Abstract
Introduction:
Heart failure with preserved ejection fraction (HFpEF) continues to be a major challenge for clinicians. Many crucial aspects of the syndrome remain unclear, including the exact pathophysiology, early diagnosis, and treatment. Patients with HFpEF are often asymptomatic late into the disease process, and treatment with medications commonly used in heart failure with reduced ejection fraction (HFrEF) has not been proven to be beneficial. In addition, the confusion of similar terms with HFpEF, such as diastolic heart failure (DHF), and diastolic dysfunction (DD), has led to a misunderstanding of the true scope of HFpEF.
Areas covered:
In this review, authors highlight the differences in terminology and critically review the current knowledge on the underlying mechanisms, diagnosis, and latest treatment strategies of HFpEF.
Expert commentary:
While significant advances have been made in the understanding of HFpEF, the definitive diagnosis of HFpEF continues to be difficult. The development of improved and standardized methods for detecting diastolic dysfunction (DD) has shown promise in identifying early HFpEF. However, even with early detection, there are few treatment options shown to provide mortality benefit warranting further investigation.
Keywords: HFpEF, HFrEF, Diastolic dysfunction, Inflammation, Biomarkers
1. Introduction
Heart failure is a common but complex syndrome, caused by a wide variety of etiologies. Historically, heart failure was primarily classified by the type of cardiac dysfunction present, either systolic or diastolic heart failure [1]. While this is a useful theoretical categorization emphasizing whether the primary problem is defective pumping or filling, in clinical practice systolic dysfunction and diastolic dysfunction (DD) are rarely isolated and often both contribute to the clinical picture [2]. More recently, heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) have become the preferred terms for describing heart failure, relying solely on the calculated ejection fraction (EF) [1]. This terminology allows for easier and reproducible classification of heart failure, without misrepresenting the complex underlying cause. A challenge with this definition has been establishing a consistent cut-off between preserved and reduced EF. While an EF of 50% is the most common cut off for HFpEF, some sources and clinical trials have used 40%, blurring the data between the two groups [3]. In addition, the new classification of heart failure with mid-range ejection fraction (HFmrEF) with an EF between 40% and 50% further adds to the confusion [4]. After clinically determining the EF, evaluation of systolic and diastolic function is crucial to the work up of both HFrEF and HFpEF in order to determine the likely etiology, as well as provide valuable information about the disease process.
While systolic and diastolic dysfunction often coexist in HFpEF, DD is recognized as the primary contributor to the development and clinical presentation of this syndrome [5]. DD is defined as impaired myocyte relaxation or increased wall stiffness, resulting in decreased filling and elevated pressures during diastole [6]. This impaired filling is then thought to contribute to the well characterized nonspecific heart failure symptoms seen in HFpEF including dyspnea on exertion, edema, and many others. DD can also be present in asymptomatic patients and HFrEF patients and is therefore not pathopneumonic of HFpEF [7]. Furthermore, there is notable deterioration of diastolic function in normal cardiac aging, making distinction in the elderly more complicated [8]. Overall, assessment of diastolic function can provide crucial insight into the development and severity of HFpEF. However, it is important to understand that while DD is an underlying contributor to HFpEF, and the resultant elevation in filling pressures, left ventricular end diastolic pressure (LVEDP), and left atrial pressure are hallmarks of HFpEF, the terms are not interchangeable.
2. Epidemiology
Throughout the world, heart failure is one of the most significant contributors to morbidity and mortality. According to the American Heart Association (AHA), 6.5 million Americans were estimated to have heart failure from 2011–2014, a number which is expected to increase to over 8 million by 2030 [7,9]. In addition, heart failure had an estimated annual cost of $30.7 billion in 2012 [9]. Determination of the true prevalence of HFpEF is complicated by missing EF data, and a wide range of values have been reported by different studies. Owan and Redfield [10] reviewed many international epidemiological studies, reporting that 40–71% of heart failure patients were classified as HFpEF, with HFpEF having an overall prevalence of 1.1–5.5% of the general population.
Several studies have compared the 5-year mortality rates between HFpEF and HFrEF, coming up with varying results. Interestingly, Lam et al. [11] noted that epidemiological cohort studies typically reported similar mortality rates between the two, whereas clinical trials often found HFpEF to have a lower mortality rate. Although widely variable, HFpEF 5-year mortality rates range from between 55–74% [11]. It is important to note that HFpEF also has a significant proportion of non-cardiovascular deaths (30–50%), especially compared to HFrEF (15–30%) [12]. This is not surprising, as HFpEF patients also have a higher prevalence of comorbidities including hypertension, diabetes mellitus, and chronic obstructive pulmonary disease (COPD) [13]. While diagnosed heart failure is common, many asymptomatic individuals have underlying preclinical heart failure and cardiac dysfunction. In fact, data from one study showed that in all individuals regardless if they were asymptomatic or had diagnosed HF, about 28% had DD (21% had mild DD with 7% having moderate or severe DD) and 6% had systolic dysfunction [7].
3. Potential mechanisms
Although the mechanism of HFpEF is still incompletely understood, many factors are thought to be involved. While HFrEF is mainly due to a volume overloaded state and systolic dysfunction, HFpEF may primarily result from systemic inflammation and its resulting effects of altered titin isoform ratios, altered phosphorylation status of titin by protein kinase A (PKA), protein kinase G (PKG), protein kinase C (PKC), hypertension, fibrosis, and DD. It is important to note that despite substantial clinical, functional, and comorbidity overlap, HFpEF and HFrEF are most likely driven by distinct disease processes as opposed to a continuum of one disorder. Interestingly, with the introduction of HFmrEF as its own sub-classification, much is still not understood about the underlying pathophysiology [4]. Continued investigation is needed to determine if HFmrEF is representative of a unique disease process or that it is more likely a combination of the processes behind HFpEF and HFrEF. However, in this section, the focus remains on the mechanisms driving the development of DD and HFpEF.
Several infiltrative processes create DD through a similar mechanism, though these are classified as restrictive cardiomyopathies and are considered a different disease group. Due to a similar clinical presentation, these will be briefly discussed here. In these diseases, there is an infiltrate – protein or otherwise – that is deposited in the myocardium, disrupting the normal cardiac architecture and causing the heart to stiffen. The result of this deposition translates to an impaired ability to relax in diastole, resulting in the development of DD. Examples of this type of HFpEF include cardiac amyloidosis, hemochromatosis, and sarcoidosis [14,15]. Hemochromatosis can also cause DD through an accumulation of iron with resultant increased oxidative stress [16]. These disease processes are fairly well characterized. Therefore, this review article primarily focuses on non-infiltrative causes of HFpEF.
Hypertension has historically been thought to cause concentric hypertrophy as an adaptive response to increased afterload [17]. This seems to be a reasonable explanation, as the increased wall thickness would decrease the wall stress of the ventricle per the Law of LaPlace. However, more recently the relationship between hypertension and heart failure has been shown to be more complex than a mere adaptive response. There is evidence that increased afterload causes initial T-tubule dysfunction and abnormal Ca2+ handling, which is seen before overt signs of heart failure are apparent [18]. This abnormal T-tubule organization and resultant improper Ca2+ handling is thought to be the initiator of both systolic and diastolic dysfunction seen in heart failure patients [18]. While the initial changes in the hypertensive heart may be due to abnormal cardiomyocyte function, evidence suggests that the progression from left ventricular hypertrophy to overt heart failure is instead caused by alterations in the extracellular matrix. In spontaneously hypertensive rats, proteins, including collagen Iα1, collagen IIIα1, TGF-β, tissue inhibitor of metalloproteinase-1, plasminogen activator inhibitor, cathepsin K, and cathepsin S are upregulated [19]. The increased collagen content and TGF-β suggest that within a failing heart there is increased deposition of ECM, while the increased protease inhibitors and cathepsins suggest increased turnover of extracellular matrix (ECM) [19]. The changes in ECM are accompanied by minimal changes to Ca2+ homeostasis and myofibril and cytoskeleton gene expression, suggesting that the development of heart failure depends more on ECM changes and less on intrinsic cardiomyocyte changes in hypertensive heart failure [19]. Thus, the increased afterload in hypertension results not only in cardiomyocyte hypertrophy but also changes in the ECM.
