Abstract
Background
Use of enzyme replacement therapy (ERT) to treat Fabry disease, caused by deficient lysosomal α-galactosidase A activity, can lead to formation of neutralizing antidrug antibodies (ADAs). These antibodies are associated with increased accumulation of plasma globotriaosylceramide (Gb3) and disease progression. Because agalsidase ERT can saturate ADA-binding sites during infusions (achieving agalsidase/antibody equilibrium), we investigated in this open cohort study whether saturated patients (who have excess agalsidase after infusions) experience better clinical outcomes compared with not saturated patients (who have excess ADAs after infusions).
Methods
We isolated ADAs from sera of 26 men with Fabry disease receiving ERT (for a median of 94 months) and determined the amount of agalsidase necessary for antibody saturation. Clinical and biochemical outcomes included measurements of eGFR, interventricular septum thickness, and lyso-Gb3.
Results
ADA titers decreased significantly in all patients during infusion. Agalsidase-α and agalsidase-β had similar ADA-binding capacity and comparable ADA saturation frequency. Fourteen patients with saturated ADAs presented with mild (but significant) loss of eGFR, stable septum thickness, and significantly decreased lyso-Gb3 levels. The 12 not saturated patients had a more pronounced and significant loss of eGFR, increased septum thickness, and a smaller, nonsignificant reduction in lyso-Gb3, over time. In three patients, dose escalation resulted in partially elevated ADA titers, but importantly, also in reduced lyso-Gb3 levels.
Conclusions
A not saturated ADA status during infusion is associated with progressive loss of eGFR and ongoing cardiac hypertrophy. Dose escalation can result in saturation of ADAs and decreasing lyso-Gb3 levels, but may lead to increased ADA titers.
Keywords: chronic kidney disease, Fabry disease, glomerular filtration rate, immune, complexes, left ventricular hypertrophy
Visual Abstract
Fabry disease (FD; Online Mendelian Inheritance in Man [OMIM] no. 301500) is a rare, X-linked (chromosome Xq22.1) inherited disorder caused by a deficiency of lysosomal α-galactosidase A (GLA; OMIM no. 300644) activity. Progressive cellular globotriaosylceramide (Gb3) accumulation results in a multisystemic disease, with stroke, heart failure, cardiac arrhythmia, and ESRD, leading to a mean reduction in lifespan of 10–15 years.1 Since 2001, treatment with two different recombinant enzyme replacement therapies (ERTs) is available (agalsidase-α [0.2 mg/kg body wt every other week] and agalsidase-β [1.0 mg/kg body wt every other week]),2,3 leading to cellular Gb3 clearance and an overall improvement of disease burden. However, a number of studies have demonstrated that ERT may cause infusion-associated reactions2,3 as well as the formation of neutralizing antidrug antibodies (ADAs).4–7 In male patients with FD, the presence of ADAs has been shown to be associated with increased cellular Gb3 depositions,8 plasma lyso-Gb3 concentrations,6,7 and harmful clinical end points including increased left ventricular mass and progressive loss of renal function.7 Neutralizing ADAs predominantly belong to the IgG4 subtype9 and titers may be decreased by general immunosuppression.10 Recently, we established an advanced inhibition assay (AIA), using purified IgGs from patients’ sera to determine individual ADAs.9 By this approach, we were able to demonstrate that the amount of circulating anti-GLA ADAs decreased individually during infusions with ERT.9 Preliminary data further suggest a better biochemical response (i.e., decreasing lyso-Gb3 levels) in patients where ADA titers were saturated during infusions.9 Interestingly, a better biochemical response in patients with ADAs, dependent on the applied ERT dose, was also suggested in a recent multicenter study.11
In this work, we aimed to analyze if the amount of infused enzyme may saturate individual neutralizing ADA titers (achieving agalsidase/antibody equilibrium). We hypothesized that saturated patients (those who have agalsidase excess after infusions) compared with not saturated (those who have antibody excess or are agalsidase-deficit after infusions) patients benefit from a better clinical outcome. We further elucidated the compensatory potential of both currently approved compounds (agalsidase-α and agalsidase-β) and assessed the influence of dose adjustment (i.e., escalation) for saturation of ADA titers.
Methods
Patients
Inclusion criteria of this open cohort study were that patients were adult men with at least 6 months of any ERT, and had tested positive for neutralizing ADAs. All investigations were performed after the approval of the Medical Association of Westphalian-Lippe and the Ethical Committee of the Medical Faculty of the University of Muenster (project no. 2011–347-f, date of report: July 7, 2011). Written informed consent of patients was obtained for analysis and publication. If not stated otherwise, time point of data assessment and determination of neutralizing ADA status was the last visit (2017–2018).
eGFR was quantified using the CKD Epidemiology Collaboration equation on the basis of serum creatinine.12 Septum thickness was measured as interventricular septum thickness in diastole (IVSd). Left ventricular hypertrophy was defined as an IVSd≥12 mm. eGFR, IVSd, and lyso-Gb3 data were assessed at ERT-naïve baseline and at the last available visit (2017–2018 follow-up visit). If no values were available for ERT-naïve baseline, the nearest values to ERT-naïve baseline were assessed and these patients were highlighted (dotted lines) within the figures. Yearly slopes for eGFR, IVSd, and lyso-Gb3 were retrospectively calculated between ERT initiation and follow-up.
Patients’ individual GLA mutations and GLA activities are presented in Supplemental Table 1.
