Abstract
Background
Atrial fibrillation (AF), the most common sustained arrhythmia in CKD, is associated with poor clinical outcomes in both patients without CKD and patients with dialysis-treated ESRD. However, less is known about AF-associated outcomes in patients with CKD who do not require dialysis.
Methods
To prospectively examine the association of new-onset AF with subsequent risks of cardiovascular disease events and death among adults with CKD, we studied participants enrolled in the Chronic Renal Insufficiency Cohort Study who did not have AF at baseline. Outcomes included heart failure, myocardial infarction, stroke, and death occurring after diagnosis of AF. We used Cox regression models and marginal structural models to examine the association of incident AF with subsequent risk of cardiovascular disease events and death, adjusting for patient characteristics, laboratory values, and medication use.
Results
Among 3080 participants, 323 (10.5%) developed incident AF during a mean 6.1 years of follow-up. Compared with participants who did not develop AF, those who did had higher adjusted rates of heart failure (hazard ratio [HR], 5.17; 95% confidence interval [95% CI], 3.89 to 6.87), myocardial infarction (HR, 3.64; 95% CI, 2.50 to 5.31), stroke (HR, 2.66; 95% CI, 1.50 to 4.74), and death (HR, 3.30; 95% CI, 2.65 to 4.12). These associations remained robust with additional adjustment for biomarkers of inflammation, cardiac stress, and mineral metabolism; left ventricular mass; ejection fraction; and left atrial diameter.
Conclusions
Incident AF is independently associated with two- to five-fold increased rates of developing subsequent heart failure, myocardial infarction, stroke, or death in adults with CKD. These findings have important implications for cardiovascular risk reduction.
Keywords: cardiovascular events, chronic kidney disease, heart failure
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide.1 Patients with CKD comprise nearly 14% of the population2 and have even greater burden of AF.3,4 The incidence of AF is estimated to be two- to three-fold higher5 in patients with CKD compared with the general population.
In the absence of CKD, it is well established that AF is associated with poor outcomes such as ischemic stroke, heart failure (HF), myocardial infarction (MI), and death.6–14 Similarly, in patients with ESRD treated with chronic dialysis, studies have reported that AF is also linked with excess risks of stroke and death.15–18 However, less is known about outcomes associated with AF in the substantially larger, nondialysis-requiring population of patients with CKD. In a study of patients with CKD in a large health care delivery system, we previously reported that incident AF was associated with greater risk of all-cause death but were not able to address other important individual cardiovascular outcomes.19
Given the growing population with CKD and AF, a better understanding of clinical outcomes in patients with CKD who develop AF would have potentially important implications for the management of this high-risk group of patients. This study aimed to determine the association of incident AF on the risk of subsequent cardiovascular events, including HF, MI, and deaths among adults with CKD. We hypothesized that CKD participants who developed incident AF would have greater risk of all types of cardiovascular events, particularly HF and stroke, compared with those who did not develop AF.
Methods
Study Population
A total of 3939 adults were enrolled into the Chronic Renal Insufficiency Cohort (CRIC) study between June 2003 and August 2008 at seven clinical centers nationally (Ann Arbor/Detroit, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland/San Francisco, CA). Men and women were eligible for the study if they were between 21 and 74 years of age and met the following age-specific eGFR criteria: 20–70 ml/min per 1.73 m2 for age 21–44 years, 20–60 ml/min per 1.73 m2 for age 45–64 years, and 20–50 ml/min per 1.73 m2 for age 65–74 years. Exclusion criteria included severe (New York Heart Association class III/IV) HF and polycystic kidney disease, among others previously described.20 Details on study design and baseline characteristics of the participants were previously published.20,21 All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites.
A total of 3080 participants were included in this analysis after excluding persons with prevalent AF at entry (n=830), which was determined by self-report or presence of AF on 12-lead electrocardiogram (ECG) at entry3 and those with missing baseline covariates (n=29).
Predictor Variable
Incident AF was the main predictor of interest and was determined by identification of hospitalizations or emergency department visits involving AF during study follow-up. Participants were asked twice yearly if they were hospitalized and electronic health records from corresponding hospitals or health care systems were queried for qualifying encounters. Diagnostic codes for AF and other arrhythmias prompted retrieval of medical records and centralized adjudicated review for the presence of AF. At least two study physicians reviewed all possible AF events by manual review of relevant medical records and ECGs, if available, using standardized criteria. All discordances were discussed by the two reviewers and resolved. AF was confirmed when both reviewers agreed upon the diagnosis of AF.
Outcomes
The primary outcomes were adjudicated HF, MI, and stroke as well as all-cause death and a composite outcome (HF, MI, stroke, or mortality) that occurred after incident AF and until March 31, 2013. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital and outpatient records, and searching Social Security Death vital status and state death certificate files, if available.
