients with CAD [5], the mortality rate remains higher in patients with DM than in those without [6, 7]. In addition, there are several data that support that DM is an important prognostic factor of mortality and complications in patients with CAD [7, 8]. However, in contemporary real-world practice, data that can be used to assess the clinical implications of DM among patients with established CAD are limited. Therefore, using the claims data of the National Health Insurance (NHI) in South Korea, the clinical impact of DM in patients with CAD who underwent percutaneous coronary intervention (PCI) in Korea was evaluated.
Methods
Data sources
As described in detail previously [9], in South Korea, all healthcare providers had to join the NHI system on a fee-for-service basis. The Health Insurance Review & Assessment Service (HIRA) is a quasi-governmental organization that systematically reviews medical fees to minimize the risk of redundant and unnecessary medical services. Accordingly, all NHI claims records are reviewed by the HIRA. The current study analyzed data from the January 2011 to June 2016 claims records of the HIRA. The diagnosis codes of the International Classification of Diseases, 10th Revision (ICD-10) were used. In addition, specific information about drugs, devices, and procedures was obtained using codes from the HIRA database [9]. Since the claims data of the HIRA were fully anonymized, this study was approved by the local institutional review board of Ulsan University Hospital, Ulsan, Korea, which waived the requirement for informed consent.
Study population
Based on the claims database of the HIRA from July 2011 to June 2015, we identified patients aged 18 years and older who had undergone PCI (M6551, M6552, M6561–4, M6571, and M6572) for the diagnosis of CAD (ICD-10 codes I20.X–I25.X). To ensure patients’ first episode of CAD, patients with at least 6 months of eligibility prior to the index day were selected. Patients were also excluded if the HIRA database indicated that patients had a previous history of CAD (ICD-10 codes I20.X–25.X) within 6 months of the index day. Furthermore, patients were categorized as either those with acute myocardial infarction (AMI) and angina pectoris, and separate analyses were conducted for each group. AMI was defined using the ICD-10 codes (I21.X–I22.X) in the hospital discharge databases of the HIRA [9].
Study variables
DM was defined as patients who were assigned with the ICD-10 codes for those with DM (E10.0, E10.1, E10.6, E10.8-E11.1, E11.6, E11.8-E12.1, E12.6, E12.8-E13.1, E13.6, E13.8-E14.1, E14.6, E14.8, and E14.9); those with DM and chronic complications (E10.2-E10.5, E10.7, E11.2-E11.5, E11.7, E12.2-E12.5, E12.7, E13.2-E13.5, E13.7, E14.2-E14.5, and E14.7); or those who use anti-diabetic medications from the medication codes in the HIRA database within 6 months of the index day [9, 10].
Within 6 months of the index day, the ICD-10 codes were used to identify other comorbidities, such as hyperlipidemia, hypertension, congestive heart failure, arrhythmia, valvular disease, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, moderate to severe liver disease, renal disease, cancer, and rheumatic disease [9–11]. The Charlson comorbidity index was obtained using the ICD-10 codes [9–11]. In the HIRA database, all prescribed medications were recorded with rigorous accuracy. Moreover, patients who use anti-hypertensive and anti-hyperlipidemic drugs were considered to have hypertension and hyperlipidemia, respectively. Furthermore, we identified the use of medications, such as antiplatelet agents, statins, beta-blockers, and angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) [9].
We assigned claims as drug-eluting stents (DES) if DES device codes (J5083XXX) were used. The claims were designated as bare metal stents (BMS) if there were BMS device codes (J5231XXX). The claims were also classified as a non-stent coronary balloon angioplasty if device codes did not include any code indicating a DES or BMS [9].
Clinical outcomes
All-cause deaths were identified by all in- and out-patient claims that indicated death. Coronary revascularizations were identified using the procedure codes of PCI (M6551, M6552, M6561-4, M6571, and M6572) and coronary artery bypass surgery (O1641, O1642, O1647, OA641, OA642, and OA647) from the HIRA database. In the current study, for the evaluation of clinical outcomes, the HIRA database was used until June 2016. In patients with multiple events, the first event was considered to be the component of the composite outcome [9, 11].