HFpEF is associated with multiple comorbidities including hypertension, COPD, obesity, and chronic kidney disease; all of which induce a systemic inflammatory state [19—23]. Another significant comorbidity is diabetes mellitus, an incredibly complex topic which is outside the scope of this article. A thorough review of the relationship between diabetes and heart failure has been covered in another review article [24].
A compelling theory put forth by Paulus and Tschöpe [25] proposes that there is an initiating event resulting in systemic inflammation. This leads to reactive oxygen species production by endothelial cells, consuming nitric oxide (NO) in the process. The decreased availability of NO for cardiomyocytes causes a decrease in PKG activity resulting in concentric hypertrophy of the myocardium in addition to decreased titin phosphorylation [25]. This contributes to the stiffer myocardium and DD seen in HFpEF. This process is summarized in Figure 1.
Figure 1:
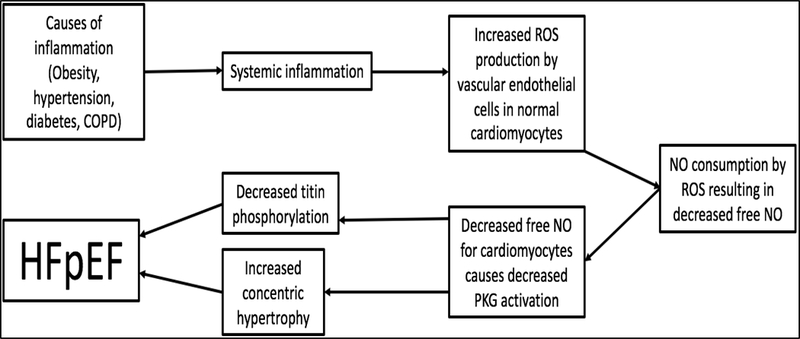
Following systemic inflammation due to obesity, hypertension, diabetes or COPD in a patient, ROS molecules build up and deplete NO, causing decreased active PKG proteins. Decreased levels of PKG cause concentric hypertrophy and decreased titin phosphorylation leading to HFpEF. COPD, chronic obstructive pulmonary disease; ROS, reactive oxygen species; NO, nitric oxide; PKG, protein kinase G; HFpEF, heart failure with preserved ejection fraction.
The inflammation is thought to decrease PKG activity through the depletion of NO by reactive oxygen species (ROS) molecules, which is indicated by the increased nitrotyrosine in HFpEF patients compared to aortic stenosis and HFrEF patients [26]. A study done on human hearts with HFpEF demonstrated that vascular cell adhesion molecule (VCAM) was upregulated and there was an increase in TGF-β expressing inflammatory cells with the markers CD3, CD11a, and CD45 in the heart of human HFpEF patients [27]. Furthermore, the TGF-β expressed by these cells was shown to increase collagen production, cause fibroblast differentiation into pathological myofibroblasts, and decrease the MMP/TIMP ratio in a cell culture with fibroblasts from HFpEF patients [27].
These various factors related to systemic inflammation such as increased inflammatory cells, increased NOX2 expression, and decreased NO availability were all researched within one comprehensive study done on rat hearts to determine their relationships to HFpEF. This study again found that HFpEF hearts had decreased NO availability leading to decreased soluble guanylyl kinase activity and an eventual decrease in PKG activity [28]. In addition, the adhesion proteins, ICAM-1 and E-selectin, were upregulated with evidence of increased macrophage recruitment in the diseased hearts with increased activity of NOX2 within the recruited macrophages [28].
4. Detection and risk stratification
4.1. Diagnostic criteria
The diagnosis of heart failure is primarily achieved clinically, followed by echocardiography to establish an EF subtype (Table 1) [29,30]. In the past, guidelines such as the Framingham criteria and Boston criteria have provided useful standardized symptoms and signs for clinical diagnosis; however, patients are often asymptomatic or oligosymptomatic until late in the pathophysiologic process, and therefore do not meet these criteria for official diagnosis [29]. Alternatively, the 2016 European Society of Cardiology (ESC) guidelines use the presence of symptoms and signs combined with clinical history, physical exam, and electrocardiography (ECG) to determine the need for further workup, but a strict number or combination of symptoms and signs is not necessary for diagnosis [4]. Instead, echocardiography findings are typically used in addition to symptoms and signs to establish a diagnosis of heart failure and determine the EF subtype [4]. This takes into consideration less typical signs of heart failure and is more widely accepted in clinical practice (Table 1).
Table 1:
Common echocardiographic parameters used in the assessment of diastolic function.
| Parameter | Definition | Application to DD |
|---|---|---|
| E | Mitral flow velocity during early diastole | Representative of the pressure gradient between atrium and ventricle. Related to ventricular relaxation and left atrial pressure. |
| A | Mitral flow velocity during late diastole | Representative of the pressure gradient between atrium and ventricle. Related to ventricular wall compliance and atrial contractility. |
| E/A ratio | Ratio between mitral flow velocities during early and late diastole | Representative of filling patterns, with low ratios suggestive of restricted filling and high ratios suggestive of poor relaxation. |
| e’ | Mitral annular velocity during early diastole | Primarily representative of ventricular relaxation. Found to be related to tau, the time constant of ventricular pressure decay. |
| E/e’ ratio | Ratio between mitral flow and mitral annular velocities during early diastole | Representative of ventricular filling pressures during diastole by correcting for ventricular relaxation. |
| TR velocity | Peak velocity of tricuspid regurgitation during systole | Representative of left atrial pressures, in absence of pulmonary hypertension. |
| LAVI | Maximum left atrial volume | Representative of structural changes in the atrium due to increased pressures. |
The above information is collected and tabulated from the publication of Nagueh et al. [30]; DD, diastolic dysfunction; TR, tricuspid regurgitation; LAVI, left atrial maximal volume index.
Specifically for HFpEF, the ESC guidelines require an EF ≥ 50% and the additional criterion of the presence of DD or relevant structural heart disease [4]. While the need for evidence of DD is controversial for formal diagnosis of HFpEF, it is clear that assessment of diastolic function can provide valuable insight into the development and progression of HFpEF, allowing for earlier detection and intervention [6]. Therefore, this section primarily focuses on the current and upcoming approaches to assessing diastolic function and their utility in evaluating HFpEF.
4.2. Diastolic dysfunction detection
Detecting DD has been a challenge, especially using noninvasive measures. Since DD is defined as impaired myocardial relaxation and/or decreased left ventricular compliance, direct measurement of left ventricular relaxation and filling pressures is an excellent way to assess diastolic function. However, this requires invasive hemodynamics using cardiac catheterization, which is not appropriate as a universal approach to all patients with suspected DD. Echocardiography with Doppler flow and Tissue Doppler Echocardiography (TDE) are the most commonly used diagnostic tools, but the effectiveness of alternative detection methods is currently under investigation. Other imaging modalities of interest include speckle tracking echocardiography (STE) and cardiovascular magnetic resonance (CMR), as well as the use of a variety of serum biomarkers. A multifaceted approach using a combination of noninvasive techniques may be helpful for distinguishing DD and HFpEF from other causes of heart failure, in addition to discovering DD prior to onset of symptoms.