Study participants were queried every 6 months during alternating in-person and telephone visits about whether they were hospitalized, experienced a possible cardiovascular disease (CVD) event, or underwent a selected set of diagnostic tests/procedures. The International Classification of Diseases, Ninth Revision discharge codes were obtained for all hospitalizations and relevant medical records were retrieved for review by at least two study physicians to ascertain events of HF, MI, and stroke.
HF events were determined on the basis of clinical symptoms, radiographic evidence of pulmonary edema, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging in hospitalized patients modeled after Framingham and Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial criteria.22,23 Diagnosis of probable or definite MI was made on the basis of symptoms consistent with acute ischemia, cardiac biomarker levels, and ECGs as recommended by a consensus statement on the universal definition of MI.24 Two neurologists reviewed all hospitalizations and emergency department visits suggestive of stroke. Outcomes included both probable and definite ischemic stroke. The latter was determined on the basis of autopsy findings or sudden onset of neurologic symptoms supported with computed tomography or magnetic resonance imaging demonstration of infarction in a territory where an injury or infarction would be expected to create those symptoms. The former was defined as sudden or rapid onset of one major or two minor neurologic signs or symptoms within a cerebrovascular distribution lasting for more than 24 hours or until the patient died, without evidence of hemorrhage or infarction on computed tomography or magnetic resonance imaging performed within 24 hours of the onset of symptoms, and with no alternative explanation.25
Covariates
At the baseline study visit and each subsequent study visit, participants provided information on their sociodemographic characteristics, medical history (including prior CVD and history of hypertension), medication usage (including antiplatelet agents and warfarin), and lifestyle behaviors (e.g., tobacco use). Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. Diabetes mellitus was defined as a fasting glucose >126 mg/dl, a nonfasting glucose >200 mg/dl, or use of insulin or other antidiabetic medication. Anthropometric measurements and BP were assessed using standard protocols.26 Body mass index (BMI) was derived as weight in kilograms divided by height in meters squared. Serum creatinine concentration was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC study Central Laboratory and standardized to isotope dilution mass spectrometry-traceable values.27–29 eGFR was calculated from serum creatinine and cystatin C using the CRIC study equation.29 Additional assays measured 24-hour urine total protein, fibroblast growth factor-23 (FGF23), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), high-sensitivity troponin T (hsTnT), and high-sensitivity C-reactive protein (hs-CRP). Transthoracic echocardiograms were performed 1 year after enrollment and provided data on left ventricular ejection fraction and left ventricular mass index,30–33 and were quantified centrally by a highly trained, registered diagnostic cardiac sonographer.
Statistical Analyses
All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). We compared baseline characteristics of participants who did versus did not develop incident AF using t tests or chi-squared methods, as appropriate. Crude rates of each outcome were calculated per 100 person years for those with and without incident AF with associated 95% confidence intervals (95% CIs).
We performed multivariable Cox proportional hazards regression to examine the association between development of incident AF during follow-up and risk of each type of CVD event and death. AF was classified as a time-updated exposure. Thus, if a patient developed AF during follow-up, they contributed time to the “no AF” exposure group before being diagnosed with incident AF. After being diagnosed with AF, they would contribute person-time to the “incident AF” exposure group.
Covariates in the models were selected on the basis of biologic plausibility. Only participants with all covariates available at baseline were included in the analyses. Baseline variables included in models were sociodemographic characteristics; clinical center; diabetes; hypertension; self-reported history of HF, coronary heart disease, or stroke (i.e., prevalent CVD); tobacco use; systolic BP; BMI; eGFR; proteinuria level; BMI; LDL cholesterol; HDL cholesterol; use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ARBs); and use of β-blockers and diuretics. For this analysis, patients were censored at occurrence of the outcome event, death, loss to follow-up, or end of administrative follow-up on March 31, 2013.
In a secondary analysis, given the concern for potential time-dependent confounding, we fit marginal structural models (MSMs), which applies inverse probability weighting in a discrete time failure causal inference model structure.34 A substantial body of work has emerged demonstrating the usefulness of statistical tools like MSMs in the areas of CKD.35,36 Briefly, a MSM is a two-step approach wherein models are first fit to predict the probability of AF during follow-up (i.e., the exposure of interest) and the probability of noncensoring in each time window, and second, inverse-probability weighted models are then fit for the outcomes. Inverse-probability weighting was also used to handle censoring events and missing data. For extreme weights, an upper and lower bound was set and any values above or below those thresholds were assigned to those limit values. In the MSM, both AF and covariates were time-updated with the exception of sex, race/ethnicity, and education level.37 Missing covariates during follow-up were assumed to be the same as the last observation. Hazards ratios (HRs) and 95% CIs were reported for all models.
