SUMMARY
Myogenic differentiation of human pluripotent stem cells (hPSCs) has been done by gene overexpression or directed differentiation. However, viral integration, long-term culture, and the presence of unwanted cells are the main obstacles. By using CRISPR/Cas9n, a double-reporter human embryonic stem cell (hESC) line was generated for PAX7/ MYF5, allowing prospective readout. This strategy allowed pathway screen to define efficient myogenic induction in hPSCs. Next, surface marker screen allowed identification of CD10 and CD24 for purification of myogenic progenitors and exclusion of non-myogenic cells. CD10 expression was also identified on human satellite cells and skeletal muscle progenitors. In vitro and in vivo studies using transgene and/or reporter-free hPSCs further validated myogenic potential of the cells by formation of new fibers expressing human dystrophin as well as donor-derived satellite cells in NSG-mdx4Cv mice. This study provides biological insights for myogenic differentiation of hPSCs using a double-reporter cell resource and defines an improved myogenic differentiation and purification strategy.
Graphical Abstract

In Brief
Wu et al. demonstrate that, by generation of a double-reporter human embryonic stem cell line for skeletal muscle lineage, cells can be screened for governing pathways and surface markers, defining myogenic induction and purification protocol and evaluation of their regenerative potential using in vivo studies in a mouse model for muscular dystrophy.
INTRODUCTION
Skeletal muscle, as the largest tissue in the human body, has excellent regeneration potential, which allows exceptional recovery after minor to moderate injuries (Serrano et al., 2011). This capacity is mainly attributed to adult muscle stem cells (satellite cells), with unique self-renewal and differentiation potential (Wang and Rudnicki, 2011). However, this repair mechanism is not capable of protecting muscle against severe muscle disorders, such as muscular dystrophies, sarcopenia, cachexia, and mass loss injuries. Such disorders eventually lead to the exhaustion and loss of satellite cell reserve, muscle atrophy, and fibrosis (Evans, 2010; Juhas and Bursac, 2013; Serrano et al., 2011). Therefore, to ameliorate the lack of muscle regenerative potential in severe disorders, muscle replacement with healthy stem cells is a potential therapeutic option. In this regard, human pluripotent stem cells (hPSCs), specially induced pluripotent stem cells (iPSCs), are among the ideal candidates, due to their host compatibility and unique self-renewal and differentiation potential (Cao et al., 2005; Darabi and Perlingeiro, 2008; Inoue et al., 2014; Madonna, 2012; Sohn and Gussoni, 2004; Takahashi et al., 2009; Takahashi and Yamanaka, 2013). Additionally, iPSCs can be used for in vitro disease modeling, gene correction, and drug screen studies.
During the past decade, several efforts have been made to differentiate PSCs toward skeletal myogenic lineage (Barberi et al., 2007; Borchin et al., 2013; Chal et al., 2015; Choi et al., 2016; Darabi et al., 2008, 2011, 2012; Kim et al., 2017; Magli et al., 2017; Shelton et al., 2014; Tanaka et al., 2013; Tedesco et al., 2012; Xi et al., 2017; Xu et al., 2013). Initial reports by collaborators using myogenic gene overexpression during differentiation provided proof of the concept, i.e., myogenic potential of murine and human PSCs (Darabi et al., 2008, 2011, 2012; Darabi and Perlingeiro, 2016; Tanaka et al., 2013). Later on, transgene-free approaches were developed using directed differentiation toward mesodermal-paraxial lineages. These efforts employed by tuning major signaling pathways governing stem cell differentiation toward mesoderm and muscle, such as WNT, BMP, and transforming growth factor β (TGF-β) (Barberi et al., 2007; Borchin et al., 2013; Caron et al., 2016; Chal et al., 2015; Hicks et al., 2018; Shelton et al., 2014; Xi et al., 2017; Xu et al., 2013). Although these methods were successful in inducing skeletal myogenesis, parallel generation of unwanted cell types hindered the purity of the myogenic cells, as recently reported by our collaborators (Kim et al., 2017). Even in the most recent methods (Hicks et al., 2018; Shelton et al., 2016), the need for a long-term culture (up to 50 days) and associated difficulties (such as mixed cultures and difficulties in maintenance and harvest) are among major obstacles.
Considering these shortcomings and to study the chronological development pattern of skeletal myogenesis during differentiation of hPSCs, we have generated a PAX7/MYF5 double-reporter human embryonic stem cell (ESC) line using CRISPR/Cas9n-mediated homologous recombination (HR). PAX7 and MYF5 were chosen based on their unique roles in muscle stem cell (satellite cells) and early skeletal muscle lineage specification, thus enabling identification of early myogenic progenitors capable of expansion, self-renewal, and differentiation. This targeting strategy, along with a P2A-mediated bicistronic expression of the reporters (Wu et al., 2016a, 2016b), provided a myriad of advantages. First, it allowed screening for appropriate differentiation condition as well as identification of early myogenic precursors using bright expression of the reporters. It also allowed studying the effect of important pathways, identification of differential gene expression profile, and screening for surface markers of myogenic progenitors. Lastly, this approach enabled to define an efficient skeletal lineage induction and purification strategy applicable to transgene and/or reporter-free hPSCs. This was done using a high-throughput screen (HTS) for a panel of 242 human surface markers guided by double reporters to identify purification and exclusion markers (CD10 and CD24). Identified markers proved superior specificity for purification of myogenic cells, validated by in vitro and in vivo experiments. CD10 expression was also confirmed on human satellite cells and myogenic progenitors-myoblasts. This approach also allowed induction and purification of skeletal myogenic progenitors in a much shorter time course (2 weeks) with considerable in vitro and in vivo myogenic potential (myofiber engraftment and satellite cell seeding).
