Introduction
Multiple sclerosis (MS) is an autoimmune-inflammatory neurodegenerative disease of the central nervous system (CNS) characterized by acute inflammation, demyelination and neuronal loss and associated with severe disruption of brain, spinal cord and oculomotor function [66]. Neuropathic pain is one of the most frequent and debilitating symptoms of MS. It is reported by over 50% of the MS population, and contributes to an overwhelming decrease in quality of life [12; 26; 29; 66]. Despite this, neither have determined the underlying mechanisms of, or yielded effective treatments for, neuropathic pain in MS [43; 87], although clinical and basic science studies have offered promising leads [6; 37; 50; 51; 55; 72; 78; 82; 86]. Cross-sectional and longitudinal data suggest that MS-associated chronic pain is refractory to older-generation disease modifying therapies (DMT) [27]. However, whether new-generation DMTs such as fingolimod are efficacious for neuropathic pain of MS is unknown.
Fingolimod (FTY720; 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol) is an FDA-approved immunosuppressive therapy for patients with MS, having shown clinical efficacy for relapsing or relapsing-remitting MS [17; 48]. In vivo, fingolimod is phosphorylated by sphingosine kinase 2 (SPHK2) to its active form fingolimod-phosphate [8; 31], a potent ligand at four of the five known S1P receptor subtypes (S1PR1, S1PR3, S1PR4, S1PR5) [9]. Like other S1PR agonists, fingolimod can regulate cellular function through high-affinity receptor binding and then activation of G-protein-coupled receptors [98], including persistent signaling from internalized S1PR1 [63]. In lymphoid organs, fingolimod has an additional mechanism of functional antagonism at S1PR1. This involves irreversible internalization after receptor activation followed by ubiquitination and subsequent degradation; this can occur within minutes and persist for days [38; 58; 69]. S1PR1 is required for chemotactic egress of lymphocytes from lymphoid organs, thus leading to peripheral lymphopenia [58], and so fingolimod-phosphate-mediated actions at S1PR1 reduces T-cell numbers in the blood. In MS, reductions in the migration of an autoreactive subset of lymphocyte into the CNS are thought to prevent attacks of the myelin sheath and thus reduce the neuromuscular deficits of MS [9; 16].
Repeated administration of fingolimod or fingolimod-phosphate reduces behavioral signs of acute and persistent pain in multiple animal models of peripheral inflammation or peripheral nerve injury, including intraplantar formalin [19; 20] or carrageenan [28], traumatic nerve injury [20; 97], or the paclitaxel-induced model of chemotherapy-induced neuropathic pain [44]. Its mechanisms of analgesic action, however, is complex and appears to involve both antagonism of pronociceptive S1PR1 signaling in the periphery [28; 44] as well as direct agonism of antinociceptive S1PR1 signaling in the spinal cord [19; 97]. Despite the fact that fingolimod produces antinociceptive effects in multiple models of peripheral inflammation or injury [19; 20; 97], its efficacy in central neuropathic pain in an MS model has not been studied. To address this gap, we assessed the effect of fingolimod on neuropathic pain and three indices of spinal physiological and cellular activity in an experimental autoimmune encephalomyelitis (EAE) mouse model.
MATERIALS AND METHODS
Animals.
A total of 144 female C57BL/6 mice were used in this study: 106 EAE mice and 5 sham at 7–9 weeks of age for behavioral and immunohistochemistry studies; and 24 EAE mice and 9 sham at 25–28 d of age for calcium imaging studies were purchased from Charles River Laboratories (Indianapolis, USA). Mice were housed four to a cage and had access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky in accordance with American Veterinary Medical Association guidelines. Mice were maintained in a temperature (68–72° F) and humidity (30 – 70 %) controlled environment on a 14:10 h light/dark cycle (Lights on: 6:00 AM, Lights off: 8:00 PM). Animals were allowed several days to habituate to the facility prior to the beginning of the study.
EAE induction.
Typical MOG35–55 models of EAE were designed to generate autoimmune-mediated motor impairment, and this requires adjuvant injection of complete Freund’s adjuvant (CFA) to enhance neuroinflammatory effects [42; 70] and pertussis toxin to increase circulating peripheral lymphocyte proliferation [84]. However, the severity of these models can compromise behavioral assessment of limb withdrawal and thus preclude measurement of hyperalgesia. To overcome this confound, Khan et al replaced CFA with Quil A and reduced pertussis toxin concentration [52] while others have reduced MOG concentration so as to induce submaximal EAE [37; 86]. Such milder forms of MOG33–55-induced EAE may be more representative of the relapsing-remitting form of multiple sclerosis [7; 37] – the form that is FDA approved for treatment with fingolimod. Similarly, we optimized our model with reductions in the doses of MOG35–55, CFA and pertussis toxin [32; 71], thus generating an EAE model with a robust pain phenotype and less severe motor paralysis, allowing assessment of withdrawal behaviors to somatosensory stimuli.
For the behavior and immunohistochemistry studies, we induced EAE at 6 weeks of age. For the Ca2+ imaging studies, we induced EAE at a younger age (25 d) so as to optimize detection of Ca2+ responses. We induced EAE with emulsified myelin oligodendrocyte glycoprotein 35–55 (MOG35–55, AnaSpec Inc, Fremont, CA) using a modified methodology from Fu and Taylor [32] and Rahn et al [71]. MOG35–55 was emulsified in a 1:1 solution of 1x phosphate buffered saline (Fisher Scientific, USA; PBS) and CFA. The emulsion was generated by pushing the 1:1 mixture back and forth between two glass syringes 20 times through an 18 gauge micro-emulsifying needle (Cole-Palmer, IL, USA). CFA was prepared at a concentration of 3 mg/ml of mycobacterium tuberculosis (Voigt Global Distribution, Lawrence, KS) in incomplete Freund’s adjuvant (IFA, Sigma Aldrich, St Louis, MO). On the afternoon following initial behavioral testing (D0), MOG35–55 (75 µg, s.c.) was bilaterally injected (100 µl) at the flank of each hindlimb. A booster injection of MOG35–55 (75 µg per flank; 100 µl) was administered on D6. Mice received MOG35–55 injections under light isoflurane (Butler Schein, USA) anesthesia. Pertussis toxin (List Biological Laboratories, Campbell, CA) was injected (200 ng/200 µl, i.p.) on D0 and D2. Age-matched mice (sham control groups) received identical CFA and pertussis toxin treatment. Following the first MOG35–55 injections at D0, animals were given access to DietGel® 76A (Clear H2O®, Portland, OR), which was replaced approximately every 4 d. As observed previously with this dosing regimen [71], no animals developed full paralysis of either limb over the 56 days of observation following EAE induction, and thus all animals could be tested for withdrawal behaviors to somatosensory stimuli.
Behavioral testing.
All behavioral measurements including motor scoring and hindlimb withdrawal were performed by a male (TI, Experiments 1–3, 7) or female investigator (SD, Experiment 8), blinded to group assignments. Mice were acclimated for 30 min/d to individual Plexiglas (4 × 4 × 10-inches) chambers placed upon an elevated stainless steel wire mesh for tests of cold and mechanical sensitivity. Mice were acclimated for 2 d prior to baseline behavioral measurement, and for 30 min on each testing day. Mechanical sensitivity was evaluated first, followed by cold sensitivity, and then neurological motor function. Baseline testing was conducted prior to and after EAE induction, and at various time points after drug injection (as indicated in the figures).
Neurological motor function.
Mice were monitored daily for signs of neurological motor deficits according to the following scale: Grade 0, absence of clinical deficits; Grade 1, hanging tail or impaired righting; Grade 2, mild paresis of one hind limb; Grade 3, paresis (weakness) of two hind limbs; Grade 4, full paralysis of one or two hind limbs/moribund; Grade 5, death [47; 71]. We never observed scores of 4 or 5 in this study, thus allowing the monitoring of mechanical and cold sensitivity in all animals.
Mechanical threshold.
Mice were acclimated for 30 – 60 min in the testing environment within a plastic box (4 × 4 × 10-inches; 3 white opaque walls and 1 clear wall) on a raised metal mesh platform. To evaluate mechanical threshold we used a logarithmically increasing set of 8 von Frey filaments (Stoelting, Illinois), ranging in gram force from 0.007 to 6.0 g. These were applied perpendicular to the ventral-medial hindpaw surface with sufficient force to cause a slight bending of the filament. A positive response was characterized as a rapid withdrawal of the paw away from the stimulus fiber within 4 s. Using the up-down statistical method [14], the 50% withdrawal threshold was calculated for each mouse and then averaged across the experimental groups.
Cold response.
Cold sensitivity was assessed using acetone drops applied to the plantar surface of the hind paw as previously described [71]. Acetone was applied to the center of the plantar surface of the hindpaw using a syringe connected to PE-90 tubing applicator, flared at the tip to a diameter of 3.5 mm. Surface tension maintained the volume of the drop at 10–12 µl on top of the applicator. Under visual guidance, the applicator was gently raised to apply the tip of the drop to the skin, with care to avoid direct contact between the skin and applicator. Acetone is extremely volatile, and quickly evaporates, lowering the skin temperature to approximately 6 °C. The duration of time the animal lifted, shook or licked its paw was recorded. Animals were observed for 60 s following each application of acetone. Three trials, with an interval of at least 1 min between each, were averaged.
Immunohistochemistry.
