Abstract
One criticism of kidney paired donation (KPD) is that easy-to-match candidates leave the registry quickly, thus concentrating the pool with hard-to-match sensitized and blood type O candidates. We studied candidate/donor pairs who registered with the National Kidney Registry, the largest US KPD clearinghouse, from 1/2012–6/2016. There were no changes in age, gender, BMI, race, ABO, or PRA of newly registering candidates over time, with consistent registration of hard-to-match candidates (59% type O and 38% PRA≥97%). However, there was no accumulation of type O candidates over time, presumably due to increasing numbers of non-directed type O donors. Although there was an initial accumulation of candidates with PRA≥97% (from 33% of the pool in 2012 to 43% in 2014, p=0.03), the proportion decreased to 17% by June 2016 (p<0.001). Some of this is explained by an increase in the proportion of candidates with PRA≥97% who received a deceased donor kidney transplant after the implementation of the Kidney Allocation System, from 8% of 2012 registrants to 17% of 2015 registrants (p=0.02). In this large KPD clearinghouse, increasing participation of non-directed donors and the Kidney Allocation System have lessened the accumulation of hard-to-match candidates, but highly sensitized candidates remain hard-to-match.
INTRODUCTION
Kidney paired donation (KPD) was developed to help transplant candidates utilize a willing, but incompatible, living donor via a more compatible kidney transplant (1). While other countries have developed centralized national KPD programs (2–4), early United States (US) KPD registries have been fragmented, with many regional and single-center clearinghouses (5–8). This fragmentation particularly affected sensitized candidates, as the likelihood of identifying a more compatible match depends directly upon the size of the donor pool (9, 10). Early reports from regional and single-center registries concluded that the benefit of KPD was limited to easy-to-match candidates, and that registries became concentrated with highly sensitized or harder-to-match patients with unfavorable blood types (5, 10). Single-center KPD programs reported small pool sizes ranging from 24–160 candidates and more than 40% with PRA>80% (6, 7). Similarly, a regional US KPD program found that their pool of candidates with PRA>80% increased significantly from 34% in 2007 to 49% in 2014 (5). The small sizes of these registries limit their usefulness in drawing broad inferences about how a large multicenter KPD clearinghouse would perform in the US.
As a striking example, Canada’s national KPD program experienced a sharp accumulation in the prevalence of candidates with a panel-reactive antibody (PRA) 97% or higher in the first 18 months after inception, from 14% to 55% of registered candidates (2). Alongside this, there has not been widespread adoption of KPD in the US (8, 9, 11). However, since then, several factors have emerged which might reduce the concentration of hard-to-match candidates in KPD registries. First, the combination of incompatible kidney transplantation (IKT) and KPD has gained favor as a treatment option to allow for “a more compatible” match for highly sensitized candidates (12–15), possibly facilitating more matches for sensitized patients. Second, the implementation of the Kidney Allocation System (KAS) in 2014, which prioritizes highly sensitized candidates for deceased donor kidneys, allowed deceased donor kidney transplantation (DDKT) to become a more feasible treatment option for the very most sensitized candidates with the proportion of recipients with PRA 100% increasing from 1.0% to 10.3% after KAS implementation (16). This possibly decreased the concentration of these extremely hard-to-match candidates in KPD clearinghouses. Third, there has been an increase in the number of non-directed donors who can initiate “dominos” or “chains” of paired donation (1, 9, 17, 18), expanding the blood type distribution of KPD donors and facilitating matches that are not necessarily reciprocal (19). Finally, the National Kidney Registry (NKR) has grown substantially in the past decade and is currently the largest multi-center KPD clearinghouse in the US.
To determine whether, in the modern era of desensitization and kidney allocation, a large multi-center KPD clearinghouse would experience similar limitations and hard-to-match accumulation as smaller US and international KPD registries, we studied temporal trends in the NKR between 2012 and 2016.