RESULTS
Medium Screen for Mesoderm-PAX7 Induction
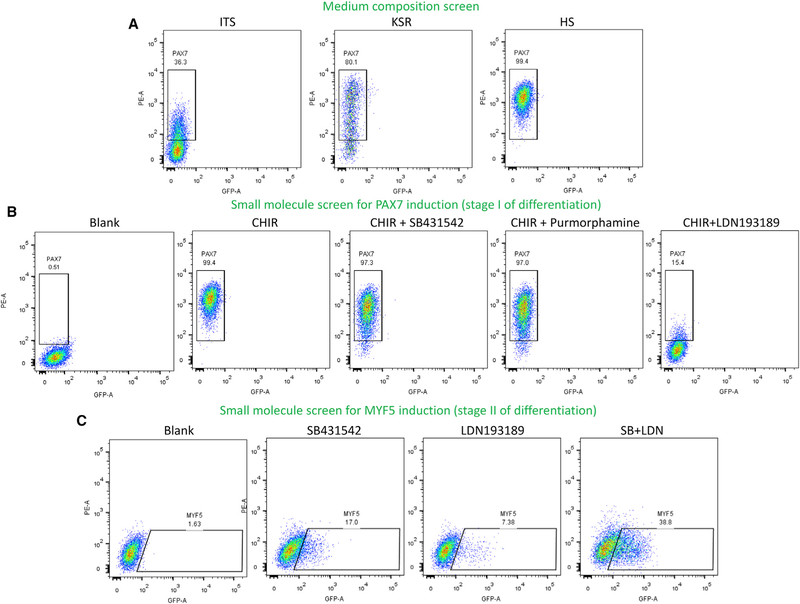
As skeletal muscle progenitors arise from mesoderm and subsequently paraxial-somite progenitors (Buckingham, 1996, 2006, 2017; Buckingham et al., 2003; Buckingham and Rigby, 2014; Tajbakhsh and Buckingham, 2000), we initially evaluated the effect of canonical WNT signaling on this process. It has been previously shown that WNT activation is sufficient to induce presomitic mesoderm formation from human ESCs (Barberi et al., 2007; Borchin et al., 2013; Borello et al., 2006; Hwang et al., 2014; Martin and Kimelman, 2012; Xu et al., 2013). However, the medium composition was different in each report and the efficacy of each condition was unclear. Therefore, we decided to screen commonly used media compositions with the addition of a WNT agonist (a glycogen synthase kinase-3 inhibitor- CHIR 99021). For the first stage of differentiation (stage I), human double-reporter ESCs (PAX7-tdTomato and MYF5-EGFP) were differentiated in different media supplemented with the WNT agonist (CHIR). Media consisted of three common compositions: two serum-free media containing ITS (insulin-transferrin-selenium) or KSR (knockout serum replacement) and one serum-containing medium supplemented with 5% horse serum (HS). Cells were differentiated in a 7-day time course study and analyzed daily by flow cytometry for induction of PAX7 (which marks early mesodermal-paraxial and/or neuroectodermal cells).
As demonstrated in Figure 1A, although serum-free conditions induced PAX7 induction to some degree (35.3% and 80.1% for ITS and KRS-based media, respectively) and both supported average cell survival, 5% HS-containing medium was the only condition which supported maximal, uniform, and bright PAX7 induction (99.4%) with excellent cell survival and expansion during next phases of expansion. Greatest PAX7 expression was observed after 4 days of induction and decreased afterward. Therefore, HS-containing medium was chosen for subsequent screening and differentiation experiments in the current study. Nevertheless, it is also important to point that, in case of the need for serum-free cell production, the protocol still can be adapted to either of the serum-free media as indicated above.
Figure 1. Small-Molecule Screen for Skeletal Myogenesis in PAX7/MYF5 Double-Reporter hESCs.
(A) Base medium screen for maximal PAX7 induction in the presence of WNT activator (CHIR). Base medium containing 5% horse serum (HS) induced maximal PAX7 expression with superior cell survival. ITS: insulin, transferrin, selenium; KSR: knockout serum replacer.
(B) Maximal PAX7 expression was achieved in the presence of CHIR after 4 days. As demonstrated in the right panel, PAX7 induction was inhibited in the presence of a BMP inhibitor, indicating the need for WNT activation along with endogenous BMP signaling for maximal induction of PAX7. CHIR: WNT activator. LDN193189: BMP inhibitor. purmorphamine: Smoothened (Smo) agonist. SB431542: TGF-β inhibitor.
(C) Screen for MYF5 induction. As demonstrated in the flow cytometry profiles, co-inhibition of BMP and TGF-β pathways created a synergistic effect on MYF5 induction. This can be explained by shifting the cells toward skeletal myogenic lineage through inhibition of sclerotome-hematopoietic formation. See also Figures S1 and S2.
Role of WNT, BMP, and TGF-β Signaling in Presomitic Mesoderm and PAX7 Induction in hESCs
Several important pathways (such as BMP, ACTIVIN/NODAL/ TGF-β, sonic hedgehog [Shh], Notch, P38 mitogen-activated protein kinase [MAPK], JAK/STAT, and cyclic AMP [cAMP]) have significant roles in initial patterning of the mesoderm toward somite and skeletal muscle lineage (Bernet et al., 2014; Buckingham, 1996, 2006, 2017; Chalamalasetty et al., 2011; Chen et al., 2016; de Angelis et al., 2005; Epperlein et al., 2007; Hagos and Dougan, 2007; Jiang et al., 2014; Keren et al., 2006; Martin and Kimelman, 2012; Price et al., 2014; Sheeba et al., 2016; Smith, 1999; Wen et al., 2012; Xi et al., 2017; Xu et al., 2013). Therefore, another screen was performed during the first stage of differentiation using WNT activation along with these pathways modifiers (Figures 1B and S2A). Interestingly, as demonstrated in Figure 1B, inhibition of endogenous BMP (using LDN193189) during first stage of differentiation (days 0–4) significantly inhibited the PAX7 expression. This finding indicated the importance of endogenous BMP signaling for initial induction of mesodermal cells (Winnier et al., 1995; Zhang et al., 2008). When compared to WNT activation (CHIR) alone, other pathway modulators did not show any significant effects on initial mesoderm-PAX7 induction. In agreement with previous reports, WNT activation alone was sufficient to induce mesodermal and/or neural PAX7-positive cells after 4 days (Borchin et al., 2013; Chal et al., 2015; Xu et al., 2013). As indicated above, at this stage, the presence of endogenous BMP signaling was a definitive requirement for maximal PAX7 induction. Another noteworthy finding was that, although co-inhibition of TGF-β at this stage did not have any effect on the percentage of PAX7+ cells compared to CHIR alone, it shortened the time needed for maximal PAX7 induction and improved subsequent MYF5 expression during the second stage of differentiation (Figure S2B). Therefore, for the first stage of differentiation (days 0–4), activation of WNT along with the presence of endogenous BMP and inhibition of TGF-β (MDM-I) was identified as the best combination for maximal PAX7 induction and subsequent myogenic differentiation of the cells.
Role of BMP and TGF-β Inhibition in Specification of Myotome and MYF5 Activation
Next, these conditions were compared during the second stage of differentiation (stage II), which was skeletal myogenic induction using the MYF5- EGFP reporter. For this stage, differentiated day 4 cells from each condition (Figure 1C) were switched to stage II of differentiation. The aim was to differentiate early mesodermal cells into somite-myotomal cells by providing appropriate signaling cues. This was accomplished by modification of potentially important governing pathways (BMP, TGF-β, Shh, Notch, P38 MAPK, and cAMP). Reporter cells were differentiated in stage II medium containing different small molecules and analyzed for MYF5 expression (which marks early-specified skeletal myogenic cells) by daily flow cytometry analysis for next ten days.
As shown in Figures 1C and S2C, although other pathways modifiers had minimal effects on MYF5 expression, inhibition of BMP or TGF-β was sufficient to induce MYF5 in the cells. This induction effect was increased synergistically by using both BMP/TGF-β inhibitors (MDM-II) and led to maximal myogenic induction in the cells between days 10 and 15 of differentiation (4 days during stage I + 6–11 days in stage II). This finding confirmed that the inhibition of BMP and TGF-β at second stage is required for directing the differentiation of early somitic mesodermal cells toward skeletal muscle lineage (Reshef et al., 1998; Tonegawa and Takahashi, 1998).