4.2.1. Invasive hemodynamics
The assessment of invasive hemodynamic using cardiac catheterization is currently the gold standard for diagnosing DD due to its ability to directly measure ventricular pressures throughout the cardiac cycle [31,32]. While this method provides a wealth of information about cardiac performance, the most useful parameters for measuring diastolic function are tau, the time constant of ventricular pressure decay, and LVEDP. Tau is an accepted measurement of myocardial relaxation, and LVEDP is helpful for determining the left ventricular wall compliance; both of which are central to the definition of DD [31,33]. With measurement of these parameters, cardiac catheterization currently provides the most detailed insight into diastolic function; however, the invasiveness and risks decrease the clinical value of this method, especially in patients with significant comorbidities and unstable patients. As a result, echocardiography has emerged as the preferred approach for diagnosing DD, though cardiac catheterization continues to serve as a useful tool for evaluating complicated cases and validating alternate methods of detection.
4.2.2. Echocardiography
A wide variety of echocardiographic techniques have been used to assess diastolic function, including Doppler flow, TDE, and speckle tracking echocardiography (STE). The American Society of Echocardiography (ASE) and European Association of Echocardiography (EAE) recently published updated recommendations for assessing DD with echocardiography. While many echocardiographic parameters were analyzed, the critical variables were noted to be mitral flow velocities (E, A, and E/A ratio), mitral annular velocity (e’), E/e’ ratio, peak velocity of tricuspid regurgitation (TR) jet, and left atrial maximum volume index (LAVI) [30]. A brief description of each variable is shown in Table 1. Using these variables, detailed algorithms for diagnosing and grading DD have been proposed by Nagueh and colleagues [30]. A recent study of patients with DD demonstrated this algorithm to be better than the previous existing guidelines for detecting elevated LVEDP and predicting poor prognosis [34].
In addition to Doppler flow and TDE, STE is a relatively new technique able to quantitatively measure ventricular movement and deformation. This is particularly useful for analyzing myocardial strain, an important factor in both HFrEF and HFpEF, and left ventricular untwisting, which is a known contributor to early diastolic filling [35—37]. While none of these parameters are specific to diastolic function, each one provides unique data that add to the clinical picture and understanding of the disease process. Although echocardiography is the most widely used modality to assess diastolic function, significant weaknesses still exist, most notably operator dependence, flow calculation errors, difficulty in obtaining clear imaging windows, and limited spatial resolution [38].
4.2.3. Cardiac magnetic resonance
Another imaging modality of interest is cardiac magnetic resonance (CMR). CMR has become the gold standard for estimating ventricular volumes and assessing systolic function [39]. However, it has not been widely used to detect DD. CMR has proven to be an effective tool for directly measuring DD parameters similar to those found with echocardiography, including E/A ratio, e’ E/e’ ratio, and peak filling rate, in addition to providing a wealth of structural information unique to CMR [40]. Studies have also investigated novel CMR parameters that correlate to DD such as mid-wall longitudinal fractional shortening (MLFS) and extracellular volume fraction [39,41]. MLFS, a correlate of long-axis systolic function, effectively distinguishes clinically significant DD (grades II and III) from mild or no DD (grades 0 and I) [39,42]. Another valuable CMR parameter is extracellular volume fraction (ECV), representing the amount of interstitial myocardial fibrosis [41]. While diffuse myocardial fibrosis is an essential player in the development of DD and HFpEF, it is also present in many other disease processes, including systolic heart failure and systemic hypertension. A recent study showed that patients with HFpEF and systolic HF had significantly higher ECVs than healthy patients and patients with hypertension, but the utility of ECV as a diagnostic tool has not been established [41]. Therefore, ECV may be useful in assessing the pathophysiological process of DD but using ECV to distinguish DD from other myofibrotic processes has not been proven. Overall, CMR can be a valuable tool for evaluating DD especially in patients who had inconclusive echocardiography results, due to the ability of CMR to measure LV filling, higher spatial resolution, and better reproducibility. However, contraindications to CMR, lack of transportability, and increased cost make it less practical as a first line investigative tool for DD.
4.2.4. Exercise stress testing
A number of studies have investigated exercise stress testing for evaluation of DD and HFpEF. In many patients with early DD, filling pressures may be normal at rest and only elevated during exercise [43]. Invasive exercise hemodynamics remains the gold standard for measuring left ventricular filling pressures, specifically pulmonary capillary wedge pressure (PCWP) and LVEDP, in response to exercise. Elevations in PCWP and LVEDP during exercise have been shown to successfully distinguish patients with DD from control patients with non-cardiac dyspnea, even with normal brain natriueritc peptide (BNP) levels and filling pressures at rest [43]. Obokata et al. [44] performed simultaneous invasive hemodynamics and echocardiography, and found a correlation between the E/e’ ratio and PCWP, both at rest and during exercise. Furthermore, measurement of exercise E/e’ ratio provided improved sensitivity but decreased specificity in testing for HFpEF when compared to resting echocardiography [44]. Finally, echocardiography also showed a smaller increase in stroke volume in response to exercise in patients with DD [45]. Therefore, measuring exercise parameters for invasive hemodynamics and echocardiography may allow for earlier detection of DD and subclinical HFpEF, especially in patients with normal resting echocardiogram. However, cardiac catheterization is not practical for routine diagnosis due to its invasiveness, and exercise echocardiography has been shown to have more benefit ruling out DD and HFpEF when combined with resting detection methods.
4.3. Biomarkers
In addition to imaging, serum biomarkers are a useful tool for assessing cardiac function. Natriuretic peptides, especially BNP and N-terminal proBNP (NT-proBNP), have been commonly used in heart failure work up due to their correlation with ventricular wall stress [46,47]. Studies have proven that BNP can be used in a clinical setting to rapidly distinguish heart failure from other causes of dyspnea, in both an outpatient and emergent settings [48,49]. BNP and NT-proBNP levels have also been shown to be correlated with heart failure severity and prognosis [50]. In regard to HFpEF, a recent study found that in patients with hypertension and echocardiographic evidence of DD, NT-proBNP levels were significantly correlated with DD echocardiographic parameters including e’ and E/e’ . However, in this study the patients were asymptomatic and had not yet developed heart failure, and the DD in the patients was already discovered [51]. Although natriuretic peptides may be a helpful tool for monitoring the progress of DD, they have not been proven useful for detection of DD or distinction from other types of heart failure. Therefore, there is a need for other biomarkers more specific to DD and its pathophysiologic mechanism (Table 2) [52—61].
Table 2:
Non-natriuretic peptide biomarkers of interest in heart failure with preserved ejection fraction and diastolic dysfunction.