To determine the effect of vehicle or drug on pERK, a light-touch stimulation protocol was initiated 30 min later. As previously described [36; 39], mice were anesthetized with isoflurane (1.5 %). The plantar surface of the left hindpaw was gently stroked in the longitudinal plane with a cotton-tip for 2 s out of every 5 s, for 5 min. After an additional 5 min under anesthesia, mice were transcardially perfused with 10% buffered formalin (Fisher Scientific, Pittsburgh, PA) and the lumbar spinal cord was dissected. The spinal cord was removed and post-fixed for 4 h in 10% buffered formalin followed by 30% sucrose in 0.1M PBS (Fisher Scientific, Pittsburgh, PA) overnight. Transverse sections (40µm) were cut on a freezing microtome and collected in 0.1M PBS. The sections were washed three times in 0.1 M PBS and then pretreated with 3% normal goat serum (Gemini Bio-Products, West Sacramento, CA) and 0.3% Triton X-100 (Sigma Aldrich, St Louis, MO) to block non-specific binding. Sections were then incubated in a primary antibody for pERK (1:500, #4370, Cell Signaling Technology, Danvers, MA), glial fibrillary acidic protein (GFAP;1:1000, ab7779, Abcam, Cambridge, MA) or ionized calcium-binding adapter molecule 1 (Iba1; 1:5000, 0191971, WAKO Chemical, CA) overnight at room temperature on a laboratory shaker. The tissue was then washed three times in 0.1 M PBS, and incubated in Alexa Fluor 568 goat anti-rabbit secondary (1:500; Molecular Probes, Grand Island, NY). The tissue was then washed in 0.1 M PBS, and mounted with Prolong Gold / DAPI mounting medium (Molecular Probes).
All images were captured with a Nikon TE-2000 microscope and Nikon Elements software. For quantification of pERK, regions of interest were selected to include lamina I-II of the left (ipsilateral to light-touch stimulation) and right (contralateral to light-touch stimulation) sides at segments L3-L4. An observer who did not know the identity of the slides / sections (e.g. blinded to treatment) manually counted punctate immunoreactive profiles in lamina I-II. For GFAP and Iba1, we quantified mean pixel intensity in lamina I-V of left and right sides of the dorsal horn at segments L3-L4. Six animals per group and 4–6 slices per animal were analyzed for pERK, GFAP, and Iba1.
Spinal cord slice preparation and fluorometric Ca2+ measurements.
Ca2+ measurements from adult transverse spinal cord dorsal horn slices were performed as previously described [23]. Mice were anesthetized with 5% isoflurane and transcardially perfused with 10 ml of ice-cold sucrose-containing artificial cerebrospinal fluid (aCSF) (sucrose-aCSF) that contained (in mM): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50, kynurenic acid 1, oxygenated with 95% O2, 5% CO2; pH 7.4. The lumbar spinal cord was isolated by laminectomy, cleaned of dura mater and glued to a block of 4% agar (Fisher Scientific, Pittsburgh, PA) on the stage of a Campden 5000mz vibratome (Lafayette, IN). Transverse slices (450 μm) from lumbar segments L3-L4 were cut in ice-cold sucrose-aCSF and incubated for 30 min at 37º C with Fura-2 AM (10 µM) and pluronic acid (0.1%) in oxygenated aCSF containing (in mM): NaCl 127, KCl 1.8, KH2PO4 1.2, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 15, followed by a 20 min de-esterification period in aCSF. Slices were kept at RT in a chamber containing aCSF prior to recording. For recording, slices were perfused at 1–2 ml/min with aCSF in an RC-25 recording chamber (Warner Instruments, Hamden, CT) mounted on a Nikon FN-1 upright microscope fitted with a 79000 ET FURA2 Hybrid filter set (Nikon Instruments, Melville, NY) and a Photometrics CoolSNAP HQ2 camera (Tucson, AZ). Relative intracellular Ca2+ levels were determined by measuring the change in ratio of fluorescence emission at 510 nm in response to excitation at 340 and 380 nm (200 ms exposure). Paired images were collected at 1–1.5 seconds/frame. Relative changes in Ca2+ levels were evaluated using Nikon Elements software by creating a region of interest over the cell body and calculating the peak change in ratio in response to a 10 s exposure to glutamate. Only cells that displayed a consistent response to 1 mM glutamate at the beginning and end of the experiment (showing a less than 40% decrease in glutamate-evoked Ca2+ transients) were included. Our previous studies characterized glutamate-responsive profiles as neuronal rather than glial responses [23], indicating that [Ca2+]i responses in the current study predominantly reflect neuronal responses.
Drug Preparation and Administration.
Fingolimod hydrochloride salt was purchased from LC Labs (MA, USA), and 5 mg was dissolved in absolute ethanol (55 μl) and 0.85% sodium chloride (55 ml, Ricca Chemical Company, TX, USA) in a 1:999 ratio for Experiments 2–7. For Experiment 8, fingolimod was dissolved in the same vehicle as SEW2871 (Cayman Chemical, MI, USA). SEW2871, a selective S1PR1 agonist that produces central effects with systemic administration [3; 95], (3.3 mg) was dissolved in absolute ethanol (165 μl) and Alkamuls EL-620 (165 μl) and sonicated for 5 min at 35º C. 1320 ul of normal saline was added for a final ratio of 1:1:8 ethanol:Alkamuls:saline. W146, a selective S1PR1 antagonist [22; 30] was purchased from Cayman Chem. (MI, USA). W146 was dissolved in 1 M Na2CO3 (Sigma Aldrich, St Louis, MO), β-cyclodextrin (Sigma Aldrich, St Louis, MO) and 0.85% sodium chloride (Ricca Chemical Company, TX, USA) in a 1:1:8 ratio, respectively.
Repeated administration of fingolimod or SEW2871 was initiated after the onset of neurological motor deficits and pain-like behaviors. Daily dosing was chosen for fingolimod because this protocol is sufficient to achieve therapeutic effects in humans [21] and mice; fingolimod has a half-life of 6–9 d in humans [21] and 19–23 h in rat after systemic administration [45; 59]. Fingolimod doses of 0.001, 0.01, 0.03, 0.1 or 1 mg/kg (i.p., 200 μl) were chosen based on their ability to exert antihyperalgesic effects in other models of acute and persistent pain, in the absence of side effects [20; 44; 58; 93]. In Experiment 7A-B, W146 was administered (i.p., 100 μl) 15 minutes before each injection of fingolimod at a dose of 1 mg/kg that does not produce overt side effects or capillary leakage in lungs from C57 mice [74; 80; 99]. Vehicle controls did not differ for mechanical hyperalgesia (F(1,4) = 0.043, P = 0.95) or thermal hyperalgesia (F(1,4) = 3.66, P = 0.13), and were combined as a single group for comparison to drug treatments (see Figure Supplemental Digital Content 1). In Experiment 7C-D, fingolimod and W146 were co-administered. In Experiment 8, FTY720, SEW2871 or vehicle were injected daily (i.p.) from D16 to 43. On D44, drug administration was discontinued. Once behavioral scores returned to pre-treatment levels, mice were challenged with a single i.p. injection of vehicle, FTY720 or SEW2871, followed by repeated assessment of mechanical and cold sensitivity. For Experiment 9 FTY720 or vehicle were injected daily (i.p.) from D15 to 29 and then spinal cords were harvested using pressure ejection. 4 mm of lumbar dorsal horn pieces were stored at −80ºC for subcellular fractionation and western blotting.
Subcellular Fractionation.
All experiments compared fractions from 2–3 independent samples, each obtained by pooling lumbar dorsal horn tissue from three mice. Samples were homogenized in 1 ml of sucrose homogenization buffer (10 mM Tris, 30 mM sucrose, pH 7.5) with phosphatase and protease inhibitors (Sigma #P0044, #P5726, #P8340) on ice and an aliquot was saved for total homogenate analysis. The samples were then centrifuged 1000 x g for 10 min to remove nuclei and debris. The resulting supernatant (S1) was centrifuged (10,000×g, 15min, 4ºC) to yield a crude membrane pellet (P2) and the cytosolic supernatant from the P2 fraction (S2). The membrane fraction (P2) was resuspended in 70 μl of 1% SDS. All samples were stored at −80ºC until protein concentration was determined using Bio-Rad DC protein assay (Hercules, CA) according to kit instructions.
Western Blotting.
Equal amounts of protein (10–20 μg for total homogenates, 5–10 μg for membrane and cytosol fractions) were analyzed by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. We used anti-S1PR1 antibody (1:500, #sc-25489, Santa Cruz Biotechnology, Dallas, TX). The specificity of this antibody has previously been defined by comparing immunostaining of the vasculature in paraffin sections of S1PR1 knockout vs. WT mouse embryos [2]. Furthermore, we found that S1PR1 immunoreactivity was absent in spinal cord tissue taken from spinal cord tissue taken from conditional S1PR1 knockout mouse (see Figure Supplemental Digital Content 2, tissue kindly provided by Laura Sim-Selley and Matthew Lazenka from Virginia Commonwealth University). Equal protein loading was ensured by probing the membranes with anti-actin antibody (1:5000, #MAB1501, Millipore Billerica, MA). Fluorescent secondary antibodies were used at 1:20,000 (anti-mouse, abcam #186699; anti-rabbit, abcam #175772, Cambride, UK). Fluorescent images were taken using an Odyssey imaging system V3.0 (LI-COR Biosciences, Lincoln, NE). Densitometry was performed using Nikon Elements software.
W146 administration and detection by LCMS/MS.