Defining In Vitro Myogenic Differentiation Protocol for Reporter hESCs
After the two-stage screening using the double-reporter line, a myogenic differentiation protocol was defined as illustrated in Figure 2A. This protocol allows for uniform and bright expression of PAX7 after 4 days of mesoderm induction, followed by the gradual myogenic fate specification in the subsequent 6–11 days. This is demonstrated by time course flow cytometry in Figure 2B. Using this protocol, maximal myogenic differentiation can be achieved by day 15 (average MYF5-EGFP expression of 50%–55%), which is remarkably shorter compared to the recent protocols (Chal et al., 2015; Hicks et al., 2018; Shelton et al., 2014). As demonstrated, both reporters demonstrated bright and well-separated expression from negative cells, which was critical for subsequent isolation and characterization of the cells.
Figure 2. Time Course Differentiation Profile of PAX7/MYF5 Double-Reporter hESCs toward Skeletal Muscle Progenitors.
(A) Schematic figure demonstrates optimized myogenic differentiation protocol in human pluripotent stem cells.
(B) Flow cytometry profile of PAX7/MYF5 reporter expressions during differentiation time course. Uniform expression of PAX7 (99%) was achieved after 4 days of differentiation. Maximal expression of MYF5 (52%) at 2nd stage of differentiation (4 + 11) can be achieved by co-inhibition of BMP and TGF-β pathways.
(C) Bright-field and direct fluorescent images of cells during differentiation time course. In bright-field images on top, cell morphology is gradually transitioned from epithelial to elongated-myogenic appearance during differentiation. Maximal PAX7-tdTomato expression is achieved after 4 days of differentiation, followed by its downregulation and subsequent upregulation of MYF5-EGFP in later time points (days 10–15). Lower panel shows merged images. Scale bars: 100 µm.
As expected, cell morphology also demonstrated typical induction of myogenic-mesenchymal fate during differentiation timeline, as epithelial-shaped cells gradually transformed toward elongated myogenic cells (Figure 2C, top). Direct fluorescent microscopy (Figure 2C, bottom) also confirmed strong and uniform induction of PAX7-tdTomato signal in the first few days (using WNT activation and TGF-β inhibition), followed by its downregulation and subsequent activation of MYF5-EGFP during the second stage of differentiation (BMP and TGF-β inhibition).
Evaluation of In Vitro Myogenic Differentiation Potential of the Reporter hESCs
To evaluate reporter and purification efficiency, differentiation potential of the cells was tested at different stages. At first, day 5 PAX7-tdTomato-positive cells were plated as bulk or single cell into myogenic differentiation medium to evaluate their myogenic potential. However, cells at this stage failed to differentiate into myotubes. This could be due to the inadequacy of short-term PAX7 expression to initiate the muscle fate program at this stage or the presence of mixed cell types expressing PAX7 (such as neural crest progenitor cells). Therefore, we shifted our assays to later time points when the cells express MYF5. Consequently, day 15 differentiated cells were sorted for MYF5-EGFP reporter and myotube differentiation potential was evaluated and compared to unsorted and MYF5-EGFP-negative cells by plating in myotube medium (MDM-III) for five days. As shown in Figure 3, although unsorted cells (Figure 3A) formed a mixed population and MYF5-EGFP-negative cells (Figure 3B, top) failed to differentiate into myotubes, MYF5-EGFP-reporter-positive cells (Figure 3B, bottom) were able to enrich for a homogeneous myogenic population with uniform myotube differentiation. Quantitative data analysis confirmed significant enrichment of myogenic cells in the MYF5-GFP-positive sorted fraction (p < 0.001 versus GFP-negative cells; Figure 3C). These cells can also be expanded exponentially in vitro while preserving myogenic potential (Figures S3A and S3B). In addition, in order to evaluate whether this differentiation protocol leads to formation of PAX7-positive reserve cells in the culture, in cohort sets of experiments, cells were kept for few more weeks in stage 2 medium and were evaluated for reactivation of PAX7-tdTomato expression. Interestingly, after five weeks of differentiation, a sub-fraction of the cells (14.7%) started to re-activate PAX7-tdTomato reporter expression, indicating the potential of current differentiation protocol to support in vitro formation of PAX7+ myogenic reserve pool (Figures S3C and S3D). Together, these results further confirmed the skeletal myogenic induction efficiency as well as validation of MYF5 reporter activity in the current setting. Compared to the recent reports (Chal et al., 2015; Hicks et al., 2018; Shelton et al., 2014), this protocol allowed shorter myogenic induction, along with a prospective screening and purification readout. Furthermore, these data clearly indicated the presence of a notable fraction of non-myogenic cells in the culture (near to 45%) and therefore the necessity for purification of the myogenic cells.
Figure 3. In Vitro and In Vivo Myogenic Differentiation Potential of the PAX7/MYF5 Double-Reporter hESCs.
(A) Image demonstrates myotube differentiation potential of unsorted cells. Myotube staining using a myosin heavy chain (MHC) antibody (red) indicates presence of unwanted non-myogenic cells in the explant (DAPI+ MHC ) in upper right image. Scale bars: 200µm.
(B) Cell sorting for MYF5-EGFP-positive and negative fractions enables purification of myogenic population and exclusion of unwanted non-myogenic cells. Terminally differentiated myotubes expressed MHC (red) and formed multinucleated myotubes in MYF5-EGFP+ fraction. Scale bars: 200µm.
(C) Graph shows myotube count after differentiation. Cells were plated in similar densities in myotube differentiation medium and the number of myotubes quantified after MHC staining (data are mean ± SEM; n = 6 images from 2 biological replicates per group). ***p < 0.001 versus other cell fractions.
(D) In vivo regeneration potential of sorted MYF5-EGFP-negative cells after injection into the TA muscles of immunodeficient NSG mice. MYF5-EGFP-negative cell nuclei were barely detectable in the muscle (stained with human-specific nuclear lamin A/C in green) and did not express terminal differentiation marker of human dystrophin (red). Scale bars: 100µm.
(E) In vivo regeneration potential of sorted MYF5-EGFP-positive cells one month after injection into the TA muscles of immunodeficient NSG mice. As shown in upper panel, these cells were able to engraft efficiently into the injected muscle and contributed in new myofiber formation with abundant expression of human-specific markers (human nuclear lamin A/C and human dystrophin). Lower panel demonstrates magnified myofibers expressing human-specific dystrophin (red) with attached human-derived nuclei marked with human nuclear lamin A/C (green). Scale bars: 100 µm.
(F) Quantification of human cell-derived myofibers after staining with a human-specific dystrophin antibody (data are mean ± SEM; n = 5 sections per mice and N = 6 mice per group). ***p < 0.001 versus GFP cells.
