Abstract
Anticoagulant-related nephropathy (ARN) is a newly recognized form of AKI in which overanticoagulation causes profuse glomerular hemorrhage, which manifests on renal biopsy as numerous renal tubules filled with red cells and red cell casts. The glomeruli show changes, but they are not sufficient to account for the glomerular hemorrhage. We were the first to study ARN, and since then, our work has been confirmed by numerous other investigators. Oral anticoagulants have been in widespread use since the 1950s; today, >2 million patients with atrial fibrillation take an oral anticoagulant. Despite this history of widespread and prolonged exposure to oral anticoagulants, ARN was discovered only recently, suggesting that the condition may be a rare occurrence. This review chronicles the discovery of ARN, its confirmation by others, and our animal model of ARN. We also provide new data on analysis of “renal events” described in the post hoc analyses of three pivotal anticoagulation trials and three retrospective analyses of large clinical databases. Taken together, these analyses suggest that ARN is not a rare occurrence in the anticoagulated patient with atrial fibrillation. However, much work needs to be done to understand the condition, particularly prospective studies, to avoid the biases inherent in post hoc and retrospective analyses. Finally, we provide recommendations regarding the diagnosis and management of ARN on the basis of the best information available.
Keywords: chronic kidney disease, nephropathy, renal biopsy
Anticoagulant-related nephropathy (ARN) just might be the most common kidney problem that clinicians have never seen. Here is the story of its emergence and why it deserves attention.
ARN is a recently discovered cause of AKI and possibly, progressive CKD as well. It occurs in patients receiving oral anticoagulant therapy, namely warfarin1,2 or a novel oral anticoagulant (NOAC; either a direct thrombin inhibitor or a factor Xa inhibitor).3–8 The first reports of ARN involved two unrelated cases in which the patients experienced unexplained AKI and gross hematuria while receiving warfarin. After reversal of their international normalized ratio (INR), which was in the INR=6–9 range at presentation, the patients underwent renal biopsy, which showed unexplained profuse glomerular hemorrhage. Specifically, there were numerous renal tubules containing red cells and red cell casts (Figure 1). The glomeruli, however, were unremarkable, except for idiopathic thin glomerular basement membrane (GBM)9 in one patient and idiopathic thick GBM in the other patient.10 As we previously reported, patients with abnormally thin or abnormally thick GBM (and no other glomerular abnormalities) can experience bouts of spontaneous gross hematuria.11 We reasoned that the AKI in these herald patients would likely be a rare occurrence, because it would require the concurrence of an extremely high INR in a patient with an unusual GBM abnormality.10
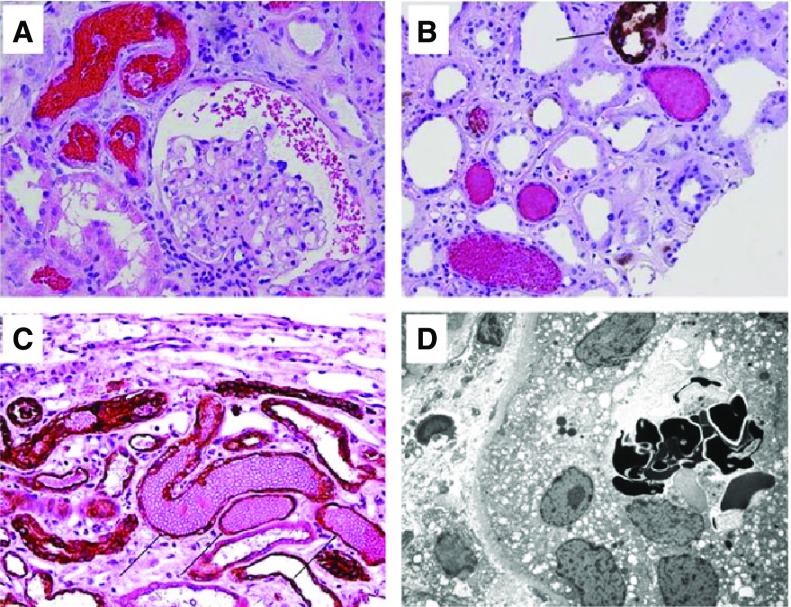
Figure 1.