We performed several additional sensitivity analyses. First, we repeated our analyses stratifying (and testing for interaction) by demographic features and prevalent CVD: age (≤ or >45 years), sex, race (black and nonblack), history of HF, history of MI, and history of stroke. Second, we adjusted for use of warfarin and antiplatelet agents to determine whether treatment for AF attenuated the observed associations. Third, we adjusted for levels of targeted biomarkers reflecting altered mineral metabolism (FGF23), cardiac stress (NT-proBNP and hsTnT), and inflammation (hs-CRP). These biomarkers have all been shown to be associated with AF in prior studies.38–42 Fourth, we adjusted for measures of subclinical HF as ascertained by echocardiograms 1 year after entry, including left ventricular hypertrophy, left ventricular ejection fraction, and left atrial diameter, because these measures are associated with incident AF and many of our outcomes of interest and thus may be important confounders. Finally, we excluded participants in whom incident AF and either HF or MI occurred during the same hospitalization.
Results
Participant Characteristics
There were 323 patients with incident AF (10.5% of study population) observed during follow-up. Participants who developed incident AF were more likely to be older, men, white, have a prior history of CVD, higher BP and BMI, and lower entry eGFR (Table 1). Furthermore, those who developed incident AF were also more likely to use antiplatelet agents or warfarin, and have higher levels of circulating FGF23, hs-CRP, NT-proBNP, and hsTnT. They also had higher left ventricular mass index, higher left atrial diameter, and lower left ventricular ejection fraction on ECG (Table 1).
Table 1.
Characteristics at study entry in participants who did or did not develop incident AF
| Characteristic | Incident AF, n=323 | No Incident AF, n=2757 | P Value |
|---|---|---|---|
| Age, mean (SD), yr | 61.6 (9.2) | 57.3 (11.2) | <0.001 |
| Women, N (%) | 129 (39.9) | 1258 (45.6) | 0.05 |
| Race/ethnicity, N (%) | |||
| Non-Hispanic white | 165 (51.1) | 1156 (41.9) | 0.009 |
| Non-Hispanic black | 119 (36.8) | 1120 (40.6) | |
| Hispanic | 30 (9.3) | 365 (13.2) | |
| Other | 9 (2.8) | 116 (4.2) | |
| Diabetes mellitus, N (%) | 171 (52.9) | 1290 (46.8) | 0.04 |
| Hypertension, N (%) | 298 (92.3) | 2341 (84.9) | <0.001 |
| Prior MI or coronary revascularization, N (%) | 103 (31.9) | 449 (16.3) | <0.001 |
| History of stroke, N (%) | 36 (11.1) | 231 (8.4) | 0.10 |
| History of HF, N (%) | 38 (11.8) | 145 (5.3) | <0.001 |
| History of peripheral vascular disease, N (%) | 24 (7.4) | 158 (5.7) | 0.22 |
| Any prior CVD, N (%) | 143 (44.3) | 723 (26.2) | <0.001 |
| Current smoker, N (%) | 41 (12.7) | 361 (13.1) | 0.84 |
| Systolic BP, mean (SD), mm Hg | 131.9 (21.9) | 127.7 (21.7) | 0.001 |
| BMI, mean (SD), kg/m2 | 33.0 (7.9) | 31.8 (7.7) | 0.01 |
| eGFR, ml/min per 1.73 m2, N (%) | |||
| <30 | 78 (24.1) | 514 (18.6) | <0.001 |
| 30–44 | 127 (39.3) | 885 (32.1) | |
| 45–59 | 81 (25.1) | 814 (29.5) | |
| ≥60 | 37 (11.5) | 544 (19.7) | |
| Urine protein, median (IQR), g/24 h | 0.2 (0.1–0.9) | 0.2 (0.1–1.0) | 0.27 |
| Aspirin use, N (%) | 167 (51.7) | 1130 (41.0) | <0.001 |
| Antiplatelet use, N (%) | 177 (54.8) | 1212 (44.0) | <0.001 |
| Warfarin use, N (%) | 20 (6.2) | 59 (2.1) | <0.001 |
| FGF23, median (IQR), RU/ml | 163.7 (109.2–255.0) | 135.5 (91.7–219.3) | <0.001 |
| hs-CRP, median (IQR), mg/L | 3.4 (1.3–7.6) | 2.4 (1.0–5.9) | <0.001 |
| NT-proBNP, median (IQR), pg/ml | 326.7 (120.2–678.2) | 120.2 (53.8–291.6) | <0.001 |
| hsTnT, median (IQR), pg/ml ECG parameters | 16.5 (9.1–32.5) | 10.7 (5.1–20.9) | <0.001 |
| Left ventricular hypertrophy, N (%) | 158 (65.3) | 969 (47.4) | <0.001 |
| Left ventricular mass index, median (IQR), g/m2 | 55.4 (46.7–64.3) | 47.8 (40.7–57.3) | <0.001 |
| Left atrial diameter, mean (SD), cm | 4.1 (0.7) | 3.9 (0.6) | <0.001 |
| Left ventricular ejection fraction, median (IQR) | 54.5 (49.0–57.8) | 55.5 (51.9–59.3) | <0.001 |
IQR, interquartile range.