W146 was injected (20 μg, retro-orbital i.v.) into uninjured and EAE mice (15 d post-induction). At either 5 or 20 minutes after injection, mice were sacrificed by direct cardiac puncture followed by light transcardial PBS/heparin perfusion (5 mL over 30s) to clear blood from the brain. Cardiac blood was collected into Eppendorf tubes containing EDTA and centrifuged at 1100 × g for 10 min within 2 hours of collection. Whole brain was harvested and flash-frozen with liquid nitrogen within 2 minutes of perfusion. All samples were stored at −80°C for 4 days until they were shipped to Cayman Chemical Company (Ann Arbor, MI) for measurement of W146 by LC-MS/MS. Brain samples were homogenized in MeOH using Precellys Evolution (Cayman Item # 16900), then centrifuged at 4000 x g for 15 min at 20 °C. 300µL supernatant was mixed with 300 µL MeOH and 75 µL of 2000 ng/mL VPC23019. VPC23019 was used in place of isotopically labeled W146, which is unavailable, as an internal standard as it is structurally similar to W146. Mouse plasma samples underwent protein precipitation with 150 µL of 300 ng/mL VPC23019 5 μL of each sample was injected on column.
Because an isotopically-labeled W146 does not exist, calibration curves were generated using either brain extract or plasma spiked with isotopically-labeled internal standard VPC23019 (Cayman Item #13240), a structurally similar analogue to W146 (0.25–1000 ng/mL, r2 = 0.998119 for brain; 1–1000 ng/mL, r2 = 0.999302 for plasma). A Waters Acquity UPLC with BEH C8 1.7 μm, 100 × 2.1 mm column was used at 25°C for separation. A solvent gradient (0.1% formic acid in water as solvent A, 0.1% formic acid in acetonitrile as solvent B) was used as follows: 0–0.5 min, 15% B; 6.5 min, 50% B, 6.6 min, 95% B, 8.7–10.7 min, 15% B). After elution, a Waters XEVO TQD tandem mass spectrometer with electrospray source in positive ion mode was used for spectrometric analysis (W146 m/z 343 ⇒ 138, VPC23019 m/z 373 ⇒ 118). Some plasma samples were outside the linear range, and their concentrations are extrapolated based on the linear range. All brain samples, except for the blind blanks, had detectable concentrations of W146 within the dynamic range. We did not see a significant difference in brain W146 concentrations between 5 and 20 minutes in either naive or EAE animals (p > 0.05) and therefore combined time points within each group.
Statistical Analysis.
For behavioral and Ca2+ imaging studies 1- or 2-way ANOVA with repeated measures was followed by pair-wise comparisons using JMP 12 (SAS, Cary, NC). For LCMS/MS results two-way ANOVA followed by Fisher’s exact test was performed using JMP 12. For immunohistochemical studies, effects of dose were analyzed using either two-way ANOVA followed by Bonferroni’s multiple comparisons (for pERK) or one-way ANOVA followed by Dunnett’s multiple comparison tests (for GFAP and Iba1) using GraphPad Prism 6 software (GraphPad Software, San Diego, CA). For western blot analysis, the relative abundance of protein in vehicle and fingolimod-treated pairs were compared using non-paired 2 tailed t-tests.
RESULTS
EAE leads to a robust pain-related hypersensitivity that peaks before hindlimb paresis
The EAE model in rodents has extended our understanding of the mechanisms underlying the motor deficits and demyelination associated with the MS disease state [4; 43; 89]. EAE is emerging as the model of choice to study MS pain, and is now routinely characterized by mechanical and cold hypersensitivity that develops before the onset of severe neurological motor deficits [43; 68; 71; 73; 96].
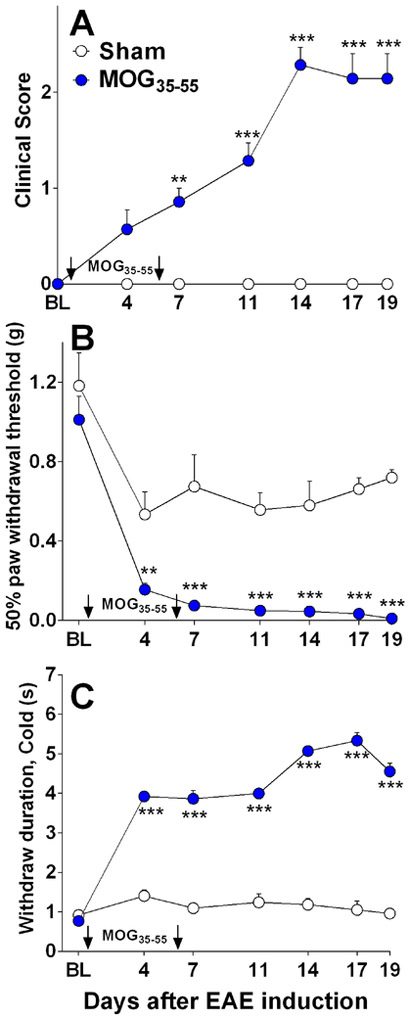
As illustrated in Fig 1A, control injections of adjuvants (CFA and pertussis toxin, Sham, n = 5) did not produce neurological motor deficits (P > 0.05). By contrast, MOG35–55 plus adjuvants (MOG35–55, n = 7) produced motor deficits (F(1, 61) = 144.13, P < 0.0001; Fig 1A) that began 7 d post-immunization and lasted throughout the duration of the study (19 d). Frank paralysis never accompanied hindlimb weakness or impaired movement, thus allowing measurement of noxious stimulus-induced withdrawal behaviors.
Figure 1. Pain-related hypersensitivity peaks before neurologic motor deficits in EAE mice.
Line graphs describing neuromuscular function (Clinical score) and pain-like behavioral responses at baseline (BL) and after sham injection of adjuvant (CFA and pertussis toxin) or after induction of EAE in C57BL/6 mice with MOG35–55. (A) Neuromuscular deficits included hanging tail, impaired righting, and/or hindlimb paresis in MOG35–55 (closed circles) but not sham (open circles) mice. (B) Mechanical sensitivity to hindlimb application of von Frey hairs in MOG35–55 and sham mice. (C) Cold sensitivity to hindlimb application of acetone in MOG35–55 and sham mice. Arrows indicate days of MOG injections. Data represent mean ± SEM from 5–7 mice per group. BL= Baseline. *P<0.05.
MS patients often report mechanical and cold hypersensitivity [90]. We show that EAE mice exhibit robust hypersensitivity to mechanical or cold stimuli, in agreement with previous studies [1; 5; 32; 52; 67; 68; 71; 73; 92; 96]. Indeed, MOG35–55 produced mechanical (F(1, 42) = 118.39, P < 0.0001; Fig 1B) and cold hypersensitivity (F(1,33) = 890.45, P < 0.0001; Fig 1C). Mechanical and cold hypersensitivity in EAE animals were significantly different from sham controls (CFA plus pertussis toxin alone) beginning 4 d after the first MOG35–55 injection (before the development of motor deficits). Compared to baseline measurements, sham treatment modestly decreased mechanical threshold (F(6, 36) = 6.02, P = 0.0002; Fig 1B), and increased cold withdrawal duration only at D4 (F(6, 38) =2.99, P = 0.017; Fig 1C). The next results are presented with the caveat that a non-adjuvant control group was not included.
Fingolimod dose-dependently decreased hyperalgesia in a reversible and repeatable manner
Previous studies indicate that fingolimod reverses signs of EAE disease including motor deficits [15; 33; 49; 75; 93], and here for the first time we tested the hypothesis that fingolimod reverses EAE-induced pain-like behavior as well. Figure 2 illustrates that fingolimod (0.1 and 1 mg/kg, n = 4–6) reduced mechanical (F(2,11) = 38.01, P ˂ 0.001; Fig 2A) and cold hypersensitivity (F(2, 11)=32.90, P ˂ 0.001; Fig 2B) in a reversible and repeatable manner. When fingolimod was discontinued on D34, pain scores gradually returned to pre-treatment levels, consistent with the long half-life of the drug.
Figure 2. Antihyperalgesic effects of fingolimod are reversible and repeatable.
(A) Mechanical and cold (B) hypersensitivity from baseline (BL) through 52 d post-EAE induction with MOG35–55. Fingolimod (FTY720) was injected daily (i.p.) from D15 to 34 and from D45 to 52 (yellow). Arrows indicate days of MOG injections. Data represent mean ± SEM. N = 3–4 for vehicle and 0.1 mg/kg groups and 4–6 for 1 mg/kg group. *P<0.05, **P<0.01, †P<0.001 by t-test compared to vehicle.
Fingolimod can decrease hyperalgesia either by direct actions at pain modulatory centers or by indirect actions secondary to amelioration of disease. To dissect these possibilities, we carefully monitored the time course of clinical motor deficits and pain scores. As expected, daily administration of fingolimod dose-dependently decreased neurological motor deficits (F(6, 35) = 11.84, P ˂ 0.0001, n = 6–7; Fig 3A), and the highest dose (1 mg/kg) yielded an onset of action at D27. Also, fingolimod dose-dependently reversed mechanical (F(6,35) = 145.78, P ˂ 0.001; Fig 3B) and cold hyperalgesia (F(6,35)=74.00, P ˂ 0.001; Fig 3C); the highest dose inhibited mechanical hyperalgesia and cold hyperalgesia within just 21 days and 18 days, respectively. Close inspection of Figures 3D-F reveals that fingolimod did not alter motor deficits from D0 to D21. Not until later time points (e.g. Day 30, Fig 3G-I) did the effect of fingolimod on motor deficits become significant (P>0.05). This temporal dissociation in the onset of action of fingolimod on motor deficits and pain-like behaviors suggest that fingolimod acts directly at pain modulatory centers to decrease EAE-induced hyperalgesia.