Biochemical Analyses
If not stated otherwise, serum samples were collected 30–60 minutes before and 30 minutes after the infusion at the opposite arm. Standard ERT inhibition assays to identify inhibition-positive patients were performed as reported previously.4,7 In brief, 5 µl of serum were preincubated with 1 ng agalsidase-α or -β for 10 minutes at room temperature. Subsequently, the remaining GLA activity was determined using 4-methylumbelliferyl-α-D-galactopyranoside (Santa Cruz Biotechnology, Heidelberg, Germany), as described elsewhere.13 N-acetylgalactosamine (Santa Cruz Biotechnology) was used as specific inhibitor of endogenous α-galactosidase B activity.14 To determine mean ERT inhibition in percent, absolute values were compared with nonserum-treated (5 µl 0.7% sodium chloride control) GLA activity of 1 ng agalsidase-α or -β, respectively. Patients with a mean ERT inhibition >50% were defined as ERT inhibition-positive.4,7 For AIAs, IgGs from serum samples were purified using the Melon Gel IgG Spin Purification Kit (Thermo Scientific, Darmstadt, Germany) according to manufacturer’s instructions as described recently.9,10 In brief, 25–50 µl of raw serum were incubated 5 minutes at room temperature with 500 µl Melon Gel for protein absorption. Subsequently, IgGs were separated by spin purification. Purified IgG content was determined by BCA analysis (Thermo Scientific) and routinely controlled by SDS-PAGE and subsequent Coomassie staining (Supplemental Figure 1). AIAs were then performed similar to standard ERT inhibition assays with the following modifications. Instead of raw sera, 10 µl of patients’ purified total IgGs was preincubated for 10 minutes with 1 ng ERT (agalsidase-α or -β). Individual ERT inhibition in picograms per microgram IgG was determined by normalizing the absolute values against the values of pooled inhibition-negative controls.9 On the basis of this method, patients with free ADAs (antibody excess) after infusions did not receive enough infused enzyme to saturate all available antibodies and their sites (agalsidase deficit). According to this definition that a distinct concentration of infused enzyme is required to saturate all available antibody sites resulting at least in an agalsidase/antibody equilibrium, patients can be distinguished as saturated (agalsidase excess) and not saturated (antibody excess/agalsidase deficit) during infusions.
For HPLC-based lyso-Gb3 determination, lyso-Ceramide was used as reference (Matreya, LLC, Pleasant Gap, PA) and D5-Fluticasone Propionate (EJY Tech, Inc., Rockville, MD) served as internal standard.
Titration of Neutralizing ADAs
To determine the amount of ERT required to saturate patients’ ADAs, AIAs were modified as follows: 5 µg purified total IgGs were preincubated with a serial dilution (0.125–52 ng) of ERT (agalsidase-α or -β) and ERT inhibition in percent was determined by normalization with inhibition-negative controls. ERT inhibition was then plotted against the amount of agalsidase. Saturation was defined as the amount of enzyme required to reduce the neutralizing capacity of 5 µg patients total IgG below the ERT neutralizing threshold of 10% (background threshold). Subsequently, the amount of agalsidase saturating 5 µg IgGs was extrapolated to the amount needed to saturate patients’ total IgGs. Calculation of patients’ total IgG was performed as previously described, assuming that male patients have, on average, approximately 3 L serum.10
Repeated Sampling and ADA Measurements during Infusion
To analyze the effect of infused agalsidase on ADA titers during infusion, repetitive serum samples (opposite arm, every 30 minutes) were drawn. Total IgGs were purified from every sample and the individual ERT inhibition in picograms per microgram IgG was determined to measure neutralizing ADA titers as described above. GLA activity was measured from every serum sample to demonstrate increasing GLA concentrations during infusion. Because the flow rate of the infusion was constant, the cumulative dose at every time point during infusion was known. Detection of circulating recombinant GLA protein during infusions was further confirmed by Western blot analysis and subsequent signal evaluation using ImageJ. In brief, 1 µl serum from individual blood drawings was used for SDS-PAGE and subsequent immobilization on PDF membranes. Membranes were blocked overnight in Tris buffered saline with 5% milk powder. Detection was performed using anti-GLA antibody (ab168341, dilution: 1:10,000; Abcam) and a horseradish-peroxidase–labeled goat anti-rabbit IgG antibody (dilution: 1:20,000; Sigma-Aldrich).
Statistical Analyses
If not stated otherwise, continuous variables were expressed as mean with SD or as median with range. Categorical data were expressed as n and relative frequencies as percent. One-sample t test or Wilcoxon signed-rank tests were used to analyze yearly slopes. Statistical significance was considered at a two-sided P<0.05.
Correlation analyses were performed using Spearman rank (r) or Pearson correlation coefficient (r2) to analyze associations between total amounts of infused enzymes and ERT neutralization from ADAs, as appropriate. GraphPad PRISM V5.0 software (GraphPad Software Inc., La Jolla, CA) was used for statistical analyses and visualization. If not stated otherwise, experiments performed with agalsidase-α and agalsidase-β showed similar results.