Typical renal biopsy findings in warfarin-related nephropathy. Red blood cells (RBCs) in different compartments of the kidney in patients on warfarin therapy with acute kidney injury. (A) Numerous RBCs and RBC occlusive casts were noticed in tubules and Bowman space. (Hematoxylin and eosin stain; original magnification ×200). (B) Immunohistochemical stain for Tamm-Horsfall protein shows that most RBC casts do not contain Tamm-Horsfall protein. (Arrow) Positively-stained thick ascending loop of Henle. (C) Immunohistochemical stain for cytokeratin AE1/AE3 (arrows, dark brown) highlights distal tubules with occlusive RBC casts. (Counterstain with hematoxylin/eosin; original magnification ×200). (D) Dysmorphic RBCs were noticed in several tubules by means of electron microscopy. (Uranyl acetate, lead citrate stain; original magnification ×3000).
Six years later, however, we encountered another patient with AKI associated with supratherapeutic warfarin therapy. In this patient, the INR was 5.2, and the renal biopsy showed numerous red blood cell casts (indicative of profuse glomerular hemorrhage), although the glomeruli showed only changes of mild diffuse diabetic glomerulosclerosis. This patient prompted us to search our native renal biopsy database of 2801 native kidney biopsies over the previous 5 years using the search term “warfarin and AKI.” This identified nine such kidney biopsies, each of which showed evidence of severe glomerular hemorrhage. The median number of tubular cross-sections containing red cells was 6.5% (range, 2.2%–9.1%). Frequently, occlusive red blood cell casts, mainly in distal nephrons, were also present.12 In each patient, the INR was elevated at the time of the AKI but only moderately so (mean INR of 4.4). Each patient had preexisting CKD on the basis of serum creatinine level. The glomeruli showed minor nonspecific changes, but otherwise, they were unremarkable by light, immunofluorescence, and electron microscopy. Therefore, the severe glomerular hemorrhage was unexplained. Also unexplained and unexpected was that most of the patients showed little or no recovery of kidney function.
We next undertook a retrospective analysis of 103 consecutive warfarin-treated patients with CKD, most with atrial fibrillation, who were followed in our nephrology program from January 2005 to June 2009. We analyzed the outcome of the 48 patients who for the first time experienced an INR>3.0 and happened to have their serum creatinine measured within 1 week of the INR measurement. Of these, 18 (37%) showed AKI by AKI Network (AKIN) criteria (Figure 2). The AKI was unexplained on the basis of detailed chart review. Remarkably, there was no difference in mean INR between those who developed AKI and those who did not (4.91±3.1 SD versus 4.21±1.3 SD; P=0.27),1 suggesting that ARN is not simply related to coagulopathy. For ARN to develop, the glomeruli must be vulnerable to profuse glomerular hemorrhage, and the glomerular hemorrhage must inflict pervasive tubular injury.
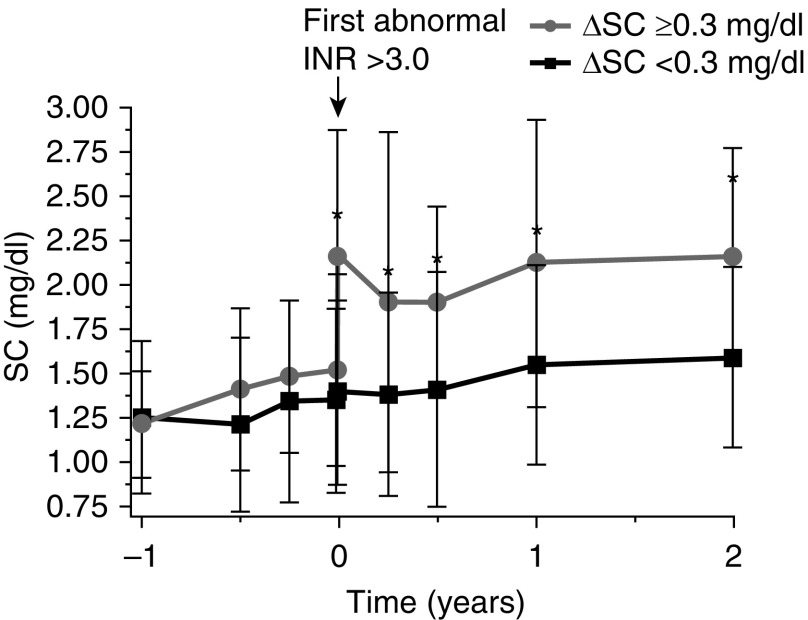
Figure 2.
Changes in serum creatinine (SC) levels associated with an international normalized ratio (INR) increase >3.0 IU in CKD with initial increase in SC that was either ≥0.3 mg/dl (upper curve) or <0.3 mg/dl (lower curve).