Figure 3. Fingolimod dose-dependently decreases hyperalgesia before it decreases motor deficits.
Line graphs describing (A) neuromuscular deficits (B) mechanical sensitivity and (C) cold sensitivity from baseline (BL) through 37 days post-EAE induction with MOG35–55. The 2nd MOG35–55 injection on D2 is not shown. Fingolimod (FTY720) was injected daily (i.p.) from D 15 to 37 (yellow). Histograms describing effect of fingolimod doses on D21 (D, E, F) and D30 (G, H, I). Arrows indicate days of MOG injections. Data represent mean ± SEM from 4–7 mice per drug treatment group. *P<0.05, **P<0.01, ***P<0.001 compared to vehicle controls.
Fingolimod inhibits physiological and cellular indices of EAE-induced central sensitization in dorsal horn
Fingolimod targets spinal nociceptive pathways in the paclitaxel, spared nerve injury (SNI), formalin and carrageenan models of persistent pain [20; 28; 44; 97]. Therefore, we first asked whether our EAE model is associated with activation of dorsal horn neurons, and then asked whether this activation could be reduced with fingolimod. To address these questions, we evaluated components of central sensitization in the dorsal horn: Ca2+ mobilization, phosphorylation of ERK, and up-regulation of GFAP and Iba1. Central sensitization refers to an exaggerated response of CNS nociceptive neurons to normal or sub-threshold afferent input, and is thought to contribute to enhanced responsiveness to sensory input following tissue injury [77]. However, the question as to whether EAE generates central sensitization remains unanswered.
An increase in intracellular Ca2+ levels is the key trigger for the initiation of activity-dependent central sensitization in dorsal horn neurons [54]. Recordings in brain slices from EAE mice prior to the emergence of motor deficits indicated that oral fingolimod reversed the increased duration and frequency of glutamate-mediated spontaneous excitatory postsynaptic currents [75]. Therefore, as our first attempt to detect central sensitization, we first evaluated EAE-induced increase in glutamate-evoked Ca2+ responses with live-cell Fura-2 ratiometric analysis in adult spinal cord slices. As described previously [23], glutamate concentration-dependently increased [Ca2+ ]i mobilization in lamina II neurons of dorsal horn slices taken from sham mice (Fig 4A). Importantly, this glutamate-evoked [Ca2+ ]i mobilization was potentiated in animals treated with MOG35–55 (F(1, 16) = 9.97, P = 0.0061; n = 9; Fig 4A). This was reversed in dorsal horn slices obtained from EAE-mice treated with 1 mg/kg fingolimod for 7–10 days (F(1, 13) = 6.80, P = 0.022; n = 7–8; Fig 4B). Our results extend our previous studies that indicate neuropathic and inflammatory injury potentiates glutamate-evoked Ca2+ signaling in the dorsal horn. This potentiation coincided with the temporal onset and resolution of hyperalgesia [18; 23].
Figure 4. Fingolimod prevents the EAE-induced potentiation of glutamate-evoked Ca2+ signaling.
(A) Concentration-dependence of glutamate-evoked Ca2+ responses in dorsal horn slices from MOG35–55-treated and sham mice. Calcium level (expressed as ΔF340/F380 as a function of increasing glutamate concentration. (B) Glutamate-evoked Ca2+ responses in EAE mice treated with vehicle or fingolimod (1 mg/kg for 7–10 days). Data represent mean ± SEM. N = 7 in vehicle groups, 8 in MOG35–55 + fingolimod group and 9 in MOG35–55 and sham group. *P<0.05, **P<0.01.
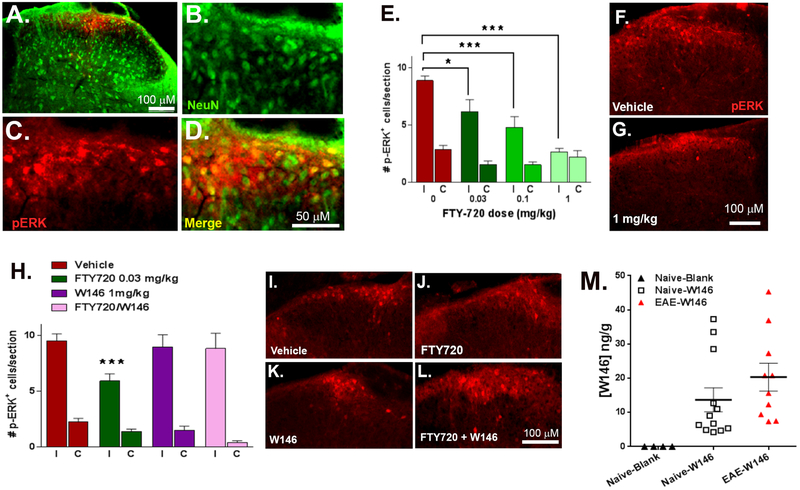
Our second approach to evaluate central sensitization was to measure EAE-induced increase in stimulus-evoked pERK in dorsal horn neurons. Dorsal horn pERK expression is widely used as a marker of central sensitization in models of persistent pain [18; 34; 36]. Figures 5A-D illustrate that light-touch stimulation evoked pERK staining in lumbar dorsal horn of EAE mice, largely in NeuN-positive profiles. Stimulus-evoked pERK was greater at the left (ipsilateral) as compared to the right (contralateral) side of stimulation (p < 0.0001 at 0 mg/kg fingolimod; Fig 5E). Figures 5E-G illustrates that fingolimod dose-dependently reduced the number of pERK positive cells on the ipsilateral (F(4, 42) = 9.90 p ˂ 0.0001; n = 4–6), but not contralateral side (p > 0.05). As illustrated in Fig 5H-L, W146 alone had no effect compared to vehicle (p>0.05), but reversed fingolimod inhibition of stimulus-evoked pERK expression in dorsal horn (F(3,58) = 5.88, p ˂ 0.001).
Figure 5. Fingolimod dose-dependently inhibits stimulus-evoked pERK in dorsal horn neurons via S1PR1 in EAE mice.
(A) Representative dorsal horn photomicrograph of NeuN and pERK immunoreactivity taken from a vehicle-treated EAE mouse ipsilateral to light touch stimulation. Enlarged images of (B) NeuN, (C) pERK and (D) NeuN/pERK merged. (E) Histogram describing the number of pERK-positive profiles at the side ipsilateral (I) and contralateral (C) to light touch hind-paw stimulation. Fingolimod (FTY720) was injected daily (i.p.) from D15–37 post-EAE induction at the doses shown and then processed for immunohistochemistry described in Methods. (F-G) Representative images of pERK immunoreactivity in ipsilateral dorsal horn from mice that received (F) vehicle or (G) 1 mg/kg fingolimod. (H) Histogram describing the number of pERK-positive profiles after daily administration of vehicle, fingolimod, W146 or fingolimod + W146. Representative images of pERK immunoreactivity from mice treated with (I) vehicle, (J) fingolimod, (K) W146 or (L) fingolimod + W146. Data represent mean ± SEM from 5–12 mice. *P<0.05, ***P<0.001. (M) Scatter plot shows detectable W146 concentrations in brain at 5 – 20 min after retro-orbital intravenous injection of W146. “Naïve-blank” mice did not receive W146, while “Naïve-W146” and “EAE-W146” mice did.
To determine whether the S1PR1-selective antagonist W146 crosses the blood brain barrier, we measured plasma and intracerebral W146 after retro-orbital intravenous injection (20 μg) in non-EAE (Naïve-W146) and EAE mice (EAE-W146; 15 d post induction). As expected, W146 measurements in plasma or brain from non-EAE mice that did not receive W146 (Naïve-blank mice) were below the limit of detection. And as expected, we found high plasma concentrations of W146 when measured 5 min after injection (2345 ± 136 ng/ml). These concentrations significantly declined within 20 min (644 ± 50 ng/ml; F(1,14) = 102.7, p < 0.0001), in accordance with its half-life [80]. There was no difference in the plasma concentration of W146 between Naïve-W146 and EAE-W146 (p > 0.05). As illustrated in Fig 5M, we observed measureable concentrations of W146 in brain from Naïve-W146 or EAE-W146 mice (Fisher’s exact test: P < 0.0001), and these concentrations were higher in EAE mice as compared to naïve controls. These data indicate that systemically administered W146 can cross the blood-brain barrier to block S1PR1 receptors in the CNS, particularly when this barrier is compromised as occurs in EAE.
