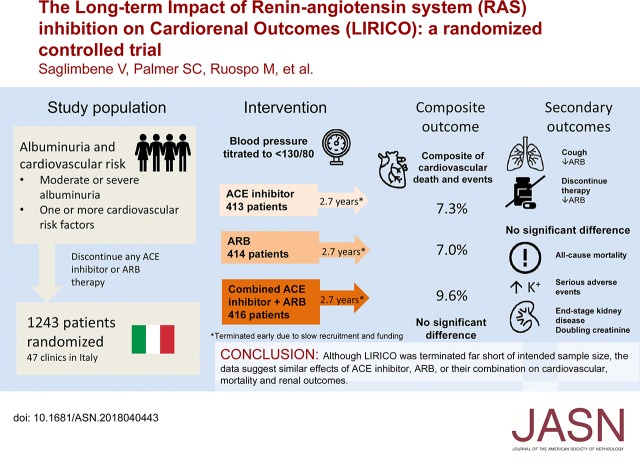
Abstract
Background
The comparative effectiveness of treatment with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or their combination in people with albuminuria and cardiovascular risk factors is unclear.
Methods
In a multicenter, randomized, open label, blinded end point trial, we evaluated the effectiveness on cardiovascular events of ACE or ARB monotherapy or combination therapy, targeting BP<130/80 in patients with moderate or severe albuminuria and diabetes or other cardiovascular risk factors. End points included a primary composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for cardiovascular causes and a revised end point of all-cause mortality. Additional end points included ESRD, doubling of serum creatinine, albuminuria, eGFR, BP, and adverse events.
Results
Because of slow enrollment, the trial was modified and stopped 41% short of targeted enrollment of 2100 participants, corresponding to 35% power to detect a 25% reduced risk in the primary outcome. Our analysis included 1243 adults, with median follow-up of 2.7 years. Efficacy outcomes were similar between groups (ACE inhibitor versus ARB, ACE inhibitor versus combination, ARB versus combination) as were rates of serious adverse events. The rate of permanent discontinuation for ARB monotherapy (6.3%) was significantly lower than for ACE inhibitor monotherapy (15.7%) or combined therapy (18.3%).
Conclusions
Patients may tolerate ARB monotherapy better than ACE inhibitor monotherapy. However, data from this trial and similar trials, although as yet inconclusive, show no trend suggesting differences in mortality and renal outcomes with ACE inhibitors or ARBs as dual or monotherapy in patients with albuminuria and diabetes or other cardiovascular risk factors.
Keywords: albuminuria, diabetic nephropathy, renin angiotensin system, mortality, end-stage renal disease, clinical trial
Visual Abstract
Whether angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), used alone or in combination, have similar beneficial effects on mortality and cardiovascular complications in patients who have diabetes or vascular disease is uncertain.1,2 Guidelines recommend ACE inhibitor or ARB therapy as first-line therapy for patients with diabetes and albuminuria.3
Trials comparing ACE inhibitor or ARB monotherapy or combination therapy among people with diabetes and CKD are generally inconclusive, or evidence is reliant on subgroup analyses.4–10 In a trial of 1448 participants with type 2 diabetes, a urinary albumin-to-creatinine ratio of 300 mg/g, and an eGFR of 30.0–89.9 ml/min (the VA-NEPHRON-D study), there was no evidence that losartan monotherapy or lisinopril combined with losartan had different effects on GFR, ESRD, or death.11 In the ALTITUDE study evaluating the addition of a direct renin inhibitor aliskiren as an adjunct to ACE inhibitor or ARB therapy, there was no evidence that treatment made any difference to a composite outcome of cardiovascular or renal outcomes.12 In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), no cardiovascular or renal benefits were observed with combination ACE inhibitor and ARB therapy (telmisartan and ramipril) compared with monotherapy.1,13
To address the residual uncertainties, we conducted a randomized trial to compare ACE inhibitor, ARB, or combined ACE inhibitor with ARB therapy for patients with diabetes or other cardiovascular risk factor and albuminuria on mortality and cardiovascular outcomes.
Methods
The design of the Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) study is reported elsewhere.14 In brief, the LIRICO study was a multicenter, randomized, open label, blinded end point (PROBE) trial of ACE inhibitor, ARB, or combined treatment with ACE inhibitor or ARB for patients with diabetes and moderate to severe albuminuria. The trial was registered on the Australian New Zealand Clinical Trials Registry with the trial identification ACTRN12607000333415.
Setting and Participants
Patients treated at 47 internal medicine clinics and nephrology units within Italy were identified and recruited. Adult men and women were eligible if they were aged 18 years of age or older, had moderate albuminuria (urinary albumin-to-creatinine ratio 30–299 mg/g) or severe albuminuria (urinary albumin-to-creatinine ratio ≥300 mg/g), and had diabetes15 or one or more cardiovascular risk factors: current or recent smoking, hypertension (systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or antihypertensive treatment), abdominal obesity, dyslipidemia, or family history of premature cardiovascular events. Patients were excluded if they were pregnant, intended to become pregnant, had active malignancy (except basal cell carcinoma), had a contraindication to ACE inhibitor or ARB, or had substantially reduced life expectancy.