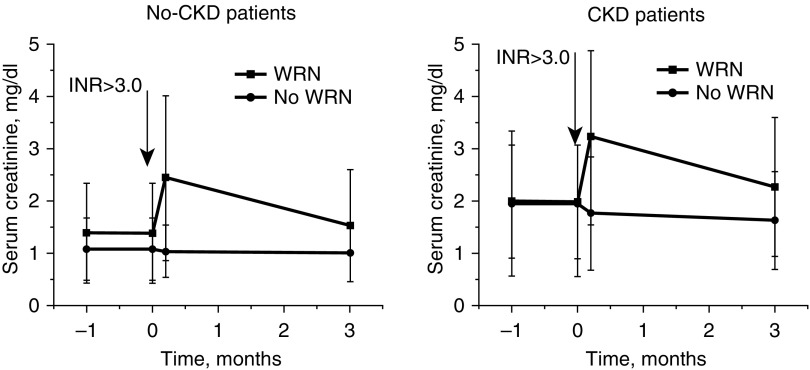
To further investigate the clinical risk factors for ARN (which we initially called warfarin-related nephropathy [WRN]), we undertook a retrospective analysis of the Ohio State University Wexner Medical Center Information Warehouse, an electronic database that stores all of the center’s clinical data since 1998. We identified 4006 distinct patients who initiated warfarin therapy, experienced for the first time an INR>3.0, and happened to have a serum creatinine measured within 1 week of having an INR>3.0. AKI (defined by AKIN criteria) occurred in 16.5% of the patients without CKD and 33% of the patients with CKD. For the cohort as a whole, 20.5% developed AKI. The subsequent changes in serum creatinine in this cohort are illustrated in Figure 3. The AKI was unexplained on the basis of a detailed analysis of International Classifications of Diseases, Ninth Revision codes. We classified these patients as having “presumptive” WRN. After CKD, the strongest risk factors for presumptive WRN were diabetes mellitus, heart failure, hypertension, and GN.13
Figure 3.
Changes in serum creatinine levels associated with international normalized ratio (INR) increase ≥3.0 in patients whose initial serum creatinine change was either ≥0.3 mg/dl (upper curve) or <0.3 mg/dl (lower curve). In patients with CKD, 33% developed AKI. In patients with no CKD, 16.5% developed AKI.2 For the cohort as a whole, 20.5% developed AKI. WRN, warfarin-related nephropathy.
In parallel with the clinical studies, we developed an animal model of ARN using warfarin or the direct thrombin inhibitor dabigatran.14–16 The animal model reproduced with fidelity the key features of human ARN. In addition, the animal model showed that only a few days of supratherapeutic warfarin or dabigatran use are needed to induce the glomerular hemorrhage of ARN.
To date, there are 39 ARN publications, including 13 that are our publications. We suggest that the existence of ARN is now accepted, but the magnitude of the problem is uncertain.17–19
Risk Factors
One key question is whether all patients who receive chronic anticoagulant therapy are susceptible to ARN. Although supratherapeutic anticoagulation seems to be essential to inducing ARN, it is likely that a second factor is required. That second factor seems to be either a substantially reduced number of nephrons (resulting in overperfusion of the surviving glomeruli and glomerular hypertension that renders them vulnerable to glomerular hemorrhage20) or acute damage to the glomeruli, allowing the glomeruli to bleed profusely under conditions of overanticoagulation.8,21
With regard to the role of glomerular overperfusion, our animal model of ARN shows that it is impossible for anticoagulation alone to induce ARN in the normal rodent. However, the condition is consistently induced in the 5/6 nephrectomy model, in which the glomeruli are histologically normal (although increased in size) but overperfused.14 At the clinical level, glomerular overperfusion is a plausible factor, because preexisting CKD approximately doubles the risk of developing presumptive ARN.1,13 It is noteworthy that the majority of the patients who developed presumptive ARN did not meet criteria for CKD on the basis of eGFR.13 However, this is an artifact of the definition of CKD (eGFR<60 ml/min per 1.73 m2); large nephron loss must occur before this level of chronic eGFR loss is reached.22
With regard to the role of an underlying acute glomerular injury in precipitating ARN, it is plausible that some instances of ARN are an “unmasking” of an underlying inflammatory glomerular disease, such as we have recently documented.8,21
Areas of Uncertainty
What Is the Scope of the ARN Problem?