See also Figure S3.
Evaluation of In Vivo Engraftment Potential of the Reporter hESCs
Considering the fact that hPSCs are one of ideal candidates for regenerative medicine, evaluation of in vivo engraftment in animal models is important. Therefore, in order to validate myogenic potential of the cells, in vivo regeneration was tested by intramuscular injection of different cell fractions into the cardiotoxin-damaged tibialis anterior (TA) muscles of immunodeficient NOD-scid IL2Rgammanull (NSG) mice. As demonstrated, although the MYF5-negative cells were unable to engraft (Figure 3D), MYF5+ cells engrafted efficiently into the damaged muscles and were able to differentiate into mature fibers expressing human-specific markers of nuclear lamin A/C and dystrophin (Figure 3E). Abundant expression of human dystrophin in the transplanted muscles (average of 116 human dystrophin-positive fibers and muscle; Figure 3F), along with their associated human nuclei, confirmed the efficiency of this differentiation protocol to generate engraftable skeletal myogenic progenitors.
Transcriptome Profiling of the Reporter hESCs during Differentiation Time Course
After establishing the differentiation protocol and to identify the transcriptional signature of the cells at different time points, a time course transcriptional profiling was done using RNA sequencing (RNA-seq). Cells were harvested at day 0 (undifferentiated), day 4 (100% PAX7 positive), day 10 sorted for MYF5-negative and positive fractions, and day 20 differentiated myotubes from MYF5-positive fraction. For each sample, 18 million mapped reads were collected and aligned to GENECODE annotated genes (median 17,894,077 reads). Genes not sufficiently quantified were filtered out, which resulted in a set of 21,249 quantified genes (for criteria, see data processing section in STAR Methods). Analysis on global patterns of gene expression across all time points using principal-component analysis (PCA) (Figure 4A) revealed that the majority of the variation in transcriptional profile comes from the variation between differentiation time points. In particular, the first two principal components explain 55.3% of the total variance and separate samples into three main clusters that can be categorized by time points (Figure 4A). This separation between time points is in sharp contrast to the tight clustering of biological replicates of each time point (Figure 4A), indicating that the major signal in RNA-seq data captures transcriptome variation during myotube differentiation, as opposed to technical or biological variations between samples. The clustering of latter time points (i.e., day 10 and beyond; top right in Figure 4A) was further separated by projecting data to principal components 4 and 5 (Figure S4A), which explains 14.4% of the total variance.
Figure 4. Transcriptome Profiling of the PAX7/MYF5 Double-Reporter hESCs during Differentiation Time Course.
Skeletal muscle lineage differentiation was analyzed at five different time points during differentiation (day 0, day 4, day 10 sorted for MYF5-positive and negative fractions, and day 20 after myogenic differentiation of MYF5-positive fraction). For each time point, 3 independent biological replicates were analyzed.
(A) Major variations in transcript level reflect time point differences. Scatterplot showing RNA-seq data projected onto the first two principal components: each data point represents an individual sample.
(B) Heatmap showing scaled expression level of lineage marker genes at each time point. Expression level (log2CPM) was scaled to a Z score for each gene. The sample clustering and branch length of the dendrogram were resulted from hierarchical clustering of the scaled expression data using Euclidian distance.
(C) Differential gene expression between MYF5-positive and negative cells. Each data point represents a gene: position along the x axis indicates log2 ratio of transcript level between MYF5-positive and MYF5-negative, position along the y axis indicates significance level, and color of each data point indicates whether the gene is significantly different between cell types at a significance cutoff of FDR 0.05 (blue, significant; gray, not significant; red, a few example genes).
(D) Upregulated genes in MYF5-positive cells (i.e., relative to MYF5-negative cells) enriched of striated skeletal-muscle-related processes. Gene ontology (GO) enrichment analysis results are shown in a bar plot for top ten significantly enriched biological process (BP) terms. For each GO term, bar height represents the proportion of upregulated genes out of the total number of quantified genes associated with the term (positive value) and the corresponding log10 p value (negative value).
(E) Expression profile of selected genes demonstrates chronological activation of mesodermal/limb genes at day 4 and subsequent skeletal myogenic program activation and terminal maturation in day 10 MYF5-positive and day 20 myotube fractions, respectively. Data are mean ± SEM of log2CPM values. See also Figures S4 and S5.
To evaluate whether differentiation protocol produces cells of the expected lineage at each stage, we next focused on expression level profile of marker genes that are known to participate in the process of multi-lineage differentiation. A list of genes known to have high expression levels in relevant cell lineages (Table S1) was compiled. Hierarchical clustering based on expression level of cell lineage marker genes clustered samples into time point groups similar to the results from PCA on the full dataset (Figures 4A and 4B). High expression level of presomitic mesoderm-somite/limb development genes (e.g., MIXL1, LMX1B, LIX1, PAX7, and PAX3; Figure 4E) as well as neural crest markers (e.g., NRXN3, DRAXIN, and TFAP2C) in day 4 (Figure S4C) indicated the induction of mesoderm and neural lineages during the first stage of differentiation.
On the other hand, day 10 samples demonstrated clear lineage distinction between MYF5-positive and negative fractions (Figures 4B–4E and S5), indicating the necessity of further purification. Although the MYF5-positive fraction demonstrated high levels of skeletal muscle lineage genes, such as MIR133B, MYF5, MYOD1, MYOG, and CKM (Figures 4B–4E and S4D), MYF5-negative cells showed high expression levels of genes associated with sclerotomal-hematopoietic-neural lineages, such as MEOX1, BMPER, CKIT, SIM1, and NELL1 (Figures 4B, 4C, and S5B). MYF-5-positive transcriptome data were also compared with ENCODE polyA RNA-seq data of the human skeletal myoblasts from the World lab (GEO: GSM958744; ENCODE Project Consortium, 2012), indicating comparable gene expression and clustering profile, as demonstrated in Figure S4B. To further characterize the cell lineage differences between MYF5-positive and MYF5-negative fractions of day 10 cells, differential expression tests were performed on the RNA-seq data. 1,540 differentially expressed genes were found between the two fractions at 5% false discovery rate (FDR) out of 21,249 genes quantified (Figure 4C). To identify biological processes that could distinguish between the two cell populations, gene ontology (GO) enrichment analysis was performed, respectively, on genes with increased expression in MYF5-positive cells (MYF5-positive genes) and genes with increased expression in MYF5-negative cells (MYF5-negative genes). Enrichment of striated muscle-related terms was found in MYF5-positive genes (Figure 4D; e.g., GO: 0006941 striated muscle contraction p < 10 22; GO: 0061061 muscle structure development p < 10 29) and MYF5-negative genes (Figure S5A) demonstrated enrichment of terms associated with general and nervous system development (Figure S5A; e.g., GO: 0048856 anatomical structure development p < 10 17; GO: 0007399 nervous system development p < 10 16). Altogether, these data confirmed the commitment of MYF5-EGFP-positive fraction to the skeletal muscle lineage and the presence of unwanted differentiating cells (sclerotomal and neural cells) in the negative fraction and therefore the need for purification of the skeletal myogenic fraction.