Rates of Cardiovascular Events after Incident AF
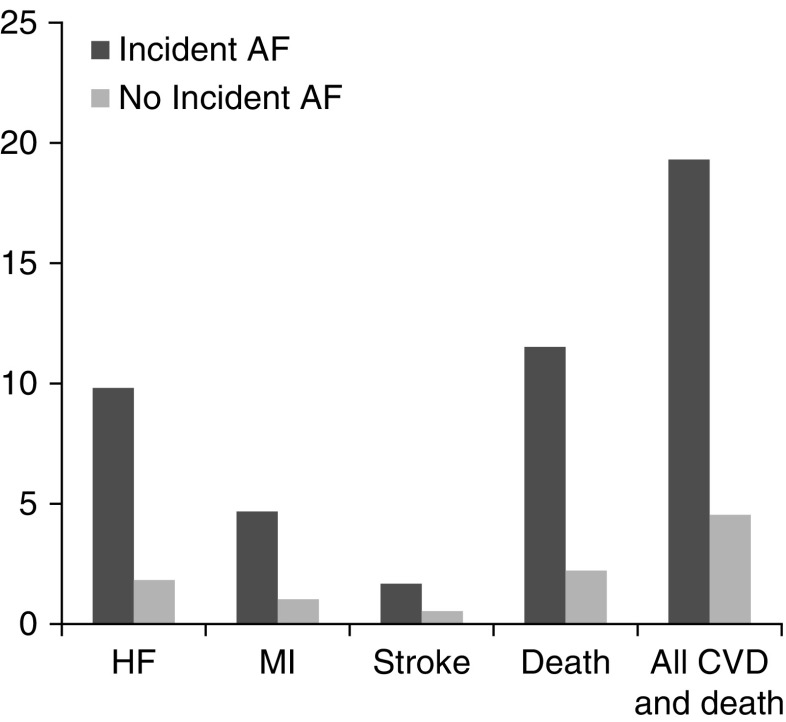
Mean (SD) follow-up time was 6.10 (2.7) years. Among patients without a history of HF, the crude rate of HF hospitalization was several-fold higher among patients who developed incident AF compared with those who did not (Figure 1, Table 2). Similarly, rates of MI and stroke were higher among incident AF participants. The overall rate of death along or the composite of death or any incident CVD event was nearly five-fold higher among participants with incident AF versus those without AF.
Figure 1.
Incidence rates of cardiovascular events and death are higher in participants who developed incident atrial fibrillation versus those who did not.
Table 2.
Association of time-updated incident AF with risk of subsequent cardiovascular events and death in adults with CKD
| Cardiovascular Events | Number of Events | Unadjusted | Model 1 | Model 2 |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| HF | ||||
| No incident AF | 344 | Ref | Ref | Ref |
| Incident AF | 66 | 5.99 (4.55–7.89) | 6.06 (4.59–8.01) | 5.17 (3.89–6.87) |
| Acute MI | ||||
| No incident AF | 198 | Ref | Ref | Ref |
| Incident AF | 36 | 4.81 (3.33–6.95) | 4.28 (2.95–6.22) | 3.64 (2.50–5.31) |
| Stroke | ||||
| No incident AF | 106 | Ref | Ref | Ref |
| Incident AF | 15 | 3.47 (1.98–6.09) | 3.14 (1.78–5.54) | 2.66 (1.50–4.74) |
| Death | ||||
| No incident AF | 461 | Ref | Ref | Ref |
| Incident AF | 111 | 4.50 (3.64–5.57) | 4.14 (3.33–5.15) | 3.30 (2.65–4.12) |
| Composite outcome of HF, acute MI, stroke, and death | ||||
| No incident AF | 827 | Ref | Ref | Ref |
| Incident AF | 112 | 4.38 (3.57–5.36) | 4.20 (3.42–5.15) | 3.68 (2.99–4.53) |
Model 1: adjusted for age, sex, race/ethnicity, clinical site. Model 2: adjusted for model 1 plus diabetes, hypertension, any history of CVD, tobacco use, systolic BP, BMI, eGFR, proteinuria, BMI, LDL cholesterol, HDL cholesterol, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, β-blocker use, and diuretic use. Ref, reference value.