Results
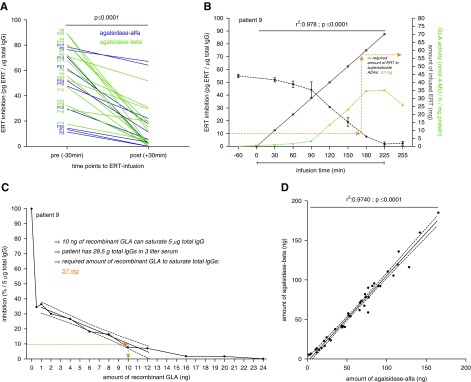
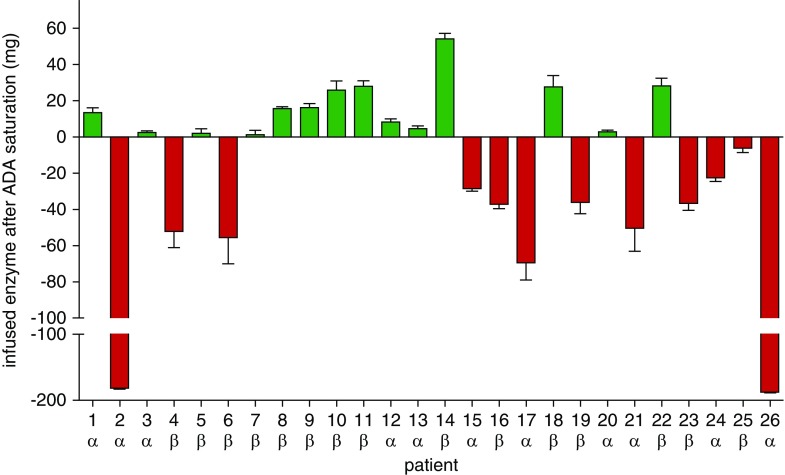
Twenty-six male patients with classic FD were consecutively recruited between 2001 and 2017 (Muenster [Germany] n=20, Wuerzburg [Germany] n=2, Muellheim [Germany] n=1, Berlin [Germany] n=1, Innsbruck [Austria] n=1, Zurich [Switzerland] n=1) (Table 1). At data assessment, mean patients’ age was 40±13 years and median time under ERT was 94 (range: 7–204) months (11 and 15 patients treated with agalsidase-α [0.2 mg/kg], agalsidase-β [1.0 mg/kg], respectively). Three patients had been switched from agalsidase-α to agalsidase-β between ERT initiation and follow-up (Table 1). Clinical baseline parameters as well as individual yearly slopes for lyso-Gb3, eGFR, and interventricular septum thickness between ERT initiation and follow-up are presented in Table 1. The majority of patients carried nonsense mutations (77%; Supplemental Table 1), and the distribution of these patients to agalsidase-β and agalsidase-α was comparable (86.7% versus 63.6%; P=0.35). Using AIAs, a significant reduction of ADA titers in all patients was achieved already during infusions, independent of the applied compound (i.e., agalsidase-α or -β; P<0.001; Figure 1A). Patients’ ADA status after ERT infusion (saturated or not saturated) is presented in Supplemental Table 2. As reported earlier,9 a continuous measurement of ADAs during infusion allows the determination of the required amount of enzyme necessary to saturate all neutralizing ADAs. An example is shown in Figure 1B for patient 9 receiving 70 mg agalsidase-β (15th infusion; Supplemental Figure 2) in total, with 57 mg agalsidase required for ADA saturation. Because these measurements require continuous blood sampling during infusions, we aimed to transfer the assessment of required enzyme to a laboratory setting using titration of a fixed amount of patients’ purified total IgGs from a single blood sample against increasing amounts of enzyme (agalsidase-α and agalsidase-β) (Figure 1C). This approach allows the calculation of the individual amount of enzyme required to saturate patients’ ADAs from one single blood sample (Figure 1C, Supplemental Table 2). Of note, by titration against agalsidase-α and agalsidase-β, we observed no difference between both compounds in the amount of enzyme required, but found a high correlation for the capacity to saturate ADAs (r2=0.97, P<0.001; Figure 1D). Patients receiving agalsidase-β tended to have higher antibody titers compared with patients treated with agalsidase-α (Supplemental Figure 3; P<0.10). Further analysis revealed also a trend toward a correlation between increasing ERT doses and increasing ADA titers (Spearman r=0.37; P=0.07). Consequently, we retrospectively analyzed whether patients’ saturation status determined during infusion affected the clinical outcome. Therefore, patients were separated by saturation status by subtracting the calculated amount of agalsidase required to saturate ADAs from the individually infused current dose (Figure 2). All patients suffered from the classic form of FD and showed decreased GLA activities (Supplemental Table 1). To exclude that the underlying genotype might have an effect on the ADA titers in our study, we first compared patients with nonsense (n=20) and missense (n=6) mutations and observed comparable amounts of recombinant enzyme required for ADA saturation in both groups (missense: 68±26 mg, nonsense: 68±1 mg; P=0.99). In general, there was no increased frequency for ADA saturation (agalsidase excess) in patients receiving agalsidase-β compared with agalsidase-α (n=9/15 [60.0%] versus n=5/11 [45.5%] patients; P=0.69). At (ERT-naïve) baseline, no significant genetic, clinical, and biochemical differences between saturated and not saturated patients were observed (Supplemental Table 3). At baseline, both groups presented with comparable eGFR and lyso-Gb3 levels (Supplemental Table 3). However, the group of saturated patients presented with an increased interventricular septum thickness at baseline, pointing toward a higher cardiac disease burden at baseline in these patients (P<0.05; Supplemental Table 3). The saturated group of patients (n=14, 53.8%) presented with a loss of eGFR of −3.0±2.0 ml/min per 1.73 m2 per year (P<0.001), a stable interventricular septum thickness (measured as IVSd) [−0.2±0.9 mm/yr, P=0.97]), and a significant decrease of plasma lyso-Gb3 levels (−24.9±35.0 ng/ml per year, P<0.001) over time (Figure 3). By contrast, not saturated patients (n=12, 46.2%) presented with a steeper decline of eGFR (−4.8±4.0 ml/min per 1.73 m2 per year, P=0.004), a significant increase in interventricular septum thickness (0.4±0.4 mm/yr, P=0.009), and a trend for decreasing plasma lyso-Gb3 values (−7.9±18.8 ng/ml per year, P=0.32) over time (Figure 3). These results indicate that a saturated status during infusion might be beneficial in terms of clinical outcome and less severe disease progression of affected patients.