METHODS
Study population
We studied all kidney transplant candidates, their paired potential donors, and non-directed donors without a specified recipient who completed registration in the National Kidney Registry (NKR) between 1/1/2012 and 6/30/2016. The potential donor who registered with a candidate as a pair is referred to as a “paired donor” while individuals who registered to donate a kidney without having a directed recipient are referred to as “non-directed donors.” Though candidates can register multiple paired donors to the NKR, for simplicity we reported on only the primary donor with whom candidates registered to the NKR. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Definition of incident and prevalent pools
We considered all new candidates and donors who registered over the course of an entire year to be the incident pool for that year. We considered all candidates awaiting a match in the registry on June 30 and December 31 of each year of the study period to be the prevalent pool on those dates. For example, a candidate who registered early in 2013 and left the registry late in 2015 would be a part of the prevalent pool on 6/30/2013, 12/31/2013, 6/30/2014, 12/31/2014, and 6/30/2015 (Figure 1).
Figure 1. Definition of incident and prevalent registry pools.
Subject A newly registered early in 2013 and left the pool late in 2015. Thus, subject A would be considered part of the incident pool in 2013, and would be a part of the prevalent pool on 6/30/2013, 12/31/2013, 6/30/2014, 12/31/2014, and 6/30/2015. Subject B would be part of the incident pool in 2013 and would be part of the prevalent pool on 12/31/2013 and 6/30/2014. Subject C would be part of the incident pool in 2014 and would be part of the prevalent pool on 6/30/2014 only.
One-year disposition of registering candidates
We described the disposition of newly registering candidates at one-year following registration, over calendar years. We categorized disposition as still waiting, KPD through the NKR, other living donor kidney transplant (LDKT), DDKT, and “other” removal from registry which included removal due to paired donor becoming unavailable, candidate died or became too sick to undergo transplantation, and removal from registry due to other or unknown reason.
Statistical analysis
Descriptive comparisons were made with t-tests, chi-square tests, and analysis of variance (ANOVA), as appropriate. We used a two-sided α of 0.05 to indicate a statistically significant difference. Statistical analysis was performed using Stata 14.2/SE for Windows (College Station, Texas).
RESULTS
Study population
There were 2209 candidate/donor pairs and 426 non-directed donors who registered with the NKR 1/1/2012 and 6/30/2016 (Table 1). Candidates were older than their paired donors (median 50 vs. 45 years, p<0.001), more likely to be male (51.3% vs. 38.9%, p<0.001), had similar body mass index (BMI, median 27 vs 26, p<0.001), were more likely to be African American (14.3% vs. 12.0%, p<0.001), and were more likely to have blood type O (58.7% vs. 31.2%, p<0.001). Candidates had a median PRA of 64% (interquartile range [IQR] 0–97%, range 0–100%). Non-directed donors were a similar age than paired donors (median 44 vs. 45 years, p=0.03), more likely to be Caucasian (84.3% vs. 70.0%, p<0.001), and more likely to have blood type O (46.2% vs. 31.2%, p<0.001). Non-directed donors increased in number over the time period, from 8 in 2012 to 129 in 2015, with no change in the proportion of non-directed donors with blood type O over time (p=0.2).
Table 1.
Characteristics of new registrations to the NKR, 1/1/2012–6/30/2016.