Randomization and Masking
Participants were randomized using an electronically generated random list created by the study statistician stratified by center and in randomly permuted blocks. Patients were allocated to study treatment by investigators via telephone contact with staff at a central study office. The allocation sequence was concealed to central office staff until after a participant was irreversibly allocated to a treatment group. Participants and physicians were not blinded to study allocation postrandomization, but outcome assessment for the primary composite outcome was carried out by an independent committee that was unaware of treatment allocation. A pragmatic study design was chosen to test the interventions within a usual care setting to maximize applicability and generalizability.
Interventions
Participants were assigned to receive an ACE inhibitor, an ARB, or combined treatment with an ACE inhibitor and ARB. Randomized medications included any commercially available drug approved for the indication. Patients discontinued any nonallocated ACE inhibitor or ARB therapy at randomization and commenced randomly allocated therapy without a washout period. Initial dosing was at the investigator’s discretion. Treatment doses were titrated to the full tolerated dose by the usual attending physician. Additional antihypertensive therapy was allowed except for ACE inhibitor or ARB for those not randomly assigned to these medications to reach a target BP of <130/80 mm Hg.16
After randomization, participants were assessed at 1 and 3 months, and then, they were assessed every 6 months unless they died, withdrew consent, or were not contactable for follow-up. Participants who were not able or willing to continue randomized treatment were asked to continue with planned trial assessments. Adherence was assessed by pill counting.
Outcomes and Follow-Up
The initial primary study outcome was the first occurrence of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for cardiovascular cause. A protocol amendment occurring in July 2010 resulted in a change in the primary outcome to all-cause mortality with a planned cumulative meta-analysis with the ONTARGET. This paper reports results of both the former composite and current all-cause mortality end points for the LIRICO study data alone. Meta-analysis in combination with ONTARGET data were the ultimate intention for the primary all-cause mortality data. The composite end point is emphasized in the most detail in this paper, because the intended meta-analysis of all-cause mortality will be the subject of a separate paper in preparation. Additional end points included each of the individual end points of the composite outcome: ESRD (permanent commencement of RRT [dialysis or kidney transplantation]), doubling of serum creatinine, eGFR, progression to severe albuminuria or regression to normal or mildy increased albuminuria, systolic and diastolic BP, and urinary albumin-to-creatinine ratio. Safety outcomes were serious adverse events, permanent discontinuation of therapy, hyperkalemia >6 mEq/L, hypotension, and cough.
Three protocol amendments in 2008, 2010, and 2011 were generated to extend the trial recruitment phase for 12 months each (Supplemental Appendix 1, Supplemental Material 1).
Ethics and Oversight
The study received institutional review board approval before participant recruitment and data collection from the Ethics Committee of the “Ospedale Policlinico Consorziale” di Bari on March 15, 2007. The study was overseen by an independent data safety monitoring board that regularly reviewed safety parameters and study conduct (Supplemental Appendix 2, Supplemental Material 1).
Statistical Analyses
The study was designed to enroll 2100 participants to provide 80% power to detect a risk reduction of 25% in the composite outcome between the intervention (combined ACE inhibitor plus ARB therapy) and the control groups. The power calculation assumed an annual incidence of the composite end point of 5% and two-sided α=0.05.14 Limited funding and slow recruitment (509 participants, including 344 with diabetes) together with release of the results of the ONTARGET resulted in a protocol amendment to limit the inclusion of participants to those with albuminuria and diabetes and reduce the sample size to 1000 participants with diabetes. This sample size was considered sufficient to combine with data involving participants with diabetes from the ONTARGET to power a study focused on all-cause mortality. Trial recruitment was terminated after inclusion of 1059 participants with diabetes. This early termination of the study was decided by the trial steering committee independent of the sponsor and according to the protocol amendment. A subsequent futility analysis assuming that future events for the composite outcome would accrue at the rate already observed in this analysis indicated that the probability of detecting a statistically significant hazard ratio (HR) of 0.75 with the originally planned study recruitment in the LIRICO study was 0%. A revised power calculation indicated that the power of the study with 1243 participants evaluable for the composite end point of cardiovascular death and nonfatal events with 2.7 years of follow-up provided 35% power to detect a risk reduction of 25% in the primary outcome between the intervention groups.
The analysis used a time-to-event approach. Time-to-event data for each treatment assignment were compared using the Cox proportional hazards model and expressed as HRs with 95% confidence intervals (95% CIs). We estimated the mean differences between the trial groups for BP, urine albumin-to-creatinine ratio, and eGFR using a generalized linear mixed model for repeated measurements with an unstructured variance-covariance matrix.17 Missing data (<3.7% for all variables with the exception of baseline values for serum lipids, creatinine, and glucose) were not imputed.