Statistical analysis
We conducted separate analyses of the angina and AMI groups. Baseline patient characteristics and comorbidities were presented as mean ± standard deviation or frequency (%) for continuous or categorical variables, respectively. Continuous variables with normality were compared using the student’s t-test, and those without normality were compared using the Mann–Whitney U test. Categorical data were compared using the chi-square or Fisher’s exact test. Cumulative incidence rates for all-cause death between the DM and non-DM groups were estimated using the Kaplan–Meier method. Furthermore, we compared the cumulative incidence rates between the DM and non-DM groups using the log-rank test. The generalized estimating equations and the Cox proportional hazards regression model were used to identify adjusted DM effects for the in-hospital mortality or the time to event data, respectively. In particular, to reduce the impact of potential confounding effects between the comparison groups, significant differences in the baseline characteristics were adjusted using the propensity score matching method. The propensity scores were obtained nonparametrically using age, gender, comorbidities, type and number of stents, and the Charlson comorbidity index. Nonparametric propensity score estimation was useful because there was no need to fit the fully corrected parametric model. Propensity score matching was performed with the nearest-neighbor matching using a caliper size of 0.2 multiplied by the standard deviation for linearly transformed propensity scores (logit transformation). The balance of covariates in the matched groups was evaluated by measuring their standardized differences in means. All standardized differences in the baseline variables were less than 0.05 (5%). Thus, we examined whether all pretreatment variables were balanced between the two comparison groups. In addition, we conducted the paired t-test or the McNemar test for continuous or categorical variables, respectively, to assess the covariate balance between the two matched groups. In the propensity score-matched cohort, the in-hospital mortality rates were compared using the generalized estimating equations. For the time to event data, the Cox regression model with robust standard errors was used to accommodate the clustering of matched pairs. The R packages of “MatchIt,” “geepack,” and “survival” were used for the propensity score matching, generalized estimating equation fitting, and Cox model fitting, respectively. All data analyses were performed using the R software version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). A p value <0.05 was considered significant for all two-sided tests.
Results
Study population and characteristics
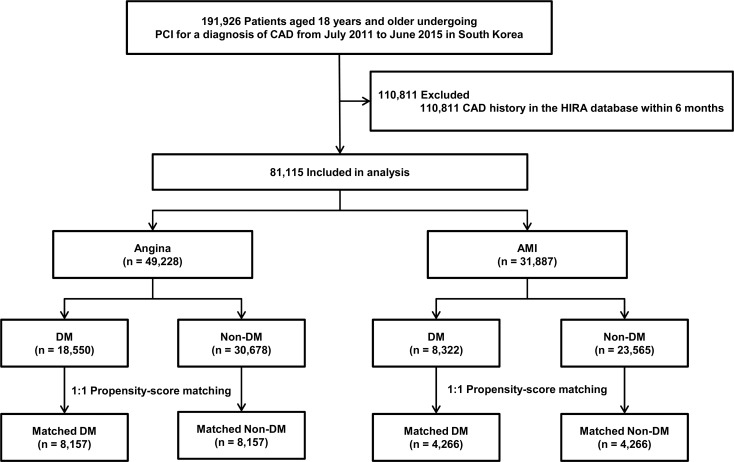
Between July 2011 and June 2015, a total of 191,926 patients aged 18 years and older who were diagnosed with CAD and underwent PCI were identified from the claims database of HIRA. Among them, 81,115 met the eligibility criteria, and they were then included in the analysis. Table 1 shows the baseline characteristics of the study participants. The mean age of the study participants was 64.4 ± 12.2 years and 56,576 (69.7%) were men. DM, hyperlipidemia, and hypertension were observed in 26,872 (33.1%), 30,675 (37.8%), and 45,389 (56.0%) patients, respectively. In PCI procedures, DESs were the most frequently used devices (n = 75,600, 93.2%), followed by balloon angioplasty (n = 4,479, 5.5%) and BMSs (n = 1,036, 1.3%). At discharge, antiplatelet agents, statins, beta-blockers, and ACEIs or ARBs were provided to 80,575 (99.3%), 71,411 (88.0%), 57,429 (70.8%), and 54,418 (67.1%) patients, respectively. According to the presence of DM, patients were classified into the DM and non-DM groups (Fig 1). In addition, specific anti-diabetic agents were presented in S1 Table. The number of diabetic patients with insulin treatment was 5,813 patients. In the overall population, during the follow-up period (median, 2.1 years; interquartile range, 1.1–3.2), 13,340 patients had 5,849 deaths and 7,881 coronary revascularizations (S2 Table).
