Abstract
Inhibitory control or the ability to refrain from incorrect responses is a critical executive function known to diminish during aging. Imaging studies have elucidated cerebral changes that may underlie the age‐related deficits. However, it remains unclear whether the structural and functional changes occur in the same brain regions and whether reduced gray matter volumes (GMV) mediate decreased activation during inhibition. Here, in a sample of 149 participants, we addressed the issues using structural and functional magnetic resonance imaging. Individual's response inhibition was evaluated by the stop signal reaction time (SSRT) in a stop signal task. The results showed that age was associated with prolonged SSRT across participants. Many cortical and subcortical regions demonstrated age‐related reduction in GMV and activation to response inhibition. Additionally, age‐related diminution in inhibitory control, as indexed by the SSRT, was associated with both shared and distinct morphometric and functional changes. Voxel‐based morphometry demonstrated age‐related reduction in GMV in the right dorsolateral prefrontal cortex and caudate head as well as bilateral insula, in association with prolonged SSRT. In a contrast of stop success versus go success trials, age was associated with lower activation in the medial and inferior frontal cortex and inferior parietal cortex. Further, reduction in GMV mediated age‐related differences in activations only of the medial prefrontal cortex, providing limited evidence for structure function association. Thus, the decline in inhibitory control, as evidenced in the stop signal task, manifest with both shared and distinct structural and functional processes during aging.
Keywords: aging, fMRI, inhibitory control, VBM
1. INTRODUCTION
Inhibitory control or the ability to refrain from incorrect responses is central to an intact cognition. Aging is associated with changes in multiple domains of cognitive functions, including inhibitory control. The ability to suppress an undesired action diminishes in the elderly especially in situations that involve uncertainty and conflicts (Liebherr, Schiebener, Averbeck, & Brand, 2017). Deficits in inhibitory control contribute to impaired daily function not only in individuals with mild cognitive impairment and dementia (O'Callaghan, Hodges, & Hornberger, 2013), but also during healthy aging (Levin, Fujiyama, Boisgontier, Swinnen, & Summers, 2014; Lustig & Jantz, 2015). The current study aimed to investigate age‐related structural and functional brain changes that underlie diminished inhibitory control in otherwise healthy individuals.
Age‐related cognitive decline is associated with decreased gray and white matter volumes (Hakun, Zhu, Brown, Johnson, & Gold, 2015). Specifically, whole brain gray matter volume (GMV) decreased on average by 0.32% (0.10%–0.54%) annually (Bourisly et al., 2015; Liu et al., 2003; Scahill et al., 2003), with the most widespread decrease noted in the medial prefrontal cortex (PFC), followed by dorsolateral prefrontal, temporal, and parietal cortical regions, hippocampus, and the caudate nucleus (Bourisly et al., 2015; Dennis, Daselaar, & Cabeza, 2007; Fjell & Walhovd, 2010). Other areas including the cerebellum and thalamus appeared to be relatively spared (Bergfield et al., 2010). Importantly, volumetric brain reduction was associated with deteriorated cognitive performance in older adults (Fleischman et al., 2014; Kramer et al., 2007; Salthouse, 1996). For instance, older adults performed poorly in signal detection in a rapid visual information processing task, in association with decreased GMV in the left putamen and insula (Pagnoni & Cekic, 2007). Many studies supported a link of higher PFC GMV to better performance in executive control (Elderkin‐Thompson, Ballmaier, Hellemann, Pham, & Kumar, 2008; Gunning‐Dixon & Raz, 2003; Sakai et al., 2012), processing speed (Staff, Murray, Deary, & Whalley, 2006), and fluid knowledge (Fjell et al., 2006) in old adults (Kaup, Mirzakhanian, Jeste, & Eyler, 2011).
In functional imaging, inhibitory control has been studied with different behavioral paradigms including the stop signal task (SST). In the SST participants respond to a frequent go signal and withdraw their response when instructed by a stop signal. Older adults demonstrated impaired inhibitory control as represented by prolonged stop signal reaction time (SSRT) in the SST (Bloemendaal et al., 2016; Coxon et al., 2016; Hsieh & Lin, 2017; Hu & Li, 2012; Sebastian et al., 2013). Aging was associated with lower frontal cortical activations during inhibitory control (Coxon et al., 2016; Hu, Chao, Winkler, & Li, 2012; Sebastian et al., 2013), providing a unique opportunity to examine the structure–function relationship. Other studies have reported not only decreased PFC GMV and activation (Brassen et al., 2009; Nyberg et al., 2010), but also lower GMV and greater activation of the PFC (see also Di, Rypma, & Biswal, 2014 for a meta‐analysis; Kalpouzos, Persson, & Nyberg, 2012; Tyler et al., 2010) in older adults exposed to cognitive challenges. Investigators have suggested the latter findings as a compensatory mechanism of older adults to improve performance (Maillet & Rajah, 2013). Overall, the structure–function relationship may depend on task performance, and the investigation of intersubject variation may provide important clues to addressing this issue.
Here, combining magnetic resonance imaging (fMRI) and an SST, the current study investigated both structural and functional changes and their interrelationship with diminished inhibitory control during aging. We posited age‐related volumetric reduction as well as lower activation during inhibitory control in frontal and parietal areas and tested two specific hypotheses in relation to structure and function. First, reduction in GMV leads to lower cortical activations and, as a result, deficits in response inhibition, as reflected in prolonged SSRT. Second, lower GMV and altered functional activations are related independently to deficits in response inhibition, either because different brain regions are involved or because the same brain regions are involved but with unrelated structural and functional changes during aging.
2. METHODS
2.1. Participants and behavioral task
A total of 149 adults (83 women) between the age of 18 and 72 (31.6 ± 11.9) years participated in the study. All participants were physically healthy with no major medical illnesses or current use of prescription medications. None of them reported having a history of head injury, neurological, or psychiatric illness. All participants signed a written consent after given a detailed explanation of the study in accordance with a protocol approved by the Yale Human Investigation Committee.
All participants performed a stop signal task (SST) in which go and stop trials were randomly intermixed in presentation with an intertrial interval of 2 s (Hu et al., 2012; Hu, Ide, Zhang, & Li, 2016; Hu, Tseng, Winkler, & Li, 2014). A fixation dot appeared on the screen to signal the beginning of each trial. After a fore‐period varying from 1 s to 5 s (uniform distribution), the dot became a circle, the “go” signal, prompting participants to press a button quickly. The circle disappeared at button press or after 1 s, if the participant failed to respond. In approximately one quarter of trials, the circle was followed by a “cross,” the stop signal, prompting participants to withhold button press. The trial terminated at button press or after 1 s, if the participant stopped the response successfully. The time between the go and stop signals, the stop signal delay (SSD), started at 200 ms, and varied from one stop trial to the next according to a staircase procedure, increasing and decreasing by 67 ms each after a successful and failed stop trial (Levitt, 1971). With the staircase procedure, we anticipated that participants would succeed in stopping half of the time. Participants were trained briefly on the task before imaging to ensure that they understood the task. They were instructed to press the button quickly when they saw the go signal while keeping in mind that a stop signal might come up in some trials. In the scanner, they completed four 10 min sessions of the task, with approximately 100 trials in each session.