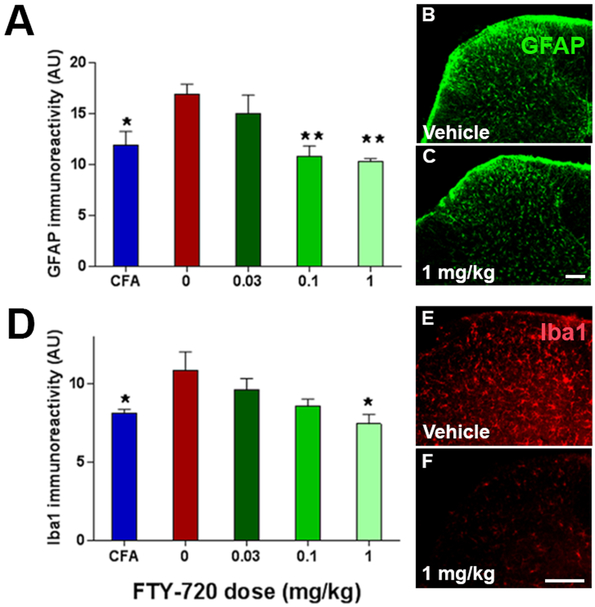
Neuronal injury leads to the activation of astrocytes (which express GFAP) and macrophage/microglia (which express Iba1) that then contribute to the development of central sensitization [35; 83]. Spinal cord glial cell activation contributes to central sensitization and pathological pain in a number of pain syndromes with different etiologies, including diabetic neuropathy, chemotherapy-induced neuropathy, peripheral nerve inflammation and trauma, and spinal cord inflammation [60]. Therefore our third approach to evaluate central sensitization was to measure EAE-induced increases in dorsal horn GFAP and Iba1. In agreement with previous studies [68], EAE increased GFAP and Iba1 immunoreactivity. Compared to EAE mice treated with vehicle, fingolimod (n = 4–6) dose-dependently reduced both dorsal horn GFAP (F(4, 15) = 5.95, p < 0.01; Fig 6A-C) and Iba1 (F(4, 17) =3.62, p ˂ 0.05; Fig 6D-F).
Figure 6. Fingolimod decreases EAE-induced activation of astrocytes and microglia.
Fingolimod (FTY720) was injected daily (i.p.) from D16 to 37 post-EAE induction and then processed for immunohistochemistry described in Methods. Lumbar L4-L5 spinal cord sections were stained with primary antibodies against (A-C) GFAP and (D-F) Iba1. (A) GFAP immunoreactivity in sham controls or MOG35–55-treated mice after daily administration of FT720 as indicated. Representative images show GFAP staining in either (B) vehicle, or (C) fingolimod-treated mice. (D) Iba1 immunoreactivity in sham controls or MOG35–55-treated mice after daily administration of FT720 as indicated. Representative images show Iba1 staining in (E) vehicle or (F) fingolimod-treated mice. Data represent mean ± SEM from 4–6 mice per group. *P<0.05, **P<0.01 compared to MOG35–55–treated animals that received vehicle (0 mg/kg fingolimod, red bar). Scale bars = 100 um.
S1PR1 receptor antagonist W146 prevents fingolimod-induced antihyperalgesia and pERK activation
To test the hypothesis that S1PR1 mediates the development of fingolimod-induced antihyperalgesia, we administered the selective antagonist, W146, 15 prior to every injection of fingolimod. As illustrated in Fig 7 and similar to Figs 2 and 3, fingolimod decreased mechanical (F(3,21) = 238.71, P < 0.0001; Fig 7A) and cold hypersensitivity (F(3,21) = 22.52, P < 0.0001; Fig 7B). When administered at a dose of 1 mg/kg, i.p., W146 prevented fingolimod inhibition of mechanical (F(1, 11)= 548.90, p ˂ 0.001) and cold hypersensitivity (F(1, 11)= 48.97, p < 0.0001 compared to fingolimod alone, n = 6–7). W146 alone had no effect compared to vehicle (p>0.05). W146 alone had no effect on either hyperalgesia or pERK (p > 0.05) arguing against a contribution of ongoing activity of S1PR1 to EAE hyperalgesia and spinal pERK expression.
Figure 7. S1PR1 antagonist W146 prevents fingolimod-induced attenuation of hyperalgesia.
(A-B) Fingolimod (FTY720) and/or W146 were injected daily (i.p.) from D27 to 41 (yellow). (A) Mechanical and (B) cold hyperalgesia were monitored prior to and through 41 days post-EAE induction. n = 6–7. (C-D) fingolimod was injected daily (i.p.) from D14 to 34 (yellow); fingolimod and W146 were co-administered D35 to 44. (C) Mechanical and (D) cold hyperalgesia were monitored prior to and through 44 days post-EAE induction (n = 4–11). Arrows indicate days of MOG injections. Data represent mean ± SEM. *P<0.05 vehicle vs. fingolimod, †P<0.05 fingolimod alone vs. fingolimod + W146.
W146 reverses fingolimod-induced antihyperalgesia
S1PRs can regulate cellular function through activation of G-protein-coupled receptors [98] or by ligand-induced functional antagonism (receptor internalization and degradation; [38; 58; 69]). To determine whether fingolimod behaves as an S1PR1 agonist rather than as a functional antagonist to inhibit EAE pain, we initiated the administration of W146 after the development of fingolimod-induced anti-hyperalgesia. We reasoned that if the fingolimod mechanism of analgesia is functional antagonism (irreversible internalization and degradation), then S1PR1s would not be available for antagonist actions of W146. On the other hand, if the fingolimod mechanism of analgesia is S1PR1 agonism as suggested by Scholich and colleagues [97], then S1PR1s should be available for binding and inhibition by W146. To test this, fingolimod was administered daily (0.03 mg/kg i.p.) from d14 to 34 to produce antihyperalgesia, and was then co-administered with W146 (1 mg/kg i.p.) from d 35 to 44. Figs 7C-D show that W146 reversed fingolimod-induced inhibition of both mechanical (F(6, 26) = 16.33, p<0.0001, n = 4 Fig 7C) and cold hypersensitivity (F(6, 25) = 3.94, p=0.0099, n = 4; Fig 7D), supporting an antinociceptive mechanisms of action of fingolimod.
Fingolimod and S1PR1 receptor agonist SEW2871 mediate antihypersensitivity
To further test the hypothesis that repeated injection of fingolimod behaves as an agonist rather than as a functional antagonist, we predicted that its antinociceptive effects would be mimicked with either: 1) a selective S1PR1 agonist that does not induce irreversible internalization and degradation such as SEW2871 [46; 79]; or 2) a single injection. Fig 8A demonstrates that repeated administration of fingolimod decreased neurological motor deficits (F(1, 9) = 5.19, P = 0.049). Importantly, both fingolimod and SEW2871 reduced mechanical (Fig 8B; F(2,13) = 10.05, p = 0.0023) and cold hypersensitivity (Fig 8C, F(2,13) = 7.28, p = 0.0075), n=5–7.
Figure 8. Antihyperalgesic effects of repeated and single dosing of fingolimod are mimicked by the S1PR1-selective agonist, SEW2871.
(A) Motor function, (B) Mechanical and (C) cold hyperalgesia were monitored prior to and through 56 days post-EAE induction. Fingolimod (FTY720), SEW2871 or vehicle were injected daily (i.p.) from D16 to 43 (yellow; n = 5–6). On D44, drug administration was discontinued. Once behavioral scores returned to pre-treatment levels, mice were given a single injection as indicated and (D) Mechanical and (E) cold hyperalgesia were monitored acutely (n = 8–10). Data represent mean ± SEM. *P<0.05 fingolimod compared to MOG35–55–treated animals that received vehicle. †P<0.05 SEW2871 compared to vehicle.
We then discontinued drug administration, allowed behavior thresholds to return to pre-treatment indices of hypersensitivity, and administered a single injection of fingolimod or SEW2871, and then evaluated just von Frey sensitivity and responses to plantar application of acetone. Both drugs reduced mechanical hypersensitivity at 30 minutes after injection (Fig 8D; F(2,26) = 5.76, p = 0.009) and both reduced cold hypersensitivity at all time points tested (Fig 8E, F(2,23) = 10.68, p = 0.0005, n = 8–10).
Fingolimod does not decrease membrane S1PR1
Functional antagonism is associated with internalization and degradation of GPCRs such as S1PR1 [10; 11; 38; 58; 69] leading to large decreases in both membrane and cytosolic compartments. By contrast, agonism leads to receptor internalization and recycling back to the cell surface. This should be associated with minimal if any changes in membrane and cytosolic compartments. To further explore whether fingolimod behaves as an agonist or as a functional antagonist in mouse spinal cord, we measured S1PR1 content in both membrane and cytosolic fractions. On day 15 post EAE induction, we confirmed behavioral hyperalgesia, administered fingolimod (0.1 mg/kg) or vehicle daily for 14 days, and then collected and pooled membrane fractions, cytosolic fractions, and total homogenate for western blot analysis. As illustrated in Fig. 9C-F, fingolimod did not change S1PR1 levels in either the membrane or cytosolic fractions of either naïve or EAE mice (P > 0.05). Fingolimod did not alter S1PR1 content in the total homogenate of naïve mice (Fig. 9G), but did decrease S1PR1 in EAE mice (Fig. 9H, 80.7 ± 3.6 % compared to control, p = 0.033). This latter effect was small (less than 20%) and so perhaps not physiologically significant.
Figure 9. S1PR1 content after fingolimod administration.
Representative blots of S1PR1 levels from (A) Naïve and (B) EAE mice. For vehicle and fingolimod-treated mice, 2–3 samples are shown for both S1PR1 and actin. Relative S1PR1 protein expression comparing vehicle to fingolimod-treated mice from naïve (left side) and EAE mice (right side) from (C-D) membrane, (E-F) cytosol and (G-H) total homogenates. Data represent mean ± SEM. *P<0.05 fingolimod compared to MOG35–55–treated animals that received vehicle.
Supplemental Digital Content 1, which demonstrates the lack of difference in responses in vehicle for fingolimod and W146.
DISCUSSION
EAE mice exhibit reliable behavioral measures of neuropathic pain
Consistent with previous studies [50; 52; 68; 71; 73; 96] we found that mechanical and cold hypersensitivity developed and peaked earlier than motor deficits. The early emergence of pain-like behavior in EAE mice suggests that pain pathways are sensitized independently of the clinical course of the disease, well before motor pathology and deficits. Taken together with the lack of hindlimb paralysis, we conclude that neuropathic pain behavior is not confounded by motor impairment in our optimized EAE mouse model.