Table 1. Characteristics of patients undergoing percutaneous coronary intervention according to the presence of diabetes mellitus.
| Angina (n = 49,228) | AMI (n = 31,887) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | DM (n = 18,550) |
Non-DM (n = 30,678) |
p value | DM (n = 8,322) |
Non-DM (n = 23,565) |
p value | |
| Age, years | 66.7 ± 10.4 | 64.2 ± 12.0 | <0.001 | 66.1 ± 11.5 | 62.1 ± 13.4 | <0.001 | |
| Gender (male), no. (%) | 11,425 (61.6%) | 21,343 (69.6%) | <0.001 | 5,572 (67.0%) | 18,236 (77.4%) | <0.001 | |
| Number of participants, no. (%) | 0.812 | 0.510 | |||||
| July 2011 to June 2012 | 4,148 (22.4%) | 6,957 (22.7%) | 2,152 (25.9%) | 5,916 (25.1%) | |||
| July 2012 to June 2013 | 4,479 (24.1%) | 7,404 (24.1%) | 2,098 (25.2%) | 5,922 (25.1%) | |||
| July 2013 to June 2014 | 4,889 (26.4%) | 8,090 (26.4%) | 2,062 (24.8%) | 5,914 (25.1%) | |||
| July 2014 to June 2015 | 5,034 (27.1%) | 8,227 (26.8%) | 2,010 (24.2%) | 5,813 (24.7%) | |||
| Comorbidities, no. (%) | |||||||
| Hyperlipidemia | 12,559 (67.7%) | 10,037 (32.7%) | <0.001 | 4,419 (53.1%) | 3,660 (15.5%) | <0.001 | |
| Hypertension | 15,237 (82.1%) | 16,014 (52.2%) | <0.001 | 6,140 (73.8%) | 7,998 (33.9%) | <0.001 | |
| Congestive heart failure | 1,916 (10.3%) | 1,626 (5.3%) | <0.001 | 500 (6.0%) | 483 (2.0%) | <0.001 | |
| Arrhythmia | 1,905 (10.3%) | 2,243 (7.3%) | <0.001 | 417 (5.0%) | 581 (2.5%) | <0.001 | |
| Valvular disease | 107 (0.6%) | 144 (0.5%) | 0.117 | 19 (0.2%) | 37 (0.2%) | 0.222 | |
| Peripheral vascular disease | 3,136 (16.9%) | 2,648 (8.6%) | <0.001 | 1,238 (14.9%) | 1,276 (5.4%) | <0.001 | |
| Cerebrovascular disease | 3,764 (20.3%) | 3,279 (10.7%) | <0.001 | 1,144 (13.7%) | 1,324 (5.6%) | <0.001 | |
| Chronic pulmonary disease | 3,630 (19.6%) | 4,624 (15.1%) | <0.001 | 1,422 (17.1%) | 2,600 (11.0%) | <0.001 | |
| Moderate to severe liver disease | 16 (0.1%) | 10 (0.03%) | 0.015 | 11 (0.1%) | 4 (0.02%) | <0.001 | |
| Renal disease | 1,941 (10.5%) | 734 (2.4%) | <0.001 | 575 (6.9%) | 230 (1.0%) | <0.001 | |
| Cancer | 769 (4.1%) | 662 (2.2%) | <0.001 | 292 (3.5%) | 373 (1.6%) | <0.001 | |
| Rheumatic disease | 52 (0.3%) | 48 (0.2%) | 0.004 | 16 (0.2%) | 34 (0.1%) | 0.336 | |
| Charlson comorbidity index | 2.48 ± 1.34 | 0.78 ± 1.03 | <0.001 | 2.21 ± 1.26 | 0.51 ± 0.84 | <0.001 | |
| Type of treatment, no. (%) | 0.021 | 0.033 | |||||
| Drug-eluting stents | 17,212 (92.8%) | 28,655 (93.4%) | 7,718 (92.7%) | 22,015 (93.4%) | |||
| Bare metal stents | 230 (1.2%) | 324 (1.1%) | 122 (1.5%) | 360 (1.5%) | |||
| Balloon angioplasty (no stent) | 1,108 (6.0%) | 1,699 (5.5%) | 482 (5.8%) | 1,190 (5.0%) | |||
| Number of stents per person | 1.46 ± 0.69 | 1.39 ± 0.65 | <0.001 | 1.42 ± 0.64 | 1.34 ± 0.59 | <0.001 | |
| Medications at discharge, no. (%) | |||||||
| Antiplatelet agents | 18,348 (98.9%) | 30,473 (99.3%) | <0.001 | 8,277 (99.5%) | 23,477 (99.6%) | 0.048 | |
| Statins | 15,561 (83.9%) | 27,049 (88.2%) | <0.001 | 7,352 (88.3%) | 21,449 (91.0%) | <0.001 | |
| Beta-blockers | 11,867 (64.0%) | 19,602 (63.9%) | 0.869 | 6,635 (79.7%) | 19,325 (82.0%) | <0.001 | |
| ACEI/ARB | 12,102 (65.2%) | 18,633 (60.7%) | <0.001 | 6,157 (74.0%) | 17,526 (74.4%) | 0.493 | |
Data were expressed as n (%) and mean ± SD.