2.2. Behavioral data analysis
A critical SSD was computed for each participant that represents the time delay required for the participant to withhold the response successfully in half of the stop trials, following a maximum‐likelihood procedure (Wetherill, Chen, & Vasudeva, 1966). Briefly, SSDs across trials were grouped into runs, with each run being defined as a monotonically increasing or decreasing series. We derived a mid‐run estimate by taking the median SSD of every second run. The critical SSD was computed by taking the mean of all mid‐run SSDs. It was reported that, except for experiments with a small number of trials (30), the mid‐run measure was close to the maximum‐likelihood estimate of ×50 (50% positive response; i.e., 50% SS in the SST; Wetherill et al., 1966). The stop signal reaction time (SSRT) was computed for each participant by subtracting the critical SSD from the median go trial reaction time (Logan, Cowan, & Davis, 1984). A longer SSRT suggests lesser capacity of inhibitory control.
2.3. MRI protocol and spatial preprocessing of brain images
Conventional T1‐weighted spin‐echo sagittal anatomical images were acquired for slice localization using a 3‐Tesla scanner (Siemens Trio, Erlangen, Germany). Anatomical images of the functional slice locations were obtained with spin‐echo imaging in the axial plane parallel to the Anterior Commissure‐Posterior Commissure (AC‐PC) line with repetition time (TR) = 300 ms, echo time (TE) = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm, and no gap. A single high‐resolution T1‐weighted gradient‐echo scan was obtained. One hundred and seventy‐six slices parallel to the AC‐PC line covering the whole brain were acquired with TR = 2,530 ms, TE = 3.66 ms, bandwidth = 181 Hz/pixel, flip angle = 7°, field of view = 256 × 256 mm, matrix = 256 × 256, 1 mm3 isotropic voxels. Functional blood oxygenation level‐dependent (BOLD) signals were then acquired with a single‐shot gradient‐echo echo‐planar imaging (EPI) sequence. Thirty‐two axial slices parallel to the AC‐PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm, and no gap. There were 300 images in each session for a total of four sessions.
Data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, United Kingdom). In the preprocessing of BOLD data, images of each participant were realigned (motion‐corrected) and corrected for slice timing. A mean functional image volume was constructed for each participant for each session from the realigned image volumes. These mean images were co‐registered with the high‐resolution structural image and then segmented for normalization to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner & Friston, 1999; Friston et al., 1995). Finally, images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half‐Maximum. Images from the first five TRs at the beginning of each session were discarded so only signals with steady‐state equilibrium between radio frequency pulsing and relaxation were included in the analyses.
2.4. General linear models and group analyses
We distinguished four trial outcomes, go success (GS), go error (GE), stop success (SS), and stop error (SE), and modeled BOLD signals by convolving the onsets of the go signals of each trial with a canonical hemodynamic response function (HRF) and the temporal derivative of the canonical HRF (Friston et al., 1995; Hu & Li, 2012). Realignment parameters in all six dimensions were entered in the model. We included the following variables as parametric modulators in the model: RT of GS trials, SSD of SS trials, and SSD of SE trials, in that order. Serial autocorrelation of the time series was corrected by a first degree autoregressive or AR(1) model (Della‐Maggiore, Chan, Peres‐Neto, & McIntosh, 2002; Friston et al., 2000). The data were high‐pass filtered (1/128 Hz cutoff) to remove low‐frequency signal drifts.
In the first‐level analysis, a contrast of “stop success > go success” (SS > GS) was made for individual participants. In the second‐level analysis, we performed a one‐sample t test on this contrast to identify regional activations to response inhibition. We then performed whole‐brain regressions against age and SSRT, respectively, with small volume correction for voxels showing significant activations to SS > GS in the one‐sample t test. Following current reporting standards, all results were evaluated for voxels meeting a threshold of voxel p < .05, corrected for familywise error (FWE) of multiple comparisons, or voxel p < .001 uncorrected in combination with cluster p < .05, FWE corrected, on the basis of Gaussian Random Field theory, as implemented in the SPM.
2.5. Voxel‐based morphometry
Voxel‐based morphometry was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) packaged in SPM 8. T1 images were first co‐registered to the Montreal Neurological Institute or MNI template space using a multiple stage affine transformation, during which the 12 parameters were estimated. Co‐registration started with a coarse affine registration using mean square differences, followed by a fine affine registration using mutual information. In this step, coefficients of the basis functions that minimize the residual square difference (between individual image and the template) were estimated. After affine transformation, T1‐images were corrected for intensity bias field and a local means denoising filter was applied, to account for intensity variations (inhomogeneity) and noise caused by different positions of cranial structures within the MRI coil. Finally, the images were segmented into cerebrospinal fluid, gray and white matters, using an adaptive maximum a posteriori method with k‐means initializations, as implemented in VBM8, generating tissue class maps (which included the gray matter or GM maps). Segmented and the initially registered tissue class maps were normalized using DARTEL, a fast diffeomorphic image registration algorithm of SPM. As a high‐dimensional nonlinear spatial normalization method, DARTEL generates mathematically consistent inverse spatial transformations. We used the standard customized tissue probability maps constructed from 550 healthy subjects to drive DARTEL normalization. Normalized GM maps were modulated to obtain the absolute volume of GM tissue corrected for individual brain sizes. Finally, the GM maps were smoothed by convolving with an isotropic Gaussian kernel. Smoothing helps to compensate for the inexact nature of spatial normalization and reduces the number of statistical comparisons (thus, making the correction for multiple comparisons less severe); however, it reduces the accuracy of localization. We used a smaller kernel size of FWHM = 8 mm to enable better localization accuracy.
In group analysis, we employed a linear whole‐brain regression to identify voxels with GMV in correlation with age and SSRT in two separate models. We did not include age and SSRT in the same regression model because the two variables were correlated (see section 3). As with BOLD data, we reported voxels meeting a threshold of voxel p < .05, corrected for familywise error (FWE) of multiple comparisons, or voxel p < .001 uncorrected in combination with cluster p < .05, FWE corrected.
2.6. Mediation analysis
We performed mediation analyses to test the hypothesis that regional GMV and activations each mediates the association between the age and SSRT, and the hypothesis that regional GMV mediates the association between age and activation to inhibitory control, using the toolbox M3 developed by Wager and Lindquist (http://www.columbia.edu/cu/psychology/tor/). The methods were detailed in our previous work (Ide & Li, 2011). Briefly, in a mediation analysis, the relation between the independent variable X and dependent variable Y, that is, X → Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by using three regression equations:
where a represents X → M, b represents M → Y (controlling for X), c’ represents X → Y (controlling for M), and c represents X → Y. In the literature, a, b, c, and c’ were referred to as path coefficients or simply paths, and we followed this notation. Variable M is said to be a mediator of connection X → Y, if (c – c’), which is mathematically equivalent to the product of the paths a × b, is significantly different from zero (MacKinnon, Fairchild, & Fritz, 2007). If the product of a × b and the paths a and b are significant, one concludes that X → Y is mediated by M. In addition, if path c’ is not significant, it indicates that there is no direct connection from X to Y and that X → Y is completely mediated by M. Note that path b represents M → Y, controlling for X, and should not be confused with the correlation coefficient between Y and M. Note also that a significant correlation between X and Y and between X and M is required for one to perform the mediation test.
3. RESULTS
3.1. Behavioral performance
Participants on average achieved a go response rate of 0.98 ± 0.03 (mean ± SD) and successfully inhibited the response in approximately half of the stop trials (stop success rate = 0.51 ± 0.03). The median go trial reaction time (goRT) and stop signal reaction time (SSRT) were 628 ± 119 ms and 215 ± 45 ms, respectively. Both goRT and SSRT were correlated positively with age (Figure 1). The correlation between SSRT and age remained significant after controlling for go RT (r = .2169, p = .0081). These results suggested that older adults are slower in executing and inhibiting a response.