| Biomarker | Description | Application to DD and HFpEF | References |
|---|---|---|---|
| Suppression of Tumorgenicity 2 (ST2) | Member of the interleukin 1 receptor family | Implicated in mechanical stress, cardiac hypertrophy, fibrosis, and ventricular dysfunction. No significant ability to distinguish HFpEF from HFrEF. Associated with worse prognosis. | Sinning et al. [52], Meluzin and Tomandl [53], Parikh et al. [54], Santhanakrishnan et al. [57] |
| Galectin-3 | β-galactoside-binding member of the lectin family | Implicated in fibrosis processes including myofibroblast proliferation, fibrogenesis, tissue repair, inflammation and ventricular remodeling. Associated with worse prognosis (mortality and hospitalizations). Possible predictive value in distinguishing HFpEF from HFrEF in patients that present with acute heart failure. | Meluzin and Tomandl [53], de Boer et al. [55], Carrasco-Sanchez et al. [56] |
| High-sensitivity troponin T (TnT) | Cardiac myocyte structural protein involved in sarcomere contraction | Related to cardiac myocyte injury and necrosis. Possible predictive value in distinguishing HFpEF from HFrEF and control groups (HFrEF have significantly higher values). | Meluzin and Tomandl [53], Santhanakrishnan et al. [57] |
| Cardiotrophin-1 (CT-1) | Interleukin 6 related pro-inflammatory cytokine | Implicated in fibrosis, inflammation, ventricular remodeling and ventricular stretch. Associated with worse prognosis (mortality and hospitalizations). No significant ability to distinguish HFpEF from HFrEF. | Meluzin and Tomandl [53], Lopez et al. [58], Celik et al. [59] |
| Growth differentiation factor-15 (GDF-15) | Member of the transforming growth factor-β family | Implicated in fibrosis and hypertrophy. Associated with worse prognosis (mortality and hospitalizations). No significant ability to distinguish HFpEF from HFrEF. | Sinning et al. [52], Santhanakrishnan et al. [57], Chan et al. [60] |
| Syndecan-1 | Member of the proteoglycan family | Implicated in fibrosis and ventricular remodeling. Associated with worse prognosis (mortality). No significant ability to distinguish HFpEF from HFrEF. | Tromp et al. [61] |
While natriuretic peptides are great indicators of myocyte stretch, biomarkers for myocyte damage, inflammation, and fibrosis may be useful additional indicators of DD. A recent study analyzing 33 biomarkers found that the profile of HFpEF consisted of elevated biomarkers of inflammation and angiogenesis, whereas HFrEF correlated more with myocyte stretch [62]. Specifically, the acute phase reactants high-sensitive C-reactive protein (hs-CRP) and pentraxin-3, as well as interleukin-6 were all found to be correlated to HFpEF, supporting the hypothesis that HFpEF is related to a pro-inflammatory state [62]. This study did not test the validity of using these biomarkers to diagnose HFpEF or distinguish from HFrEF; however, the study did identify neuropilin (a marker for angiogenesis) and osteopontin (a marker for remodeling) as independent predictors of all-cause mortality and readmission for HFpEF [62]. A list of other current biomarkers of interest is provided in Table 2, along with a brief description of their potential use [52—61]. A simplified diagram of the upregulation, secretion and effects of biomarkers of interest is shown in Figure 2.
Figure 2:
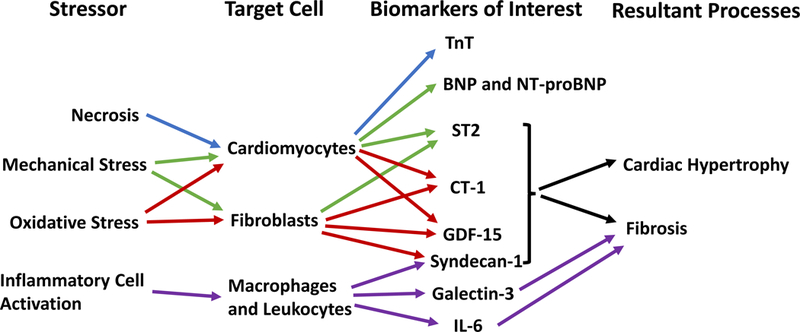
The release of biomarkers of interest and their potential role in the development of diastolic dysfunction and heart failure with preserved ejection fraction. Tnt, high-sensitivity troponin T; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; ST2, suppression of tumorgenicity 2; CT-1, cardiotrophin-1; GDF-15, growth differentiation factor-15; IL-6, interleukin 6.
Although there are many potential biomarkers, there is no unequivocal data to support a specific biomarker that provides the best information in a clinical setting. Currently, the ACC/AHA guidelines recommend the measurement of natriuretic peptides and troponin for diagnosis and prognosis of heart failure, and the measurement of Suppression of Tumorgenicity 2 (ST2) and galectin-3 for additional risk stratification and prognosis [46]. However, these recommendations are for heart failure in general and are not targeted for DD or HFpEF. Neither ST2 nor galectin-3 have shown any independent discriminatory power in diagnosing DD or HFpEF, but both are correlated with worse prognosis irrespective of treatment [63]. While no single biomarker has shown to effectively distinguish HFpEF from HFrEF, Sinning and colleagues [52] demonstrated that the use of combined biomarker indices, specifically (CRP + Growth differentiation factor-15 (GDF-15) + ST2)/ NT-proBNP as well as GDF-15/NT-proBNP were able to successfully predict HFpEF from HFrEF in patients with diagnosed heart failure [52].
Biomarkers provide significant information about DD parameters such as filling pressures, myocardial fibrosis, and ventricular remodeling without requiring invasive techniques like cardiac catheterization and endocardial biopsy. In addition, many of these biomarkers have been shown to have strong prognostic value, successfully predicting future hospitalization and mortality. Unfortunately, elevated filling pressures occur in a variety of cardiac disease processes, and markers of inflammation and fibrosis are not specific to HFpEF. Even markers more tailored to DD have not been shown to independently predict HFpEF, severely restricting their diagnostic utility. However, the use of combined biomarker indices have shown promise in better differentiation between HFpEF and HFrEF [52]. Further investigations are warranted to improve biomarker indices for detection of DD and HFpEF, as well as to expand their prognostic value.
5. Treatment of HFpEF
Current methods to treat HFpEF have proven to be relatively unsuccessful when compared to the treatment for HFrEF. Many studies have evaluated the effect of drugs shown to improve morbidity and mortality in HFrEF, which have ultimately failed to show the same beneficial effects in HFpEF patients.
5.1. β-blockers
Many studies have investigated multiple different β-blockers in the treatment of HFpEF, all of which have produced insignificant decreases in morbidity and mortality. For example, in the SENIORS clinical study, there was no effect of nebivolol to decrease the rate of all-cause mortality or cardiovascular hospitalization in patients older than 70 [64]. Additionally, the ELANDD study demonstrated the ineffectiveness of increasing even the exercise capacity of HFpEF patients on nebivolol compared to placebo, and actually was shown to increase the 6-minute walk test by a smaller margin that the placebo group [65]. The OPTIMIZE-HF study compared patients put on any β-blocker to placebo on mortality over a year. It was found that in patients with preserved EF there was no significant difference in mortality or re-hospitalizations between the treatment and placebo groups [66]. A smaller study named J-DHF looked at the effectiveness of carvedilol in decreasing mortality and hospitalizations in Japanese patients. Again, it was found that there was no decrease in these primary endpoints in the carvedilol group compared to the placebo group [67]. It is clear from these various studies that β-blockers, which have proven to be extremely valuable in other heart conditions, do not have the same benefit in HFpEF, necessitating other treatment options.
5.2. Mineralocorticoid receptor inhibitor
Due to the beneficial effects of spironolactone in HFrEF, it was originally thought there may be an equally beneficial response in patients with HFpEF. Unfortunately, this has not been proven to be true. The TOPCAT study demonstrated that, while spironolactone may significantly improve exercise tolerance in a subset of patients with a poor diastolic response to exercise [68], there is no decrease in cardiovascular death or hospitalization over placebo [69]. The TOPCAT study had unfortunate flaws that may have affected the results. A significant issue was the difference in baseline characteristics between the placebo groups and the treatment groups. There were regional differences in event rates, standards for hospitalization, and medical practices. Another significant problem this study faced was the number of participants that discontinued the medication in the trial, totaling around 1/3 of total participants. Finally, there were multiple analyses performed on the final data, increasing the risk that the final statistical findings were due to chance. Additional studies have further shown the futility of spironolactone in improving exercise capacity and quality of life [70] or other measures such as arterial mass, left ventricular stiffness, and remodeling in older patients [71].