Prespecified subgroups for analyses were sex, type of diabetes, presence or absence of hypertension, family history of cardiovascular disease, presence or absence of prior cardiovascular event, microalbuminuria or macroalbuminuria, hemoglobin A1C (above or below 7.5%), serum cholesterol (above or below 4.7 mmol/L [180 mg/dl]), and baseline GFR (above or below 60 ml/min per 1.73 m2). Subgroup analysis for other prespecified subgroups of patients (type 1 diabetes and those with previous cardiovascular events) gave results that were unreliable due to few events within a group.
Results
Participants
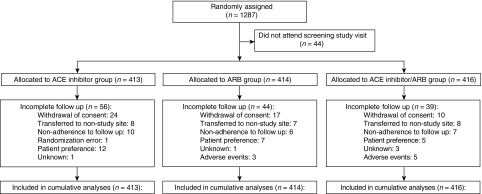
From November 22, 2007 to March 26, 2013, 1287 participants with moderate or severe albuminuria and diabetes or other cardiovascular risk factors were randomized (Figure 1). Forty-four participants did not attend the baseline assessment; 1243 were included in primary analyses.
Figure 1.
Overall, 1287 participants were randomized to the LIRICO trial. Consolidated Standards of Reporting Trials flow diagram of the Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes study. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
At baseline, the mean age of study participants was 62.8 years old (SD, 10.6), and 28.3% were men. The mean systolic BP was 138.0 (SD, 16.4) mm Hg, and the mean eGFR was 67.9 (SD, 27.9) ml/min per 1.73 m2. Overall, 890 (73.9%) participants had moderate albuminuria, and 314 (26.0%) had severe albuminuria. At baseline, 539 participants (43.4%) were taking an ACE inhibitor, and 579 (46.6%) were prescribed an ARB. Baseline characteristics were similar between allocated groups (Table 1). During the median follow-up of 2.7 years, 139 participants (11.2%) had discontinued follow-up.
Table 1.
Baseline characteristics
| Characteristic | ACE Inhibitor, n=413 | ARB, n=414 | Combination, n=416 |
|---|---|---|---|
| Age at randomization, yr, mean (SD) | 62.2 (11.2) | 62.7 (10.7) | 63.4 (10.0) |
| Sex, n (%) | |||
| Women | 290 (71.3) | 288 (71.5) | 295 (72.5) |
| Men | 117 (28.7) | 115 (28.5) | 112 (27.5) |
| Ethnicity, n (%) | |||
| Black | 4 (1.0) | 6 (1.5) | 2 (0.5) |
| Other | 399 (99.0) | 399 (98.5) | 407 (99.5) |
| Diabetes, n (%) | 353 (85.5) | 351 (84.8) | 355 (85.3) |
| Type 1 | 11 (3.2) | 11 (3.2) | 10 (2.9) |
| Type 2 | 331 (96.8) | 329 (96.8) | 337 (97.1) |
| Albuminuria, n (%) | |||
| Moderate albuminuria | 291 (70.5) | 295 (71.3) | 304 (73.1) |
| Severe albuminuria | 103 (24.9) | 109 (26.3) | 102 (24.5) |
| Smoker, n (%) | |||
| Current | 96 (23.2) | 96 (23.2) | 95 (22.8) |
| Former | 122 (29.5) | 103 (24.9) | 134 (32.2) |
| Body mass index, kg/m2, mean (SD) | 30.5 (5.6) | 30.8 (5.5) | 30.5 (5.4) |
| Weight, kg, mean (SD) | 84.3 (16.4) | 85.1 (17.1) | 83.7 (16.9) |
| Waist circumference, cm, mean (SD) | 105.0 (14.3) | 105.0 (12.6) | 104.7 (13.1) |
| Heart rate, min, mean (SD) | 75.6 (10.5) | 74.5 (10.3) | 74.0 (9.0) |
| BP, mm Hg, mean (SD) | |||
| Systolic | 138.0 (16.7) | 138.2 (15.7) | 137.8 (16.8) |
| Diastolic | 80.6 (9.4) | 80.0 (9.0) | 80.4 (9.8) |
| Fasting glucose, mg/dl, mean (SD) | 138.1 (46.4) | 143.2 (52.0) | 139.5 (47.6) |
| HbA1C, %, mean (SD) | 7.5 (1.6) | 7.6 (1.7) | 7.5 (1.5) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 70.2 (28.0) | 68.0 (27.7) | 65.5 (27.8) |
| eGFR<60 ml/min per 1.73 m2, n (%) | 144 (34.9) | 155 (37.4) | 174 (41.8) |
| Serum creatinine, mg/dl, mean (SD) | 1.10 (0.73) | 1.14 (0.81) | 1.15 (0.59) |