Table 1.
Patients’ characteristics and clinical data at ERT-naïve baseline
| Patient No. | Age, yr | Initial ERT | Current ERT | Infused ERT (current), mg | Time under ERT, mo | GLA Mutation | ACEi/ARB, Yes/No | eGFR, ml/min per 1.73 m2 | LVH, yes/no | Lyso-Gb3, ng/ml | Lyso-Gb3, ng/ml per yr | eGFR, ml/min per 1.73 m2 per yr | IVSd, mm/yr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | α | α | 17.5 | 63 | p.R301Q | Yes | 65.0 | Yes | 26.7 | −1.9 | −5.4 | +1.7 |
| 2 | 49 | α | α | 14 | 54 | p.S247P | Yes | 45.0 | Yes | 57.8 | +13.1 | −10.9 | 0.0 |
| 3 | 48 | α | α | 14 | 46 | c.1090_1103del | Yes | 6.5 | Yes | 55.0 | −4.7 | −0.1a | +0.2 |
| 4 | 47 | β | β | 70 | 114 | p.L294S | Yes | 37.9 | No | 19.5 | −1.5 | −16.3b | +1.5 |
| 5 | 44 | β | β | 70 | 192 | p.R220X | No | 99.7 | Yes | 30.4 | −2.1 | −0.5 | −0.1 |
| 6 | 42 | β | β | 70 | 192 | p.R220X | No | 95.4 | Yes | 70.0 | −4.7 | −2.0 | +0.3 |
| 7 | 30 | β | β | 70 | 190 | c.762ins282bp | No | 148.8 | No | 21.5 | +2.1 | −1.1 | −0.2 |
| 8 | 47 | α | β | 70 | 152 | p.C94S | No | 108.5 | No | 45.7 | −5.3 | −3.1 | −0.1 |
| 9 | 31 | β | β | 70 | 7 | c.723dupT | No | 92.2 | N/A | 112.0 | N/A | −11.8 | N/A |
| 10 | 46 | α | β | 70 | 145 | p.R227X | Yes | 122.7 | Yes | 110.0 | −18.6 | −0.1 | +0.2 |
| 11 | 19 | β | β | 70 | 8 | p.R227X | No | 148.9 | Yes | 133.0 | N/A | −3.4 | +0.7 |
| 12 | 22 | α | α | 14 | 43 | c.702_709del | No | 146.8 | Yes | 155 | −17.4 | −0.9 | +0.1 |
| 13 | 31 | α | α | 14 | 92 | c.IVS2–2A>G | No | 148.1 | No | 46.9 | −1.7 | −2.6 | −0.4 |
| 14 | 50 | β | β | 70 | 130 | p.R220X | Yes | 77.8 | Yes | 55.0 | N/A | −0.4 | −0.2 |
| 15 | 42 | α | α | 14 | 95 | p.M1T | No | 118.4 | Yes | 105.0 | N/A | −3.4 | +0.6 |
| 16 | 31 | β | β | 70 | 204 | p.R227X | No | 145.0 | No | 41.4 | +0.4 | −1.7 | +0.1 |
| 17 | 24 | α | α | 14 | 13 | p.Y151X | No | 124.6 | No | 197.0 | −10.1 | −1.3 | +0.1 |
| 18 | 53 | β | β | 105 | 122 | p.Y173X | No | 47.0 | Yes | 46.9 | −13.1 | −3.9 | +0.2 |
| 19 | 43 | β | β | 70 | 162 | Deletion E7 | Yes | 117.8 | No | 29.9 | +0.5 | −2.9 | +0.1 |
| 20 | 39 | α | α | 14 | 47 | p.W349X | Yes | 104.6 | Yes | 164.0 | −28.1 | −3.6 | +0.2 |
| 21 | 50 | α | α | 17.5 | 191 | p.R220X | Yes | 110.3 | Yes | 107.0 | −12.6 | −3.6 | +0.3 |
| 22 | 22 | α | β | 55 | 13 | p.C202Y | Yes | 140.0 | Yes | 76.8 | −53.6 | −7.5 | −2.8 |
| 23 | 47 | β | β | 80 | 24 | p.R227X | Yes | 61.8 | No | 170.0 | −58.4 | −1.7 | 0.0 |
| 24 | 19 | α | α | 14 | 12 | p.I91T | No | 152.4 | N/A | 12.9 | N/A | N/A | N/A |
| 25 | 52 | β | β | 70 | 181 | c.1208delAAG | Yes | 120.6 | Yes | 66.2 | −2.9 | −2.2 | +0.2 |
| 26 | 49 | α | α | 14 | 46 | c.718_719delAA | Yes | 90.5 | Yes | 65.1 | +4.9 | −9.5 | +0.5 |
| Total | |||||||||||||
| 26 | 44 [19–70] | 14 α; 12 β | 11 α; 15 β | 70 [14–105] | 94 [7–204] | 6 missense/20 nonsense | 13 yes; 13 no | 109.4 [6.5–152.4] | 16 yes/8 no | 74.6 [12.9–197.0] | −4.7 [−58.4 to +13.1] | −3.0 [−16.3 to –0.1] | 0.2 [−2.8 to 1.7] |
Values are given as total or mean with range. If no values at ERT-naïve baseline were available, the earliest value is presented. Albuminuria: 0=none, 1=microalbuminuria, 2=macroalbuminuria. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVH, left ventricular hypertrophy; N/A, not available.