5.3. Angiotensin converting enzyme inhibitors
Angiotensin converting enzyme inhibitors (ACE-I) and angiotensin II receptor blockers (ARBs) have also been considered in the treatment of HFpEF. In the I-Preserve study, Ibersartan was studied in relation to mortality and quality of life measures and showed no significant improvement in these two areas [72]. Similarly, while the CHARM-Preserved study looking at the effects of Candesartan on HFpEF patients did significantly decrease hospitalizations, there was no significant decrease in cardiovascular mortality [73]. In the PEP-CHF study aimed to determine the efficacy of perindopril in treating HFpEF in elderly patients, there was no significant decrease in mortality or hospitalizations [74].
5.4. Cardiac sodium funny channel inhibitors
Cardiac sodium funny channels are another potential target to treat HFpEF. There are conflicting results regarding the use of ivabradine in these patients. One randomized trial looked at the effect of ivabradine on filling pressures, exercise capacity, NT-proBNP levels, and found no significant change over placebo [75]. In contrast, an earlier randomized trial found that the use of ivabradine improved exercise tolerance over placebo, indicating its usefulness in symptomatic treatment on exertion [76].
5.5. Altering concentrations of nitric oxide
More recently, the phosphodiesterase-5 inhibitor sildenafil was studied in its ability to treat HFpEF. The RELAX trial demonstrated again that sildenafil was not useful in treating this condition, failing to improve clinical status or exercise capacity in these patients [77]. Another study looked at sildenafil and its relation to cardiac structure, response to exercise, quality of life, and laboratory values in HFpEF, finding again that sildenafil did not improve any of these measures [78]. The direct NO donor isosorbide mononitrate was investigated as well, but showed a significant decrease in activity levels in the isosorbide mononitrate group compared to placebo with no significant changes in proBNP, 6 minute walk, or quality of life between the groups [79].
An alternative method of increasing NO availability was studied using the drug vericuguat. The mechanism of action of vericuguat involves direct activation of guanylyl cyclase, differing from the ineffective drugs that focus on inhibiting PDE-5. Despite not demonstrating a significant difference in the primary endpoints of left atrial volume and proBNP, the drug did demonstrate significant differences in the KCCQ scale over placebo [80]. This suggests the possibility of using vericuguat in symptomatic control to improve quality of life for patients with HFpEF. There are several issues with this study, including a short duration of treatment of 8–10 weeks at the full doses 5 mg and 10 mg, respectively, and a four-week recruitment window allowing for natural patient recovery from the initial exacerbation [80].
5.6. Angiotensin receptor neprilysin inhibitors
An additional therapeutic avenue that shows promise in treating HFpEF lies in the angiotensin receptor neprilysin inhibitor (ARNI) class with the drug known as LCZ696. The original trial in 2012 determined that this drug significantly decreases NT-proBNP levels when compared to valsartan alone [81]. As decreased NT-proBNP levels are correlated with improved clinical outcomes in regard to HFpEF, LCZ696 shows potential to be a good therapeutic drug, however upon review of the literature there have been scant studies directly measuring clinical outcomes with LCZ696 treatment. Due to the efficacy of this drug in reducing NT-proBNP, it would be interesting to investigate this relationship directly.
5.7. Statins
A recent study has shown potential benefits in using statins to decrease all-cause and cardiovascular mortality, especially in patients with no history of ischemic heart disease [82]. Although there were problems with the study – two big concerns being it was a post hoc analysis using TOPCAT study data and the statin doses were unknown – there were promising results showing decreased all-cause and cardiovascular-related deaths in HFpEF patients [82]. A separate meta-analysis again demonstrated mortality benefits, although no cause-specific mortality benefit could be determined [83]. A third study looking at the Swedish Heart Failure Registry found that the use of statins in patients with HFpEF and an EF of >= 50% had improved mortality associated with a hazard ratio of 0.80 [84]. Future studies should perform randomized control trials using different doses of statins to determine if these drugs truly are beneficial to this patient population and, if they are, what benefit they truly provide.
The drugs studied up until this point have largely proved to be futile in decreasing morbidity and mortality in patients with HFpEF. However, a recent double-blinded crossover phase 1 trial involving anakinra demonstrated that a 14-day treatment with this drug significantly improved the peak VO2 max and decreased CRP compared to placebo [85].
5.8. Exercise therapy
An additional effective treatment for HFpEF may be exercise therapy (ET). Edelmann et al. [86] demonstrated that patients who underwent ET showed significant improvement in multiple parameters over standard care, including peak V02, left atrial volume index, and the E/e’ ratio. These findings suggested a decrease in filling pressures and improvement in diastolic function, having the potential to reverse cardiac remodeling. While the exact mechanism for diastolic improvement with ET is not clear, previous studies have proposed that a combination of improved oxidative capacity, vascular function, and even a decrease in system inflammation with ET may contribute to improvement of diastolic function [87,88]. The finding of decreased systemic inflammation is particularly interesting in light of the potential inflammatory mechanism of HFpEF. The benefit of ET in HFpEF was further supported by a meta-analysis done in 2014 which demonstrated improved peak oxygen uptake and quality of life measures, though this study failed to show improved systolic or diastolic function over control groups [89]. Unfortunately, this study had a small sample size despite being a meta-analysis, and the findings require further clarification with a more encompassing sample group. Overall, these findings especially from Edelmann et al. [86] are extremely promising, suggesting potential reversal of disease process, not shown with any current medication. Longer-term studies will need to be performed to determine if ET conveys a mortality reduction, but even the improvement of quality of life is a step up from other available therapies.
5.9. Intra-atrial shunt placement
An interesting alternative therapy to medical management for HFpEF is an intra-atrial shunt placement. The REDUCE LAP-HF randomized control trial placed these shunts in 64 eligible patients. The results of this trial were promising though modest – decreased left atrial pressure, improved quality of life, and improved functional capacity [90,91]. This is certainly a potential future treatment option for HFpEF, although with it this treatment option carries other significant considerations such as the risks of surgery, the need for anticoagulation after the procedure, and whether there truly is a mortality benefit. Future studies should determine whether the benefits to the patient are worth the risks of this type of surgery.
It is clear from the multitude of unsuccessful trials to treat HFpEF, there is much we do not know about this condition. The initial promising results of treatment with anakinra suggest that the underlying etiology may in fact be inflammatory in nature, and thus the treatment for HFpEF may lie in targeting this inflammation. More research will be necessary to investigate the precise underlying mechanisms to determine a target for better therapeutic potential.
6. Expert commentary
Significant progress has been made toward the understanding of HFpEF, but many questions and challenges remain, particularly in early diagnosis and treatment. As discussed above, patients with HFpEF are often asymptomatic late into the disease process, making early diagnosis difficult. Additionally, indicators of HFpEF and DD such as decreased ventricular relaxation and wall compliance are difficult to measure without invasive testing. A huge effort has been made to investigate different imaging modalities for DD; however, in clinical practice, it is uncommon to evaluate DD beyond 2D echocardiography. While echocardiography is the recommended initial imaging tool, it has significant weaknesses and often cannot significantly distinguish preclinical DD (grade I) from normal diastolic function. Other modalities, including TDE, STE, and CMR, have shown to provide valuable information regarding diastolic function, but still have not provided a gold standard for detecting preclinical DD. Further investigation is needed before these alternative modalities are practical for clinical evaluation of DD.
Various biomarkers have been used as diagnostic and prognostic indicators for HFpEF and HFrEF; however, the mechanisms underlying DD and HFpEF involve a complex combination of nonspecific factors including inflammation, fibrosis, and ventricular remodeling. Therefore, discovering a biomarker specific for HFpEF has been difficult. Although no biomarker has proven to independently diagnose preclinical HFpEF, use of a panel of biomarkers may be helpful to distinguish HFpEF from HFrEF, as well as provide valuable insight into disease severity and prognosis. Further research into novel biomarkers and standardized algorithms using a set of biomarkers is needed to improve their clinical utility.