ACEI = angiotensin-converting enzyme inhibitor; AMI = acute myocardial infarction; ARB = angiotensin receptor blocker; DM = diabetes mellitus
Fig 1. Overview of the study population.
CAD = coronary artery disease; DM = diabetes mellitus; HIRA = Health Insurance Review & Assessment Service; AMI = acute myocardial infarction; PCI = percutaneous coronary intervention.
DM versus non-DM in angina
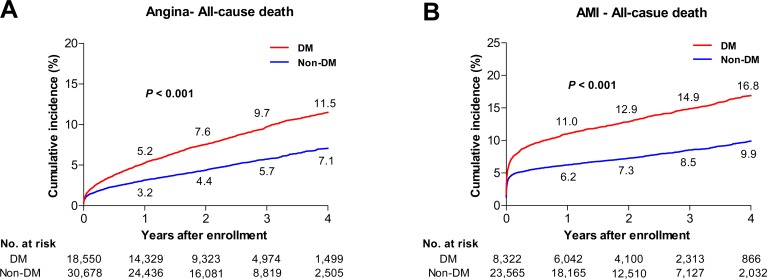
According to diagnosis, the study participants were classified as patients with angina pectoris (n = 49,228). Among them, patients were categorized into the DM (n = 18,550) and non-DM (n = 30,678) groups. Patients with DM were older and had more comorbidities than those without DM (Table 1). Fig 2 shows the cumulative incidence rates for all-cause deaths between the two groups. After the propensity score matching, there were 8,157 matched pairs. In the matched cohort, no significant differences in terms of covariates were observed between the two groups (Table 2). During the follow-up period (median, 2.1 years; interquartile range, 1.1–3.1), 2,435 patients had 1,088 deaths and 1,414 coronary revascularizations (S3 Table). There was no significant difference in terms of the incidence of in-hospital mortality between the two groups (adjusted odds ratio [aOR] of DM: 1.31; 95% confidence interval [CI]: 0.99–1.74; p = 0.062). However, the occurrence of all-cause death (adjusted hazard ratio [aHR] of DM: 1.30; 95% CI: 1.16–1.47; p<0.001) and the composite of death and recurrent coronary revascularization (aHR: 1.20; 95% CI: 1.11–1.30; p<0.001) was significantly higher in the DM group than in the non-DM group (Table 3). In DM group, matched diabetic patients with insulin treatment had poorer short- and long-term clinical outcomes compared with those without (Table 4).
Fig 2. Cumulative incidence rates for all-cause deaths in the study population.
Cumulative incidence rates for (A) all-cause death in patients with angina and (B) in those with acute myocardial infarction. The numbers in each figure represent the cumulative incidence rates at each time point. AMI = acute myocardial infarction.
Table 2. Characteristics of the propensity score-matched patients according to the presence of diabetes mellitus.
| Angina (n = 8,157 pairs) | AMI (n = 4,266 pairs) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | DM (n = 8,157) |
Non-DM (n = 8,157) |
p value | DM (n = 4,266) |
Non-DM (n = 4,266) |
p value | ||
| Age, years | 66.6 ± 10.5 | 66.8 ± 11.4 | 0.860 | 65.8 ± 11.7 | 66.1 ± 12.5 | 0.360 | ||
| Gender (male), no. (%) | 5,080 (62.3%) | 5,109 (62.6%) | 0.327 | 2,902 (68.0%) | 2,895 (67.9%) | 0.570 | ||
| Comorbidities, no. (%) | ||||||||
| Hyperlipidemia | 4,702 (57.6%) | 4,410 (54.1%) | 0.426 | 1,857 (43.5%) | 1,661 (38.9%) | 0.896 | ||
| Hypertension | 6,142 (75.3%) | 5,852 (71.7%) | 0.131 | 2,749 (64.4%) | 2,609 (61.2%) | 0.244 | ||
| Congestive heart failure | 745 (9.1%) | 694 (8.5%) | 0.164 | 214 (5.0%) | 197 (4.6%) | 0.155 | ||
| Arrhythmia | 839 (10.3%) | 844 (10.3%) | 0.759 | 197 (4.6%) | 199 (4.7%) | 0.718 | ||
| Valvular disease | 47 (0.6%) | 47 (0.6%) | 0.606 | 11 (0.3%) | 13 (0.3%) | 0.540 | ||
| Peripheral vascular disease | 1,197 (14.7%) | 1,130 (13.9%) | 0.297 | 546 (12.8%) | 484 (11.3%) | 0.712 | ||
| Cerebrovascular disease | 1,484 (18.2%) | 1,428 (17.5%) | 0.788 | 500 (11.7%) | 509 (11.9%) | 0.094 | ||
| Chronic pulmonary disease | 1,647 (20.2%) | 1,680 (20.6%) | 0.032 | 697 (16.3%) | 747 (17.5%) | 0.927 | ||
| Moderate to severe liver disease | 7 (0.1%) | 6 (0.1%) | 0.096 | 4 (0.1%) | 4 (0.1%) | 0.724 | ||