Dialysis treatment.
Kidney transplantation.
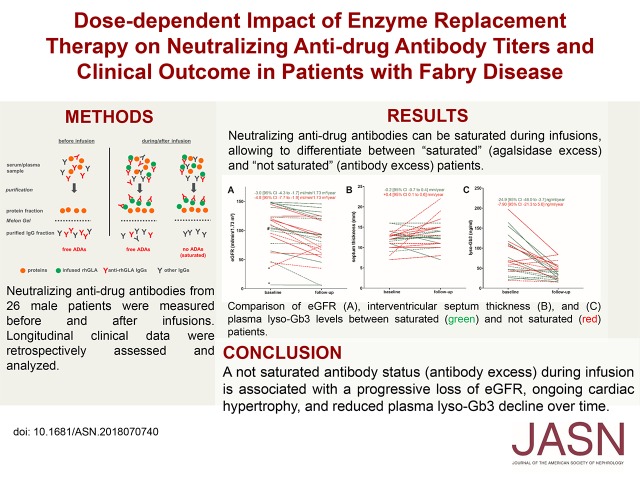
Figure 1.
The required amount of recombinant enzyme for antibody saturation can be calculated using patients' purified total IgGs. (A) Determination of the individual antibody status before and after infusion. (B) Effect of infused ERT amounts (gray) on antibody titer (black) during infusion and agalsidase activity (green). The longitudinal measurement of ADA titers during infusions allows estimating the amount of enzyme required to saturate ADAs. (C) Titration of purified total IgG against ERT allows calculating the required amount of enzyme from one serum sample. The determination of the individual amount of recombinant GLA to saturate ADAs was performed using titration and subsequent calculation. First, a titration was performed to identify the required amount of enzyme to saturate 90% of 5 µg total IgGs (see Methods). ERT inhibition was plotted against the amount of enzyme. The amount required can be identified on the y-axis (here 10 ng, dashed orange line), showing that 2 ng is sufficient to saturate 1 µg total IgG. In the example shown, the patient’s measured IgG concentration was 9.5 mg/ml serum. Because each patient has approximately 3 L of serum, the total amount of patient’s IgGs per total volume of serum was calculated with 28.5 g. Therefore, the amount of enzyme required to saturate 90% of all IgGs was 57 mg (28.5 g×2/1000=57 mg). (D) Crosstitration of agalsidase-α and agalsidase-β demonstrates similar ADA saturation of either type of enzyme.
Figure 2.
Antibody titer determination allows a classification in saturated and not saturated patients during infusion. Positive values represent residual infused enzyme after ADA saturation (green, agalsidase excess). Negative values represent additional theoretical infused enzyme necessary to saturate ADA titers (red, antibody excess).
Figure 3.
Antibody saturation due to enzyme excess is associated with better outcomes over time. (A) Change in eGFR. (B) Change of interventricular septum thickness. (C) Change of plasma lyso-Gb3 levels. Green lines: saturated patients with agalsidase excess during infusion. Red lines: not saturated patients with antibody excess (agalsidase deficit) during infusion. Solid lines represent ERT-naïve patients at baseline. * indicates patients excluded for eGFR calculations because of renal transplantation or dialysis. # indicates a patient excluded from eGFR calculation because of newly adjusted renin-angiotensin-aldosterone system blockers. 95% CI, 95% confidence interval.
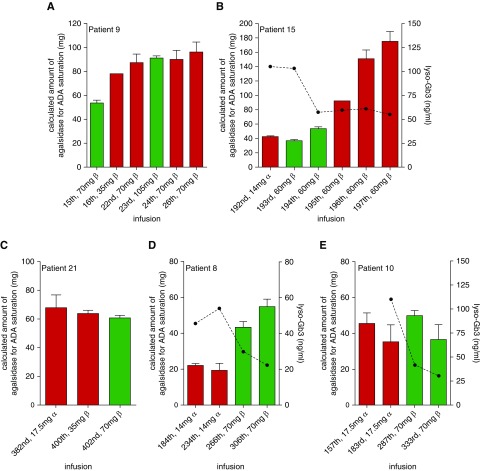
Because effects of an approved dose increase on saturation of ADAs are yet unknown, we increased the infused dose of enzyme in three patients (patients 9, 15, and 21) as a proof-of-concept study (Figure 4, A–C). A single-dose increase of additional 35 mg (from 70 to 105 mg) agalsidase-β in patient 9 resulted in a saturation of ADAs at the 23rd infusion and no significant changes in ADA titers (22nd versus 24th infusion; Figure 4A). A switch from 14 mg agalsidase-α to 60 mg agalsidase-β (patient 15) also resulted in saturation of ADAs during the first two infusions after the switch (193rd and 194th versus 192nd infusion; Figure 4B). However, an increase of ADA titer was already detected at the second infusion with agalsidase-β (194th infusion), resulting in at least a three-fold increase of ADA titers at the 197th infusion (compared with titers before switch), which could not be saturated after the 195th infusion by an approved dose of agalsidase-β (Figure 4B). In patient 21, a stepwise escalation from 17.5 mg agalsidase-α to 35 mg agalsidase-β (from the 396th infusion) and subsequently 70 mg agalsidase-β (402nd infusion) resulted in a saturation of ADA titers at the highest dose but no measurable increase of ADA titers (Figure 4C). To retrospectively analyze the long-term effect of a dose increase on ADA titers, we analyzed two patients (patients 8 and 10) who switched from agalsidase-α (14 and 17.5 mg, respectively) to agalsidase-β (70 mg) (Figure 4, D and E). Titration measurements of frozen serum samples revealed that ADA titers in both patients were not fully saturated by the infused amount of 14 and 17.5 mg agalsidase-α, respectively. After the patients had been switched to 70 mg agalsidase-β, ADA titers in patient 8 increased to some extent but could still be saturated, whereas ADA titers in patient 10 seemed to remain stable (Figure 4, D and E). Most importantly, after dose increase and saturation of ADAs, plasma lyso-Gb3 values markedly decreased in patients (Figure 4, B, D, and E).