| Urinary albumin-to-creatinine ratio, median (IQR), mg/g | 108 (55–302) | 110 (52–316) | 128 (57–325) |
| Serum potassium, mEq/L, mean (SD) | 4.49 (0.61) | 4.54 (0.56) | 4.55 (0.63) |
| Total cholesterol, mg/dl, mean (SD) | 180.1 (41.8) | 178.0 (38.9) | 176.0 (42.1) |
| LDL cholesterol, mg/dl, mean (SD) | 103.6 (36.2) | 102.6 (34.0) | 101.3 (33.6) |
| Triglycerides, mg/dl, mean (SD) | 154.0 (88.0) | 144.8 (77.1) | 146.0 (81.5) |
| Symptomatic neuropathy, n (%) | 62 (15.0) | 47 (11.3) | 55 (13.2) |
| Diabetic retinopathy, n (%) | 97 (26.4) | 88 (21.3) | 109 (26.2) |
| Previous cardiovascular event, n (%) | 94 (22.8) | 101 (24.4) | 102 (24.5) |
| Family history of cardiovascular disease, n (%) | 40 (9.7) | 42 (10.1) | 43 (10.3) |
| Medications before randomization, n (%) | |||
| BP lowering | 346 (83.8) | 346 (83.8) | 369 (88.7) |
| ACE inhibitor | 176 (42.6) | 176 (42.6) | 187 (45.0) |
| ARB | 174 (42.1) | 174 (42.1) | 209 (50.2) |
| ACE inhibitor or ARB | 317 (76.8) | 317 (76.8) | 342 (82.2) |
| β-Blocker | 99 (24.0) | 99 (24.0) | 78 (18.8) |
| Calcium channel blocker | 110 (26.6) | 110 (26.6) | 129 (31.0) |
| Diuretic | 150 (36.3) | 150 (36.3) | 180 (43.3) |
| Lipid lowering | 234 (56.7) | 234 (56.7) | 251 (60.3) |
| Statin | 214 (51.8) | 214 (51.8) | 227 (54.6) |
| Ezetimibe | 18 (4.4) | 18 (4.4) | 19 (4.6) |
| Fibrate | 12 (2.9) | 12 (2.9) | 14 (3.4) |
| Omega-3 PUFA | 33 (8.0) | 33 (8.0) | 42 (10.1) |
| Platelet aggregation inhibitors | 161 (39.0) | 161 (39.0) | 170 (40.9) |
| Acetylsalicylic acid | 142 (34.4) | 142 (34.4) | 151 (36.3) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HbA1C, hemoglobin A1C; PUFA, Polyunsaturated fatty acids.
Interventions
Doses of ACE inhibitor and ARB in each of the monotherapy groups and in the combined therapy group at baseline and final study visit were similar between groups (Supplemental Table 1). During follow-up, 65 (16.7%) permanently discontinued ACE inhibitor therapy (P<0.001 versus ARB; P=0.32 versus combination), six (1.5%) permanently discontinued ARB therapy (P<0.001 versus combination), and 55 (13.2%) permanently discontinued combination therapy. Of those participants who continued treatment, adherence to prescribed treatment and follow-up was estimated at 92.1% for ACE inhibitor therapy, 98.0% for ARB therapy, and 90.7% for combined therapy. Treatments did not lead to different systolic or diastolic BPs during follow-up (Supplemental Figure 1).
Outcomes
Composite Outcome
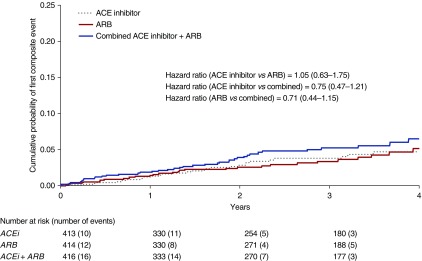
Treatment group did not seem to influence the risk of the composite outcome of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for cardiovascular causes (30 [7.3%] in ACE inhibitor group [HR, 1.05; 95% CI, 0.63 to 1.75 versus ARB monotherapy; HR, 0.75; 95% CI, 0.47 to 1.21 versus combination], 29 [7.0%] in the ARB group [HR, 0.71, 95% CI, 0.44 to 1.15 versus combination], and 40 [9.6%] in the combined group) (Figure 2, Table 2).
Figure 2.
Treatment group did not seem to influence the risk of the composite outcome of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for cardiovascular causes. Kaplan–Meier estimates of composite outcome according to treatment allocation. Number of events refers to the number of participants experiencing their first event of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for cardiovascular cause. ACE, angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 2.