Clinical investigators have only recently turned their attention to the problem of ARN in anticoagulated patients. The focus has been on patients with atrial fibrillation, the largest single category of patients requiring anticoagulation to prevent stroke (Tables 1 and 2 show the key publications).
Table 1.
AKI and CKD events identified in retrospective analysis of administrative databases that compared warfarin with a novel oral anticoagulant in patients with atrial fibrillation
| Ref. and Patient No. | Treatmenta | AKI Rateb | Hazard Ratioc | |
|---|---|---|---|---|
| AKId | CKDd | |||
| Chan et al.26 | ||||
| >20,000 (total) | Warfarin | CKD2 (6.8 to 26.0) | NR | NR |
| Warfarin | No CKD (2.0 to 6.2) | NR | NR | |
| Dabigatran | CKD (2.9 to 3.95) | 0.62 (0.49 to 0.77) | NR | |
| Dabigatran | No CKD (1.7 to 2.6) | 0.56 (0.46 to 0.69) | NR | |
| Yao et al.23 | ||||
| 9769 | Warfarin | 10.3 | 0.69 to 0.84 | 0.46 to 0.88 |
| All NOACs | 5.9 to 9.2 | |||
| Shin et al.27 | ||||
| 6412 | Warfarin | 9.49 | ||
| All NOACs | 7.5 | 0.79 (0.68 to 0.92) | NR | |
These studies did not take into account whether coagulopathy (international normalized ratio >3.0 or significant bleeding) was present when the AKI was identified. NR, not reported; NOAC, novel oral anticoagulant.
At baseline.
AKI events per year shown as 95% confidence interval (Chan et al.26), AKI rate per year of follow-up (Chan et al.,26 Yao et al.,23 and Shin et al.27), or hazard ratio (Chan et al.,26 Yao et al.,23 and Shin et al.27).
Hazard ratios calculated as AKI or CKD in patients on NOAC/patients on warfarin. All hazard ratios were significantly <1.0 except for the ARISTOTLE trial.
Variously defined (e.g., on-treatment eGFR decline >20%–30% per year).
Table 2.
AKI and CKD events identified in post hoc analysis of randomized trials that compared warfarin with a novel oral anticoagulant in patients with atrial fibrillation
| Trials | Patient No. | Treatment | AKI + CKD Rate | Hazard Ratio |
|---|---|---|---|---|
| RE-LY17 | 18,113 | Warfarin versus dabigatran | NRa | 0.81 (0.69 to 0.96) |
| ROCKET28 | 12,612 | Warfarin versus rivaroxaban | NRb | NR but described as “consistent” with RE-LY (see above) |
| ARISTOTLE29 | 16,869 | Warfarin versus apixaban | 13.6%b | Not different from 1.0 |
These studies did not take into account whether coagulopathy (international normalized ratio >3.0 or significant bleeding) was present when the AKI was identified. RE-LY, Randomized Evaluation of Long Term Anticoagulation Therapy; NR, not reported; ROCKET, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibitor Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation Trial; ARISTOTLE, APIXABAN for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation.
Inferred from renal events described as on-treatment “substantial deterioration of eGFR (>25% loss from baseline),” which were assigned a hazard ratio.
On-treatment decrease in creatinine clearance >20% per year.
The three studies listed in Table 1 are retrospective analyses of large administrative databases. In these studies, researchers identified AKI, variously defined, by electronic search of medical records of patients with atrial fibrillation receiving either warfarin or an NOAC. The development of CKD, variously defined (e.g., on-treatment eGFR loss of >30 ml/min per 1.73 m2 per year), was also reported.
The three studies listed in Table 2 are post hoc analyses of the pivotal clinical trials of patients with atrial fibrillation who were randomized to either warfarin or an NOAC. The study designs did not use efficient mechanisms for identifying AKI or to adjudicate its cause. The greatest limitation was that serum creatinine levels, which were measured at prespecified intervals, were assessed only a few times each year at most. These post hoc analyses were better able to identify CKD defined as substantial on-treatment GFR decline (e.g., >30 ml/min per 1.73 m2 over 6–12 months). Nevertheless, the total duration of follow-up was only a few years, and therefore, it would have been relatively easy to overlook patients whose chronic GFR loss was >50% after 2 years of follow-up.
Regardless of these concerns, the data shown in Tables 1 and 2 are remarkable in two respects. First, compared with the NOACs, warfarin was more strongly associated with the risk of renal events—namely AKI, CKD, or both. The single exception among the randomized trials was the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial, in which the hazard ratio for renal events comparing warfarin with apixaban was not significantly different from 1.0. Among the retrospective analyses, the greater renal risks of warfarin were associated with greater INR variability.23 Therefore, out-of-range INR may explain, at least in part, the greater number of renal events in the warfarin cohorts.