Figure 1.
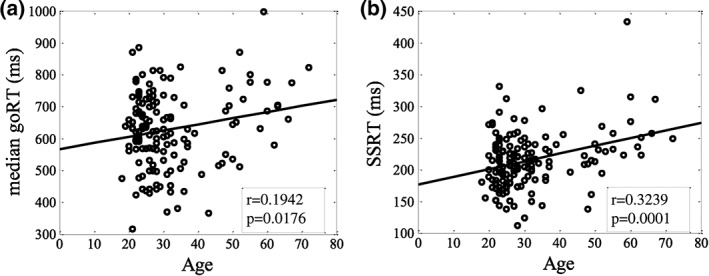
Linear correlation between age and (a) median go reaction time (goRT) and (b) stop signal reaction time (SSRT) in the stop signal task. Each data point represents one participant and the line shows the regression fit
3.2. Structural changes during aging
We computed the mean whole‐brain GMV for individual participants. In linear regressions whole‐brain GMV was correlated with age (r = −.6587, p = .0000) and SSRT (r = −.2352, p = .0039). Age‐related reduction in areal GMV was identified with whole‐brain linear regression in medial and lateral frontal cortical regions, middle cingulate gyrus, bilateral insula extending to inferior frontal cortex, temporal cortical regions, parietal and occipital cortex, basal ganglia, basal forebrain, and cerebellum (Figure 2a, Table 1). In another whole‐brain regression, lower GMVs of bilateral insula, right caudate head and right inferior and middle frontal gyri (IFG/MFG) were associated with longer SSRT (Figure 2b, Table 1). Next, we identified clusters that showed reductions in GMV in linear correlation both with age and with SSRT. Specifically, in voxel‐wise linear regression against SSRT, we examined the results with inclusive masking of the voxels that were significantly correlated with age. These clusters included bilateral insula, right caudate head, and right DLPFC, involving largely the same voxels without masking (Figure 2c, Table 1). In mediation analysis, we showed that reduction in the GMV of these regions completely mediated the age‐related differences in SSRT (Figure 2d).
Figure 2.
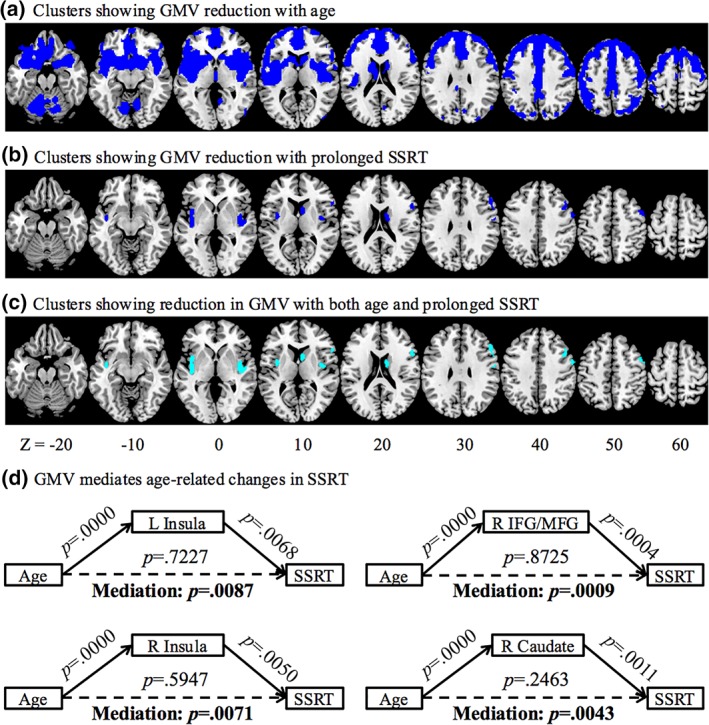
Gray matter volume (GMV) reduction with (a) age and (b) prolonged SSRT (blue), and (c) both age and prolonged SSRT (cyan). (d) GMV reduction in bilateral insula, right inferior and middle frontal gyri, and right caudate head completely mediated the relationship between age and prolonged SSRT [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Brain regions showing GMV reduction with age, SSRT, and both age and SSRT
| Contrast | Region | Cluster | Cluster p | Peak voxel | MNI coordinate (mm) | ||
|---|---|---|---|---|---|---|---|
| Size | value | Z value | X | Y | Z | ||
| Age (neg.) | MPFC/LPFC/mCC/Pre‐CG/OFC/BG/insula/TG | 101,552 | 0.000 | Inf | 3 | 33 | 36 |
| L CBL | 576 | 0.001 | 7.07 | −35 | −82 | −39 | |
| L CBL | 8,578 | 0.000 | 6.50 | −8 | −70 | −14 | |
| L CBL | 143 | 0.000 | 6.31 | −39 | −40 | −42 | |
| R CBL | 184 | 0.000 | 6.24 | 38 | −40 | −42 | |
| L cuneus | 260 | 0.000 | 5.97 | −8 | −78 | 40 | |
| R MOG | 233 | 0.000 | 5.62 | 44 | −85 | 9 | |
| R STG | 55 | 0.003 | 5.05 | 68 | −12 | 16 | |
| R CBL | 48 | 0.004 | 5.03 | 38 | −79 | −39 | |
| L MOG | 27 | 0.009 | 4.97 | −51 | −81 | 6 | |
| SSRT (neg.) | R MFG/IFG | 1,310 | 0.001 | 4.27 | 51 | 26 | 31 |
| R caudate | 605 | 0.045 | 4.25 | 8 | 5 | 13 | |
| R insula | 877 | 0.011 | 4.24 | 41 | −9 | −3 | |
| L insula | 1,161 | 0.003 | 4.09 | −41 | −7 | 0 | |
| Age ∩ SSRT | L insula | 1,107 | 0.002 | Inf | −45 | −3 | 3 |
| R IFG | 1,219 | 0.001 | Inf | 54 | 18 | 25 | |
| R MFG | Inf | 48 | 18 | 42 | |||
| R insula | 854 | 0.008 | Inf | 42 | −3 | 0 | |
| R caudate | 487 | 0.000^ | 6.78 | 8 | 14 | 10 | |
Abbreviations: MPFC/LPFC = medial/lateral prefrontal cortex; mCC = middle cingulate cortex; PreCG = precentral gyrus; OFC = orbitofrontal cortex; BG = basal ganglia; CBL = cerebellum; TG = temporal gyrus; MOG = middle occipital gyrus; STG = superior temporal gyrus; MFG/IFG = middle/inferior frontal gyrus; Inf = infinity; neg. = negative correlation.