Association of Incident AF with Subsequent Cardiovascular Events
In multivariable Cox models, incident AF was significantly associated with three- to six-fold greater rate of HF, MI, stroke, and death in all models (Table 2). After adjustment for demographics, comorbidity, medication use, and kidney function level, incident AF was associated with substantially higher adjusted rates of HF (HR, 5.17; 95% CI, 3.89 to 6.87), MI (HR, 3.64; 95% CI, 2.50 to 5.31), and stroke (HR, 2.66; 95% CI, 1.50 to 4.74). Furthermore, the adjusted rate of all-cause death and the composite of death or CVD event was greater among participants who developed incident AF (adjusted HR, 3.30 [95% CI, 2.65 to 4.12] and 3.68 [95% CI, 2.99 to 4.53], respectively).
Incident AF and Subsequent Cardiovascular Events using MSMs
Using MSM, the direction and strength of associations of incident AF with subsequent CVD events and death were similar to those observed using our primary analytic approach (Supplemental Table 1).
Sensitivity Analyses: Subgroup Analyses
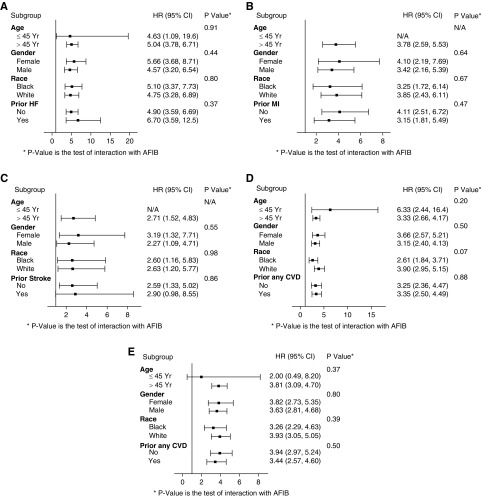
We stratified and tested for potential interactions by age (≤ or >45 years), sex, race (black and nonblack), history of HF, history of MI, and history of stroke. The association of incident AF with HF, MI, stroke, and death were consistent across participant subgroups, and none of the interaction tests were statistically significant (Figure 2, A, D, and E).
Figure 2.
Adjusted association of incident atrial fibrillation with risk of heart failure is similar across patient subgroups. (B) Adjusted association of incident atrial fibrillation with risk of acute myocardial infarction is similar across patient subgroups. (C) Adjusted association of incident atrial fibrillation with risk of stroke is similar across patient subgroups. (D) Adjusted association of incident atrial fibrillation with risk of death from any cause is similar across patient subgroups. (E) Adjusted association of incident atrial fibrillation with risk of the composite of heart failure, acute myocardial infarction, stroke or death is similar across patient subgroups. All models adjusted for age, sex, race/ethnicity, clinical site, diabetes, hypertension, any history of cardiovascular disease, tobacco use, systolic blood pressure, BMI, eGFR, proteinuria, BMI, LDL cholesterol, HDL cholesterol, ACEi/ARB use, β-blocker use, and diuretic use. ACEi, angiotensin-converting enzyme inhibitor; AFIB, atrial fibrillation; ARB, angiotensin receptor blocker; N/A, no events observed in subgroup.
Sensitivity Analyses: Incident AF and Subsequent Cardiovascular Events, Adjusting for Potential Mediators
We first adjusted for longitudinal use of antiplatelet and anticoagulant agents in addition to the fully adjusted models, and this yielded very similar associations of incident AF with subsequent incident CVD events (Table 3). With adjustment for circulating biomarkers of inflammation, mineral metabolism, and cardiac biomarkers of stress, the associations of incident AF with subsequent CVD events remained statistically significant. Adjustment for left ventricular hypertrophy, left ventricular ejection fraction, and left atrial diameter led to modest attenuation in the models; however, even with these additional adjustments, the associations of incident AF with HF, MI, and death remained statistically significant, whereas the association with stroke did not (Table 3).
Table 3.
Association of time-updated incident AF with risk of subsequent cardiovascular events and death after adjustment for potential mediators among adults with CKD
| Cardiovascular Events | Adjusted Modela | Adjusted Modela Plus Adjustment for Warfarin and Antiplatelet Agent Use | Adjusted Modela Plus Adjustment for Baseline hs-CRP, NT-proBNP, hsTnT, and FGF23 Levels | Adjusted Modela Plus Adjustment for ECG Measures: LVM, LVEF, and LA Diameter |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| HF | ||||
| No incident AF | Ref | Ref | Ref | Ref |
| Incident AF | 5.17 (3.89–6.87) | 5.17 (3.89–6.88) | 4.91 (3.68–6.56) | 4.54 (3.23–6.39) |
| Acute MI | ||||
| No incident AF | Ref | Ref | Ref | Ref |
| Incident AF | 3.64 (2.50–5.31) | 3.65 (2.50–5.34) | 3.60 (2.47–5.26) | 3.05 (1.94–4.78) |
| Stroke | ||||
| No incident AF | Ref | Ref | Ref | Ref |
| Incident AF | 2.66 (1.50–4.74) | 2.57 (1.44–4.61) | 2.71 (1.52–4.84) | 1.86 (0.91–3.77) |
| Death | ||||
| No incident AF | Ref | Ref | Ref | Ref |
| Incident AF | 3.30 (2.65–4.12) | 3.28 (2.63–4.09) | 3.14 (2.51–3.93) | 3.12 (2.36–4.11) |
| Composite outcome of HF, acute MI, stroke, and death | ||||
| No incident AF | Ref | Ref | Ref | Ref |
| Incident AF | 3.68 (2.99–4.53) | 3.69 (2.99–4.55) | 3.45 (2.79–4.26) | 3.40 (2.64–4.38) |
LVM, left ventricular mass; LVEF, left ventricular ejection fraction; LA, left atrial; Ref, reference value.