Second, the rates of renal events shown in Tables 1 and 2 (AKI versus CKD or AKI plus CKD) are substantial, particularly among patients with CKD. It is important to note that these renal events are expressed as events per year of follow-up; if these events were ongoing, they would result in large loss of renal function within only a few years of treatment. Indeed, the Chronic Renal Insufficiency Cohort (CRIC) study recently showed that atrial fibrillation is a strong risk factor for developing ESRD (11.8% per year in the cohort with CKD versus 3.4% per year in the cohort without CKD).24 Although this work did not consider whether ARN contributed to this risk, it is nevertheless plausible that ARN was largely responsible for the ESRD in the patients with atrial fibrillation in the CRIC study, possibly mediated by multiple small ARN “hits” that escaped detection.
Another perspective on the renal risk of anticoagulation is shown in Table 3. In contrast to Tables 1 and 2, which analyzed renal events (AKI and CKD) over specific periods of time, Table 3 assessed the risk of AKI (as warfarin or dabigatran related) at the time that the patient first became coagulopathic from warfarin or dabigatran. Using this approach, the risks of AKI shown in Table 3 are greater than those shown in Tables 1 and 2.
Table 3.
AKI risk in patients whose coagulopathy was induced by warfarin or dabigatran
| Ref. | Type | Patient No.a | %WRNb | Mean INRc | Mean SCr increase, mg/dl | ↑ CKD Progression | ↑ Mortality in WRN | ||
|---|---|---|---|---|---|---|---|---|---|
| WRN | No WRN | WRN | No WRN | ||||||
| Brodsky et al.1 | R | 49 | 37 | 4.91±3.1 | 4.21±1.3 | 0.61±0.44 | 0.04±0.19 | Yes | ND |
| Brodsky et al.13 | R | 4006 | 20.5 | 4.44±2.46 | 4.15±2.15 | 1.13±1.01 | 0.09±0.56 | Yes | Yes |
| Lim and Campbell30 | P | 150 | 63d | Mean ∆ 4.8±2.2e | NR | NR | NR | NR | |
| An et al.31 | R | 1297 | 19.3 | 4.19±1.56 | 3.82±1.14 | 0.81±0.94 | −0.05±0.29 | Yes | Yes |
| Berger et al.32 | R | 25Wf | 42 | NR | NR | 39.9±10g | NR | NR | Yes |
| 15Dh | 53 | NR | NR | NR | Yes | ||||
WRN, warfarin-related nephropathy; IRN, international normalized ratio; SCr, serum creatinine; R, retrospective; ND, not done; P, prospective; NR, not reported; W, warfarin; D, dabigatran.
Number of patients with acute increase in INR>3.0 and SCr measured within 1 week of INR>3.0.
Percentage of patients with INR>3.0 who developed AKI on the basis of the AKIN criteria (see text). D-treated patients are reported as the percentage of patients with AKI (27).
At the discovery of AKI.
This study reported that 30.7% of the warfarin-treated patients developed AKI. However, of those with INR>3.0, 63% developed AKI. That is the value reported here, because it conforms to the criteria for WRN used in the other four studies.
The INRs of the AKI and no AKI groups were not reported separately, but the mean difference between the group was reported as NS (P=0.34).
Number of patients with coagulopathy attributed to warfarin therapy.
Reported as percentage changes from baseline.
Number of patients with coagulopathy attributed to D therapy.
What can explain the differences in renal risk shown in Tables 1 and 2 compared with Table 3? Estimates in Tables 1 and 2 are renal events per year of follow-up, whereas estimates in Table 3 are renal events discovered at the first episode of coagulopathy (warfarin, INR>3.0; dabigatran, bleeding). Therefore, if the AKI events are more likely to occur in the first several months of the start of anticoagulation therapy, such as has been suggested,1 data in Table 3 would have a bias to overestimate the magnitude of the ARN/WRN problem per year of follow-up. Nevertheless, there are numerous other confounding factors that could lead to either overestimating or underestimating the magnitude of the ARN problem. For example, in the retrospective studies, ascertainment bias may have exaggerated the magnitude of ARN; sicker patients are more likely to have their AKI detected, because they are tested more frequently. However, in the post hoc analyses of the pivotal randomized trials, infrequent serum creatinine testing may have underestimated the magnitude of ARN.