3.3. Regional activations to inhibitory control during aging
To examine age‐related differences in the functional correlates of inhibitory control, we performed linear regressions of the contrast “stop success (SS) > go success (GS)” on age and SSRT, respectively. As age and SSRT were significantly correlated, they were not included in the same regression model. We examined these results, focusing only on the voxels that showed a significant difference in SS > GS in one‐sample t test for the whole‐brain (Figure 3a). The results showed age‐related increases in activation in the right middle occipital gyrus (MOG), and decreases in activation in the medial prefrontal cortex (MPFC) including the anterior presupplementary motor area (pre‐SMA) and dorsal and rostral anterior cingulate cortex (ACC), right superior frontal gyrus (SFG), and bilateral inferior frontal gyrus pars opercularis/anterior insula (IFGpo/AI), as well as bilateral inferior parietal lobule (IPL) (Figure 3b, Table 2). MPFC, including the pre‐SMA and ACC, bilateral SFG, right IFGpo/AI, left insula, bilateral but predominantly right IPL extending to middle temporal gyrus (MTG), bilateral caudate head, and thalamus showed lower activation in association with longer SSRT (Figure 3c, Table 2). We identified clusters that showed both age and SSRT‐related reduction in activation; that is, clusters that showed linear correlation both with age and with SSRT. Specifically, in voxel‐wise linear regression against SSRT, we examined the results with inclusive masking of the voxels that were significantly correlated with age. The results showed clusters in the MPFC, including the preSMA and ACC, right IFGpo/AI, as well as bilateral IPL (Figure 3d, Table 2). In mediation analysis, right IFGpo/AI activation completely mediated the correlation between age and SSRT, and MPFC activation partially mediated the correlation between age and SSRT (Figure 3e).
Figure 3.
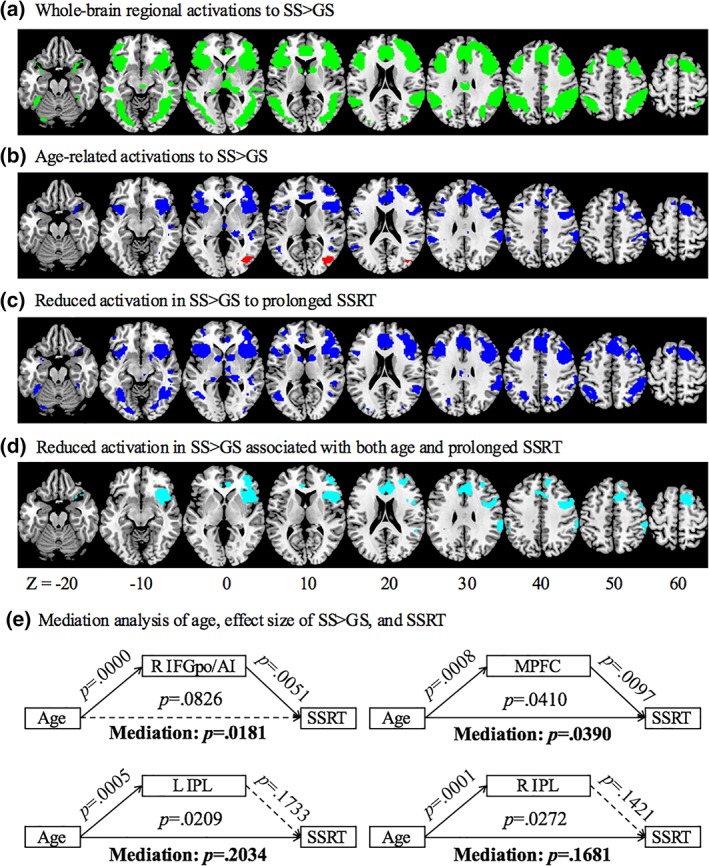
(a) Whole‐brain activations of SS > GS (green); (b) clusters showing altered activation with age in linear regression (red/blue: positive/negative correlation); (c) clusters showing decreased activation with prolonged SSRT in linear regression (blue); (d) clusters showing decreased activation both with age and prolonged SSRT (cyan); and (e) mediation analysis of age, effect size of SS > GS, and SSRT. BOLD activations of the right IFGpo/AI and MPFC each completely and partially mediates the correlation between age and SSRT [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain regions showing reduction in SS > GS effect size with age, SSRT, and both age and SSRT
| Contrast | Region | Cluster | Corrected | Peak voxel | MNI coordinate (mm) | ||
|---|---|---|---|---|---|---|---|
| size | p value | Z value | X | Y | Z | ||
| Age (pos.) | R MOG | 123 | 0.027^ | 4.29 | 42 | −76 | 4 |
| Age (neg.) | IFGpo/AI/preSMA/ACC/SFG/caudate | 2,435 | 0.005 | 5.18 | 42 | 8 | −8 |
| L IPL/OG | 83 | 0.001^ | 5.13 | −63 | −37 | 31 | |
| R IPL/OG | 277 | 0.027^ | 4.29 | 60 | −25 | 34 | |
| R MCC | 21 | 0.038^ | 4.20 | 9 | −22 | 40 | |
| SSRT (neg.) | R IPL/MTG | 911 | 0.009^ | 4.56 | 42 | −28 | −5 |
|
MPFC/caudate/ R SFG/IFGpo/AI |
3,304 | 0.001 | 4.42 | 54 | 14 | 40 | |
| Age ∩ SSRT | R IFGpo/AI | 1,132 | 0.001^ | 5.18 | 42 | 8 | −8 |
| L IPL | 19 | 0.003^ | 4.82 | −60 | −37 | 34 | |
| R preSMA/ACC | 754 | 0.008^ | 4.58 | 9 | 5 | 61 | |
| R IPL | 166 | 0.027^ | 4.29 | 60 | −25 | 34 | |
Abbreviations: MOG = middle occipital gyrus; IFGpo/AI = inferior frontal gyrus pars opercularis/anterior insula; IPL = inferior parietal lobule; ACC/MCC = anterior/middle cingulate cortex; MTG = middle temporal gyrus; OG = occipital gyrus; SFG/MFG = superior/middle frontal gyrus; pre‐SMA = presupplementary motor area. ^ = peak voxel p value. L = Left; R = Right; pos. = positive correlation; neg. = negative correlation.
To address the issue whether the age‐related change (in Figure 3b) may reflect higher activation to GS but not SS, we performed age correlation each on SS and GS beta weights for each region. The results showed that SS beta weights were correlated negatively with age for all regions (R IFGpo/AI: r = −.2945, p = .0003; L IPL: r = −.1880, p = .0217; R IPL: r = −.2568, p = .0016; MCC: r = −.2956, p = .0003), while GS beta weights were not correlated with age for any regions (R IFGpo/AI: r = −.0972, p = .2385; L IPL: r = .0265, p = .7481; R IPL: r = −.0706, p = .3921; MCC: r = −.1351, p = .1004).
3.4. Relationship between age‐related differences in GMV and BOLD contrast
We also identified brain regions showing both a reduction in GMV and BOLD activation of SS > GS during aging. In voxel‐wise linear regression of SS > GS against age, we examined the results with inclusive masking of the brain regions where the GMV's were significantly reduced with age. The results showed that the right IFGpo/AI (x = 42, y = 8, z = −8, p = .001 FWE, Z = 5.18, k = 599) and a cluster encompassing the MPFC (x = 9, y = 5, z = 61, p = .008 FWE, Z = 4.58, k = 725) including the pre‐SMA, rostral ACC and right SFG and LPFC, but not other brain regions, showed age‐related differences in both GMV and BOLD activations (Figure 4a). To test for the structure–function relationship, a mediation analysis showed that the GMV of the MPFC but not the right IFGpo/AI mediate the correlation between age and BOLD contrast (Figure 4b).
Figure 4.