The current treatment options for HFpEF are very limited and unsuccessful. To improve treatment, it is first necessary to elucidate the exact mechanism(s) involved in the development of this syndrome. The systemic inflammatory mechanism proposed by Paul and Tschöpe seems promising based on the current knowledge; however there still appear to be gaps in this theory. For example, if the released ROS molecules truly do cause a decrease in the activity of PKG by depleting NO, why is it that the treatment with the PDE5 inhibitor sildenafil showed no positive effect on morbidity or mortality? Future studies will need to determine if decreased PKG activity truly does play an active role in the pathogenesis of HFpEF as current studies suggest. If PKG is actively involved, it is necessary to determine why treatment with sildenafil does not produce a beneficial response. Is there a role of another endogenous molecule other than PDE5 that could regulate PKG?
While most of the current treatment options offer little benefit, statins and early studies using anakinra has shown improvement in specific outcomes regarding HFpEF. It will be necessary to follow the progress of the latest phase 2 clinical trials of anakinra to determine if there truly is a benefit for HFpEF patients. If the theory of systemic inflammation does explain the development of HFpEF, the next area of treatment studies should focus on decreasing this systemic inflammation. Potential drugs may include other inflammatory cytokine inhibitors such as TNF inhibitors, in addition to anakinra and other immunomodulatory therapies.
7. Five-year view
As the understanding of the underlying mechanism and development of HFpEF continues to improve, strategies for diagnosis and treatment can become more targeted. Biomarkers for inflammation, fibrosis, and remodeling have already shown promise as prognostic factors, but development of more sophisticated combined biomarker indices may allow for earlier detection and intervention for DD and HFpEF. While many imaging modalities exist for evaluating DD, improvement in imaging technology as well as further development of standardized approaches should allow for more precise evaluation of diastolic function. Finally, the theory of systemic inflammation as a possible mechanism of HFpEF should drive future research into targeted treatment options including anti-inflammatory medications such as immunomodulatory drugs, and drugs targeting other systemic inflammatory processes. Ideally targeted therapy may provide significantly greater efficacy for decreased morbidity and mortality as well as relief of symptoms in HFpEF patients.
Key issues.
HFpEF is a common cause of morbidity and mortality, especially in the elderly population.
The mechanism of HFpEF is poorly understood, with recent investigations pointing towards systemic inflammation as the primary cause.
No diagnostic tools exist to definitively detect preclinical HFpEF, resulting in most cases being discovered late in the disease process.
Assessment of diastolic function can provide valuable insight into the development and progression of HFpEF, allowing for earlier detection and intervention.
Echocardiography has become the preferred imaging modality for evaluating DD, with invasive hemodynamics, STE, and CMR playing supplementary roles.
Biomarker panels correlated to DD parameters such as filling pressures, myocardial fibrosis, and ventricular remodeling have been used to predict hospitalizations and mortality, as well as to distinguish HFpEF from HFrEF.
Historically, methods that have successfully treated HFrEF have not proven beneficial in HFpEF. Interestingly, recent studies have shown a potential benefit of statins, but future investigation is needed to evaluate treatment options.
Acknowledgments
Funding
This work was supported by NIH grants R01HL112597, R01HL116042, and R01HL120659 to DK Agrawal from the NHLBI. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
*of interest
**of considerable interest
- [1].Alagiakrishnan K, Banach M, Jones LG, et al. Update on Diatolic Heart Failure or Heart Failure With Preserved Ejection Fraction in the Older Adults. Ann Med. 2013;45:37–50. [DOI] [PubMed] [Google Scholar]
- [2].De Keulenaer GW, Brutsaert DL. Are Systolic and Diastolic Heart Failure Overlapping Phenotypes Within the Heart Failure Spectrum. Circ. 2011;123:1996–2004. [DOI] [PubMed] [Google Scholar]
- [3].2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239. [DOI] [PubMed] [Google Scholar]
- [4].Ponikowski P, Voors AA, Anker SD, et al. 2016. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- [5].Lam CSP, Roger VL, Rodeheffer RJ, et al. Cardiac Structure and Ventricular-Vascular Function in Persons With Heart Failure and Preserved Ejection Fraction From Olmsted County, Minnesota. Circ. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vasan RS, Levy D. Defining Diastolic Heart Failure – A Call for Standardized Diagnostic Criteria. Circ. 2000;101:2118–2121. [DOI] [PubMed] [Google Scholar]
- [7].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics – 2017 Update: A Report From the American Heart Association. Circ. 2017;135:e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parikh JD, Hollingsworth KG, Wallace D, et al. Normal Age-Related Changes in Left Ventricular Function: Role of Afterload and Subendocardial Dysfunction. Int J Cardiol. 2016;223:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association. Circ Heart Fail. 2013;6;606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Owan TE, Redfield MM. Epidemiology of Diastolic Heart Failure. Prog Cardiovasc Dis. 2005;47:320–332. [DOI] [PubMed] [Google Scholar]
- [11].Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and Clinical Course of Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail. 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan MMY, Lam CSP. How Do Patients With Heart Failure with Preserved Ejection Fraction Die? Eur J Heart Fail. 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- [13].Upadhya B, Kitzman DW. Heart Failure with Preserved Ejection Fraction in Older Adults. Heart Fail Clin. 2017;13:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Siddiqi OK, Ruberg FL. Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nihoyannopoulos P, Dawson D. Restrictive Cardiomyopathies. Eur J Echocardiogr. 2009;10:iii23-iii33. [DOI] [PubMed] [Google Scholar]
- [16].Shizukuda Y, Bolan CD, Tripodi DJ, et al. Does Oxidative Stress Modulate Left Ventricular Diastolic Function in Asymptomatic Subjects With Hereditary Hemochromatosis? Echocardiography. 2009;26:1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oktay AA, Shah SJ. Current Perspectives on Systemic Hypertension in Heart Failure With Preserved Ejection Fraction. Curr Cardiol Rep. 2014;16:545. [DOI] [PubMed] [Google Scholar]
- [18].Shah SJ, Aistrup GL, Gupta DK, et al. Ultrastructural and Cellular Basis for the Development of Abnormal Myocardial Mechanics During the Transition from Hypertension to Heart Failure. Am J Physiol Heart Circ Physiol. 2014;306:H88-H100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rysä J, Leskinen H, Ilves M, et al. Distinct Upregulation of Extracellular Matrix Genes in Transition From Hypertrophy to Hypertensive Heart Failure. Hypertension. 2005;45:927–933. [DOI] [PubMed] [Google Scholar]
- [20].Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac Comorbidities in Heart Failure With Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B Cells Promote Inflammation in Obesity and Type 2 Diabetes Through Regulation of T-Cell Function and an Inflammatory Cytokine Profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rovina N, Koutsoukou A, Koulouris NG. Inflammation and Immune Response in COPD: Where Do We Stand? Mediators Inflamm. 2013;2013:413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ter Maaten JM, Damman K, Verhaar MC, et al. Connecting Heart Failure With Preserved Ejection Fraction and Renal Dysfunction: The Role of Endothelial Dysfunction and Inflammation. Eur J Heart Fail. 2016;18:588–598. [DOI] [PubMed] [Google Scholar]
- [24].Pappachan JM, Varughese GI, Sriraman R, et al. Diabetic Cardiomyopathy: Pathophysiology, Diagnostic Evaluation and Management. World J Diabetes. 2013;4:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271.**-This article provides the first synthesis of information in support of systemic inflammation being the etiology of HFpEF. The hypothesis of their paper provided a plausible explanation of the mechanistic relationship between inflammation the end result of heart failure with preserved ejection fraction.