Surface Marker Screen for Purification of Skeletal Myogenic Progenitors from hPSCs
One of the main goals of this study was to define an efficient myogenic induction and purification protocol in a prospective manner and applicable to the transgene and/or reporter-free hPSCs. Therefore, after establishing the differentiation protocol using reporter cells, an unbiased and comprehensive surface marker screen was done to identify purification markers for skeletal myogenic progenitors. Cells were stained at different time points expressing either reporters (day 4 PAX7-tdTomato and day 10 MYF5-EGFP) for a comprehensive panel of human surface marker monoclonal antibodies containing 242 antibodies (Figure 5A). This list contains a broad range of validated human monoclonal antibodies, including a majority of the previously reported markers for myogenic cells. The samples were then analyzed using a HTS flow cell analyzer to identify any surface marker overlapping or excluding the reporters. As expected, there was no single specific marker only expressed by the myogenic progenitors. Nevertheless, we were able to identify two markers (CD10 and CD24) as positive and negative selection for purification of skeletal myogenic progenitors at the emergence point of MYF5-EGFP-positive cells. As demonstrated in Figure 5B, MYF5-EGFP-positive cells overlapped mainly with CD10+CD24 fraction, suggesting these two markers can be used for purification of the myogenic progenitors. Next, time course expression profiling by flow cytometry confirmed gradual increase of this fraction from day 10 with the peak at day 15 of differentiation.
Figure 5. High-Throughput Surface Marker Profiling Identifies CD10 and CD24 as Markers for Skeletal Myogenic Lineage Enrichment during hESC/iPSC Differentiation.
(A) Schematic figure demonstrating surface marker screening process at days 4 and 10 during PAX7 or MYF5 expression time points.
(B) Double staining of the day 15 differentiated cells for CD10 and CD24 demonstrates co-localization and/or overlap of MYF5-EGFP-positive cells in CD10+CD24 fraction.
(C) Sorting the four cell fractions of differentiated hESCs for CD10 and CD24 confirms skeletal myogenic enrichment of the cells within CD10+CD24 fraction. Sorted cells were plated with similar densities in myotube differentiation medium for 5 days and stained for MHC (red) to mark differentiated myotubes. Scale bars: 100µm.
(D) Graph shows myotube count after differentiation. Cells were plated in similar densities in myotube differentiation medium and the number of myotubes quantified after MHC staining (data are mean ± SEM; n = 6 images from 2 biological replicates per group). ***p < 0.001 versus other cell fractions.
(E) Myogenic enrichment potential of CD10+CD24 fraction tested in transgene and/or reporter-free hPSCs further validates the specificity of the identified markers to enrich myogenic cells. Scale bars: 100µm.
(F) Immunostaining and quantification of CD10 on human muscle biopsy cross-section demonstrates expression of CD10 (green) on human satellite cells co-expressing PAX7 (red). Bar graph on right demonstrates quantification of positive satellite cells for CD10 in the stained muscle sections (data are mean ± SEM; n = 6 images from a human muscle biopsy). Scale bars: 100µm.
(G) Evaluation of CD10 and CD24 expression on human primary myoblasts indicates uniform expression of CD10 and the lack of CD24 expression on human primary myoblasts. See also Figure S6.
To confirm specificity of the markers to enrich for myogenic cells, differentiated cells were sorted from all four fractions after staining for CD10 and CD24 and evaluated for in vitro myotube differentiation potential (Figures 5C and 5D). As demonstrated, although other fractions hardly differentiated into myotubes, CD10+CD24 cells were able to significantly enrich the majority of the myogenic cells and uniformly differentiated into myotubes (MHC+DAPI+). Quantification data (Figure 5D) indicated significant enrichment of myotubes in differentiated cultures of CD10+CD24 fraction compared to others (average of 272 myotubes/low magnification image; p < 0.001 compared to other fractions). Next, for further validation, differentiation and purification was done on three other transgene and/or reporter-free hESC/iPSC lines using CD10 and CD24 surface markers. The cells were differentiated according to the protocol and sorted for all four fractions at day 15 and then tested for myotube differentiation. As demonstrated (Figures 5E and S6), only CD10+CD24 sorted fractions from other cell lines were also able to enrich skeletal myogenic cells capable of myotube differentiation. Next, in order to determine the expression of CD10 on satellite cells, human muscle sections were stained for PAX7 and CD10. As demonstrated in Figure 5F, results indicated the expression of CD10 in majority of human satellite cells (average of 80%; based on quantification of stained sections for PAX7 and CD10). These markers were also evaluated on primary human skeletal myoblasts (HSKM Skeletal Myoblasts; Gibco), which demonstrated uniform expression of the CD10 and the absence of CD24 on human primary myoblasts (Figure 5G).
Next, in order to determine the specificity of these markers compared to the previously reported ones, they were compared with ERBB3 and NGFR (Hicks et al., 2018). Using a 50-day differentiation method (Shelton et al., 2014), the authors had compared ERBB3/NGFR to previously identified markers (NCAM/HNK) and concluded their advantage for identification of the hPSC-derived myogenic progenitors (Hicks et al., 2018). Therefore, we initially tried the 50-day differentiation protocol (Hicks et al., 2018; Shelton et al., 2014, 2016). As demonstrated in the Figure S7, the culture led to the formation of a dense, multi-layered, mixed cell population. As expected, fluorescent staining also indicated the presence of a mixed culture containing myoblasts-myotubes (mainly expressing late markers, such as MYOGENIN and myosin heavy chain [MHC]) and neural cell aggregates (expressing b-tubulin-TuJ-1; Figure S7B). Subsequently, presence of mixed cell types led to partial detachment of the cells during myotube differentiation phase and significant difficulty of the cell isolation in our hands (Figures S7B and S7C).
As an alternative, we next evaluated the enrichment potential of ERBB3 and NGFR using current short-term protocol. Therefore, 2-week myogenic differentiation was performed and expression of ERBB3/NGFR was evaluated with reporter signals (PAX7-tdTomato and MYF5-EGFP). These data are demonstrated in Figure 6A. As demonstrated, myogenic cells (MYF5-EGFP-positive cells) were similarly distributed in all three sub-fractions of ERBB3/NGFR without major enrichment (flow cytometry dot plot in Figure 6A, right). In addition, similar non-specific pattern was detected at day 5 using PAX7-tdTomato reporter (Figure 6A). Subsequently, to test the myogenic enrichment potential, sorted fraction of ERBB3/NGFR from days 5 and 15 were plated in similar densities, subjected to myotube differentiation, and stained for MHC. Day 5 sorted cell fractions could not differentiate into myotubes. On the other hand, day 15 sorted cell fractions of ERBB3/NGFR (Figure 6B) contained mixed populations of myotubes (MHC+DAPI+) along with evident presence of non-myogenic cells (MHC DAPI+) in all three sorted cell fractions. Finally, quantification data (Figure 6C) confirmed significantly lower levels of myotube formation in each fraction (average of 120–170 myotubes per field) when compared to CD10+CD24 cells (average of 272; p < 0.001; Figure 6C). These data confirmed advantage of CD10/CD24 cocktail for enrichment of myogenic cells while using current short-term protocol.