Efficacy outcomes
| Outcome | ACE Inhibitor, n=413, n (%) | ARB, n=414, n (%) | ACE Inhibitor + ARB, n=416, n (%) | ACE Inhibitor Versus ARB, Hazard Ratio (95% CI) | ACE Inhibitor Versus Combination, Hazard Ratio (95% CI) | ARB Versus Combination, Hazard Ratio (95% CI) |
|---|---|---|---|---|---|---|
| Composite (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, hospitalization secondary to cardiovascular cause) | 30 (7.3) | 29 (7.0) | 40 (9.6) | 1.05 (0.63 to 1.75) | 0.75 (0.47 to 1.21) | 0.71 (0.44 to 1.15) |
| All-cause mortality | 15 (3.6) | 20 (4.8) | 18 (4.3) | 0.76 (0.39 to 1.48) | 0.84 (0.42 to 1.67) | 1.11 (0.59 to 2.10) |
| Cardiovascular death | 6 (1.5) | 7 (1.7) | 4 (1.0) | 0.87 (0.29 to 2.58) | 1.51 (0.43 to 5.36) | 1.75 (0.51 to 5.97) |
| ESRD | 6 (1.5) | 2 (0.5) | 4 (1.0) | 3.04 (0.61 to 15.0) | 1.53 (0.43 to 5.44) | 0.50 (0.09 to 2.76) |
| Nonfatal myocardial infarction | 4 (1.0) | 4 (1.0) | 10 (2.4) | 1.00 (0.25 to 4.01) | 0.41 (0.13 to 1.29) | 0.40 (0.13 to 1.28) |
| Nonfatal stroke | 4 (1.0) | 2 (0.5) | 5 (1.2) | 2.02 (0.37 to 11.0) | 0.81 (0.22 to 3.01) | 0.40 (0.08 to 2.05) |
| Hospitalization for cardiovascular cause | 25 (6.1) | 20 (4.8) | 34 (8.2) | 1.27 (0.71 to 2.29) | 0.74 (0.44 to 1.25) | 0.58 (0.34 to 1.01) |
| Doubling of serum creatinine | 21 (5.1) | 19 (4.6) | 23 (5.5) | 1.12 (0.60 to 2.08) | 0.95 (0.53 to 1.74) | 0.85 (0.46 to 1.57) |
| Progression to eGFR<60 ml/min per 1.73 m2a | 71 (30.1) | 75 (33.0) | 65 (31.7) | 0.88 (0.63 to 1.21) | 0.97 (0.70 to 1.37) | 1.11 (0.79 to 1.55) |
| Progression to severe albuminuria | 46 (14.4) | 49 (15.2) | 41 (13.0) | 0.86 (0.57 to 1.29) | 1.04 (0.68 to 1.59) | 1.21 (0.80 to 1.83) |
| Regression to normal or mildly increased albuminuria | 83 (20.6) | 86 (21.6) | 92 (22.7) | 0.94 (0.69 to 1.27) | 0.90 (0.67 to 1.22) | 0.96 (0.72 to 1.29) |
Counts correspond to the number of participants who experienced a specific outcome event at least once. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval.
In participants with an eGFR>60 ml/min per 1.73 m2 at baseline (ACE inhibitor, n=236; ARB, n=227; ACE inhibitor + ARB, n=205).
Cardiovascular and Mortality Outcomes
Treatment assignment had very uncertain effects on all-cause mortality (15 [3.6%] in the ACE inhibitor group [HR, 0.76; 95% CI, 0.39 to 1.48 versus ARB monotherapy; HR, 0.84; 95% CI, 0.42 to 1.67 versus combination], 20 [4.8%] in the ARB group [HR, 1.11; 95% CI, 0.59 to 2.10 versus combination], and 18 [4.3%] in the combination group). Risks of the individual end points of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for cardiovascular causes were not statistically significantly different between the treatment groups (Supplemental Figures 2 and 3, Table 2).
Renal Outcomes
Nine (0.9%) participants required dialysis for ESRD. Treatment had very uncertain effects on ESRD (Table 2). For the 668 participants who had an eGFR>60 ml/min per 1.73 m2 recorded at baseline, the rate of progression to an eGFR<60 ml/min per 1.73 m2 was not different between treatment groups (Table 2). Doubling of serum creatinine occurred in 63 (5.1%) participants and was not different between groups (Table 2).
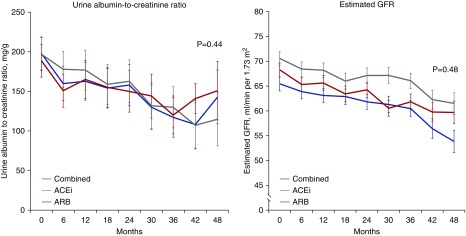
Progression to severe albuminuria occurred in 46 (14.4%) participants assigned to ACE inhibitor (HR, 0.86; 95% CI, 0.57 to 1.29 versus ARB; HR, 1.04; 95% CI, 0.68 to 1.59 versus combination), 49 (15.2%) assigned to ARB therapy (HR, 1.21; 95% CI, 0.80 to 1.83 versus combination), and 41 (13.0%) assigned to combined treatment (Table 2). Regression to normal or mildly increased albuminuria occurred in 83 (20.6%) on ACE inhibitor (HR, 0.94; 95% CI, 0.69 to 1.27 versus ARB; HR, 0.90; 95% CI, 0.67 to 1.22 versus combination), 86 (21.6%) on ARB (HR, 0.96; 95% CI, 0.72 to 1.29 versus combination), and 92 (22.7%) on combination therapy (Table 2). During follow-up, there was no evidence that the urinary albumin-to-creatinine ratio or eGFR was different between groups at any time point (Figure 3).