- [26].van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low Myocardial Protein Kinase G Activity in Heart Failure With Preserved Ejection Fraction. Circulation. 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- [27].Westermann D, Lindner D, Kasner M, et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients With Heart Failure and Normal Ejection Fraction. Circ Heart Fail. 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- [28].Franssen C, Chen S, Unger A, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–324. **-This paper took the theories proposed by Paulus and Tschope (citation 24) and tested the multiple variables in a systematic and evidence based fashion. It provides supporting evidence for the theory of inflammation causing HFpEF. [DOI] [PubMed] [Google Scholar]
- [29].Yturralde FR, Gaasch WH. Diagnostic Criteria for Diastolic Heart Failure. Prog Cardiovasc Dis. 2005;47:314–319. [DOI] [PubMed] [Google Scholar]
- [30].Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. **-This article gives a detailed background into useful echocardiographic parameters applicable to DD, and provides an algorithm for diagnosing and staging DD. [DOI] [PubMed] [Google Scholar]
- [31].Eskander M, Kern MJ. Invasive Hemodynamics of Myocardial Disease: Systolic and Diastolic Dysfunction (and Hypertrophic Obstructive Cardiomyopathy). Intervent Cardiol Clin. 2017;6:297–307.*-This article provides a review of invasive hemodynamics, and provides a necessary background into the different parameters useful in assessing diastolic function. [DOI] [PubMed] [Google Scholar]
- [32].Huis in’t Veld AE, de Man FS, van Rossum AC, et al. How to Diagnose Heart Failure With Preserved Ejection Fraction: The Value of Invasive Stress Testing. Neth Heart J. 2016;24:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zile MR, Baicu CF, Gaasch WH, et al. Diastolic Heart Failure – Abnormalities in Active Relaxation and Passive Stiffness of the Left Ventricle. N Engl J Med. 2004;350:1953–1959. *-This article showed that abnormal myocardial relaxation and decreased ventricular wall compliance were central to the definition of DD, as well as the development of HFpEF. [DOI] [PubMed] [Google Scholar]
- [34].Sato K, Grant ADM, Negishi K, et al. Reliability of Updated Left Ventricular Diastolic Function Recommendations in Predicting Elevated Left Ventricular Filling Pressure and Prognosis. Am Heart J. 2017;189:28–39. [DOI] [PubMed] [Google Scholar]
- [35].Morris DA, Ma X, Belyavskiy E, et al. Left Ventricular Longitudinal Systolic Function Analysed by 2D Speckle-Tracking Echocardiography in Heart Failure with Preserved Ejection Fraction: A Meta-Analysis. Open Heart. 2017;4:e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Park S, Miyazaki C, Bruce CJ, et al. Left Ventricular Torsion by Two-Dimensional Speckle Tracking Echocardiography in Patents with Diastolic Dysfunction and Normal Ejection Fraction. J Am Soc Echocardiogr. 2008;21:1129–1137. [DOI] [PubMed] [Google Scholar]
- [37].Zhou W, Benharash P, Ho J, et al. Left Ventricular Twist and Untwist Rate Provide Reliable Measures of Ventricular Function in Myocardial Ischemia and a Wide Range of Hemodynamic States. Physiol Rep. 2013;1:e00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leong DP, De Pasquale CG, Selvanayagam JB, et al. Heart Failure With Normal Ejection Fraction: The Complementary Roles of Echocardiography and CMR Imaging. JACC Cardiovasc Imaging. 2010;3:409–420. [DOI] [PubMed] [Google Scholar]
- [39].Dusch MN, Thadani SR, Dhillon GS, et al. Diastolic Function Assessed By Cardiac MRI Using Longitudinal Left Ventricular Fractional Shortening. Clin Imaging. 2014;38:666–668. [DOI] [PubMed] [Google Scholar]
- [40].Wu V Chyou JY, Chung S, et al. Evaluation of Diastolic Function by Three-Dimensional Volume Tracking of the Mitral Annulus With Cardiovascular Magnetic Resonance: Comparison With Tissue Doppler Imaging. J Cardiovasc Magn Reson. 2014;16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Su MM, Lin L, Tseng YE, et al. CMR-Verified Diffuse Myocardial Fibrosis Is Associated With Diastolic Dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. [DOI] [PubMed] [Google Scholar]
- [42].Kurita A, Kono K, Morita H. Diastolic Cardiac Function Is Synonymous with Long-Axis Systolic Function: A Novel Concept in Cardiac Function. Echocardiography. 2012;29:397–402. [DOI] [PubMed] [Google Scholar]
- [43].Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Obokata M, Kane GC, Reddy YN, et al. The Role of Diastolic Stress Testing in the Evaluation for HFpEF: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Van Iterson EH, Olson TP, Borlaug BA, et al. Comparisons of Noninvasive Methods Used to Assess Exercise Stroke Volume in Heart Failure with Preserved Ejection Fraction. Med Sci Sports Exerc. 2017;49:1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Card Fail. 2017;23:628–651. **-These guidelines by the ACC/AHA/HFSA recommend the measurement of BNP and NT-proBNP to support the diagnosis HF and provide information on disease severity and prognosis. In addition, they support the measurement of ST2 and galectin-3 for additional prognosis and risk stratification information.28461259 [Google Scholar]
- [47].Fu S, Ping P, Wang F, et al. Synthesis, Secretion, Function, Metabolism, and Application of Natriuretic Peptides in Heart Failure. J Biol Eng. 2018;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- [49].Kelder JC, Cramer MJ, van Wijngaarden J, et al. The Diagnostic Value of Physical Examination and Additional Testing in Primary Care Patients With Suspected Heart Failure. 2011;124:2865–2873. [DOI] [PubMed] [Google Scholar]
- [50].van Veldhuisen DJ, Linssen GCM, Jaarsma T, et al. B-Type Natriuretic Peptide and Prognosis in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Coll Cardiol. 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- [51].Uraizee I, Cheng S, Hung C, et al. Relation of N-Terminal Pro-B-Type Natriuretic Peptide With Diastolic Function in Hypertensive Heart Disease. Am J Hypertens. 2013;26:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sinning C, Kempf T, Schwarzl M, et al. Biomarkers for Characterization of Heart Failure – Distinction of Heart Failure With Preserved and Reduced Ejection Fraction. Int J Cardiol. 2017;15:272–277. **-This article provides evidence that the use of a biomarker panels can successfully distinguish HFpEF and HFrEF. By being able to differentiate disease processes, biomarker indices may allow for earlier detection of DD and HFpEF. [DOI] [PubMed] [Google Scholar]
- [53].Meluzin J, Tomandl J. Can Biomarkers Help to Diagnose Early Heart Failure With Preserved Ejection Fraction. Dis Markers. 2015;2015:426045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Parikh RH, Seliger SL, Christenson R, et al. Soluble ST2 for Prediction of Heart Failure and Cardiovascular Death in an Elderly, Community-Dwelling Population. J Am Heart Assoc. 2016;5:e003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].de Boer RA, Edelmann F, Cohen-Solal A, et al. Galectin-3 in Heart Failure With Preserved Ejection Fraction. Eur J Heart Fail. 2013;15:1095–1101. [DOI] [PubMed] [Google Scholar]
- [56].Carrasco-Sanchez FJ, Aramburu-Bodas O, Salamanca-Bautista P. Predictive Value of Serum Galectin-3 Levels in Patients With Acute Heart Failure With Preserved Ejection Fraction. Int J Cardiol. 2013;169:177–182. [DOI] [PubMed] [Google Scholar]
- [57].Santhanakrishnan R, Chong JP, Ng TP, et al. Growth Differentiation Factor 15, ST2, High-Sensitivity Troponin T, and N-Terminal Pro Brain Natriuretic Peptide in Heart Failure With Preserved Vs. Reduced Ejection Fraction. Eur J Heart Fail. 2012;14:1338–1347. [DOI] [PubMed] [Google Scholar]