| Renal disease | 500 (6.1%) | 339 (4.2%) | 0.183 | 165 (3.9%) | 108 (2.5%) | 0.478 | ||
| Cancer | 302 (3.7%) | 302 (3.7%) | 0.478 | 126 (3.0%) | 108 (2.5%) | 0.460 | ||
| Rheumatic disease | 21 (0.3%) | 22 (0.3%) | 0.760 | 9 (0.2%) | 14 (0.3%) | 0.677 | ||
| Charlson comorbidity index | 1.96 ± 1.14 | 1.87 ± 1.02 | 0.256 | 1.73 ± 1.01 | 1.68 ± 0.92 | 0.663 | ||
| Type of treatment, no. (%) | ||||||||
| Drug-eluting stents | 7,583 (93.0%) | 7,614 (93.3%) | 0.711 | 3,959 (92.8%) | 3,962 (92.9%) | 0.502 | ||
| Bare metal stents | 104 (1.3%) | 99 (1.2%) | 0.160 | 65 (1.5%) | 67 (1.6%) | 0.027 | ||
| Number of drug-eluting stents | 1.35 ± 0.74 | 1.33 ± 0.71 | 0.156 | 1.33 ± 0.70 | 1.31 ± 0.67 | 0.549 | ||
Data were expressed as n (%) and mean ± SD.
ACEI = angiotensin-converting enzyme inhibitor; AMI = acute myocardial infarction; ARB = angiotensin receptor blocker; DM = diabetes mellitus
Table 3. Clinical outcomes in patients who underwent percutaneous coronary intervention according to the presence of diabetes mellitus.
| Propensity score matching analysis | Angina (n = 8,157 pairs) | AMI (n = 4,266 pairs) | ||
|---|---|---|---|---|
| DM compared with non-DM | DM compared with non-DM | |||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| In-hospital mortality | 1.31 (0.99–1.74) | 0.062 | 1.41 (1.16–1.70) | <0.001 |
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| All-cause death | 1.30 (1.16–1.47) | <0.001 | 1.35 (1.19–1.53) | <0.001 |
| Death/coronary revascularization | 1.20 (1.11–1.30) | <0.001 | 1.32 (1.21–1.45) | <0.001 |
AMI = acute myocardial infarction; CI = confidence interval; DM = diabetes mellitus
Table 4. Clinical outcomes in patients with diabetes mellitus who underwent percutaneous coronary intervention according to the treatment of insulin.
| Propensity score matching analysis | Angina (n = 3,948 pairs) | AMI (n = 1,452 pairs) | ||
|---|---|---|---|---|
| DM with insulin treatment compared with DM without | DM with insulin treatment compared with DM without | |||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| In-hospital mortality | 1.55 (1.11–2.16) | 0.010 | 1.48 (1.11–1.97) | 0.008 |
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| All-cause death | 1.54 (1.34–1.78) | <0.001 | 1.61 (1.36–1.91) | <0.001 |
| Death/coronary revascularization | 1.33 (1.20–1.47) | <0.001 | 1.39 (1.22–1.58) | <0.001 |
AMI = acute myocardial infarction; CI = confidence interval; DM = diabetes mellitus
DM versus non-DM in AMI
We also analyzed the clinical outcomes in patients with AMI (n = 31,887). Patients were classified into the DM (n = 8,322) and non-DM (n = 23,565) groups. Patients with DM were older and had more comorbidities than those without DM (Table 1). The cumulative incidence rates for all-cause deaths between the two groups are presented in Fig 2. Among the 4,266 propensity score-matched pairs, no significant differences were observed in terms of covariates between both groups (Table 2). During the follow-up period (median, 2.0 years; interquartile range, 1.0–3.2), 1,863 patients had 966 deaths and 967 coronary revascularizations (S3 Table). The incidence of all-cause death was significantly higher in patients with DM (aHR: 1.35; 95% CI: 1.19–1.53; p<0.001) than in those without. In addition, the occurrence of in-hospital mortality (aHR: 1.41; 95% CI: 1.16–1.70; p<0.001) and composite of death and recurrent coronary revascularization (aHR: 1.32; 95% CI: 1.21–1.45; p<0.001) was higher in patients with DM than in those without (Table 3). In DM group, the poorer short- and long-term clinical outcomes were observed in matched diabetic patients with insulin treatment compared with those without (Table 4).