| Candidate (n=2209) | Paired Donor (n=2209) | Non-directed Donor (n=426) | p-value between candidates and their donors | p-value between paired donors and non-directed donors | |
|---|---|---|---|---|---|
| Age at registration in years, median (IQR) | 50 (38–60) | 45 (35–54) | 44 (33–53) | <0.001 | 0.03 |
| Male gender, n (%) | 1127 (51.3%) | 854 (38.9%) | 162 (38.0%) | <0.001 | 0.7 |
| BMI, median (IQR) | 27 (23–31) | 26 (24–29) | 26 (23–28) | <0.001 | <0.001 |
| Race, n (%) | <0.001 | <0.001 | |||
| Caucasian | 1455 (66.1%) | 1540 (70.0%) | 359 (84.3%) | ||
| African American | 315 (14.3%) | 263 (12.0%) | 26 (6.1%) | ||
| Hispanic/Latino | 243 (11.0%) | 224 (10.2%) | 23 (5.4%) | ||
| Asian | 101 (4.6%) | 92 (4.2%) | 11 (2.6%) | ||
| Other | 86 (3.9%) | 81 (3.7%) | 7 (1.6%) | ||
| ABO, n (%) | <0001 | <0.001 | |||
| A | 543 (24.6%) | 1003 (45.4%) | 158 (37.1%) | ||
| B | 319 (14.4%) | 398 (18.0%) | 61 (14.3%) | ||
| AB | 50 (2.3%) | 118 (5.3%) | 10 (2.4%) | ||
| O | 1297 (58.7%) | 688 (31.2%) | 197 (46.2%) | ||
| PRA, median % (IQR) | 64 (0–97) | ||||
| PRA category, n (%) | |||||
| 0% | 619 (23.9%) | ||||
| 1–50% | 366 (14.1%) | ||||
| 51–96% | 617 (23.8%) | ||||
| 97–100% | 986 (38.1%) | ||||
Incident registration
Over the study period, there were no statistically significant differences in candidate age (median 50 years), gender (51.3% male), BMI (median 27 units), race (66.1% Caucasian), proportion of blood type O (58.7%), or PRA category (38.1% with PRA≥97%), or in donor age (median 45 years), gender (38.8% male), BMI (median 26 units), race (72.3% Caucasian), or proportion of blood type O (33.6%).
One-year disposition of registering candidates
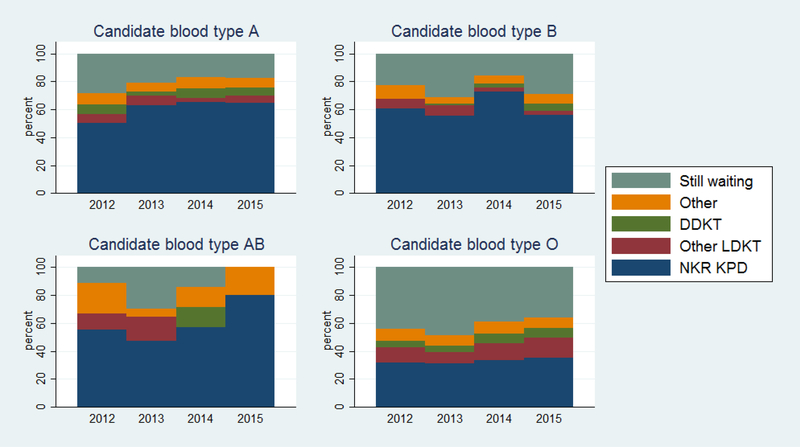
One year after registering, 45.0% of candidates matched into a KPD through the NKR, 8.6% received another LDKT, 5.1% received a DDKT, 7.2% left the registry for other reasons, and 34.2% were still waiting for a match. Candidates with blood type O were more likely to be still waiting at one year than candidates with other blood types (42.6% for type O vs. 21.2% for type A, 24.1% type B, 24.0% type AB) (Figure 2), although this improved over the study period from 44% of type O 2012 registrants still waiting at one year to 36% of 2015 registrants still waiting at one year (p=0.05).
Figure 2. Disposition of candidates at one-year after registration, by candidate ABO blood type.
More than half of candidates with blood types A and B matched into a KPD within a year of registration. Candidates with blood type O were more likely to be still waiting for a match a year after registration, compared to candidates with other blood types.
Candidates with PRA≥97% were more likely to be still waiting at one year after registration than candidates with less sensitization (57.5% for PRA≥97% vs. 22.0% for PRA 51–96%, 28.4% for PRA 1–50%, 28.4% for PRA 0%) (Figure 3), and this did not improve over the study period (p=0.09). However, the proportion of candidates with PRA≥97% who received a DDKT increased with time, from 8% of those who registered in 2012 to 17% who registered in 2016 (p=0.02).
Figure 3. Disposition of candidates at one-year after registration, by candidate PRA.