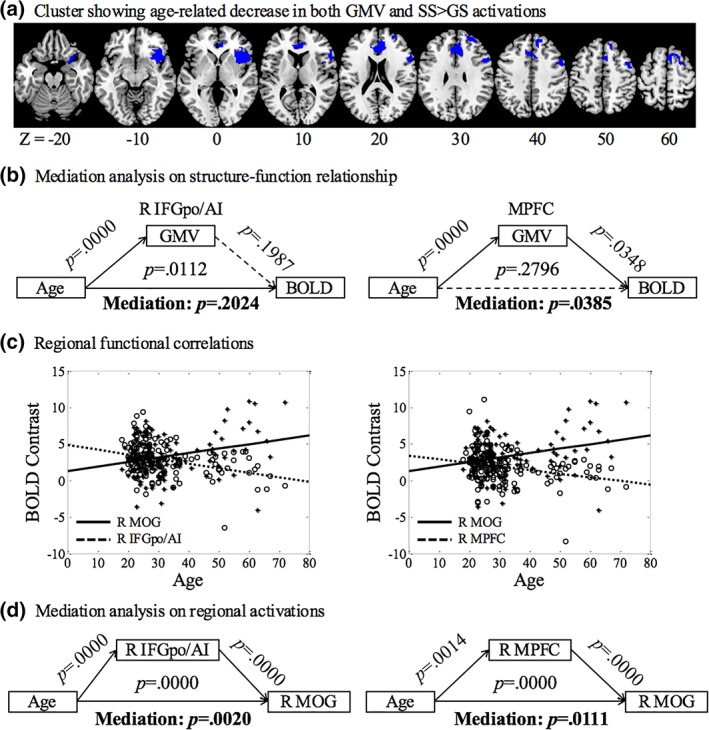
(a) Brain regions showing age‐related decreased GMV and activation in SS > GS; (b) mediation analysis showed that GMV reduction in the medial prefrontal cortex (MPFC) is a complete mediator of age‐related decrease in activation during inhibitory control; (c) linear regression of BOLD contrast against age for the right IFGpo/AI, MPFC and MOG to highlight the contrasting patterns of age‐related change; and (d) mediation analyses showing that the activities both of the right IFGpo/AI and MPFC partially mediated age‐related increases in activation of the MOG [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. Relationship in regional activations during aging
Most brain regions showed age‐related decreases in activation. At the same threshold, a cluster in the right MOG demonstrated age‐related increases in activation (Figure 3b), potentially reflecting a compensatory process during aging. Following the literature (Brassen et al., 2009; Kalpouzos et al., 2012; Rajah, Languay, & Valiquette, 2010; Steffener, Gazes, Habeck, & Stern, 2016), we examined whether the GMV and activations to inhibitory control were correlated in the MOG and whether decreased activations in other brain regions may relate to greater activations of the MOG during inhibitory control. Linear regressions showed that GMV and BOLD contrast of the MOG were not significantly correlated across subjects (r = .0046, p = .9558). However, the BOLD contrasts of the right IFGpo/AI (r = .2202, p = .0070) and MPFC (r = .2334, p = .0042) were negatively correlated with the BOLD contrast of the right MOG. Figure 4c highlighted the contrasting patterns of age‐related differences in BOLD activation for the right IFGpo/AI, MPFC, and right MOG. Further, a mediation analysis showed that activations of the right IFGpo/AI and MPFC both partially mediated age‐related greater activation in the right MOG (Figure 4d).
4. DISCUSSIONS
Age was positively correlated with go trial reaction time (RT) and stop signal reaction time (SSRT), suggesting diminished capacity in response execution and inhibition during aging. In voxel‐based morphometry, the gray matter volume (GMV) showed age‐related reduction in lateral and medial prefrontal, cingulate and parietal cortices as well as the striatum and insula. In linear regression with the SSRT, bilateral insula, right inferior and middle frontal gyri (IFG/MFG), and caudate nucleus showed reduction in GMV in correlation with prolonged SSRT. A linear regression of the fMRI contrast of stop success versus go success each against age and SSRT showed decreased activations in brain regions that overlapped in the right inferior frontal gyrus, pars opercularis and anterior insula (IFGpo/AI), medial PFC (MPFC), including the presupplementary motor area (pre‐SMA), and bilateral inferior parietal lobule (IPL), regions that have previously been implicated in response inhibition (Cai et al., 2016; Duann, Ide, Luo, & Li, 2009; Sharp et al., 2010). Both GMV's and BOLD contrasts of the IFGpo/AI and MPFC mediated age‐related differences in SSRT. Further, reduction in GMV mediated age‐related reduction in activation during response inhibition in the MPFC. We highlighted these main findings in the discussion.
4.1. Age‐related decrease in activation to inhibitory control
The results showed age‐related decreases in activation during inhibitory control (stop success > go success) in a network of brain regions including the pre‐SMA, right IFGpo/AI, and bilateral IPL, consistent with previous research of the SST (Bloemendaal et al., 2016; Coxon et al., 2016; Sebastian et al., 2013). For instance, Coxon et al. (2016) showed age‐related decreases in activation of the preSMA, caudate, putamen, right cingulate cortex during inhibitory control in a modified SST involving bimanual response. In particular, the right IFGpo/AI activation was only present in young but not older adults. However, some studies have reported age‐related increases in activations in fronto‐striatal regions during inhibitory control (Hong, Sun, Bengson, & Tong, 2014; Langenecker & Nielson, 2003; Vallesi, McIntosh, & Stuss, 2011), mostly in the go/no‐go task. Sebastian et al. (2013) examined age‐related differences in go/no‐go, Simon, and stop signal tasks, and reported age‐related increases in activation in the go/no‐go and Simon tasks but decreased activation in the SST, especially in the IFGpo/AI and pre‐SMA. Importantly, inhibitory control as indexed by commission errors was not correlated with age in go/no‐go or Simon task; in contrast, SSRT was correlated positively with age. The authors suggested that the SST was the most challenging among the three tasks because participants needed to cancel an ongoing motor response. Thus, age‐related over‐activation in the other two tasks may reflect a compensatory process to support behavioral performance. The current results thus accord with previous work, in support of an inhibition process that is more demanding and less amenable to functional compensation in the SST during aging.
We contrasted successful stop and go trials, in that this contrast captures the saliency process evoked by the stop signal that is necessary to elicit the inhibitory process (Cai et al., 2016; Duann et al., 2009; Hu, Zhang, Chao, Krystal, & Li, 2016). Two SST studies reported age‐related increases in activations in the motor and frontal regions when inhibitory control was examined by contrasting successful and unsuccessful stop trials (Bloemendaal et al., 2016; Kleerekooper et al., 2016). However, these age‐related differences in activations were not correlated with behavioral performance. In fact, in the work of Bloemendaal et al. (2016), the authors also reported age‐related decreased activation in the fronto‐striatal regions with a contrast of successful stop and go trials. These considerations suggest the importance of carefully evaluating the various fMRI contrasts that target seemingly the same psychological construct in imaging studies.
4.2. Gray matter volumetric reduction in association with aging and reduced inhibitory control
Age‐related cerebral atrophy occurs predominantly in the prefrontal cortex, subcortical, and medial temporal areas, in link with age‐related decline in cognitive, motor, and memory functions (Fjell, McEvoy, Holland, Dale, & Walhovd, 2014; Hoffstaedter et al., 2015; Raz et al., 2005). The current patterns of age‐related reduction in cerebral GMV mirrored this literature. In relation to behavioral performance, GMVs of bilateral insula and right MFG decreased both with age and in association with prolonged SSRT, in accord with previous research linking GMV of the frontal/insular system to age‐related cognitive decline (Muller, Merillat, & Jancke, 2016). An earlier study of young adults reported better performance in interference control in a flanker's task in those with greater GMV in the right MFG and bilateral insula (Liu et al., 2015), suggesting that the contribution of PFC and insula GMV to cognitive performance may not be limited to older adults.