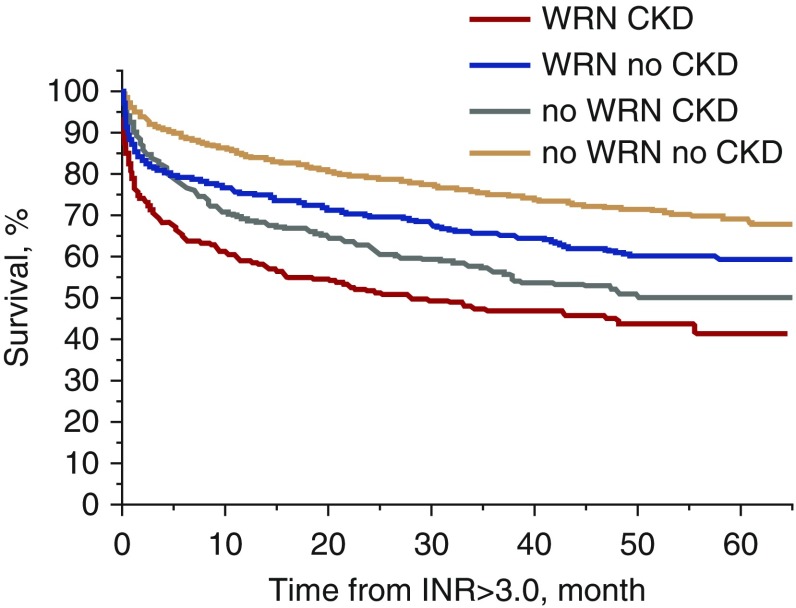
What Might Cause the 25% Spike in Mortality in the First 4 Weeks of the Onset of “Presumptive” ARN among Warfarin-Treated Patients with CKD?
This mortality spike is displayed in Figure 4, which is taken from our retrospective analysis of 4006 warfarin-treated patients (mostly patients with atrial fibrillation), as discussed above. Multivariate analysis documented that the spike in mortality was independent of other risk factors, including age, sex, race, hemorrhage, atrial fibrillation, heart failure, and diabetes mellitus.13 Determining whether presumptive ARN is present in a patient presenting with an INR>3.0 requires an element of interpretation, unless a renal biopsy is done. However, determining whether death has occurred within 1 month of the onset of an INR>3.0 and AKI is indisputable. The question is whether these deaths are an integral part of the ARN syndrome in patients with CKD. For example, it might be possible that the capillary hemorrhage that caused ARN in warfarin-treated patients with CKD also caused brain/heart capillary hemorrhage that led to death.
Figure 4.
Survival rate in patients with and without warfarin-related nephropathy (WRN). INR, international normalized ratio.
Absence of Published Guidelines Addressing ARN
The relevant professional societies have not yet offered guidance on the ARN problem. The original randomized trials of anticoagulation (warfarin versus aspirin), which in the early 1990s, established anticoagulation as standard of care to prevent stroke in atrial fibrillation, did not report serum creatinine or BUN values. Details of ARN did not begin to emerge until the early 2000s.
Given that warfarin therapy has been in widespread clinical use since 1952, how is it possible that ARN has escaped detection until very recently? We suggest that the following issues may have contributed to delaying recognition of ARN. (1) Presumptive ARN (related to warfarin or an NOAC) occurs mainly in patients who already have multiple risk factors for AKI (e.g., CKD, cardiovascular disease, diabetes, or older age). Thus, for the typical patient with ARN, it is relatively easy to default to an AKI diagnosis of “multifactorial.” (2) ARN is a diagnosis of exclusion unless a kidney biopsy is done. Nephrologists are naturally cautious regarding kidney biopsy in patients who require anticoagulant therapy, although as we have recently described, biopsy in such patients is an established procedure.25 (3) Presumptive ARN/WRN is associated with a marked increase in acute mortality rate (Figure 4). Therefore, the individuals who are most vulnerable to ARN may be under-represented in the general population of patients receiving chronic oral anticoagulant therapy. (4) Until recently, ARN has not been a part of the lexicon of the nephrologist or the nephropathologist. This bias leads to underestimation of ARN risk.