Candidates with PRA≥97% were more likely to be still waiting for a match a year after registration, compared to lesser sensitization. The proportion of candidates with PRA 97% or higher who received a DDKT within a year of registration increased from 4% in 2013 to 17% in 2015.
Prevalent candidate pool over time
There were no changes in the ABO blood type composition of the prevalent pool over the course of the study period (Figure 4). There was no increase in the proportion of the prevalent pool with recipient-paired donor ABO incompatibility, from 70.1% in 2012 to 75.2% in 2016 (p=0.3) (Table 2). While there was an increase in the proportion of candidates with PRA≥97% from 33% on 6/30/2012 to 43% on 12/31/2014 (p=0.03) (Figure 5), this subsequently decreased to 17% of the pool on 6/30/2016 (p<0.001).
Figure 4. ABO blood type of candidates in the prevalent registry pool over time.
There were no changes in the ABO blood type composition of the prevalent pool over the course of the study period.
Table 2.
Proportion of recipient and paired donor ABO type of the prevalent pool on 6/30 of each year.
| 2012 | 2013 | 2014 | 2015 | 2016 | 2012 | 2013 | 2014 | 2015 | 2016 | 2012 | 2013 | 2014 | 2015 | 2016 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dA | dB | dAB | dO | |||||||||||||||||
| rA | 6.1 | 10.0 | 7.9 | 9.4 | 9.0 | 2.7 | 2.5 | 3.5 | 1.4 | 2.1 | 0.7 | 1.6 | 0.4 | 0.7 | 2.8 | 6.8 | 6.2 | 5.3 | 4.7 | 5.5 |
| rB | 5.4 | 3.7 | 4.8 | 4.3 | 4.1 | 3.4 | 2.8 | 3.3 | 3.6 | 0.7 | 2.0 | 0.6 | 0.9 | 0.7 | 1.4 | 3.4 | 0.6 | 2.2 | 2.5 | 4.1 |
| rAB | 2.0 | 0.6 | 0.9 | 0.7 | 0 | 0 | 0.3 | 0.4 | 0.4 | 0 | 0 | 0.6 | 0.2 | 0 | 0.7 | 0.7 | 2.8 | 0 | 0 | 1.4 |
| rO | 34.0 | 34.3 | 38.8 | 42.1 | 35.2 | 10.9 | 11.5 | 11.6 | 11.9 | 16.6 | 1.4 | 2.5 | 2.4 | 2.2 | 2.1 | 20.4 | 19.3 | 17.1 | 14.7 | 14.5 |
Percent of total prevalent pool shown. Abbreviations: dA donor A, dB donor B, dAB donor AB, dO donor O. rA recipient A, rB recipient B, rAB recipient AB, rO recipient O.
Figure 5. PRA categories of candidates in the prevalent registry pool over time.
While there was an increase in the proportion of candidates with PRA≥97% from 33% on 6/30/2012 to 43% on 12/31/2014 (p=0.03), this subsequently decreased to 17% of the pool on 6/30/2016 (p<0.001).
DISCUSSION
In this study of 2209 NKR registrants, we found that, with continued new registration of highly sensitized candidates, there was an initial concentration of the prevalent registry pool with candidates with PRA 97%+. However, with an increase in the proportion of sensitized candidates who received a DDKT following implementation of KAS, the proportion of sensitized candidates in the prevalent registry pool decreased to 17% in mid-2016. Additionally, non-directed donors increased over time, from 8 in 2012 to 129 in 2015, and were more likely to have blood type O than paired donors (46.2% vs. 31.2%).
Our finding of an accumulation of highly sensitized candidates in the early years of the registry reaffirms the experience of other KPD programs (2, 5–7). The Canadian experience of a national KPD matching, in contrast to the NKR, did not include IKT as an option (2), though it has become more common in the US over the past decade (13, 20). In the Australian national KPD registry, the use of IKT was critical to find matches for sensitized candidates and those with blood type O, and a retrospective analysis found that 19 of 48 kidney transplants over 2 years would not have occurred without IKT (21). In the NKR experience, KAS has mitigated the continued accumulation of sensitized candidates in the prevalent registry pool by increasing the DDKT rate for these highly sensitized candidates (16). Another mitigating factor, particularly for ABO incompatibility, was the increase in non-directed donors over time (17). Non-directed donors, who were more likely to have blood type O, increased each year from 8 in 2012 to 129 in 2015.