Reduction in GMV of the right caudate head also mediated age‐related impairment in inhibitory control. Interconnected with prefrontal cortical structures, the caudate nucleus has been implicated in a wide range of cognitive functions (Haber, 2016; Zhang, Ide, & Li, 2012). Studies have also reported volumetric changes of the caudate in neurological and psychiatric conditions, including mild cognitive impairment (Moretti, Paternico, Binetti, Zanetti, & Frisoni, 2012) and Alzheimer's disease (Jiji, Smitha, Gupta, Pillai, & Jayasree, 2013; Yang, Pan, Song, & Shang, 2012), but the literature of healthy aging is sparse. An earlier study reported volumetric reduction of the right caudate in relation to association memory deficits in older healthy adults (Bauer, Toepper, Gebhardt, Gallhofer, & Sammer, 2015). The current findings add to this literature by demonstrating an additional role of caudate GMV in inhibitory control.
4.3. Structural and functional changes in the aging brain
The relationship between structural and functional changes is complex. GMV decrement can result from loss of neurons, synaptic connections, glia, and cerebrospinal fluid (Fjell & Walhovd, 2010), which may not all be reflected in functional changes during aging. Nonetheless, without considering behavioral performance in the SST, age‐dependent differences in activation to inhibitory control appeared to be largely confined to structures that demonstrated reduction in GMV, paralleling recent findings in structural and functional connectivity (Zimmermann et al., 2016). We identified brain regions where GMV and BOLD activations were correlated with age‐related differences in inhibitory control. Mediation analyses showed that the GMV completely mediated age‐related differences in BOLD activations in the medial PFC (Figure 4b), highlighting a potential case of structural determinant of functional activity. On the other hand, age‐related gray matter shrinkage is not necessarily linear (Fjell et al., 2014; Fotenos, Snyder, Girton, Morris, & Buckner, 2005; Terribilli et al., 2011), and Pearson regression may not capture nonlinear relationships between age‐related differences in structure and function. Further, individual brain regions partake in multiple cognitive functions (Park & Friston, 2013; Sala‐Llonch, Bartres‐Faz, & Junque, 2015), and one cannot rule out the possibility that the brain regions not showing a structure–function correlation in inhibitory control may demonstrate such a relationship in other capacities.
Earlier studies have examined the structure–function relationship in the aging brain. Both dorsomedial PFC (DMPFC) and dorsolateral PFC (DLPFC) showed age‐related reduction in GMV and increases in BOLD activations in participants performing a working memory task (Kalpouzos et al., 2012). GMV reduction was correlated with over‐activation in the DLPFC but not DMPFC as a compensatory process to support performance. In face recognition, older adults showed poorer behavioral performance and a correlation between lower GMV of the right middle frontal gyrus (MFG) and increased parahippocampal activation (Rajah et al., 2010). In another study, GMV was associated with PFC activation to face recognition across young and old participants; however, in older adults only, lower PFC GMV was associated with increased activation in the medial temporal lobe (Brassen et al., 2009). Steffener and colleagues demonstrated decreased GMV and increased activations in the frontal, parietal, and cerebellum areas along with higher switch cost (indicative of deficits) during task switching in the elderly (Steffener et al., 2016). In a review of multiple behavioral tasks, Di et al. (2014) reported a relationship between decreased GMV and increased activation in the DLPFC. Together, reduction of frontal GMV seems accompanied predominantly by increases in activation, whether in the same frontal or other regions, as a mechanism of functional compensation. Notably, the abovementioned findings were obtained largely from behavioral tasks that demand working memory, in contrast to the SST. Here, we showed greater right MOG activation to response inhibition, along with age‐related decreases in GMV and activation of a wide swath of cortical regions, again in contrast with studies that involve working memory (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Grady, 2012; Li et al., 2015; Nyberg et al., 2010). Combined studies of the SST and a working memory task would confirm the influence of behavioral paradigm on the structural and functional brain changes during aging.
4.4. Limitations of the study and conclusions
There are a few limitations to consider. First, the current study was based on a convenience sample with only 18 participants over 50 years of age. Second, participants were engaged in a single behavioral task, and age‐related differences in structure and function were addressed only in relation to response inhibition in the SST. Thus, the current findings need to be replicated with a larger sample of older adults and extended with more behavioral tests. Third, age‐dependent differences in regional volume and activation may not be linear, and studies are warranted to investigate nonlinear, including quadratic, models of change (Narvacan, Treit, Camicioli, Martin, & Beaulieu, 2017). Finally, we described shared and distinct functional and structural correlates solely in terms of whether the voxels overlapped in GMV and BOLD results. It is possible that changes in local structure may affect the functions of nonoverlapping regions, an issue that needs to be investigated further with structural and functional connectivity analyses. In conclusion, aging‐related deterioration in inhibitory control, as evaluated by the stop signal reaction time, is associated with distinct changes in regional morphometry and activations to inhibitory control. Although the reduction of many brain regions in GMV and BOLD contrasts of stop success versus go success mediated prolonged inhibition time in the stop signal task, these structural and functional correlates did not appear to overlap. Further, among many brain regions showing volume and activation changes, the medial prefrontal cortex represents the only structure where gray matter volume mediated age‐related differences in activation to inhibitory control. Together, these findings suggest largely independent age‐related differences in cerebral structure and function, perhaps as a result of neural plasticity to accommodate cognitive challenges.
CONFLICTS OF INTEREST
We have disclosed all research support and do not have conflicts of interest in the current work.
ACKNOWLEDGMENTS
This study is supported by National Institutes of Health grants DA023248, AA021449, and MH113134, as well as a VA Merit Award CX001301. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the VA.
Hu S, Ide JS, Chao HH, et al. Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum Brain Mapp. 2018;39:5085–5096. 10.1002/hbm.24347
Funding information National Institute of Mental Health, Grant/Award Number: MH113134; National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: AA021449; National Institute on Drug Abuse, Grant/Award Number: DA023248; Department of Veterans Affairs, Grant/Award Number: CX001301
Contributor Information
Sien Hu, Email: sien.hu@oswego.edu.
Chiang‐shan R. Li, Email: chiang-shan.li@yale.edu.