Adjusted for age, sex, race/ethnicity, clinical site, diabetes, hypertension, any history of CVD, tobacco use, systolic BP, BMI, eGFR, proteinuria, BMI, LDL cholesterol, HDL cholesterol, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, , β-blocker use, and diuretic use.
Sensitivity Analysis: Exclusion of Participants with Incident AF and MI or HF during the Same Hospitalization
A total of 28 participants had a diagnosis of incident AF and HF and 20 participants had a diagnosis of incident AF and MI during the same hospitalization. Excluding these participants, the adjusted multivariable association of incident AF with HF remained robust (adjusted HR, 2.87; 95% CI, 2.02 to 4.08). The association of incident AF with MI was attenuated and no longer statistically significant (adjusted HR, 1.56; 95% CI, 0.92 to 2.65).
Discussion
Among a large, well characterized, and diverse prospective cohort of patients with CKD without AF, we found strong associations of incident AF with subsequent CVD events. Incident AF was associated with a two- to six-fold increased rate of HF, MI, stroke, and death, even accounting for a broad range of potential confounders. These associations were consistent across demographic and clinical characteristics and remained robust even after adjustment for use of antiplatelet and anticoagulant therapies; circulating biomarkers of inflammation, cardiac stress, and mineral metabolism; and measures of structural cardiac impairment that may mediate the observed associations.
We found that incident AF was associated with a nearly six-fold increased adjusted rate of HF and a four-fold increased adjusted rate of MI in a large, multicenter CKD population, in whom the clinical consequences of AF have been less well defined. Our findings are novel in that we focused on other outcomes (beyond stroke and death) such as HF and MI, which are less studied in relation to AF, particularly in CKD. One recent study of 1.4 million patients from Ontario with eGFR<90 ml/min per 1.73 m2 (mean eGFR 69 ml/min per 1.73 m2) also found that development of incident AF was significantly associated with greater risk of HF, MI, and mortality, particularly in the first 6 months from diagnosis.43 The findings were largely similar to our study, with some key differences. Our study population had more advanced CKD with higher burden of comorbidity such as diabetes and hypertension compared with the Ontario population, likely contributing to the higher event rate in our study. The events in our study were ascertained by physician adjudication versus use of administrative codes in the Ontario study. We were able to adjust for a larger number of systematically collected covariates including relevant medications, key laboratory values including proteinuria, and measures from transthoracic echocardiograms. Our study materially extends the literature by providing more rigorous support for the strong association between incident AF and subsequent CVD events in adults with CKD.
Prior studies in the non-CKD population have also reported strong links between AF and HF.6–12,44,45 Among participants in the Framingham study, AF was associated with a two-fold increased risk of incident HF,46 and HF was the most common nonfatal CVD outcome after AF in Medicare beneficiaries.14 Other studies in non-CKD cohorts have also noted an association between AF and risk of MI.7,47 In a meta-analysis of 104 cohorts, AF was associated with a 1.5- to five-fold increased risk of death, stroke, coronary heart disease, and HF.6 In the Ontario study, there was a statistically significant interaction by eGFR level, with stronger associations of incident AF with CVD events among those with higher eGFR/without CKD, particularly for the outcome of HF within the first 6 months of diagnosis of AF for which the risk was 14-fold higher compared with those without AF.43 It is unclear why the magnitude of this association is so large compared with previous studies of patients without CKD. Possible reasons include residual confounding because some key covariates were not available, differences in treatment among patients with lower eGFR (greater use of cardioprotective medications), or misclassification of the outcome at lower eGFR (HF versus volume overload for example).
There are several plausible mechanisms to explain our findings. AF and HF have shared risk factors such as abnormal left ventricular structure/function, cardiac stretch from volume expansion, and inflammation, all of which are more common in CKD.48–52 Thus, it is possible that AF may reflect impending clinical HF. Patients with CKD have greater burden of vascular calcification, which in combination with greater cardiac demand, may contribute to subsequent MI.53 It is also possible that shared risk factors between MI and AF, such as hypertension and others, also help to explain the observed associations. Alternatively, AF over time may lead to structural and functional cardiac abnormalities and thus may be a precipitating factor for both HF and MI. Our study supports further efforts to elucidate these mechanisms to reduce AF-related complications in patients with CKD.