Figure 6. Evaluation of ERBB3 and NGFR Markers for Purification of Myogenic Progenitors Using Double-Reporter hESC Line.
(A) ERBB3/NGFR staining of double-reporter hESC line at day 0, day 5 (maximal PAX7-tdTomato expression), and day 15 (maximal MYF5-EGFP expression) using current protocol. Flow cytometry plots demonstrate distribution of color reporters (PAX7-tdTomato and MYF5-EGFP) for stained markers at different days.
(B) Day 15 sorted cell fractions for ERBB3/NGFR show mixed myogenic differentiation and relative lack of enrichment during current differentiation protocol. After the sort, cells were plated in similar densities in myotube differentiation medium for terminal differentiation. Resulted myotubes were then stained for MHC for confirmation and quantification. Scale bars: 100µm.
(C) Graph shows myotube quantification after differentiation for ERBB3/NGFR sorted fractions. Cells were plated in similar densities in myotube differentiation medium and the number of myotubes quantified after MHC staining. Data from CD10+CD24 fraction (from Figure 5) are also included for side-by-side com-parison (data are mean ± SEM; n = 6 images from 2 biological replicates per group). ***p < 0.001 versus other cell fractions. See also Figure S7.
Evaluation of In Vivo Regeneration and Self-Renewal Potential of hPSC-Derived Myogenic Progenitors
In order to compare engraftment potential of the cells, in vivo regeneration potential of CD10/CD24- versus ERBB3/NGFR-sorted cells was evaluated in 6–8 weeks old NSG-mdx4Cv mice. Cells were differentiated using current protocol, sorted for either surface markers, and injected (2.5 3 105 cells/10 mL PBS) into the cardiotoxin-damaged TA muscles (non-irradiated). We chose to transplant the cells with two considerations: (1) us-ing lower cell numbers (compared to Hicks et al., 2018 report), thus avoiding oversaturation of TA muscle with donor cells as a limiting factor for engraftment, and (2) using non-irradiated muscles to be able to accurately quantify donor-cell engraftment and self-renewal in the presence of host satellite cells. This also allows competing with host muscle stem cells, which is a more realistic situation in case of cell therapy in patients with muscle disorders. One month after injections, muscles were harvested and subjected to immunofluorescence staining for human cells and dystrophin, and quantification using serial sectioning (Figures 7A–7D). As demonstrated, intramuscular injection of the enriched fractions for CD10+CD24 into the muscle of the dystrophin-deficient NSG-mdx4Cv mice resulted in proper engraftment of the cells into myofibers expressing human markers (maximum of 50–60 fibers positive for human dystrophin and lamin A/C; Figures 7B and 7D). In comparison, with equal number of transplanted cells, ERBB3/NGFR fractions were able to form maximum of 10–15 myofibers positive for human dystrophin and lamin A/C, which is significantly less when compared to CD10+CD24 cells (p values less than 0.05– 0.001; Figures 7C and 7D). If compared to the published report of average of 137 fibers for ERBB3 fraction (Hicks et al., 2018), current level of engraftment (10–15 fibers, i.e., 8–10 times less) is approximately proportional to the reported range, considering using lower cell number (one-eighth) and the lack of muscle irradiation in current experimental setting.
Figure 7. Comparing In Vivo Engraftment and Satellite Cell Seeding Potential of Transgene and/or Reporter-free hESC/iPSCs Using CD10/CD24 versus ERBB3/NGFR Surface Markers.
(A) Schematic view of myogenic differentiation, sort, and intramuscular transplantation of a transgene and/or reporter-free hESC(H9) into NSG-mdx4Cv mice using the current protocol.
(B) One month after intra-muscular (IM) injection of CD10+CD24 sorted cells, abundant human-derived myofibers can be identified at the site of injection. Sections are stained with human-specific dystrophin and lamin A/C antibodies to mark donor-derived fibers and nuclei. Scale bars: 100µm.
(C) Engraftment of sorted cells for ERBB3/NGFR one month after IM injection. Scale bars: 100µm.
(D) Quantification of human nuclei and human dystrophin-positive fibers using serial sectioning along the injection site. Schematic figure on the top-right demonstrates the sectioning and quantification interval, spanning the injection site. Graphs show quantification of human nuclei (positive for human lamin A/C) and myofibers (positive for human dystrophin) in different sorted cell groups (data are mean ± SEM; n = 6 serial muscle sections per mice and N = 6 mice per group). Vp < 0.05 versus ERBB3 /NGFR ; ϮϮp < 0.01 versus ERBB3+/NGFR+; ***p < 0.001 versus ERBB3 /NGFR+.
(E) Identification of donor-derived satellite cells expressing human lamin A/C and Pax7 indicates the ability of the CD10+CD24 cells to seed satellite cells in vivo after transplantation. Magnified image in lower right demonstrates a donor-derived satellite cell with nuclear expression of Pax7 in red and human lamin A/C in green. Pie graph indicates quantification of percent PAX7-positive human nuclei (PAX7+ hLamin A/C+) among donor-derived cells (hLamin A/C+) one month after intramuscular transplantation of CD10+CD24 cells (data are mean; n = 6 muscle sections). Scale bars: 100 µm.
(F) Engraftment of CD10+CD24 purified fraction of transgene and/or reporter-free hPSCs and formation of new fibers expressing human-specific dystrophin (red) and hLamin A/C (green). Scale bars: 100µm.
Finally, analysis of the engrafted regions in CD10+CD24– group confirmed the ability of the cells to seed into satellite cell niche and identification of donor-derived satellite cells expressing human markers (Figure 7E). Quantification data indicated the presence of 12% ± 1.9% (mean ± SEM) of human PAX7+ nuclei-satellite cells in the engrafted region. This percent is very similar to the percent of PAX7-positive reserve cells (14%), which arise during long-term culture (Figures S3C and S3D). This finding indicated the ability of the CD10+CD24 cells to restore the pool of muscle stem cells after transplantation. We were not able to identify PAX7+ human nuclei in ERBB3/NGFR cell fractions after transplantation, which might be due to low level of engraftment and the presence of unwanted cells in the sorted fractions here.
At last, this strategy was further validated by CD10+CD24–purification and engraftment of different transgene and/or reporter-free hPSC clones in NSG-mdx4Cv mice, leading to the formation of donor-derived myofibers in the transplanted muscles, as demonstrated in Figure 7F. Altogether, these data indicated the application potential of the current strategy for skeletal myogenic induction and purification from transgene and/or reporter-free hPSCs and their in vivo regeneration potential.