Discussion
In the present analysis that used data from the NHI claims database in South Korea, the presence of DM was associated with poorer clinical outcomes in patients who underwent PCI for established CAD regardless of clinical presentations.
In the recent decades, there have been remarkable advancements in adjuvant pharmacologic agents and devices for treating CAD. Optimal medical therapy has been suggested as an initial treatment strategy and recommended for all patients with CAD [12, 13]. Particularly, in patients with stable CAD, optimal medical therapy had comparable clinical benefits with coronary revascularization [14, 15]. Furthermore, in those with acute coronary syndrome, new P2Y12 agents significantly improved clinical outcomes [16, 17]. On the other hand, there has also been a significant improvement in stent design and the development of new drugs and the drug-carrier systems of devices. Based on these enhanced properties, contemporary DESs had better clinical efficacy and safety than BMSs and early-generation DESs [18]. Previous studies have shown that DM had a greater adverse clinical impact on short- and long-term clinical outcomes [6, 7, 19–23]. However, considering the contemporary real-world practice, there are limited data to evaluate the clinical implications of DM. Therefore, the present study was designed. Well-controlled and reliable data from the HIRA database in Korea (i.e., a quasigovernmental organization) enabled qualified analyses for the clinical impact of DM in patients undergoing PCI for the established CAD [9, 11, 24].
For patients with angina, coronary angioplasty in diabetic patients showed a similar procedural success without increased in-hospital mortality compared with non-diabetic patients. However, DM was associated with a worse long-term prognosis [19]. Even in the PCI era, the procedural success rates in patients with DM and those without DM are comparable. However, patients with DM had a higher incidence of long-term adverse clinical outcomes [20]. Consistent with these studies, the present study showed that in-hospital mortality in patients with angina was comparable between the DM and non-DM groups. However, long-term clinical outcomes were poorer in DM group. On the contrary, in earlier studies on AMI, patients with DM showed not only increased in-hospital mortality but also worse long-term prognosis [21, 22]. Despite an improvement in the treatment of AMI, in-hospital and long-term mortality rates were still higher in patients with DM [7, 23]. Moreover, the present study, which reflects contemporary practice, showed that patients with concurrent DM and AMI had increased in-hospital and long-term mortalities. Therefore, in patients with both DM and angina, we focused on post-PCI management. In patients with AMI, preemptive prevention, early detection, and proper treatment for DM are required to improve prognosis.
DM adversely affects the outcome and course of CAD. In patients with DM, platelet hyperactivity, reduced fibrinolytic capacity, increased concentrations of hemostatic proteins, and endothelial dysfunction promote atherosclerosis and increase the risk of thrombotic vascular events [25–27]. However, an even more important thing is that the adherence to guidelines could improve clinical outcomes in patients with DM. A previous long-term study has shown that a targeted, intensified, multifactorial intervention reduces the risk of cardiovascular and microvascular events in patients with DM [28]. A randomized trial has also observed the resolution of myocardial ischemia that resulted from a more intensive treatment of cardiovascular risk factors [29]. Therefore, to prevent future CAD in this high-risk population, aggressive preventive strategies according to current guidelines must be implemented.
The anatomical SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score was developed to help physicians decide the optimal revascularization modality in patients with complex CAD [30]. However, this lesion-based scoring system showed to have a lower ability to predict mortality compared with scoring systems using clinical variables. To overcome these limitations, the clinical SYNTAX score combining the SYNTAX score with a simple clinical risk score incorporating age, ejection fraction, and creatinine clearance was advocated [31]. However, in a validation study with a patient-level pooled analysis of 6,081 patients treated with DES (75% newer-generation DES), both SYNTAX score and DM were associated with 2-year major adverse cardiac events defined as the composite of cardiac death, myocardial infarction, and clinically indicated target lesion revascularization (p <0.001 and p = 0.028, respectively). In addition, compared with patients without DM, those with DM had higher risks of 2-year major adverse cardiac events (aHR: 1.25; 95% CI: 1.03–1.53; p = 0.026) without significant interaction with SYNTAX score [32]. Therefore, these findings imply that DM is still an important risk factor in patients with complex CAD.