Spinal nociceptive neurons are sensitized in EAE mice
Clinical studies suggest that MS is associated with dysfunction of dorsal horn pain modulatory centers that contribute to neuropathic pain [43]. We observed for the first time that EAE potentiated three markers of central sensitization in the dorsal horn: 1. glutamate-evoked Ca2+ signaling in dorsal horn slices; 2. light-touch stimulation-evoked pERK expression, consistent with previous studies showing that EAE increases the dorsal horn expression of FOS, another marker of cellular activity [67]; and 3. GFAP and Iba1 immunoreactivity, consistent with previous studies indicating that both astrocyte and macrophage/microglial activation coincide with the onset of mechanical hypersensitivity [56; 68].
Our glutamate bath-application likely overrides plasticity at pre-synaptic glutamatergic terminals, leading us to speculate that the enhanced Ca2+ signaling observed in EAE mice indicates plasticity at post-synaptic sites. This is consistent with enhanced glutamate-mediated excitatory postsynaptic currents, increased expression of GluA1 and phosphorylated GluA1 in the post-synaptic fraction, and increased post-synaptic AMPAR signaling in corticostriatal slices of EAE mice [13; 75]. EAE also induces pre-synaptic changes that could contribute to central sensitization. Indeed, neuropathological data indicate that EAE animals exhibit overactive presynaptic NMDA receptors, decreased excitatory amino acid transporter-2 and increased glutamate type 1 transporter [67; 72].
Fingolimod inhibits EAE-associated hyperalgesia via S1PR1 agonism
Our data is the first to demonstrate that S1PR1 ligands reverse hyperalgesia (mechanical and cold hypersensitivity) and spinal nociceptive transmission (pERK expression and Ca2+ mobilization in spinal neurons) in EAE. Off-target effects are unlikely since the antihyperalgesic effects of fingolimod were mimicked by SEW2871 and both behavioral and biochemical effects of fingolimod were blocked by W146. Fingolimod mechanisms of analgesic action are complex and appear to involve both antagonism of pronociceptive S1PR1 [28; 44] to create a pharmacological “S1PR1-null state” as it does in lymphocytes [58], as well as direct agonism of antinociceptive S1PR1 signaling [19; 97]. Accordingly when fingolimod is acting as a functional antagonist, we see similar effects with a competitive antagonist [91]. However, our pharmacological data suggests that fingolimod evokes S1PR1 agonism in spinal nociceptive processing and pain in EAE that is antinociceptive rather than pronociceptive. First, the S1PR1-selective antagonist W146 had no effect alone but blocked fingolimod-induced decreases in pERK expression. Our studies confirm that the BBB is perturbed in our EAE model at 15d post-induction, thus providing a means for W146 to act centrally. Second, W146 had no effect on nociceptive behavior, but blocked fingolimod-induced decreases in both mechanical and cold hypersensitivity. Third, administration of SEW2871, an S1PR1-selective agonist that does not irreversibly internalize S1PR1, mimicked the antihyperalgesic effect of fingolimod is both chronic and single injection paradigms. Fourth, fingolomod did not change the membrane content of S1PR1, consistent with an agonist mechanism. Our data are consistent with previous reports indicating that intrathecal fingolimod-phosphate inhibited hyperalgesia in the SNI model via S1PR activation rather than irreversible receptor internalization associated with functional antagonism [97]. Our data are consistent with the idea that fingolimod acts as a functional antagonist at lymphocytes to reduce the clinical symptoms of EAE/MS, and as an S1PR1 agonist in the CNS to reduce the hyperalgesia of EAE/MS.
Antinociceptive and pronociceptive actions of S1PR agonists
We report that fingolimod and SEW2871 produce antihyperalgesic effects in the EAE model of MS. Pain is a problem in multiple types of MS [29], and these results may translate not only to the primary progressive form, but also to the relapsing remitting form of MS that is targeted by fingolimod [29]. Our results are consistent with previous studies demonstrating antihyperalgesic effects of fingolimod in multiple models of acute nociception, peripheral inflammation and peripheral nerve injury [19; 20; 28; 97]. For example, systemic administration of fingolimod produced antihyperalgesic effects in the SNI model that was abolished by intrathecal pre-treatment with W146 [97]. Similar results were observed after intrathecal administration of fingolimod-phosphate: inhibition of nociception and mechanical hypersensitivity after intraplantar formalin [20] or SNI [97], respectively. S1PR1 signaling in the brain may also be antinociceptive, as intracerebroventricular administration of SEW2871 reduced noxious heat sensitivity, and this was attenuated by the S1PR1/3 competitive antagonist VPC44116 [85]. Conversely, however, S1PR1 agonists can exert pronociceptive actions when administered at peripheral sites or by intrathecal injection [24; 25; 28; 44; 57; 76]. For example, Salvemini and colleagues reported that intrathecal injection of S1P and SEW2871 (0.8 nmol, at 2 h post-injection) produced heat hypersensitivity that could be blocked by local administration of W146 but not its inactive isomer W140 [28; 44] and that intrathecal SEW2871 produced mechanical hypersensitivity in uninjured rats [44]. Furthermore, intrathecal W146 (documented as an S1PR1 antagonist) or systemic fingolimod (presumptively acting as a functional S1PR1 antagonist) attenuated hyperalgesia in the paclitaxel model of chemotherapy-induced painful peripheral neuropathy [44]. We suggest that the direction of effect of fingolimod and other S1PR1 agents depends on the pain model and perhaps site of action. Further studies using site-specific drug administration are required to determine whether the antinociceptive effects of fingolimod in the pain of MS are mediated via supraspinal receptors and/or directly on spinal sites. Regardless, the present results comprise the first demonstration of an effect of S1PR1 agent in a model of central neuropathic pain.
Argument against mechanism of lymphocyte egress.
Kuner and colleagues recently reported that oligodendrocyte ablation produced a hyperalgesia that coincided with early axonal pathology in the spinothalamic tract, and suggested that oligodendrocyte function is required for normosensitivity to somatosensory stimuli [40]. In the same study, fingolimod administration at doses that inhibited lymphocyte infiltration did not affect the development or maintenance of nociceptive hypersensitivity. Consistent with this idea, in the current study fingolimod reduced pain behavior more quickly than it ameliorated motor deficits. Second, intrathecal fingolimod produced antinociceptive effects in both formalin and spared nerve injury models without alterations in blood lymphocyte count [20]. Third, fingolimod decreased astrocyte and microglial activation in a non-T-cell model of demyelination, the cuprizone model, that does not involve lymphocyte trafficking [53]. We conclude that fingolimod efficacy in EAE-induced pain is produced by central mechanisms that do not involve inhibition of lymphocyte egress/T-cell migration into the spinal cord.
Fingolimod may reduce pain by targeting astrocytes and/or microglia
In addition to its antihyperalgesic actions, we report that fingolimod normalized EAE-associated elevations of GFAP and Iba1 in the dorsal horn. This suggests that fingolimod inhibits the activation of astrocytes and/or macrophage/microglia. Fingolimod readily crosses the BBB and may therefore have direct effects on neurons, astrocytes and microglia, which readily express S1PR1s [16; 31; 59]. Supporting evidence indicates that fingolimod targets astrocytes and microglia in EAE and MS [13; 15; 44; 53; 61; 65; 75]. First, fingolimod efficacy on clinical score is lost in mice lacking S1PR1 receptors specifically on astrocytes [15]. Thus it is plausible that fingolimod imparts inhibitory effects on hyperalgesia via direct actions on spinal astrocytes. Second, S1PR1 receptors in cultured microglia bind fingolimod to downregulate production of pro-inflammatory cytokines including tumor necrosis factor-α (known to be associated with AMPAR-mediated glutamate transmission in EAE [13]), interleukin-1β, and interleukin-6 [65]. Our data suggest the intriguing hypothesis that in addition to its disease modifying actions on T cell function, fingolimod also exerts neuropathic pain inhibitory actions at spinal glia in MS.
Clinical/Translational Relevance
There are many similarities between EAE and MS including clinical course, pathological CNS lesions, glial activation, and axonal demyelination [41; 62]. Post-mortem tissue from MS patients reveal abnormal levels of glutamate and its receptors [64; 81; 88], suggesting alterations of glutamate transmission similar to that seen in EAE. MS lesions in humans are characterized by marked reductions in glutamate metabolizing enzymes, glutamate dehydrogenase and glutamine synthetase, and the major glutamate transporter GLT-1 [94]. Thus it is plausible that the pain associated with both EAE and MS results from imbalanced glutamate homeostasis. Accordingly, the inhibitory effects of fingolimod on central sensitization, spinal glial activation, and hyperalgesia in our murine model of EAE may be ideally suited to treat MS patients with neuropathic pain.
Supplementary Material
Supplemental Figure 1. Vehicle controls for both the fingolimod and W146 groups do not differ. Vehicle control for fingolimod (ethanol:0.85% sodium chloride 1:999) and W146 (1 M Na2CO3 b-cyclodextrinand 0.85% sodium chloride 1:1:8) were injected daily ( i.p.) from D27 to 41 (yellow). (A) Mechanical and (B) cold hyperalgesia were monitored prior to and through 41 days post-EAE induction. Data represent mean ± SEM. n= 3 mice per group. P>0.05 for both mechanical and cold hyperalgesia.