DISCUSSION
Although pluripotency potential of the hPSCs is a major reason for their consideration in regenerative medicine, it also carries the risk of the generation of mixed or unwanted cell population. Therefore, parallel formation of impure lineage progenitors jeopardizes their therapeutic application (Okano et al., 2013; Shi et al., 2017). In order to overcome this major problem, precise identification of differentiation cues for each lineage is necessary.
In the case of skeletal myogenesis, this can be done by proper induction of paraxial mesoderm in iPSCs and subsequent derivation of myotomal-skeletal muscle progenitors. So far, previous studies have suffered from the lack of prospective readout to guide the differentiation path and subsequently purify myogenic progenitors and therefore carrying unwanted or mixed cell populations (Kim et al., 2017). Here, we have taken the advantage of generating a double-reporter hESC line for muscle lineage. This approach allowed screening important pathways, defining a clear temporal induction protocol and, subsequently, identification and isolation of skeletal muscle progenitors.
The current study also confirmed the significant role of WNT activation along with endogenous BMP signaling for efficient induction of presomitic mesoderm during early stages of differentiation. Interestingly, we also demonstrated the need for TGF-β inhibition during this stage to allow robust myogenic induction in later stages. Compared to previous reports using WNT activation alone for first stage (Borchin et al., 2013; Chal et al., 2015; Xi et al., 2017), the presence of the reporter system allowed to improve the efficiency of the somatic mesoderm induction by addition of TGF-β inhibitor as well as preserving endogenous BMP signaling at the first stage. Furthermore, we have demon-strated the pivotal role of BMP through its inhibition at the second stage for proper induction of skeletal myogenesis by blocking lateral plate and sclerotome formation. Skeletal myogenic induction at this stage was further enhanced by TGF-β inhibition, which acted synergistically to further inhibit sclerotomal derivatives, in agreement with a recent report (Xi et al., 2017).
In addition, transcriptome profiling indicated commitment of PAX7-expressing cells to somitic mesoderm/limb or neural crest development fate at the first stage of differentiation. Interestingly, as differentiation moved toward the second stage of differentiation, neural cells were enriched in the MYF5-negative fraction, which also contained other non-myogenic derivatives of the mesoderm. RNA-seq and GO- biological process (BP) analysis also demonstrated well separation of striated muscle lineage fate (MYF5+) from non-myogenic sclerotomal-neural cells (MYF5 ) at the 2nd stage of differentiation. Furthermore, in vitro and in vivo myogenic commitment of the myogenic cells was confirmed by uniform myotube formation, alongside with significant engraftment of cells after intramuscular injection into NSG mice.
As purification of hPSC-derived lineage-specific progenitors is important for disease modeling as well as regenerative medicine (Miura et al., 2009; Okano et al., 2013; Shi et al., 2017), another major goal of the current strategy was to define a clear prospective approach for purification of myogenic cells using an unbiased HTS surface marker screen and without the need for reporters. As indicated in the results, we identified two positive and negative markers of CD10 and CD24, which, though non-specific if used alone, if used with the current protocol were able to mark MYF5+ myogenic progenitors. Indeed, CD10+CD24 fraction appropriately overlapped with the MYF5-EGFP+ reporter cells. To further strengthen this purification approach, we have applied this purification strategy to other transgene and/or reporter-free human PSCs.
It is also important to note that, although previous reports (Borchin et al., 2013; Magli et al., 2017) have identified other useful and important markers (such as ACHR, NCAM, CD54, and SDC2) for purification of myogenic progenitors, different culture condition and duration, gene overexpression, and cytokine use might be responsible for identification of different markers in these studies. In addition, side-by-side in vitro and in vivo comparison with ERBB3/NGFR markers demonstrated advantage of CD10/CD24 in the current differentiation protocol. This can also be justified by differences in differentiation protocols and duration of the culture. Despite this, our finding highlights the advantage of using prospective readouts and/or reporters for differentiation and screening studies.
Regarding identified markers in the current study, CD10 (neprilysin) is a type II transmembrane zinc-containing metallo-endopeptidase (MME) glycoprotein important for small peptide cleavage (Connelly et al., 1985; Erdo¨s and Skidgel, 1988, 1989). Pathways relevant to CD10 activity include hematopoietic and B cell-innate immune system development (Maguer-Satta et al., 2011). Interestingly, although CD10 expression has been reported on other cell types, such as B cells and epithelial-stromal cells (Be´ne´, 2005; Mishra et al., 2016), it has never been evaluated on human satellite cells and myoblasts. Here, immunofluorescent staining on human muscle biopsies and flow cytometry data using human primary myoblasts indicated the presence of CD10 on the human muscle progenitors and myoblasts. Therefore, it might be useful for isolation of human myogenic progenitors and myoblasts when used in combination with CD24 and other lineage exclusion markers.
CD24 (nectardin), on the other hand, encodes a sialo-glycoprotein, a cell adhesion molecule expressed on mature granulocytes and B cells that is important for their development and differentiation (Tan et al., 2016). Additionally, CD24 is expressed on neural progenitors (Fang et al., 2010; Gilliam et al., 2017; Poncet et al., 1996). Therefore, it can be speculated that CD24 serves as an exclusion marker for eliminating non-myogenic cells (such as hematopoietic or neural derivatives) from the culture. Validation of this purification protocol on different transgene and/or reporter-free hPSCs further confirmed its usefulness for enrichment of skeletal myogenic progenitors.
Another important aspect of the current study is the significant formation of donor-derived PAX7+ satellite cells after transplantation experiments, which confirms their ability to support muscle stem cell pool restoration in vivo. Lastly, short myogenic induction time frame (2 weeks) and expansion ability of the myogenic cells indicate its suitability for in vitro assays, such as disease modeling or screening as well as regenerative purposes.
Taken together, the myogenic differentiation approach described here is one of the most relevant methods, due to directed differentiation with prospective readouts using a bright double-reporter hESC line and inclusion of a systematic pathway and surface marker screen. This study also serves as a classical example for generation of multiple gene reporters in hPSCs using CRISPR/Cas9n system, which can be used for generation of similar reporters for other lineages.