A previous large randomized trial compared CABG and PCI with DES in 1,900 patients with DM and multi-vessel disease. CABG showed a significantly lower 5-year event rates for the composite of all-cause death, MI, and stroke compared to PCI with DES (18.7% in the CABG group versus 26.6% in the PCI group, p = 0.005) [33]. In a prespecified subgroup analysis for 452 patients with DM of the SYNTAX trial, the 5-year event rates of the composite of death, stroke, MI and repeat revascularization were also significantly lower in the CABG group compared with PCI group (29.0% versus 46.5%, p<0.001) [34]. Consequently, the current guideline recommends CABG as the revascularization modality of choice in patients with DM and multi-vessel or left main disease [35]. However, evidence supporting the guideline was largely based on the early-generation DES. A recent network meta-analysis suggested that PCI with everolimus-eluting stent was associated with similar risk of long-term death compared with CABG [36]. Therefore, future randomized clinical trials are required to compare CABG and PCI with newer-generation DES in patients with DM.
The present study had several limitations. First, our study was based on administrative data from the HIRA database in South Korea. The present study lacked clinical data and medical examination results, which is similar to previous studies that used data from administrative databases. Thus, our findings might be limited by uncertainties in terms of unmeasured confounding variables that may affect the management of patients [9, 11]. Second, although we used a database from a quasi-governmental organization, there is a possibility that these data did not fully reflect patient outcomes. In addition, we did not specify the cause of death. Finally, the present study only included a population in Korea. Ethnic differences in CAD and clinical differences in patients with DM have been observed between the Asian and Western populations [37]. Therefore, this may limit the generalizability of our findings to other ethnic groups.
Conclusions
Despite a significant progress in the treatment of patients with CAD, DM is still an independent risk factor for adverse clinical outcomes in patients who underwent contemporary PCI in Korea. Our findings highlight the importance of appropriate and aggressive strategies that reduce the risk of CAD in patients with DM.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- AMI
acute myocardial infarction
- ARB
angiotensin receptor blocker
- BMS
bare metal stent
- CAD
coronary artery disease
- CI
confidence interval
- DES
drug-eluting stent
- DM
diabetes mellitus
- NHI
National Health Insurance
- HIRA
Health Insurance Review & Assessment Service
- HR
hazard ratio
- ICD-10
International Classification of Diseases, 10th Revision
- OR
odds ratio
- PCI
percutaneous coronary intervention
Data Availability
The present study analyzed the NHI claims data in South Korea. The data of the NHI Cover Letter claims are accessible to any researchers after permission of the Health Insurance Review & Assessment Service in South Korea (http://opendata.hira.or.kr/home.do). In our study, after acceptance of our proposal, we analyzed data of the HIRA from January 2011 to June 2016. Due to personal information protection law of the HIRA, results can be exported. However, data cannot be exported. Therefore, there are some restrictions in data availability.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1D1A3B07043344 to G.M.P.) and the Ministry of Science, ICT & Future Planning (2017R1C1B1006717 to S.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011; 378: 31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014; 129: e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301: 2129–2140. 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 4.Park GM, Lee SW, Cho YR, Kim CJ, Cho JS, Park MW, et al. Coronary computed tomographic angiographic findings in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014; 113: 765–771. 10.1016/j.amjcard.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 5.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012; 366: 54–63. 10.1056/NEJMra1112570 [DOI] [PubMed] [Google Scholar]
- 6.Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB Sr., Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009; 119: 1728–1735. 10.1161/CIRCULATIONAHA.108.829176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norhammar A, Lindback J, Ryden L, Wallentin L, Stenestrand U, Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA). Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007; 93: 1577–1583. 10.1136/hrt.2006.097956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB Sr., et al. Trends in cardiovascular complications of diabetes. JAMA. 2004; 292: 2495–2499. 10.1001/jama.292.20.2495 [DOI] [PubMed] [Google Scholar]
- 9.Han S, Park GM, Kim YG, Park MW, Her SH, Lee SW, et al. Trends, characteristics, and clinical outcomes of patients undergoing percutaneous coronary intervention in Korea between 2011 and 2015. Korean Circ J. 2018; 48: 310–321. 10.4070/kcj.2017.0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 11.Park GM, Kim YH, Yun SC, Ahn JM, Choi HI, Roh JH, et al. Anatomic or functional evaluation as an initial test for stable coronary artery disease: a propensity score analysis. J Nucl Med. 2016; 57: 1364–1369. 10.2967/jnumed.115.169318 [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP, et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation. 2015; 131: 1269–1277. 10.1161/CIRCULATIONAHA.114.013042 [DOI] [PubMed] [Google Scholar]