Figure 4.
Dose increase results in antibody saturation but may also trigger further ADA formation. (A) Saturation of ADAs due to dose increase. (B) Short-term effect of dose increase due to switch of product. (C) Effect of dose increase due to stepwise escalation/product switch (396th infusion). (D) Long-term effect of dose increase due to product switch (260th infusion) resulted in increased ADA titers, but titers could be saturated. (E) Long-term effect of dose increase due to product switch (185th infusion) resulted in stable ADA titers, which could be saturated. Green bars indicate saturation (agalsidase excess), red bars indicate no saturation (antibody excess) during infusion. Dotted lines represent plasma lyso-Gb3 levels.
Discussion
In this study, we investigated the effect of neutralizing ADA titers on disease progression in 26 male patients with FD. We characterized the compensatory effects of infused enzyme dose on neutralizing ADA titers and the clinical outcome of affected patients. Our main findings are: (1) individual ADA titers and the required amount of enzyme to saturate ADA titers can be determined from one single blood sample, (2) ADA saturation (agalsidase excess) during infusion is associated with improved renal and cardiac outcome, as well as an improved biochemical response, and (3) a dose escalation may result in a saturation, but may also lead to increased ADA titers over time.
Previous studies demonstrated the presence of neutralizing ADAs in up to 70% of male patients with FD, which has been suggested to limit therapy success.4–9,11 However, the absence of a consistent assay to determine patients’ antibody status is a limitation in the comparison of results from individual studies,15 and might explain controversial findings.5,16–18 Furthermore, the acute effects of infused amounts of enzyme on ADA titers so far remain elusive. Recently, we established AIAs to demonstrate that ADA titers in affected patients may already decrease during infusions, suggesting that ADAs can be saturated.9 These preliminary data suggested that patients may be stratified into saturated (agalsidase excess) and not saturated (antibody excess/agalsidase deficit). This was further supported by the findings of Arends et al.,11 demonstrating that patients tested positive for neutralizing ADAs show a better biochemical response in terms of a steeper decline of lyso-Gb3 levels over time when treated with agalsidase-β (1.0 mg/kg) compared with agalsidase-α (0.2 mg/kg). In general, our previous data suggested that a saturation of ADAs does not depend on the individual enzyme used (i.e., agalsidase-α or agalsidase-β), but on the total amount of infused enzyme and the individual ADA titer. To provide a potential tool for a more optimized and personalized treatment, we performed titration analyses in 26 ADA-positive male patients with FD, enabling the determination of the individual amount of ERT required to saturate ADAs. By comparing calculated amounts on the basis of single blood sample measurements with infused doses, we were able to distinguish between saturated and not saturated patients. Of note, the titrations against agalsidase-α and agalsidase-β revealed no difference in the capacity to saturate ADAs between both drugs. Saturated (agalsidase excess) compared with not saturated (antibody excess) patients presented with better renal and cardiac outcomes as well as biochemical response, whereas the disease load at ERT-naïve baseline was comparable. This may indicate that in patients with ADAs a clinical effect of infused enzyme dose depends on ADA titers and their saturation during infusion (Figure 5).
Figure 5.
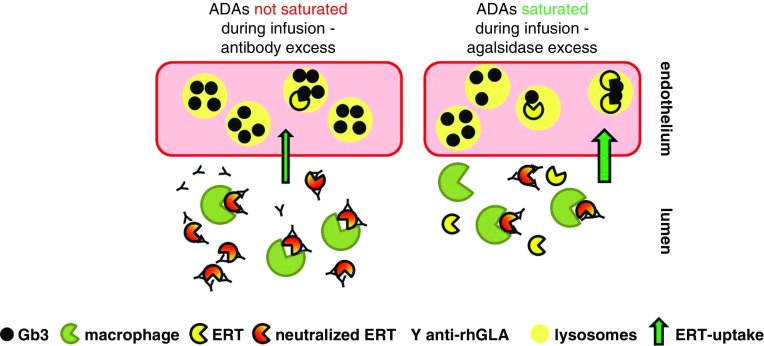
Schematic model of ERT and neutralizing antibodies during infusion. If antibodies are present, they neutralize ERT activity by binding. In addition, IgG-tagged agalsidase will be internalized and digested by macrophages. If more antibodies than ERT are present (antibody excess/agalsidase deficit), this results in a decreased cellular Gb3-clearance (left). If ERT overcomes antibody titers (agalsidase excess), more ERT can enter lysosomes of target cells (right).