We found that incident AF was associated with ischemic stroke and death in patients with CKD. Among patients on dialysis, prior reports have also confirmed an association between AF and ischemic stroke18,54; however, evidence in nondialysis-requiring CKD is limited. Our study augments previous literature reporting an association between incident AF and ischemic stroke in patients with CKD. In addition to stroke, studies of CKD and non-CKD populations have also shown a strong association of AF with risk of death.19,44,55
We adjusted for several biologic shared risk factors that may contribute to the association of incident AF with subsequent CVD events. Inflammation and cardiac stress have been implicated in the pathogenesis of AF and other types of CVD, yet our results remained strong with adjustment for hs-CRP,NT-proBNP, and hsTnT.38–42 Alterations in mineral metabolism represent a novel mechanism linking kidney disease and AF and HF.39,56,57 The observed associations remained strong with adjustment for FGF23. Adjustment for echocardiographic measures led to the greatest attenuation of the observed associations, although they remained statistically significant. Prior reports have also noted strong associations of elevated left ventricular mass and decreased left ventricular ejection fraction with risk of developing AF.58,59 As noted above, it is possible that ventricular dysfunction may lead to AF, or alternatively, AF may contribute to ventricular dysfunction.
The strong associations of incident AF with HF, MI, stroke, and death were consistent across subgroups by age, sex, race, and prevalent CVD. These findings are interesting because older adults, men and white patients are at significantly higher risk for developing AF.60–64 Our findings showing similar results in white and black patients with CKD are in contrast with certain previous studies suggesting a higher risk of AF-related complications in blacks compared with whites in the general population.7,54
Our study was strengthened by examination of a carefully followed cohort with extensive cardiovascular measures and long follow-up time. Incident AF and the CVD events of interest were systematically ascertained and confirmed by adjudication using standardized criteria. We were able to adjust for a large number of relevant covariates, including echocardiographic measures, as well as multiple circulating biomarkers that may be important confounders or mediators. In sensitivity analyses using MSMs, we did not find evidence of strong time-dependent confounding. We also recognize several limitations. The number of outcomes that occurred after development of incident AF was modest, particularly for stroke, which may have affected the precision for that outcome. We were not able to characterize the type of AF (e.g., paroxysmal, persistent, etc.). This was a cohort of patients with CKD only, so we were not able to directly compare our findings in patients without CKD. We did not have detailed data on adherence to or dosages of medications (including antiplatelet and anticoagulant medications). As an observational study, we cannot determine causality, and given that our study involved research volunteers and a significant proportion of participants were followed by nephrologists, our results may not be generalizable to all CKD populations.
In conclusion, incident AF was independently associated with notably higher rates of HF, MI, stroke, and death in a diverse adult population with CKD. Further studies should delineate mechanisms that may link AF with these cardiovascular complications and to investigate whether AF therapies can mitigate the observed excess risks of adverse outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
N.B. and A.S.G. designed the study. D.X. and D.S. conducted statistical analyses. N.B., D.X., D.S., and A.S.G. were responsible for interpretation of data. N.B., D.X., D.S., L.J.A., R.D., H.I.F., J.H., K.J., J.W.K., S.M., S.D.N., M.R., A.C.R., E.Z.S., R.T., and A.S.G. drafted and revised the manuscript. All authors approved the final version of the manuscript.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK103612 (to N.B.). Funding for the Chronic Renal Insufficiency Cohort study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH) (UL1TR000003), Johns Hopkins University (UL1TR-000424), University of Maryland General Clinical Research Center (M01RR-16500), Clinical and Translational Science Collaborative of Cleveland NCATS/NIH and NIH roadmap for Medical Research (UL1TR000439), Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago Clinical and Translational Science Awards (UL1RR029879), Tulane Centers of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases (P20 GM109036), and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco-Clinical Translational Science Awards (UL1 RR-024131).