- 13.Goyal A, Alexander JH, Hafley GE, Graham SH, Mehta RH, Mack MJ, et al. Outcomes associated with the use of secondary prevention medications after coronary artery bypass graft surgery. Ann Thorac Surg. 2007; 83: 993–1001. 10.1016/j.athoracsur.2006.10.046 [DOI] [PubMed] [Google Scholar]
- 14.Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015; 373: 1937–1946. 10.1056/NEJMoa1505532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009; 360: 2503–2515. 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357: 2001–2015. 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 17.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361: 1045–1057. 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 18.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012; 379: 1393–1402. 10.1016/S0140-6736(12)60324-9 [DOI] [PubMed] [Google Scholar]
- 19.Stein B, Weintraub WS, Gebhart SP, Cohen-Bernstein CL, Grosswald R, Liberman HA, et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation. 1995; 91: 979–989. [DOI] [PubMed] [Google Scholar]
- 20.Mehran R, Dangas GD, Kobayashi Y, Lansky AJ, Mintz GS, Aymong ED, et al. Short- and long-term results after multivessel stenting in diabetic patients. J Am Coll Cardiol. 2004; 43: 1348–1354. 10.1016/j.jacc.2003.04.004 [DOI] [PubMed] [Google Scholar]
- 21.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997; 126: 296–306. [DOI] [PubMed] [Google Scholar]
- 22.Mak KH, Moliterno DJ, Granger CB, Miller DP, White HD, Wilcox RG, et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997; 30: 171–179. [DOI] [PubMed] [Google Scholar]
- 23.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007; 298: 765–775. 10.1001/jama.298.7.765 [DOI] [PubMed] [Google Scholar]
- 24.Park GM, Han S, Kim SH, Jo MW, Her SH, Lee JB, et al. Model for assessing cardiovascular risk in a Korean population. Circ Cardiovasc Qual Outcomes. 2014; 7: 944–951. 10.1161/CIRCOUTCOMES.114.001305 [DOI] [PubMed] [Google Scholar]
- 25.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003; 26: 2181–2188. [DOI] [PubMed] [Google Scholar]
- 26.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007; 3: 46–56. 10.1038/ncpendmet0366 [DOI] [PubMed] [Google Scholar]
- 27.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013; 34: 2436–2443. 10.1093/eurheartj/eht149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mick MJ, Piedmonte MR, Arnold AM, Simpfendorfer C. Risk stratification for long-term outcome after elective coronary angioplasty: a multivariate analysis of 5,000 patients. J Am Coll Cardiol. 1994; 24: 74–80. [DOI] [PubMed] [Google Scholar]
- 29.Wackers FJ, Chyun DA, Young LH, Heller GV, Iskandrian AE, Davey JA, et al. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007; 30: 2892–2898. 10.2337/dc07-1250 [DOI] [PubMed] [Google Scholar]
- 30.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005; 1: 219–227. [PubMed] [Google Scholar]
- 31.Garg S, Sarno G, Garcia-Garcia HM, Girasis C, Wykrzykowska J, Dawkins KD, et al. A new tool for the risk stratification of patients with complex coronary artery disease: the Clinical SYNTAX Score. Circ Cardiovasc Interv. 2010; 3: 317–326. 10.1161/CIRCINTERVENTIONS.109.914051 [DOI] [PubMed] [Google Scholar]
- 32.Koskinas KC, Siontis GC, Piccolo R, Franzone A, Haynes A, Rat-Wirtzler J, et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016; 9: e003255 10.1161/CIRCINTERVENTIONS.115.003255 [DOI] [PubMed] [Google Scholar]
- 33.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012; 367: 2375–2384. 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 34.Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013; 43: 1006–1013. 10.1093/ejcts/ezt017 [DOI] [PubMed] [Google Scholar]
- 35.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018. 10.1093/eurheartj/ehy394 30165437 [Google Scholar]
- 36.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus eluting stents versus coronary artery bypass graft surgery for patients with diabetes mellitus and multivessel disease. Circ Cardiovasc Interv. 2015; 8: e002626 10.1161/CIRCINTERVENTIONS.115.002626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SH, Park GM, Lee SW, Yun SC, Kim YH, Cho YR, et al. Long-term prognostic value of coronary CT angiography in asymptomatic Type 2 diabetes mellitus. JACC Cardiovasc Imaging. 2016; 9: 1292–1300. 10.1016/j.jcmg.2016.01.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The present study analyzed the NHI claims data in South Korea. The data of the NHI Cover Letter claims are accessible to any researchers after permission of the Health Insurance Review & Assessment Service in South Korea (http://opendata.hira.or.kr/home.do). In our study, after acceptance of our proposal, we analyzed data of the HIRA from January 2011 to June 2016. Due to personal information protection law of the HIRA, results can be exported. However, data cannot be exported. Therefore, there are some restrictions in data availability.