- [58].Lopez B, Gonzalez A, Querejeta R, et al. Association of Cardiotrophin-1 With Myocardial Fibrosis in Hypertensive Patients With Heart Failure. Hypertension. 2014;63:483–489. [DOI] [PubMed] [Google Scholar]
- [59].Celik A, Sahin S, Koc F, et al. Cardiotrophin-1 Plasma Levels Are Increased in Patients With Diastolic Heart Failure. Med Sci Monit. 2012;18:CR25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chan MMY, Santhanakrishnan R, Chong JPC, et al. Growth Differentiation Factor 15 in Heart Failure With Preserved Vs. Reduced Ejection Fraction. Eur J Heart Fail. 2016;18:81–88. [DOI] [PubMed] [Google Scholar]
- [61].Tromp J, van der Pol A, Klip IT, et al. Fibrosis Marker Syndecan-1 and Outcome in Patients With Heart Failure With Reduced and Preserved Ejection Fraction. Circ Heart Fail. 2014;7:457–462. [DOI] [PubMed] [Google Scholar]
- [62].Tromp J, Khan MA, Klip IT, et al. Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Heart Assoc. 2017;6:e003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Edelmann F, Holzendorf V, Wachter R, et al. Galectin-3 in Patients With Heart Failure With Preserved Ejection Fraction: Results from the Aldo-DHF Trial. Eur J Heart Fail. 2015;17:214–223. [DOI] [PubMed] [Google Scholar]
- [64].Flather MD, Shibata MC, Coats AJ, et al. Randomized Trial to Determine the Effect of Nebivolol on Mortality and Cardiovascular Hospital Admission in Elderly Patients With Heart Failure (SENIORS). Eur Heart J. 2005;26:215–225. [DOI] [PubMed] [Google Scholar]
- [65].Conraads VM, Metra M, Kamp O, et al. Effects of the Long-Term Administration of Nebivolol on the Clinical Symptoms, Exercise Capacity, and Left Ventricular Function of Patients With Diastolic Dysfunction: Results of the ELANDD Study. Eur J Heart Fail. 2012;14:219–225. [DOI] [PubMed] [Google Scholar]
- [66].Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical Effectiveness of Beta-Blockers in Heart Failure: Findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yamamoto K, Origasa H, Hori M, et al. Effects of Carvedilol on Heart failure with Preserved Ejection Fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013;15:110–118. [DOI] [PubMed] [Google Scholar]
- [68].Kosmala W, Rojek A, Przewlocka-Kosmala M, et al. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:1823–1834. [DOI] [PubMed] [Google Scholar]
- [69].Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for Heart Failure With Preserved Ejection Fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- [70].Edelmann F, Wachter R, Schmidt AG, et al. Effect of Spironolactone on Diastolic Function and Exercise Capacity in Patients With Heart Failure With Preserved Ejection Fraction: The Aldo-DHF Randomized Controlled Trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- [71].Upadhya B, Hundley WG, Brubaker PH, et al. Effect of Spironolactone on Exercise Tolerance and Arterial Function in Older Adults with Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2017;65:2374–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients With Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- [73].Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of Candesartan in Patients with Chronic Heart Failure and Preserved Left-Ventricular Ejection Fraction: The CHARM-Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- [74].Cleland JG, Tendera M, Adamus J, et al. The Perindopril in Elderly People With Chronic Heart Failure (PEP-CHF) Study. Eur Heart J. 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- [75].Komajda M, Isnard R, Cohen-Solal A, et al. Effect of Ivabradine in Patients With Heart Failure With Preserved Ejection Fraction: The EDIFY Randomized Placebo-Controlled Trial. Eur J Heart Fail. 2017;19:1495–1503. [DOI] [PubMed] [Google Scholar]
- [76].Kosmala W, Holland DJ, Rojek A, et al. Effect of If-Channel Inhibition on Hemodynamic Status and Exercise Tolerance in Heart Failure With Preserved Ejection Fraction: A Randomized Trial. J Am Coll Cardiol. 2013;62:1330–1338. [DOI] [PubMed] [Google Scholar]
- [77].Redfield MM, Chen HH, Borlaug BA, et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu LC, Hummel YM, van der Meer P, et al. Effects of Sildenafil on Cardiac Structure and Function, Cardiopulmonary Exercise Testing and Health-Related Quality of Life Measures in Heart Failure Patients With Preserved Ejection Fraction and Pulmonary Hypertension. Eur J Heart Fail. 2017;19:116–125. [DOI] [PubMed] [Google Scholar]
- [79].Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Filippatos G, Maggioni AP, Lam CSP, et al. Patient-Reported Outcomes in the Sluble Guanylate Cyclase Stimulator in Heart Failure Patient with Preserved Ejection Fraction (SOCRATES-PRESERVED) Study. Eur J Heart Fail. 2017;19:782–791. [DOI] [PubMed] [Google Scholar]
- [81].Solomon SD, Zile M, Pieske B, et al. Prospective Comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction: a Phase 2 Double-Blind Randomised Controlled Trial. Lancet. 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- [82].Tsujimoto T, Kajio H. Favorable Effects of Statins in the Treatment of Heart Failure With Preserved Ejection Fraction in Patients Without Ischemic Heart Disease. Int J Cardiol. 2018;255:111–117. [DOI] [PubMed] [Google Scholar]
- [83].Fukuta H, Goto T, Wakami K, et al. The Effect of Statins on Mortality in Heart Failure With Preserved Ejection Fraction: A Meta-Analysis of Propensity Score Analyses. Int J Cardiol. 2016;214:301–306. *-This study shows a correlation between statins and decreased mortality in patients with HFpEF, which is good support using statins as a future treatment. [DOI] [PubMed] [Google Scholar]
- [84].Alehagen U, Benson L, Edner M, Dahlström U, et al. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail. 2015;8:862–870. [DOI] [PubMed] [Google Scholar]
- [85].Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of Interleukin-1 Blockade With Anakinra on Aerobic Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction (From the D-HART Pilot Study). Am J Cardiol. 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Edelmann F, Gelbrich G, Düngen HD, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients with Heart Failure with Preserved Ejection Fraction: Results of the Ex-DHF (Exercise Training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. [DOI] [PubMed] [Google Scholar]
- [87].Smart N, Haluska B, Jeffriess L, et al. Exercise Training in Systolic and Diastolic Dysfunction: Effects on Cardiac Function, Functional Capacity, and Quality of Life. Am Heart J. 2007;153:530–536. [DOI] [PubMed] [Google Scholar]
- [88].Adamopoulos S, Parissis J, Kroupis C, et al. Physical Training Reduces Peripheral Markers of Inflammation in Patients With Chronic Heart Failure. Eur Heart J. 2001;22:791–797. [DOI] [PubMed] [Google Scholar]
- [89].Pandey A, Parashar A, Kumbhani D, et al. Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: Meta-Analysis of Randomized Control Trials. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Feldman T, Mauri L, Kahwash R, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circ. 2018;137:364–375. *-This article provides an alternative approach to treating HFpEF through a surgical approach instead of a medical approach. The study may open the door to discussions into surgical treatments for this condition. [DOI] [PubMed] [Google Scholar]
- [91].Hasenfuß G, Hayward C, Burkhoff D, et al. A Transcatheter Intracardiac Shunt Device for Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF): A Multicentre, Open-Label, Single-Arm, Phase 1 Trial. Lancet. 2016;387:1298–1304. [DOI] [PubMed] [Google Scholar]