An important limitation of this study is the use of administrative data from registration to a single KPD program. We do not know the details of NKR matching algorithms nor how they differ from other US KPD matching algorithms. We also do not comment on the impact of changes in the NKR’s matching algorithms or center-level programmatic changes over time. We are limited in our ability to comment on what portion of NKR matches required desensitization in order to proceed, as this happens at the transplant center and is not necessarily reported to the NKR. Finally, this study may not be generalizable to other countries with different DDKT allocation systems, and where non-directed donation might not be legal, such as the experience in India as reported by Kute et al. (22, 23). Still, this study provides insight into recent trends in transplant candidates registering to the largest KPD clearinghouse in the US.
We have demonstrated that in the setting of a large KPD clearinghouse, despite continued registration of highly sensitized candidates and those with blood type O, the prevalent registry pool does not become more hard-to-match over time. The Kidney Allocation System has mitigated accumulation of candidates with PRA≥97% in the registry pool over time by increasing the DDKT rate for these candidates. Non-directed donors who are more likely to have blood type O have also increased over time, mitigating accumulation of candidates with blood type O and providing the initial donation to facilitate chains of transplants. Transplant providers should encourage continued participation in KPD clearinghouses in order to facilitate more compatible transplantation, and also provide realistic counseling about likelihood of KPD matching and wait times
ACKNOWLEDGMENTS
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the US Government. Funding for this study was provided in part by the National Kidney Registry (NKR).
This work was supported by grant numbers F32DK109662 (Holscher), F32DK113719 (Jackson), F32DK105600 (DiBrito), K01DK101677 (Massie), K24DK101828 (Segev), and R01DK098431 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases, grant number F32AG053025 (Haugen) from the National Institute on Aging, and by an American College of Surgeons Resident Research Scholarship (Holscher).
Abbreviations:
- BMI
body mass index
- DDKT
deceased donor kidney transplantation
- IKT
incompatible kidney transplantation
- KAS
Kidney Allocation System
- KPD
kidney paired donation
- LDKT
living donor kidney transplantation
- NKR
National Kidney Registry
- PRA
panel-reactive antibody
- US
United States
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.SD L, GS E, WD S, B. R, MR A Kidney paired donation and optimizing the use of live donor organs. Jama 2005;293(15):1883–1890. [DOI] [PubMed] [Google Scholar]
- 2.Cole EH, Nickerson P, Campbell P, Yetzer K, Lahaie N, Zaltzman J et al. The Canadian kidney paired donation program: a national program to increase living donor transplantation. Transplantation 2015;99(5):985–990. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari PCL, Ta J, Woodroffe C, DʼOrsogna L, Holdsworth R. Providing Better-Matched Donors for HLA Mismatched Compatible Pairs Through Kidney Paired Donation. Transplantation 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Glorie K, Haase-Kromwijk B, van de Klundert J, Wagelmans A, Weimar W. Allocation and matching in kidney exchange programs. Transpl Int 2014;27(4):333–343. [DOI] [PubMed] [Google Scholar]
- 5.Fumo DEKV, Reece LJ, Stepkowski SM, Kopke JE, Rees SE, Smith C, Roth AE, Leichtman AB, Rees MA. Historical Matching Strategies in Kidney Paired Donation: The 7-Year Evolution of a Web-Based Virtual Matching System. Am J Transplant 2015;15(10):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauvais N, Tambur A, Warren E, Leventhal J, Friedewald J. Kidney paired donation at Northwestern Memorial Hospital. Clinical transplants 2011:291–298. [PubMed] [Google Scholar]