Supplemental Figure 2. S1PR1 antibody specificity. Specificity for S1PR1 antibodies was determined using total homogenate of whole spinal cord from female Nestin-S1PR1 cKO and WT mice. Lanes from each image were loaded with 20 mg protein. (A) Alomone #ASR-011 anti-Sphingosine 1-Phosphate Receptor 1 antibody (1:200). Vendor suggested band at 48 kDa. Positive reactivity was observed in lanes 1, 3, 5 and 7 (PNGase treated) in both cKO (lanes 1 and 3) as well as WT (lanes 5 and 7). No bands were observed in the absence of PNGase treatment to remove post-translational glycolsylation (lanes 2, 4, 6 and 8). (B) Abcam #ab11424 Anti-EDG1 antibody (1:20,000). Vendor suggested band at 44 kDa. Positive immunoreactivity was observed in both cKO (lanes 1 and 2) as well as WT (lanes 3 and 4). (C). EDG-1 Antibody (H-60; 1:500). Vendor suggested band at 37 kDa. Note the lack of immunoreactivity in cKO tissue (lanes 1, 2) compared to positive immunoreactivity in WT tissue (lanes 3, 4).
Acknowledgements:
This work was supported by R01NS62306 (BKT), K01DA031961 (SD), University of Kentucky Start-Up funds (BKT), and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number KL2TR000116. We thank Professors Dawn Ehde (University of Washington) and Stella Elkabes (Rutgers) for critical readings of the manuscript, Professor Patrick Sheets (Indiana University South Bend) for help in designing and analyzing the experiments of Figures 7–8, Professors Laura Sim-Selley and Matthew Lazenka for critical discussions on S1PR1 antibody specificity and for providing Nestin-S1PR1 cKO tissue, and Professor Erhard Bieberich (University of Kentucky) for generously providing the Santa Cruz anti-S1PR1 antibody, and Justin Pinson for his help with quantification of immunohistochemistry.
References
- [1].Aicher SA, Silverman MB, Winkler CW, Bebo BF Jr. Hyperalgesia in an animal model of multiple sclerosis. Pain 2004;110(3):560–570. [DOI] [PubMed] [Google Scholar]
- [2].Akiyama T, Sadahira Y, Matsubara K, Mori M, Igarashi Y. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 in vascular and lymphatic endothelial cells. J Mol Histol 2008;39(5):527–533. [DOI] [PubMed] [Google Scholar]
- [3].Asle-Rousta M, Oryan S, Ahmadiani A, Rahnema M. Activation of sphingosine 1-phosphate receptor-1 by SEW2871 improves cognitive function in Alzheimer’s disease model rats. EXCLI J 2013;12:449–461. [PMC free article] [PubMed] [Google Scholar]
- [4].Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nature reviews Immunology 2007;7(11):904–912. [DOI] [PubMed] [Google Scholar]
- [5].Begum F, Zhu W, Cortes C, MacNeil B, Namaka M. Elevation of tumor necrosis factor alpha in dorsal root ganglia and spinal cord is associated with neuroimmune modulation of pain in an animal model of multiple sclerosis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 2013;8(3):677–690. [DOI] [PubMed] [Google Scholar]
- [6].Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE). Exp Neurol 2015;271:279–290. [DOI] [PubMed] [Google Scholar]
- [7].Berard JL, Wolak K, Fournier S, David S. Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia 2010;58(4):434–445. [DOI] [PubMed] [Google Scholar]
- [8].Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 2003;278(48):47408–47415. [DOI] [PubMed] [Google Scholar]
- [9].Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010;9(11):883–897. [DOI] [PubMed] [Google Scholar]
- [10].Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 2004;4(7):1019–1025. [DOI] [PubMed] [Google Scholar]
- [11].Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002;277(24):21453–21457. [DOI] [PubMed] [Google Scholar]
- [12].Brochet B, Deloire MS, Ouallet JC, Salort E, Bonnet M, Jove J, Petry KG. Pain and quality of life in the early stages after multiple sclerosis diagnosis: a 2-year longitudinal study. Clin J Pain 2009;25(3):211–217. [DOI] [PubMed] [Google Scholar]
- [13].Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D’Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29(11):3442–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [15].Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 2011;108(2):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical neuropharmacology 2010;33(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L, Group TS. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362(5):402–415. [DOI] [PubMed] [Google Scholar]
- [18].Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013;341(6152):1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coste O, Brenneis C, Linke B, Pierre S, Maeurer C, Becker W, Schmidt H, Gao W, Geisslinger G, Scholich K. Sphingosine 1-phosphate modulates spinal nociceptive processing. The Journal of biological chemistry 2008;283(47):32442–32451. [DOI] [PubMed] [Google Scholar]
- [20].Coste O, Pierre S, Marian C, Brenneis C, Angioni C, Schmidt H, Popp L, Geisslinger G, Scholich K. Antinociceptive activity of the S1P-receptor agonist FTY720. J Cell Mol Med 2008;12(3):995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clinical pharmacokinetics 2012;51(1):15–28. [DOI] [PubMed] [Google Scholar]
- [22].Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 2005;280(11):9833–9841. [DOI] [PubMed] [Google Scholar]
- [23].Doolen S, Blake CB, Smith BN, Taylor BK. Peripheral nerve injury increases glutamate-evoked calcium mobilization in adult spinal cord neurons. Mol Pain 2012;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Doyle T, Chen Z, Obeid LM, Salvemini D. Sphingosine-1-phosphate acting via the S1P(1) receptor is a downstream signaling pathway in ceramide-induced hyperalgesia. Neurosci Lett 2011;499(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Doyle T, Finley A, Chen Z, Salvemini D. Role for peroxynitrite in sphingosine-1-phosphate-induced hyperalgesia in rats. Pain 2011;152(3):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Drulovic J, Basic-Kes V, Grgic S, Vojinovic S, Dincic E, Toncev G, Kezic MG, Kisic-Tepavcevic D, Dujmovic I, Mesaros S, Miletic-Drakulic S, Pekmezovic T. The Prevalence of Pain in Adults with Multiple Sclerosis: A Multicenter Cross-Sectional Survey. Pain medicine 2015;16(8):1597–1602. [DOI] [PubMed] [Google Scholar]
- [27].Fiest KM, Fisk JD, Patten SB, Tremlett H, Wolfson C, Warren S, McKay KA, Berrigan L, Marrie RA, Epidemiology CTit, Impact of Comorbidity on Multiple S. Comorbidity is associated with pain-related activity limitations in multiple sclerosis. Mult Scler Relat Disord 2015;4(5):470–476. [DOI] [PubMed] [Google Scholar]
- [28].Finley A, Chen Z, Esposito E, Cuzzocrea S, Sabbadini R, Salvemini D. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PloS one 2013;8(1):e55255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S, MacLeod MR, Fallon MT. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain 2013;154(5):632–642. [DOI] [PubMed] [Google Scholar]
- [30].Foss FW Jr., Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorganic & medicinal chemistry 2007;15(2):663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther 2007;323(2):469–475. [DOI] [PubMed] [Google Scholar]
- [32].Fu W, Taylor BK. Activation of cannabinoid CB receptors reduces hyperalgesia in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neuroscience letters 2015;595:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther 2003;305(1):70–77. [DOI] [PubMed] [Google Scholar]
- [34].Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2009;2:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 2010;126(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem 2010;115(2):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grace PM, Loram LC, Christianson JP, Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam AM, Mahoney MJ, Maier SF, Chavez RA, Watkins LR. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun 2017;59:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 2004;18(3):551–553. [DOI] [PubMed] [Google Scholar]
- [39].Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK. Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARgamma mechanisms. Pain 2015;156(3):469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gritsch S, Lu J, Thilemann S, Wortge S, Mobius W, Bruttger J, Karram K, Ruhwedel T, Blanfeld M, Vardeh D, Waisman A, Nave KA, Kuner R. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nature communications 2014;5:5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Handel AE, Lincoln MR, Ramagopalan SV. Of mice and men: experimental autoimmune encephalitis and multiple sclerosis. Eur J Clin Invest 2011;41(11):1254–1258. [DOI] [PubMed] [Google Scholar]
- [42].Hui J, Zhang ZJ, Zhang X, Shen Y, Gao YJ. Repetitive hyperbaric oxygen treatment attenuates complete Freund’s adjuvant-induced pain and reduces glia-mediated neuroinflammation in the spinal cord. J Pain 2013;14(7):747–758. [DOI] [PubMed] [Google Scholar]
- [43].Iannitti T, Kerr BJ, Taylor BK. Mechanisms and pharmacology of neuropathic pain in multiple sclerosis. Current topics in behavioral neurosciences 2014;20:75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Janes K, Little JW, Li C, Bryant L, Chen C, Chen Z, Kamocki K, Doyle T, Snider A, Esposito E, Cuzzocrea S, Bieberich E, Obeid L, Petrache I, Nicol G, Neumann WL, Salvemini D. The Development and Maintenance of Paclitaxel-Induced Neuropathic Pain Requires Activation of the Sphingosine 1-Phosphate Receptor Subtype 1. J Biol Chem 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jin J, Hu J, Zhou W, Wang X, Xiao Q, Xue N, Yin D, Chen X. Development of a selective S1P1 receptor agonist, Syl930, as a potential therapeutic agent for autoimmune encephalitis. Biochemical pharmacology 2014;90(1):50–61. [DOI] [PubMed] [Google Scholar]
- [46].Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 2005;12(6):703–715. [DOI] [PubMed] [Google Scholar]
- [47].Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron 2004;41(3):323–335. [DOI] [PubMed] [Google Scholar]
- [48].Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, Group FS. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. The New England journal of medicine 2010;362(5):387–401. [DOI] [PubMed] [Google Scholar]