The CRIC Study Investigators are Lawrence J. Appel (Johns Hopkins University), Harold I. Feldman (University of Pennsylvania), Alan S. Go (Kaiser Permanente Northern California), Jiang He (Tulane University), John W. Kusek (National Institutes of Health), James P. Lash (University of Chicago), Akinlolu Ojo (University of Arizona), Mahboob Rahman (Case Western Reserve University), Raymond R. Townsend (University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018050514/-/DCSupplemental.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al.: Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 129: 837–847, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al.: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102–1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, et al.: The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 81: 469–476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al.: Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946–2953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA: Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 354: i4482, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al.: Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 174: 107–114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D: Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 98: 946–952, 1998 [DOI] [PubMed] [Google Scholar]
- 9.O’Neal WT, Salahuddin T, Broughton ST, Soliman EZ: Atrial fibrillation and cardiovascular outcomes in the elderly. Pacing Clin Electrophysiol 39: 907–913, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY: Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: The Belgrade Atrial Fibrillation Study. Eur J Heart Fail 15: 415–424, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Hart CL, Hole DJ, McMurray JJ: A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 113: 359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, et al.: GARFIELD-AF Investigators : Two-year outcomes of patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Eur Heart J 37: 2882–2889, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haywood LJ, Davis BR, Piller LB, Cushman WC, Cutler JA, Ford CE, et al.: ALLHAT Collaborative Research Group : Influence of prevalent and incident atrial fibrillation on post-trial major events in ALLHAT. J Natl Med Assoc 109: 172–181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, et al.: Clinical course of atrial fibrillation in older adults: The importance of cardiovascular events beyond stroke. Eur Heart J 35: 250–256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, et al. : Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm 11: 1752–1759, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, et al.: Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis 51: 255–262, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S: The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 22: 349–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih CJ, Ou SM, Chao PW, Kuo SC, Lee YJ, Yang CY, et al.: Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: A competing-risk analysis of a nationwide cohort. Circulation 133: 265–272, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Bansal N, Fan D, Hsu CY, Ordonez JD, Go AS: Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc 3: e001303, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB: The natural history of congestive heart failure: The Framingham study. N Engl J Med 285: 1441–1446, 1971 [DOI] [PubMed] [Google Scholar]
- 23.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, et al.: ALLHAT Collaborative Research Group : The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) heart failure validation study: Diagnosis and prognosis. Am Heart J 153: 42–53, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al.: Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction : Universal definition of myocardial infarction. Circulation 116: 2634–2653, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al.: Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 30: 736–743, 1999 [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics (NCHS) : National Health and Nutrition Examination Survey Anthropometry Procedures Manual, Atlanta, GA, Centers for Disease Control and Prevention, 2000 [Google Scholar]
- 27.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al.: Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al.: CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al.: Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal NKM, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, et al. : Evolution of left ventricular structure from CKD through ESRD: The CRIC Study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al.: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Robins JM, Hernán MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Hernán MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Brunelli SM, Joffe MM, Israni RK, Yang W, Fishbane S, Berns JS, et al.: History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol 3: 777–782, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole SR, Hernán MA: Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168: 656–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, et al.: Fibroblast growth factor-23 and incident atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation 130: 298–307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the chronic renal insufficiency cohort study. JAMA Cardiol 1: 548–556, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al.: N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: The Cardiovascular Health Study. Circulation 120: 1768–1774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, et al.: N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: The effects of age, sex and ethnicity. Heart 99: 1832–1836, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al.: ARISTOTLE and RE-LY Investigators : The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: A derivation and validation study. Lancet 387: 2302–2311, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Massicotte-Azarniouch D, Kuwornu JP, Carrero JJ, Lam NN, Molnar AO, Zimmerman D, et al.: Incident atrial fibrillation and the risk of congestive heart failure, myocardial infarction, end-stage kidney disease, and mortality among patients with a decreased estimated GFR. Am J Kidney Dis 71: 191–199, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, et al.: Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: A community-based study from the Netherlands. J Am Coll Cardiol 66: 1000–1007, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, et al.: ORBIT-AF Investigators and Patients : Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 5: 44–52, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al.: Atrial fibrillation begets heart failure and vice versa: Temporal associations and differences in preserved versus reduced ejection fraction. Circulation 133: 484–492, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang ZM, et al.: Atrial fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 131: 1843–1850, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SC, Chang JM, Liu WC, Huang JC, Tsai JC, Lin MY, et al.: Echocardiographic parameters are independently associated with increased cardiovascular events in patients with chronic kidney disease. Nephrol Dial Transplant 27: 1064–1070, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, et al.: CRIC Study Investigators : A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, et al.: High-sensitivity troponin T and N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: The Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol 26: 946–956, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Naidoo S, Dickens C, et al.: Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol 86: 131–135, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O’Donnell CJ, et al.: Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant 26: 920–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Bragt KA, Nasrallah HM, Kuiper M, Luiken JJ, Schotten U, Verheule S: Atrial supply-demand balance in healthy adult pigs: Coronary blood flow, oxygen extraction, and lactate production during acute atrial fibrillation. Cardiovasc Res 101: 9–19, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, et al.: Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 23: 112–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, et al.: Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 305: 2080–2087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al.: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, et al.: Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 63: 2007–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, et al.: Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 296: 1242–1248, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM: Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 128: 2470–2477, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al.: Candidate-Gene Association Resource (CARe) Study : European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation 122: 2009–2015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, et al.: Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 173–181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al.: Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 158: 111–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, et al.: Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ 532: h7013, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.