- 7.Bingaman AW, Wright FH Jr., Kapturczak M, Shen L, Vick S, Murphey CL. Single-center kidney paired donation: the Methodist San Antonio experience. Am J Transplant 2012;12(8):2125–2132. [DOI] [PubMed] [Google Scholar]
- 8.Massie AB GS, Montgomery RA, Bingaman AA, Segev DL. Center-level utilization of kidney paired donation. Am J Transplant 2013;13(5):1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. American journal of kidney diseases : the official journal of the National Kidney Foundation 2011;57(1):144–151. [DOI] [PubMed] [Google Scholar]
- 10.Segev DL KL, Gentry SE, Montgomery RA. Utilization and outcomes of kidney paired donation in the United States. Transplantation 2008;86(4):502–510. [DOI] [PubMed] [Google Scholar]
- 11.Melcher ML BC, Baxter-Lowe LA, Delmonico FL, Gentry SE, Leishman R, Knoll GA, Leffell MS, Leichtman AB, Mast DA, Nickerson PW, Reed EF, Rees MA, Rodrigue JR, Segev DL, Serur D, Tullius SG, Zavala EY, Feng S. Dynamic challenges inhibiting optimal adoption of kidney paired donation: findings of a consensus conference. Am J Transplant 2013;13(4):851–860. [DOI] [PubMed] [Google Scholar]
- 12.Garonzik Wang JM, Montgomery RA, Kucirka LM, Berger JC, Warren DS, Segev DL. Incompatible live-donor kidney transplantation in the United States: results of a national survey. Clinical journal of the American Society of Nephrology : CJASN 2011;6(8):2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant 2014;14(7):1573–1580. [DOI] [PubMed] [Google Scholar]
- 14.Golconda MS, de Mattos AM, Prather J, Olyaei AJ, Fletcher L, Head MA et al. Renal transplantation at Oregon Health and Science University: recent results and protocols. Clinical transplants 2003:149–154. [PubMed] [Google Scholar]
- 15.Montgomery RA ZA, Ratner LE, Segev DL, Hiller JM, Houp J, Cooper M, Kavoussi L, Jarrett T, Burdick J, Maley WR, Melancon JK, Kozlowski T, Simpkins CE, Phillips M, Desai A, Collins V, Reeb B, Kraus E, Rabb H, Leffell MS, Warren DS. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA 2005;294(13):1655–1663. [DOI] [PubMed] [Google Scholar]
- 16.Massie AB, Luo X, Lonze BE, Desai NM, Bingaman AW, Cooper M et al. Early Changes in Kidney Distribution under the New Allocation System. Journal of the American Society of Nephrology : JASN 2016;27(8):2495–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar K, Holscher CM, Luo X, Garonzik Wang J, Anjum S, King EA et al. Persistent regional and racial disparities in nondirected living kidney donation. Clin Transplant 2017;31(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentry SE MR, Swihart BJ, Segev DL. The roles of dominos and nonsimultaneous chains in kidney paired donation. Am J Transplant 2009;9(6):1330–1336. [DOI] [PubMed] [Google Scholar]
- 19.Melcher ML, Veale JL, Javaid B, Leeser DB, Davis CL, Hil G et al. Kidney transplant chains amplify benefit of nondirected donors. JAMA surgery 2013;148(2):165–169. [DOI] [PubMed] [Google Scholar]
- 20.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. The New England journal of medicine 2016;374(10):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari P HP, Cohney SJ, Woodroffe C, Fidler S, D’Orsogna L. ABO-incompatible matching significantly enhances transplant rates in kidney paired donation. Transplantation 2013;96(9):821–826. [DOI] [PubMed] [Google Scholar]
- 22.Kute VB, Shah PS, Vanikar AV, Gumber MR, Patel HV, Engineer DP et al. Increasing access to renal transplantation in India through our single-center kidney paired donation program: a model for the developing world to prevent commercial transplantation. Transpl Int 2014;27(10):1015–1021. [DOI] [PubMed] [Google Scholar]
- 23.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ et al. Seventy-seven kidney paired donation transplantations at a single transplant centre in India led to an increase in living donor kidney transplantations in 2015. Clin Kidney J 2017;10(5):709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]