REFERENCES
- Ashburner, J. , & Friston, K. J. (1999). Nonlinear spatial normalization using basis functions. Human Brain Mapping, 7, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, E. , Toepper, M. , Gebhardt, H. , Gallhofer, B. , & Sammer, G. (2015). The significance of caudate volume for age‐related associative memory decline. Brain Research, 1622, 137–148. [DOI] [PubMed] [Google Scholar]
- Bergfield, K. L. , Hanson, K. D. , Chen, K. , Teipel, S. J. , Hampel, H. , Rapoport, S. I. , … Alexander, G. E. (2010). Age‐related networks of regional covariance in MRI gray matter: Reproducible multivariate patterns in healthy aging. NeuroImage, 49, 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendaal, M. , Zandbelt, B. , Wegman, J. , van de Rest, O. , Cools, R. , & Aarts, E. (2016). Contrasting neural effects of aging on proactive and reactive response inhibition. Neurobiology of Aging, 46, 96–106. [DOI] [PubMed] [Google Scholar]
- Bourisly, A. K. , El‐Beltagi, A. , Cherian, J. , Gejo, G. , Al‐Jazzaf, A. , & Ismail, M. (2015). A voxel‐based morphometric magnetic resonance imaging study of the brain detects age‐related gray matter volume changes in healthy subjects of 21‐45 years old. The Neuroradiology Journal, 28, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen, S. , Buchel, C. , Weber‐Fahr, W. , Lehmbeck, J. T. , Sommer, T. , & Braus, D. F. (2009). Structure‐function interactions of correct retrieval in healthy elderly women. Neurobiology of Aging, 30, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Cai, W. , Chen, T. , Ryali, S. , Kochalka, J. , Li, C. S. , & Menon, V. (2016). Causal interactions within a frontal‐cingulate‐parietal network during cognitive control: Convergent evidence from a multisite‐multitask investigation. Cerebral Cortex, 26, 2140–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon, J. P. , Goble, D. J. , Leunissen, I. , Van Impe, A. , Wenderoth, N. , & Swinnen, S. P. (2016). Functional brain activation associated with inhibitory control deficits in older adults. Cerebral Cortex, 26, 12–22. [DOI] [PubMed] [Google Scholar]
- Davis, S. W. , Dennis, N. A. , Daselaar, S. M. , Fleck, M. S. , & Cabeza, R. (2008). Que PASA? The posterior‐anterior shift in aging. Cerebral Cortex, 18, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della‐Maggiore, V. , Chan, W. , Peres‐Neto, P. R. , & McIntosh, A. R. (2002). An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. NeuroImage, 17, 19–28. [DOI] [PubMed] [Google Scholar]
- Dennis, N. A. , Daselaar, S. , & Cabeza, R. (2007). Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging, 28, 1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , Rypma, B. , & Biswal, B. B. (2014). Correspondence of executive function related functional and anatomical alterations in aging brain. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 48, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann, J. R. , Ide, J. S. , Luo, X. , & Li, C. R. (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and Presupplementary motor area in stop signal inhibition. The Journal of Neuroscience, 29, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin‐Thompson, V. , Ballmaier, M. , Hellemann, G. , Pham, D. , & Kumar, A. (2008). Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology, 22, 626–637. [DOI] [PubMed] [Google Scholar]
- Fjell, A. M. , McEvoy, L. , Holland, D. , Dale, A. M. , & Walhovd, K. B. (2014). What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Progress in Neurobiology, 117, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , & Walhovd, K. B. (2010). Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences, 21, 187–221. [DOI] [PubMed] [Google Scholar]
- Fjell, A. M. , Walhovd, K. B. , Reinvang, I. , Lundervold, A. , Salat, D. , Quinn, B. T. , … Dale, A. M. (2006). Selective increase of cortical thickness in high‐performing elderly‐‐structural indices of optimal cognitive aging. NeuroImage, 29, 984–994. [DOI] [PubMed] [Google Scholar]
- Fleischman, D. A. , Leurgans, S. , Arfanakis, K. , Arvanitakis, Z. , Barnes, L. L. , Boyle, P. A. , … Bennett, D. A. (2014). Gray‐matter macrostructure in cognitively healthy older persons: Associations with age and cognition. Brain Structure & Function, 219, 2029–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos, A. F. , Snyder, A. Z. , Girton, L. E. , Morris, J. C. , & Buckner, R. L. (2005). Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology, 64, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Friston, K. , Holmes, A. P. , Worsley, K. J. , Poline, J. B. , Frith, C. D. , & Frackowiak, R. (1995). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2, 189–210. [Google Scholar]
- Friston, K. J. , Ashburner, J. , Frith, C. D. , Poline, J. B. , Heather, J. D. , & Frackowiak, R. S. J. (1995). Spatial registration and normalization of images. Human Brain Mapping, 3, 165–189. [Google Scholar]
- Friston, K. J. , Josephs, O. , Zarahn, E. , Holmes, A. P. , Rouquette, S. , & Poline, J. B. (2000). To smooth or not to smooth? Bias and efficiency in fMRI time‐series analysis. NeuroImage, 12, 196–208. [DOI] [PubMed] [Google Scholar]
- Grady, C. (2012). The cognitive neuroscience of ageing. Nature Reviews. Neuroscience, 13, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon, F. M. , & Raz, N. (2003). Neuroanatomical correlates of selected executive functions in middle‐aged and older adults: A prospective MRI study. Neuropsychologia, 41, 1929–1941. [DOI] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun, J. G. , Zhu, Z. , Brown, C. A. , Johnson, N. F. , & Gold, B. T. (2015). Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI‐DTI study. Neuropsychologia, 71, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter, F. , Grefkes, C. , Roski, C. , Caspers, S. , Zilles, K. , & Eickhoff, S. B. (2015). Age‐related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Structure & Function, 220, 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, X. , Sun, J. , Bengson, J. J. , & Tong, S. (2014). Age‐related spatiotemporal reorganization during response inhibition. International Journal of Psychophysiology, 93, 371–380. [DOI] [PubMed] [Google Scholar]
- Hsieh, S. , & Lin, Y. C. (2017). Strategies for stimulus selective stopping in the elderly. Acta Psychologica, 173, 122–131. [DOI] [PubMed] [Google Scholar]
- Hu, S. , Chao, H. H. , Winkler, A. D. , & Li, C. S. (2012). The effects of age on cerebral activations: Internally versus externally driven processes. Frontiers in Aging Neuroscience, 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Ide, J. S. , Zhang, S. , & Li, C. R. (2016). The right superior frontal gyrus and individual variation in proactive control of impulsive response. The Journal of Neuroscience, 36, 12688–12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , & Li, C. S. R. (2012). Neural processes of preparatory control for stop signal inhibition. Human Brain Mapping, 33, 2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Tseng, Y. C. , Winkler, A. D. , & Li, C. S. (2014). Neural bases of individual variation in decision time. Human Brain Mapping, 35, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Zhang, S. , Chao, H. H. , Krystal, J. H. , & Li, C. S. (2016). Association of Drinking Problems and Duration of alcohol use to inhibitory control in nondependent young adult social drinkers. Alcoholism, Clinical and Experimental Research, 40, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, J. S. , & Li, C. S. (2011). Error‐related functional connectivity of the habenula in humans. Frontiers in Human Neuroscience, 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiji, S. , Smitha, K. A. , Gupta, A. K. , Pillai, V. P. , & Jayasree, R. S. (2013). Segmentation and volumetric analysis of the caudate nucleus in Alzheimer's disease. European Journal of Radiology, 82, 1525–1530. [DOI] [PubMed] [Google Scholar]
- Kalpouzos, G. , Persson, J. , & Nyberg, L. (2012). Local brain atrophy accounts for functional activity differences in normal aging. Neurobiology of Aging, 33, 623 e1–623 e13. [DOI] [PubMed] [Google Scholar]