On the basis of the above information, we suggest that current nephrology practice and the pivotal trials of warfarin versus direct oral anticoagulant treatment in patients with atrial fibrillation have an inherent bias to underestimate ARN. However, the retrospective clinical studies of presumptive ARN, including our own studies, have an inherent bias to overestimate ARN. This bias occurs, because sicker patients are more likely to develop complications, have poor INR control, and undergo laboratory testing, and only those who underwent serum creatinine testing were included in the analyses. We suggest that the only clear path to the truth regarding ARN is to study it prospectively.
Conclusions and Recommendations
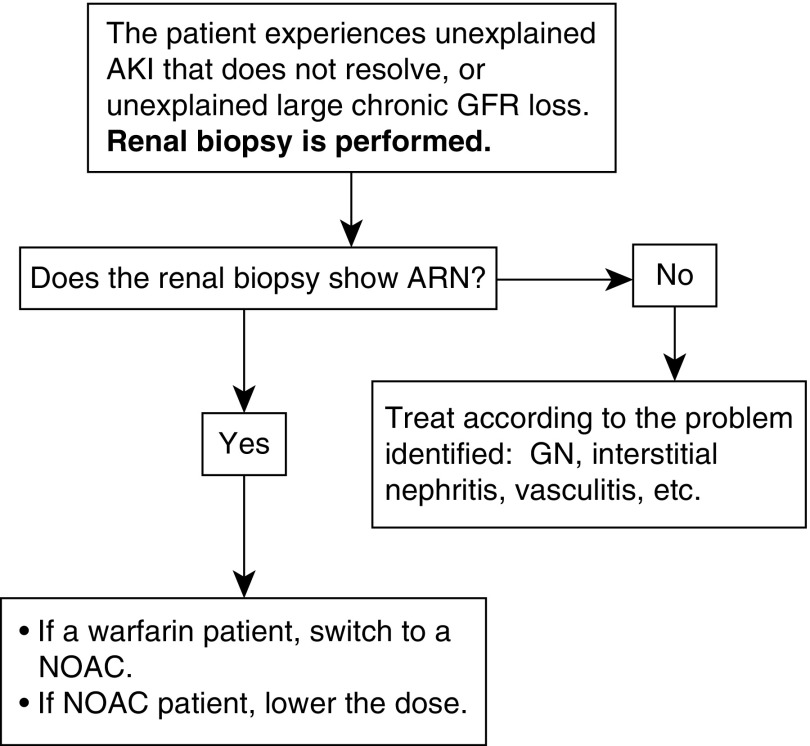
Until better information becomes available, we suggest an algorithm (Figure 5) that depicts a diagnostic and therapeutic strategy for managing the anticoagulated patient with atrial fibrillation who develops unexplained AKI or unexplained progressive CKD. Strategic use of renal biopsy is central to the approach. If the biopsy documents ARN, a patient taking warfarin is switched to an NOAC, and a patient receiving an NOAC has their dose reduced. If the renal biopsy reveals a primary or secondary glomerulopathy but does not reveal a superimposed ARN (profuse glomerular hemorrhage disproportionate to the degree of glomerular injury), it may be sufficient to continue anticoagulation while treating the glomerular inflammation.
Figure 5.
Suggested management for the anticoagulated patients with atrial fibrillation whose kidney biopsy shows anticoagulant-related nephropathy (ARN). These recommendations pertain to both ARN that is AKI and ARN that is large chronic eGFR loss. NOAC, novel oral anticoagulant.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, et al. : Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract 115: c142–c146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golbin L, Vigneau C, Touchard G, Thervet E, Halimi JM, Sawadogo T, et al. : Warfarin-related nephropathy induced by three different vitamin K antagonists: Analysis of 13 biopsy-proven cases. Clin Kidney J 10: 381–388, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansky L, Mukkamala P, Jebakumar D, Rao A, Goldson TM, Forjuoh SN: Acute kidney injury and undiagnosed immunoglobulin A nephropathy after dabigatran therapy. Proc Bayl Univ Med Cent 31: 321–323, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaitzidis RG, Duni A, Liapis G, Balafa O, Xiromeriti S, Rapsomanikis PK, et al. : Anticoagulant-related nephropathy: A case report and review of the literature of an increasingly recognized entity. Int Urol Nephrol 49: 1401–1407, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Escoli R, Santos P, Andrade S, Carvalho F: Dabigatran-related nephropathy in a patient with undiagnosed IgA nephropathy. Case Rep Nephrol 2015: 298261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafi ST, Negrete H, Roy P, Julius CJ, Sarac E: A case of dabigatran-associated acute renal failure. WMJ 112: 173–175, 2013 [PubMed] [Google Scholar]
- 7.Bachellerie B, Ruiz S, Conil JM, Crognier L, Seguin T, Georges B, et al. : [Patient with acute renal injury presenting dabigatran overdose: Hemodialysis for surgery]. Ann Fr Anesth Reanim 33: 44–46, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Brodsky SV, Mhaskar NS, Thiruveedi S, Dhingra R, Reuben SC, Calomeni E, et al. : Acute kidney injury aggravated by treatment initiation with apixaban: Another twist of anticoagulant-related nephropathy. Kidney Res Clin Pract 36: 387–392, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abt AB, Carroll LE, Mohler JH: Thin basement membrane disease and acute renal failure secondary to gross hematuria and tubular necrosis. Am J Kidney Dis 35: 533–536, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kabir A, Nadasdy T, Nadasdy G, Hebert LA: An unusual cause of gross hematuria and transient ARF in an SLE patient with warfarin coagulopathy. Am J Kidney Dis 43: 757–760, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Spetie DN, Nadasdy T, Nadasdy G, Agarwal G, Mauer M, Agarwal AK, et