The risk of developing ADAs has been suggested to be increased in patients treated with agalsidase-β.11 According to our data, patients with agalsidase-β also seem to have slightly higher antibody titers compared with patients treated with agalsidase-α. However, the calculated mean amount required to saturate ADAs (approximately 74 mg agalsidase-β versus approximately 60 mg agalsidase-α) was found to be closer to the standard dose for the treatment of a standard patient, with 70 kg body wt, and treated with agalsidase-β (70 mg) instead of agalsidase-α (14 mg). This might explain the better outcome observed by Arends et al.11 Of note, there was no general increased risk for saturation in patients receiving agalsidase-β in our cohort.
Previous studies raised the question of whether a dose escalation (i.e., switch from agalsidase-α to agalsidase-β) in patients with ADAs would have beneficial effects on clinical outcome.6,18 However, literature concerning ADA titers due to dose escalation is scarce. Smid and colleagues reported an increase of ADA titers in four out of nine patients when transferred to higher doses, but no original data have been presented.18 Our current data suggest that a switch from agalsidase-α to agalsidase-β can result in a general increase of ADAs in individual patients, at least over a short-term period. Although this switch resulted in high ADA titers that were not saturable, the stepwise dose escalation and the retrospective analysis of a previously switched patient suggest that the individual reactions on dose escalation in terms of ADA formation might also be mild to moderate. Most importantly, a saturation of ADAs due to a dose increase of ERT was associated with decreasing lyso-Gb3 levels, demonstrating a biochemical response and potential beneficial therapeutic effects. Prospective studies are now warranted for the stratification of patients’ individual ADA reaction according to adapted enzyme amounts, their saturation status, and clinical outcome including lyso-Gb3 levels. Furthermore, it would be of interest to reanalyze previous studies on the basis of saturated ADA titers.
In this study, our focus was on neutralizing ADAs, but a humoral response can include the formation of neutralizing and non-neutralizing antibodies, which has been confirmed by a recent study in affected patients with FD.19 Because IgG-tagged ERT molecules are also internalized and digested by macrophages,4 future studies are warranted to analyze a potential effect of neutralizing as well as non-neutralizing ADAs on cellular ERT uptake of antigen-ADA complexes in macrophages (for elimination) and endothelial cells (for lysosomal Gb3 clearance).
Clinical Impact
The minimal amount of ERT above the individual threshold to saturate ADAs, which has yet a beneficial effect is currently unknown and should be the scope of future longitudinal prospective studies. However, antigen/antibody recognition is a biologic process that does not happen immediately, but is rather influenced by individual concentrations. It can be assumed that not saturated patients still have a better outcome than comparable patients without any ERT, because there will always be a small amount of infused enzyme that enters target cells. The natural history of FD demonstrates a yearly eGFR decline of up to 12 ml/min per 1.73 m2 in untreated males with FD.20 Not saturated patients presented with a yearly decline of 5 ml/min per 1.73 m2, indicating at least a small beneficial effect of ERT.
Prospective studies are warranted to analyze the use of the assay and effect of the saturation of neutralizing ADAs on disease progression and clinical outcomes. As a clinical consequence, we suggest regular determination of ADA titers in all male patients with FD under ERT. If ADA titers are saturated by the current dose and patients present with a stable disease course, patients should stay on their current compound and dose. If not, affected patients might benefit from an approved dose escalation or a switch to alternative therapy options (e.g., chaperone, if carrying amenable GLA mutations).21,22 Treating physicians should be aware of the potential risk of increasing ADA titers due to higher ERT doses. If ADA titers cannot be saturated by the approved standard dose, the discussed off-label use of the combination of ERT and chaperone (migalastat-HCl) might be an option to stabilize and increase the t1/2 of the infused enzyme.23 Current approaches such as substrate reduction therapy24,25 or novel plant-based ERTs26,27 with a longer t1/2 or potentially increased ADA-binding capacities might also represent future alternatives. Finally, immunosuppressive therapy according to current protocols10,28–32 in the effort to decrease ADA titers could be the final option for increasing therapy efficiency and improvement of patients’ outcome.
Limitations
Because of the retrospective cross-sectional study design, not all data were available for all patients at ERT-naïve baseline, which might be a limitation. Further, our method also includes the purification of non-neutralizing anti-GLA antibodies, and an additional effect of these antibodies on cellular ERT uptake cannot be fully excluded. Because of the time-delayed biologic processes of antigen and antibody binding, even in patients without ADA saturation due to an ERT deficit, a small amount of ERT will probably enter target cells. Future studies are warranted to investigate if lyso-Gb3 can be used as an appropriate marker indicating the presence of neutralizing ADAs.
Disclosures
A.N. is a member of the Fabry Registry European Board of Advisors and received speaker fees and research support from Sanofi-Genzyme, Shire, and Amicus Therapeutics. C.W. has received research support from Sanofi-Genzyme, is a consultant for Actelion Pharmaceuticals, Protalix, Boehringer Ingelheim, and Sanofi-Genzyme and is a member of the European Advisory Board of the Fabry Registry. E.B. received speaker fees and research grants from Sanofi-Genzyme, Shire, and Amicus Therapeutics. J.L. received speaker fees from Shire and research grants from Centogene AG. M.C. received speaker fees and travel grands from Shire, Amicus, Actelion, and Abbvie. M.L. received speaker fees and research grants from Amicus Therapeutics, Sanofi-Genzyme, and Shire. M.R. received speaker honoraria, congress support, and research grants from Sanofi-Genzyme, Shire, and Amicus Therapeutics. P.N. received speaker fees/travel assistance and research grants from Amicus Therapeutics, Idorsia, Sanofi-Genzyme, and Shire. S.C.-K. received speaker fees from Sanofi-Genzyme, Shire, Amicus Therapeutics, and Chiesi. S.-M.B. received speaker fees and research grant from Shire. L.P.N. and B.S. have nothing to declare.