- [49].Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, Chiba K. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cellular & molecular immunology 2005;2(6):439–448. [PubMed] [Google Scholar]
- [50].Khan N, Gordon R, Woodruff TM, Smith MT. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharmacol Res Perspect 2015;3(3):e00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Khan N, Smith MT. Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology 2014;22(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khan N, Woodruff TM, Smith MT. Establishment and characterization of an optimized mouse model of multiple sclerosis-induced neuropathic pain using behavioral, pharmacologic, histologic and immunohistochemical methods. Pharmacol Biochem Behav 2014;126:13–27. [DOI] [PubMed] [Google Scholar]
- [53].Kim HJ, Miron VE, Dukala D, Proia RL, Ludwin SK, Traka M, Antel JP, Soliven B. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J 2011;25(5):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lisi L, Navarra P, Cirocchi R, Sharp A, Stigliano E, Feinstein DL, Dello Russo C. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. Journal of neuroimmunology 2012;243(1–2):43–51. [DOI] [PubMed] [Google Scholar]
- [56].Lu J, Kurejova M, Wirotanseng LN, Linker RA, Kuner R, Tappe-Theodor A. Pain in experimental autoimmune encephalitis: a comparative study between different mouse models. J Neuroinflammation 2012;9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubi-Robles M, Quarta S, Biasio W, Kuner R, Gibbins IL, Kress M, Haberberger RV. Genetic evidence for involvement of neuronally expressed S1P(1) receptor in nociceptor sensitization and inflammatory pain. PloS one 2011;6(2):e17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427(6972):355–360. [DOI] [PubMed] [Google Scholar]
- [59].Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(−4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos 2006;34(9):1480–1487. [DOI] [PubMed] [Google Scholar]
- [60].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature reviews Neuroscience 2009;10(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod). Journal of the neurological sciences 2008;274(1–2):13–17. [DOI] [PubMed] [Google Scholar]
- [62].Mix E, Meyer-Rienecker H, Hartung HP, Zettl UK. Animal models of multiple sclerosis--potentials and limitations. Prog Neurobiol 2010;92(3):386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 2009;5(6):428–434. [DOI] [PubMed] [Google Scholar]
- [64].Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T. Glutamate receptor expression in multiple sclerosis lesions. Brain pathology 2008;18(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. Journal of neuroimmunology 2013;256(1–2):13–18. [DOI] [PubMed] [Google Scholar]
- [66].O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 2008;137(1):96–111. [DOI] [PubMed] [Google Scholar]
- [67].Olechowski CJ, Parmar A, Miller B, Stephan J, Tenorio G, Tran K, Leighton J, Kerr BJ. A diminished response to formalin stimulation reveals a role for the glutamate transporters in the altered pain sensitivity of mice with experimental autoimmune encephalomyelitis (EAE). Pain 2010;149(3):565–572. [DOI] [PubMed] [Google Scholar]
- [68].Olechowski CJ, Truong JJ, Kerr BJ. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 2009;141(1–2):156–164. [DOI] [PubMed] [Google Scholar]
- [69].Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 2007;282(12):9082–9089. [DOI] [PubMed] [Google Scholar]
- [70].Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 2004;20(2):467–473. [DOI] [PubMed] [Google Scholar]
- [71].Rahn EJ, Iannitti T, Donahue RR, Taylor BK. Sex differences in a mouse model of multiple sclerosis: neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biology of sex differences 2014;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience 2010;169(4):1888–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rodrigues DH, Sachs D, Teixeira AL. Mechanical hypernociception in experimental autoimmune encephalomyelitis. Arquivos de neuro-psiquiatria 2009;67(1):78–81. [DOI] [PubMed] [Google Scholar]
- [74].Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunological reviews 2008;223:221–235. [DOI] [PubMed] [Google Scholar]
- [75].Rossi S, Lo Giudice T, De Chiara V, Musella A, Studer V, Motta C, Bernardi G, Martino G, Furlan R, Martorana A, Centonze D. Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br J Pharmacol 2012;165(4):861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Salvemini D, Doyle T, Kress M, Nicol G. Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol Sci 2013;34(2):110–118. [DOI] [PubMed] [Google Scholar]
- [77].Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009;89(2):707–758. [DOI] [PubMed] [Google Scholar]
- [78].Sanna MD, Quattrone A, Galeotti N. Silencing of the RNA-binding protein HuR attenuates hyperalgesia and motor disability in experimental autoimmune encephalomyelitis. Neuropharmacology 2017;123:116–125. [DOI] [PubMed] [Google Scholar]
- [79].Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 2004;279(14):13839–13848. [DOI] [PubMed] [Google Scholar]
- [80].Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2006;2(8):434–441. [DOI] [PubMed] [Google Scholar]
- [81].Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Archives of neurology 2003;60(8):1082–1088. [DOI] [PubMed] [Google Scholar]
- [82].Schmitz K, de Bruin N, Bishay P, Mannich J, Haussler A, Altmann C, Ferreiros N, Lotsch J, Ultsch A, Parnham MJ, Geisslinger G, Tegeder I. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol Med 2014;6(11):1398–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10(11):1361–1368. [DOI] [PubMed] [Google Scholar]
- [84].Sewell WA, Andrews P. Inhibition of lymphocyte circulation in mice by pertussis toxin. Immunology and cell biology 1989;67 (Pt 5):291–296. [DOI] [PubMed] [Google Scholar]
- [85].Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE. Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem 2009;110(4):1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun 2009;23(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Solaro C, Uccelli MM. Management of pain in multiple sclerosis: a pharmacological approach. Nature reviews Neurology 2011;7(9):519–527. [DOI] [PubMed] [Google Scholar]
- [88].Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005;128(Pt 5):1016–1025. [DOI] [PubMed] [Google Scholar]
- [89].Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Annals of neurology 2006;60(1):12–21. [DOI] [PubMed] [Google Scholar]
- [90].Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain 2005;114(3):473–481. [DOI] [PubMed] [Google Scholar]
- [91].Tarrason G, Auli M, Mustafa S, Dolgachev V, Domenech MT, Prats N, Dominguez M, Lopez R, Aguilar N, Calbet M, Pont M, Milligan G, Kunkel SL, Godessart N. The sphingosine-1-phosphate receptor-1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. International immunopharmacology 2011;11(11):1773–1779. [DOI] [PubMed] [Google Scholar]
- [92].Thibault K, Calvino B, Pezet S. Characterisation of sensory abnormalities observed in an animal model of multiple sclerosis: a behavioural and pharmacological study. Eur J Pain 2011;15(3):231 e231–216. [DOI] [PubMed] [Google Scholar]
- [93].Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. Journal of neuroimmunology 2004;153(1–2):108–121. [DOI] [PubMed] [Google Scholar]
- [94].Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Annals of neurology 2001;50(2):169–180. [DOI] [PubMed] [Google Scholar]
- [95].Ye Y, Zhao Z, Xu H, Zhang X, Su X, Yang Y, Yu X, He X. Activation of Sphingosine 1-Phosphate Receptor 1 Enhances Hippocampus Neurogenesis in a Rat Model of Traumatic Brain Injury: An Involvement of MEK/Erk Signaling Pathway. Neural Plast 2016;2016:8072156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yuan S, Shi Y, Tang SJ. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 2012;7(4):904–913. [DOI] [PubMed] [Google Scholar]
- [97].Zhang DD, Linke B, Suo J, Zivkovic A, Schreiber Y, Ferreiros N, Henke M, Geisslinger G, Stark H, Scholich K. Antinociceptive effects of FTY720 during trauma-induced neuropathic pain are mediated by spinal S1P receptors. Biol Chem 2015;396(6–7):783–794. [DOI] [PubMed] [Google Scholar]
- [98].Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol 2006;96(3):1042–1052. [DOI] [PubMed] [Google Scholar]
- [99].Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood 2010;115(23):4963–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Vehicle controls for both the fingolimod and W146 groups do not differ. Vehicle control for fingolimod (ethanol:0.85% sodium chloride 1:999) and W146 (1 M Na2CO3 b-cyclodextrinand 0.85% sodium chloride 1:1:8) were injected daily ( i.p.) from D27 to 41 (yellow). (A) Mechanical and (B) cold hyperalgesia were monitored prior to and through 41 days post-EAE induction. Data represent mean ± SEM. n= 3 mice per group. P>0.05 for both mechanical and cold hyperalgesia.
Supplemental Figure 2. S1PR1 antibody specificity. Specificity for S1PR1 antibodies was determined using total homogenate of whole spinal cord from female Nestin-S1PR1 cKO and WT mice. Lanes from each image were loaded with 20 mg protein. (A) Alomone #ASR-011 anti-Sphingosine 1-Phosphate Receptor 1 antibody (1:200). Vendor suggested band at 48 kDa. Positive reactivity was observed in lanes 1, 3, 5 and 7 (PNGase treated) in both cKO (lanes 1 and 3) as well as WT (lanes 5 and 7). No bands were observed in the absence of PNGase treatment to remove post-translational glycolsylation (lanes 2, 4, 6 and 8). (B) Abcam #ab11424 Anti-EDG1 antibody (1:20,000). Vendor suggested band at 44 kDa. Positive immunoreactivity was observed in both cKO (lanes 1 and 2) as well as WT (lanes 3 and 4). (C). EDG-1 Antibody (H-60; 1:500). Vendor suggested band at 37 kDa. Note the lack of immunoreactivity in cKO tissue (lanes 1, 2) compared to positive immunoreactivity in WT tissue (lanes 3, 4).