- Kaup, A. R. , Mirzakhanian, H. , Jeste, D. V. , & Eyler, L. T. (2011). A review of the brain structure correlates of successful cognitive aging. The Journal of Neuropsychiatry and Clinical Neurosciences, 23, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerekooper, I. , van Rooij, S. J. H. , van den Wildenberg, W. P. M. , de Leeuw, M. , Kahn, R. S. , & Vink, M. (2016). The effect of aging on fronto‐striatal reactive and proactive inhibitory control. NeuroImage, 132, 51–58. [DOI] [PubMed] [Google Scholar]
- Kramer, J. H. , Mungas, D. , Reed, B. R. , Wetzel, M. E. , Burnett, M. M. , Miller, B. L. , … Chui, H. C. (2007). Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology, 21, 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker, S. A. , & Nielson, K. A. (2003). Frontal recruitment during response inhibition in older adults replicated with fMRI. NeuroImage, 20, 1384–1392. [DOI] [PubMed] [Google Scholar]
- Levin, O. , Fujiyama, H. , Boisgontier, M. P. , Swinnen, S. P. , & Summers, J. J. (2014). Aging and motor inhibition: A converging perspective provided by brain stimulation and imaging approaches. Neuroscience and Biobehavioral Reviews, 43, 100–117. [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). Transformed up‐down methods in psychoacoustics. The Journal of the Acoustical Society of America, 49(Suppl 2), 467. [PubMed] [Google Scholar]
- Li, H. J. , Hou, X. H. , Liu, H. H. , Yue, C. L. , Lu, G. M. , & Zuo, X. N. (2015). Putting age‐related task activation into large‐scale brain networks: A meta‐analysis of 114 fMRI studies on healthy aging. Neuroscience and Biobehavioral Reviews, 57, 156–174. [DOI] [PubMed] [Google Scholar]
- Liebherr, M. , Schiebener, J. , Averbeck, H. , & Brand, M. (2017). Decision making under ambiguity and objective risk in higher age ‐ a review on cognitive and emotional contributions. Frontiers in Psychology, 8, 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. S. , Lemieux, L. , Bell, G. S. , Sisodiya, S. M. , Shorvon, S. D. , Sander, J. W. , & Duncan, J. S. (2003). A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. NeuroImage, 20, 22–33. [DOI] [PubMed] [Google Scholar]
- Liu, W. J. , Yin, D. Z. , Cheng, W. H. , Fan, M. X. , You, M. N. , Men, W. W. , … Zhang, F. (2015). Abnormal functional connectivity of the amygdala‐based network in resting‐state FMRI in adolescents with generalized anxiety disorder. Medical Science Monitor, 21, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, G. D. , Cowan, W. B. , & Davis, K. A. (1984). On the ability to inhibit simple and choice reaction‐time responses ‐ a model and a method. J Exp Psychol Human, 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Lustig, C. , & Jantz, T. (2015). Questions of age differences in interference control: When and how, not if? Brain Research, 1612, 59–69. [DOI] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Fairchild, A. J. , & Fritz, M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet, D. , & Rajah, M. N. (2013). Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Research Reviews, 12, 479–489. [DOI] [PubMed] [Google Scholar]
- Moretti, D. V. , Paternico, D. , Binetti, G. , Zanetti, O. , & Frisoni, G. B. (2012). Analysis of grey matter in thalamus and basal ganglia based on EEG alpha3/alpha2 frequency ratio reveals specific changes in subjects with mild cognitive impairment. ASN Neuro, 4, e00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, A. M. , Merillat, S. , & Jancke, L. (2016). Small changes, but huge impact? The right anterior Insula's loss of connection strength during the transition of old to very old age. Frontiers in Aging Neuroscience, 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvacan, K. , Treit, S. , Camicioli, R. , Martin, W. , & Beaulieu, C. (2017). Evolution of deep gray matter volume across the human lifespan. Human Brain Mapping, 38, 3771–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, L. , Salami, A. , Andersson, M. , Eriksson, J. , Kalpouzos, G. , Kauppi, K. , … Nilsson, L. G. (2010). Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences of the United States of America, 107, 22682–22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan, C. , Hodges, J. R. , & Hornberger, M. (2013). Inhibitory dysfunction in frontotemporal dementia: A review. Alzheimer Disease and Associated Disorders, 27, 102–108. [DOI] [PubMed] [Google Scholar]
- Pagnoni, G. , & Cekic, M. (2007). Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging, 28, 1623–1627. [DOI] [PubMed] [Google Scholar]
- Park, H. J. , & Friston, K. (2013). Structural and functional brain networks: From connections to cognition. Science, 342, 1238411. [DOI] [PubMed] [Google Scholar]
- Rajah, M. N. , Languay, R. , & Valiquette, L. (2010). Age‐related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex, 46, 535–549. [DOI] [PubMed] [Google Scholar]
- Raz, N. , Lindenberger, U. , Rodrigue, K. M. , Kennedy, K. M. , Head, D. , Williamson, A. , … Acker, J. D. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15, 1676–1689. [DOI] [PubMed] [Google Scholar]
- Sakai, H. , Takahara, M. , Honjo, N. F. , Doi, S. , Sadato, N. , & Uchiyama, Y. (2012). Regional frontal gray matter volume associated with executive function capacity as a risk factor for vehicle crashes in normal aging adults. PLoS One, 7, e45920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala‐Llonch, R. , Bartres‐Faz, D. , & Junque, C. (2015). Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology, 6, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse, T. A. (1996). The processing‐speed theory of adult age differences in cognition. Psychological Review, 103, 403–428. [DOI] [PubMed] [Google Scholar]
- Scahill, R. I. , Frost, C. , Jenkins, R. , Whitwell, J. L. , Rossor, M. N. , & Fox, N. C. (2003). A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of Neurology, 60, 989–994. [DOI] [PubMed] [Google Scholar]
- Sebastian, A. , Baldermann, C. , Feige, B. , Katzev, M. , Scheller, E. , Hellwig, B. , … Kloppel, S. (2013). Differential effects of age on subcomponents of response inhibition. Neurobiology of Aging, 34, 2183–2193. [DOI] [PubMed] [Google Scholar]
- Sharp, D. J. , Bonnelle, V. , De Boissezon, X. , Beckmann, C. F. , James, S. G. , Patel, M. C. , & Mehta, M. A. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America, 107, 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff, R. T. , Murray, A. D. , Deary, I. J. , & Whalley, L. J. (2006). Generality and specificity in cognitive aging: A volumetric brain analysis. NeuroImage, 30, 1433–1440. [DOI] [PubMed] [Google Scholar]
- Steffener, J. , Gazes, Y. , Habeck, C. , & Stern, Y. (2016). The indirect effect of age group on switch costs via gray matter volume and task‐related brain activity. Frontiers in Aging Neuroscience, 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terribilli, D. , Schaufelberger, M. S. , Duran, F. L. , Zanetti, M. V. , Curiati, P. K. , Menezes, P. R. , … Busatto, G. F. (2011). Age‐related gray matter volume changes in the brain during non‐elderly adulthood. Neurobiology of Aging, 32, 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, L. K. , Shafto, M. A. , Randall, B. , Wright, P. , Marslen‐Wilson, W. D. , & Stamatakis, E. A. (2010). Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age‐related atrophy. Cerebral Cortex, 20, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi, A. , McIntosh, A. R. , & Stuss, D. T. (2011). Overrecruitment in the aging brain as a function of task demands: Evidence for a compensatory view. Journal of Cognitive Neuroscience, 23, 801–815. [DOI] [PubMed] [Google Scholar]
- Wetherill, G. B. , Chen, H. , & Vasudeva, R. B. (1966). Sequential estimation of quantal response curves: A new method of estimation. Biometrika, 53, 439–454. [Google Scholar]
- Yang, J. , Pan, P. , Song, W. , & Shang, H. F. (2012). Quantitative meta‐analysis of gray matter abnormalities in semantic dementia. Journal of Alzheimer's Disease, 31, 827–833. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Ide, J. S. , & Li, C. S. (2012). Resting‐state functional connectivity of the medial superior frontal cortex. Cerebral Cortex, 22, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, J. , Ritter, P. , Shen, K. , Rothmeier, S. , Schirner, M. , & McIntosh, A. R. (2016). Structural architecture supports functional organization in the human aging brain at a regionwise and network level. Human Brain Mapping, 37, 2645–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]