Figure 3.
There was no evidence that the urinary albumin-to-creatinine ratio or eGFR was different between groups at any time point. Change in urine albumin-to-creatinine ratio and eGFR from baseline to study end. Data are expressed as estimated mean with 95% confidence interval. Comparative analyses are on the basis of a mixed model for repeated measurements, comparing the values over time between groups and accounting for within-participant correlation. P value for interaction between groups over time is shown. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Safety Outcomes
During the study, 41 (9.9%) in the ACE inhibitor group experienced one or more serious adverse events (P value >0.99 versus ARB; P value =0.50 versus combination), 41 (9.1%) in the ARB group experienced one or more serious adverse events (P value =0.50 versus combination), and 48 (11.5%) in the combined ACE inhibitor and ARB group experienced one or more serious adverse events (Table 3). Twenty-two participants experienced one or more episodes of hyperkalemia (serum potassium >6 mEq/L; six in the ACE inhibitor group, seven in the ARB group, and five in the combination group). Cough was experienced by 22 (5.6%) in the ACE inhibitor group, one (0.3%) in the ARB group, and eight (1.9%) in the combined group.
Table 3.
Safety outcomes
| Outcome | ACE Inhibitor, n=413, n (%) | ARB, n=414, n (%) | ACE Inhibitor + ARB, n=416, n (%) | ACE Versus ARB P Valuea | ACE Versus Combination P Valuea | ARB Versus Combination P Valuea |
|---|---|---|---|---|---|---|
| Serious adverse event | 41 (9.9) | 41 (9.1) | 48 (11.5) | >0.99 | 0.50 | 0.50 |
| Permanent discontinuation of therapy | 65 (15.7) | 26 (6.3) | 75 (18.3) | <0.001 | 0.40 | <0.001 |
| Hyperkalemia | 6 (1.4) | 7 (1.6) | 9 (2.1) | >0.99 | 0.60 | 0.80 |
| Hypotension | 3 (0.7) | 2 (0.5) | 2 (0.5) | 0.69 | 0.69 | >0.99 |
| Cough | 22 (5.6) | 1 (0.3) | 8 (1.9) | <0.001 | 0.01 | 0.04 |
Serious adverse events were defined as any unfavorable sign, symptom, or medical event, regardless of whether due to study intervention, that resulted in death, life-threatening illness, hospitalization or prolongation of hospitalization, persistent or significant disability, or a serious medical event in the opinion of the responsible investigator. The reasons for discontinuation of medication were adverse event (29.5%), BP not at target (10.2%), cough (2.4%), hospitalization (4.8%), end of study (3.0%), end point (4.2%), hyperkalemia (0.6%), patient decision (0.6%), physician decision in primary care (13.9%), physician decision in cardiology (11.5%), physician decision in nephrology (1.8%), unknown (6.0%), and worsening kidney function (2.4%). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Number of participants experiencing events was compared using the two-sided Fisher exact test.
Sensitivity and Subgroup Analyses
Among 1059 participants with diabetes and albuminuria, there was no evidence that treatment assignment influenced the risk of any outcome (Supplemental Tables 2–4).
In subgroup analysis, there was no evidence of different intervention effects on the composite outcome on the basis of sex, presence of type 2 diabetes, hypertension, cardiovascular disease, family history of cardiovascular disease, hemoglobin A1C, or eGFR (Supplemental Table 5). Interactions between treatment assignment and the subgroups of moderate and severe albuminuria at baseline were observed.