al. : Proposed pathogenesis of idiopathic loin pain-hematuria syndrome. Am J Kidney Dis 47: 419–427, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. : Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: A report of 9 cases. Am J Kidney Dis 54: 1121–1126, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. : Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 80: 181–189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware K, Brodsky P, Satoskar AA, Nadasdy T, Nadasdy G, Wu H, et al. : Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol 22: 1856–1862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar AA, et al. : 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol 35: 356–364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, et al. : Warfarin-related nephropathy is the tip of the iceberg: Direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant 29: 2228–2234, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Böhm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, et al. : Changes in renal function in patients with atrial fibrillation: An analysis from the RE-LY trial. J Am Coll Cardiol 65: 2481–2493, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Wheeler DS, Giugliano RP, Rangaswami JR: Anticoagulation-related nephropathy. J Thromb Haemost 14: 461–467, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV: Oral anticoagulants and risk of nephropathy. Drug Saf 38: 527–533, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Parikh S, Haddad N, Hebert LA: Retarding progression of kidney disease. In: Comprehensive Clinical Nephrology, 5th Ed., edited by Floege J, Fehally J, Johnson RJ, Philadelphia, Elsievier, 2014, pp 931–947 [Google Scholar]
- 21.Brodsky S, Hebert L: Warfarion-related nephropathy, response to letter by Naimark DMJ. Kidney Int 81, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Hebert PL, Nori US, Bhatt UY, Hebert LA: A modest proposal for improving the accuracy of creatinine-based GFR-estimating equations. Nephrol Dial Transplant 26: 2426–2428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, et al. : Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 70: 2621–2632, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Bansal N, Xie D, Tao K, Chen J, Deo R, Horwitz E, et al. : CRIC Study: Atrial fibrillation and risk of ESRD in adults with CKD. Clin J Am Soc Nephrol 11: 1189–1196, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh SV, Diez A, Ayoub I, Alvarado A, Rovin B: Renal biopsy: The nephrologist’s viewpoint. In: Silva’s Diagnostic Renal Pathology, 2nd Ed., edited by Jin X, Laszik ZG, Nadasdy T, D'Agati VD, Cambridge, United Kingdom, Cambridge University Press, 2017, pp 57–68 [Google Scholar]
- 26.Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, et al. : Acute kidney injury in Asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol 68: 2272–2283, 2016. 27884245 [Google Scholar]
- 27.Shin JI, Luo S, Alexander GC, Inker LA, Coresh J, Chang AR, et al. : Direct oral anticoagulants and risk of acute kidney injury in patients with atrial fibrillation. J Am Coll Cardiol 71: 251–252, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, et al. : ROCKET AF Steering Committee and Investigators: On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: Insights from ROCKET AF. Circulation 134: 37–47, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. : Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: Insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol 1: 451–460, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Lim AK, Campbell DA: Haematuria and acute kidney injury in elderly patients admitted to hospital with supratherapeutic warfarin anticoagulation. Int Urol Nephrol 45: 561–570, 2013 [DOI] [PubMed] [Google Scholar]
- 31.An JN, Ahn SY, Yoon CH, Youn TJ, Han MK, Kim S, et al. : The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One 8: e57661, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger R, Salhanick SD, Chase M, Ganetsky M: Hemorrhagic complications in emergency department patients who are receiving dabigatran compared with warfarin. Ann Emerg Med 61: 475–479, 2013 [DOI] [PubMed] [Google Scholar]