Supplementary Material
Acknowledgments
We thank the patients for participation and their physicians for supporting us with serum samples and detailed infusion protocols, which made this work possible. The technical assistance of Samira Schiwek, Irina Schumacher, Anne Huster, and Jutta Beilker is gratefully acknowledged.
M.L. and E.B. designed the study. M.L. and L.P.N. carried out the experiments. M.L., L.P.N., B.S., S.-M.B., and E.B. analyzed the data. M.R., P.N., S.C.-K., A.N., M.C., J.L., C.W., M.L., L.P.N., and E.B. acquired data. M.L., B.S., S.-M.B., and E.B. drafted and revised the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070740/-/DCSupplemental.
References
- 1.Zarate YA, Hopkin RJ: Fabry’s disease. Lancet 372: 1427–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al.: International Collaborative Fabry Disease Study Group : Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, et al.: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Linthorst GE, Hollak CEM, Donker-Koopman WE, Strijland A, Aerts JMFG: Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int 66: 1589–1595, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, et al.: Treatment of Fabry disease with different dosing regimens of agalsidase: Effects on antibody formation and GL-3. Mol Genet Metab 94: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Rombach SM, Aerts JMFG, Poorthuis BJHM, Groener JE, Donker-Koopman W, Hendriks E, et al.: Long-term effect of antibodies against infused alpha-galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One 7: e47805, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E: Serum-mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol 27: 256–264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bénichou B, Goyal S, Sung C, Norfleet AM, O’Brien F: A retrospective analysis of the potential impact of IgG antibodies to agalsidase beta on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab 96: 4–12, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Lenders M, Schmitz B, Brand SM, Foell D, Brand E: Characterization of drug-neutralizing antibodies in patients with Fabry disease during infusion. J Allergy Clin Immunol 141: 2289–2292.e7, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Lenders M, Oder D, Nowak A, Canaan-Kühl S, Arash-Kaps L, Drechsler C, et al.: Impact of immunosuppressive therapy on therapy-neutralizing antibodies in transplanted patients with Fabry disease. J Intern Med 282: 241–253, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Arends M, Biegstraaten M, Wanner C, Sirrs S, Mehta A, Elliott PM, et al.: Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study. J Med Genet 55: 351–358, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. : CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W: Fabry’s disease: Enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab ClinMed 81: 157–171, 1973 [PubMed] [Google Scholar]
- 14.Mayes JS, Scheerer JB, Sifers RN, Donaldson ML: Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry’s disease. Clin Chim Acta 112: 247–251, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Mauhin W, Lidove O, Masat E, Mingozzi F, Mariampillai K, Ziza JM, et al.: Innate and adaptive immune response in Fabry disease. JIMD Rep 22: 1–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO: Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant 21: 345–354, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, et al.: Fabry Disease Clinical Trial Study Group : Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med 146: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Smid BE, Hoogendijk SL, Wijburg FA, Hollak CE, Linthorst GE: A revised home treatment algorithm for Fabry disease: Influence of antibody formation. Mol Genet Metab 108: 132–137, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Mauhin W, Lidove O, Amelin D, Lamari F, Caillaud C, Mingozzi F, et al.: Deep characterization of the anti-drug antibodies developed in Fabry disease patients, a prospective analysis from the French multicenter cohort FFABRY. Orphanet J Rare Dis 13: 127, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, et al.: Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, et al. : Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 375: 545–555, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Hughes DA, Nicholls K, Shankar SP, Sunder-Plassmann G, Koeller D, Nedd K, et al. : Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet 54: 288–296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnock DG, Bichet DG, Holida M, Goker-Alpan O, Nicholls K, Thomas M, et al. : Oral migalastat HCl leads to greater systemic exposure and tissue levels of active a-galactosidase A in Fabry patients when coadministered with infused agalsidase. PLoS One 10: e0134341, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashe KM, Budman E, Bangari DS, Siegel CS, Nietupski JB, Wang B, et al.: Efficacy of enzyme and substrate reduction therapy with a novel antagonist of glucosylceramide synthase for Fabry disease. Mol Med 21: 389–399, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guérard N, Oder D, Nordbeck P, Zwingelstein C, Morand O, Welford RWD, et al.: Lucerastat, an iminosugar for substrate reduction therapy: Tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin Pharmacol Ther 103: 703–711, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Kizhner T, Azulay Y, Hainrichson M, Tekoah Y, Arvatz G, Shulman A, et al.: Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metab 114: 259–267, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Shen JS, Busch A, Day TS, Meng XL, Yu CI, Dabrowska-Schlepp P, et al.: Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metab Dis 39: 293–303, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady RO, Murray GJ, Oliver KL, Leitman SF, Sneller MC, Fleisher TA, et al.: Management of neutralizing antibody to Ceredase in a patient with type 3 Gaucher disease. Pediatrics 100: E11, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS: Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med 360: 194–195, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Markic J, Polic B, Kuzmanic-Samija R, Marusic E, Stricevic L, Metlicic V, et al.: Immune modulation therapy in a CRIM-positive and IgG antibody-positive infant with Pompe disease treated with alglucosidase alfa: A case report. JIMD Rep 2: 11–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elder ME, Nayak S, Collins SW, Lawson LA, Kelley JS, Herzog RW, et al.: B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 163: 847–54.e1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banugaria SG, Prater SN, McGann JK, Feldman JD, Tannenbaum JA, Bailey C, et al.: Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: Lessons learned from Pompe disease. Genet Med 15: 123–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.