Discussion
In this randomized, open label, blinded end point trial in patients with diabetes or cardiovascular risk factor and albuminuria treated to the same BP target, the risks of mortality and cardiovascular or renal outcomes seemed similar regardless of whether an ACE inhibitor, an ARB, or their combination was used. ARB monotherapy had a lower incidence of withdrawal from therapy than ACE inhibitor alone or when the two treatments were combined. These findings support existing evidence that ACE inhibitors, ARB therapy, or their combination may have similar effects on mortality and cardiovascular outcomes for people with high-risk diabetes or cardiovascular risk.1
Our results are consistent with a recent network meta-analysis showing no evidence of benefit for combination ACE inhibitor and ARB therapy compared with monotherapy for mortality and cardiovascular events among people with diabetes and kidney disease.18 Our results are also concordant with the ONTARGET, which showed no evidence for different effects between ACE and ARB or combination on cardiovascular events and fewer adverse effects with ARB monotherapy.1 In the LIRICO study, we did not observe differential effects of treatment on intermediary renal outcomes, such as eGFR, proteinuria, or ESRD. This contrasts with evidence from the ONTARGET, in which patients assigned to dual ACE inhibitor and ARB therapy had a higher risk of renal impairment, a greater decline in eGFR, and a smaller increase in urinary albumin excretion than those treated with ACE inhibitor alone. Similarly, lower-risk patients assigned to combination ACE inhibitor plus ARB therapy in the VA-NEPHRON trial experienced greater lowering of the urinary albumin-to-creatinine ratio and higher risk of AKI than those assigned to ACE inhibitor monotherapy.11 The different effects on kidney function and albumin excretion between these studies may be a consequence of the fixed doses of treatment used in the VA-NEPHRON trial and the ONTARGET, leading to a relatively greater BP lowering with combination therapy, which was not observed in this trial. The findings of the LIRICO study are unable to confirm or refute the European Medicines Agency–endorsed restrictions on combining medicines that act on the renin-angiotensin system, including ACE inhibitors, ARBs, and direct renin inhibitors.19
On the basis of the cumulative evidence from randomized trials, ACE inhibitor and ARB therapy or their combination might be used interchangeably for BP lowering among people with high-risk diabetes or other cardiovascular risk factor and albuminuria, although there remains no definitive evidence that treatment lowers all-cause mortality or cardiovascular events. ARB monotherapy may be a preferred treatment option, because this approach is apparently better tolerated than ACE inhibitor monotherapy.11,13
The strengths of the LIRICO study include a multicenter, pragmatic design; direct head-to-head comparison of ACE inhibitor, ARB, and combination therapy; well balanced treatment groups; and achievement of similar BP control across treatment groups. Limitations include protocol amendments and the small number of events for many outcomes, limiting statistical power and leading to uncertainty in treatment effects for these outcomes. In addition, the participating cohort had relatively lower levels of albuminuria and renal impairment than other similar studies, which may have reduced the power to detect treatment effects on renal outcomes.11,12 This study characteristic may have explained the lower rate of hyperkalemia observed in this trial compared with other studies.
In conclusion, although the LIRICO study comparing ACE inhibitor, ARB, and combination therapy for patients with albuminuria and diabetes was terminated far short of the intended sample size, number of events, and statistical power, the observed data suggested similar effects on cardiovascular, mortality, or renal outcomes or intermediary renal events when similar BP targets were achieved and showed no beneficial trend for dual therapy relative to either monotherapy.
Disclosures
J.H. is an employee of Diaverum Renal Services Group outside the submitted work. S.M. reports grants and nonfinancial support from AstraZeneca, Eli Lilly, and Takeda. G. Pugliese reports consultancy fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Shire and speaker’s honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Mylan, Sigma-Tau, and Takeda outside of the submitted work. D.W.J. reports receiving consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from AstraZeneca; travel sponsorships from Amgen; and an Australian Government National Health and Medical Research Practitioner Fellowship outside the submitted work. M.T. reports being a member of the Kidney Disease Improving Global Outcomes executive committee outside the submitted work. G.F.M.S. is a consultant for Diaverum Renal Services Group outside the submitted work. Authors not named here have disclosed no conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by Agenzia Italiana del Farmaco (Italian Medicines Agency) project grant N. FARM537JNE. S.C.P. is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand. Partial funding for statistical analyses was provided by Diaverum Renal Services. Data management support was received by Michele Sacco (CORESEARCH).
An abstract of this study was published as free oral communication SA-OR115 for the American Society of Nephrology Kidney Week in New Orleans, Louisiana from October 31 to November 5, 2017.
Individual participant data that underlie the results reported in this article will be available after deidentification beginning 3 months and ending 5 years after article publication to researchers who provide a methodologically sound proposal. Proposals should be directed to G.F.M.S. To gain access, data requestors will need to sign a data access agreement. The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
The the Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators are Mauro Cignarelli (University of Foggia), Maurizio Di Mauro (University of Catania), Giancarlo Tonolo (Azienda Sanitaria ASL 2 Olbia), Luigi Elio Adinolfi (Second University of Naples), Alfonso Gigante (Azienda Tutela Salute ASSL Nuoro), Luciano Carboni (University of Cagliari), Roberto Anichini (General Hospital, Pistoia), Cecilia Marino (Ospedale Gubbio), Mario Querques (Ospedale Foggia), Silvana Manfrini (Centro di Diabetologia e Malattie del Ricambio, Senigallia), Bruno Cianciaruso (University Federico II, Napoli), Giuseppe Grandaliano (University of Foggia), Stefano Del Prato (University of Pisa), Francesco Giorgino (University of Bari), Paolo Cavallo Perin (University of Torino), Fabio Malberti (Azienda Ospedaliera Istituti Ospitalieri di Cremona), Alfio Nardo (University of Catania), Cecilia Invitti (Istituto Auxologico Italiano, Milano), Immacolata Panettieri (University of Foggia), Mario Bonomini (Università degli Studi G. d'Annunzio Chieti e Pescara), Giorgio Sesti (University of Catanzaro), Emanuele Altomare (University of Foggia), Rosa Giordano (Ospedale Martina Franca), Alessandro Iacono (Nicosia Hospital), Tiziano Lusenti (Azienda Ospedaliera S. Maria Nuova di Reggio Emilia), Carlo Jovane (ASST Valle Olona P.O. “S. Antonio Abate”, Gallarate), Ivana Zavaroni (University of Parma), Luigi Vernaglione (Azienda Sanitaria Locale Brindisi), Juliette Grosso (Ospedale di Castel di Sangro), Piero Stratta (University “Maggiore della Carita” di Novara), Antonia Andriani (University of Bari), Alessio Montanaro (Azienda Sanitaria Locale BR sita in Brindisi), Agostino Di Ciaula (Hospital of Bisceglie), Giorgio Triolo (University of Torino), Antonio Santoro (S. Orsola Malpighi Hospital, Bologna), Silvio Spada (San Severo Hospital), Antonio Di Benedetto (University of Messina), Vito Borzì (University of Catania), Carla Tortul (Azienda per i Servizi Sanitari N. 2 Isontina), Mario Schiavoni (Centro Emofilia e Coagulopatie Rare, Scorrano), Cesare Cavalera (Ospedaliero “F. Ferrari” Casarano), Rossella Iannarelli (Ospedale San Salvatore, L'Aquila), Giovanni Mileti (U.O Medecina, Fasano), Salvatore Tardi (Azienda Sanitaria locale di Matera), Salvatore Di Rosa (Azienda Ospedali Riunti di Palermo).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/DOI:10.1681/ASN.2018040443/-/DCSupplemental.
Supplemental Material
Supplemental Material 1. Study personnel and amendments.
Supplemental Appendix 1. Protocol amendments.
Supplemental Appendix 2. Study administration and investigators.
Supplemental Material 2. Supplemental tables and figures.
Supplemental Table 1. Doses of medications at baseline and final observation.
Supplemental Table 2. Baseline characteristics of 1059 participants with diabetes.
Supplemental Table 3. Incidence of primary and secondary outcomes in 1059 participants with diabetes.
Supplemental Table 4. Adverse events in 1059 participants with diabetes.
Supplemental Table 5. Subgroup analyses for the primary composite outcome.
Supplemental Figure 1. Change in systolic and diastolic BP from baseline to study end.
Supplemental Figure 2. Kaplan–Meier estimates of all-cause mortality according to treatment allocation.
Supplemental Figure 3. Kaplan–Meier estimates of cardiovascular death according to treatment allocation.
References
- 1.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al.: ONTARGET Investigators : Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Wu HY, Huang JW, Lin HJ, Liao WC, Peng YS, Hung KY, et al.: Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: Systematic review and bayesian network meta-analysis. BMJ 347: f6008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, et al.: Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care 40: 1273–1284, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al.: Diabetics Exposed to Telmisartan and Enalapril Study Group : Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351: 1952–1961, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cheung R, Lewanczuk RZ, Rodger NW, Huff MW, Oddou-Stock P, Botteri F, et al.: The effect of valsartan and captopril on lipid parameters in patients with type II diabetes mellitus and nephropathy. Int J Clin Pract 53: 584–592, 1999 [PubMed] [Google Scholar]
- 6.Ko GT, Tsang CC, Chan HC: Stabilization and regression of albuminuria in Chinese patients with type 2 diabetes: A one-year randomized study of valsartan versus enalapril. Adv Ther 22: 155–162, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Lacourcière Y, Bélanger A, Godin C, Hallé JP, Ross S, Wright N, et al.: Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int 58: 762–769, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Schram MT, van Ittersum FJ, Spoelstra-de Man A, van Dijk RA, Schalkwijk CG, Ijzerman RG, et al.: Aggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: Effects on albumin excretion, endothelial function and inflammation in a double-blind, randomized clinical trial. J Hum Hypertens 19: 429–437, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD: Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 20: 2641–2650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzoni D, Porteri E, De Ciuceis C, Sleiman I, Rodella L, Rezzani R, et al.: Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin-dependent diabetes mellitus. Hypertension 45: 659–665, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al.: VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Parving H-H, Brenner BM, McMurray JJV, de Zeeuw D, Haffner SM, Solomon SD, et al.: ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al.: ONTARGET investigators : Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, et al.: LIRICO study group : Protocol of the Long-term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) randomized trial. J Nephrol 20: 646–655, 2007 [PubMed] [Google Scholar]
- 15.American Diabetes Association : Standards of medical care in diabetes--2006. Diabetes Care 29[Suppl 1]: S4–S42, 2006 [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al.: National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee : The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Singer J, Willett J: Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence, Oxford, United Kingdom, Oxford University Press, 2003 [Google Scholar]
- 18.Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al.: Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: A network meta-analysis. Lancet 385: 2047–2056, 2015 [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency : Combined Use of Medicines Affecting the Renin-Angiotensin System (RAS) to Be Restricted—CHMP Endorses PRAC Recommendation, 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Renin-angiotensin_system_(RAS)-acting_agents/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500167419.pdf. Accessed October 21, 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.