STAR*METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Pax7 | R&D Systems | Cat# MAB1675, RRID:AB_2159833 |
| Rabbit anti-Myf5 | Santa Cruz Biotechnology | Cat# sc-302, RRID:AB_631994 |
| Mouse anti-MYH1 | DSHB | Cat# MF 20, RRID:AB_2147781q |
| Mouse anti-human dystrophin | Millipore | Cat# MABT827 |
| Rabbit anti-human lamin A/C | Abcam | Cat# ab108595, RRID:AB_10866185 |
| Mouse anti-human CD10 | BioLegend | Cat# 312202, RRID:AB_314913 |
| Mouse anti-human CD24 | BD Biosciences | Cat# 562789, RRID:AB_2737796 |
| Mouse anti-human CD271 | BD Biosciences | Cat# 562562, RRID:AB_2737657 |
| Mouse anti-human erbB3/HER-3 | BioLegend | Cat# 324708, RRID:AB_2099567 |
| BD Lyoplate Human Cell Surface Marker Screening Panel | BD Biosciences | Cat# 560747, RRID:AB_1953343 |
| Mouse monoclonal anti-Pax7 | DSHB | Cat# pax7, RRID:AB_528428 |
| Goat anti-Rabbit Secondary Antibody 647 | Thermo Fisher Scientific | Cat# A-21244, RRID:AB_2535812 |
| Goat anti-Mouse Secondary Antibody 555 | Thermo Fisher Scientific | Cat# A-21424, RRID:AB_141780 |
| Goat anti-Mouse Secondary Antibody 488 | Thermo Fisher Scientific | Cat# A-11029, RRID:AB_2534088 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| CHIR99021 | Selleck Chemical LLC | Cat# S2924 |
| SB431542 | Selleck Chemical LLC | Cat# S1067 |
| LDN193189 | Stemgent | Cat# 04–0074 |
| hr-EGF | PeproTech Inc | Cat# AF-100–15 |
| hr-HGF | PeproTech Inc | Cat# 100–39H |
| hr-FGF | PeproTech Inc | Cat# AF-100–18B |
| IGF-1 | PeproTech Inc | Cat# 100–11 |
| Horse serum | Thermo Fisher Scientific | Cat# 26050088 |
| knockout serum replacement | Thermo Fisher Scientific | Cat# 10828028 |
| Human insulin | Sigma-Aldrich | Cat# I9278–5ML |
| Deposited Data | ||
| RNA-seq data | This paper | GEO: GSE121154 |
| Human myoblast RNA-seq data | (ENCODE Project Consortium, 2012) | GEO: GSM958744 |
| Experimental Models: Cell Lines | ||
| Human H9 ESC | WiCell | Cat# WA09 |
| Human H1 ESC | WiCell | Cat# WA01 |
| Human iPSC1 | This paper | N/A |
| Experimental Models: Organisms/Strains | ||
| NSG mice | The Jackson Laboratory | Stock No: 005557 |
| NSG-mdx4Cv mice | (Arpke et al., 2013) | N/A |
| Other | ||
| QIAGEN miRNeasy RNA isolation kit | QIAGEN | Cat# 217004 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Growth and myogenic differentiation of hPSC lines
Human PSC lines were maintained on Matrigel-coated dishes (BD Biosciences) in mTeSR1 medium (STEMCELL Technologies). For skeletal muscle differentiation, human pluripotent stem cells were harvested using Accutase (Stem Cell Technologies) and seeded on Matrigel-coated plates (10,000 cells/cm2) in myogenic differentiation medium-I (MDM-I) and maintained for 4 days. MDM-I medium consisted of IMDM supplemented with 5% horse serum, 3 µM CHIR99021 (Selleck Chemical LLC), 2 µM SB431542 (Selleck Chemical LLC), 10 ng/ml hr-EGF (PeproTech Inc), 10 mg/ml insulin (Sigma- Aldrich), 0.4 mg/ml dexamethasone (Sigma-Aldrich) and 200 µM L-ascorbic acid (Sigma-Aldrich). After 4 days of induction, cells were harvested using Accutase and seeded on Matrigel-coated plates (10,000 cells/cm2) in myogenic differentiation medium-II (MDM-II). MDM-II medium contained IMDM supplemented with 5% horse serum, 10 mg/ml insulin (Sigma- Aldrich), 10 ng/ml hr-EGF (Pepro Tech Inc), 20 ng/ml hr-HGF (Pepro Tech Inc), 20 ng/ml hr-FGF (Pepro Tech Inc), 10 ng/ml IGF-1 (Pepro Tech Inc), 2 µM SB431542 (Selleck Chemical LLC), 0.5 µM LDN193189 (Stemgent), 200 µM L-ascorbic acid (Sigma-Aldrich). The cells were cultured in MDM-II medium for 6–11 days. At day 10 to 15 of differentiation (4 + 6–11), the cells were harvested and sorted for EGFP or surface markers. Sorted fractions were seeded on Matrigel-coated plates with similar densities (300,000/well of a 6 well plate) and expanded in MDM-II medium for an additional 2–3 days (or longer for expansion time-course experiments). For terminal myotube differentiation, once cells reached appropriate confluency (80%–90%), the medium was switched to MDM-III medium. MDM-III medium consisted of IMDM (Invitrogen), 15% knockout serum replacement (Invitrogen) and 10 ng/ml IGF-1. After 5 days of differentiation and formation of myotubes, cells were fixed and stained for MHC. For quantification, images were taken using low magnification (10x magnification, n = 5 images/well/experiment) and used for nuclei and myotube count.
In vivo transplantation experiments
Mice experiments were performed in adherence to protocols approved by the University of Texas Health Medical Center at Houston and Institutional Animal Care and Use Committee (IACUC), which comply with the requirements of the guidelines of US National Institutes of Health (NIH) for the humane care of animals. To test the engraftment potential, cells were injected into the non-irradiated tibialis anterior (TA) muscles of immunodeficient NSG or NSG-mdx4Cv mice (6–8 weeks old mice with equal male to female ratio, randomly divided into experimental groups, N = 6 / per experimental group) as described previously (Darabi et al., 2012). Two days before cell transplantation, TA muscles of 6–8 weeks old mice were damaged by Cardiotoxin injection (Darabi et al., 2012). After 48 hours, cells were harvested from culture flasks and resuspended in PBS (2.5 3 105 cells/10ml of PBS). Damaged TAs were exposed using a 2µm incision and cells injected into the belly of TA muscles using a 26 gauge Hamilton syringe. Wounds were closed using nylon sutures and mice recovered accordingly. Muscles were harvested five weeks later and stained for human specific markers (human nuclear Lamin A/C and dystrophin) to identify donor-derived cell engraftment.
METHOD DETAILS
Generation and characterization of double reporter cell line
For generation of double reporter cells, a Cas9 nickase mediated homologous recombination (HR) strategy was used as described recently in our recent publications (Wu et al., 2016a, 2016b). The positive selection and fluorescent protein genes in the original 2A-GFP-loxP-EF1a-RFP-2A-Puro-loxP fragment of the targeting constructs were modified as previously reported (Wu et al., 2016a, 2016b). In the PAX7 targeting construct, GFP was replaced with tdTomato-IRES-Neo and RFP-2A-Puro with Puromycin. In the MYF5 targeting construct, EGFP-2A-Hygromycin was inserted before the stop codon of MYF5 and RFP-2A-Puro was replaced with Neomycin. Using similar gene targeting methods (Wu et al., 2016a, 2016b), PAX7-tdTomato single reporter human H9 ESC clones were generated and confirmed by PCR and DNA sequencing. Next, a homozygous PAX7 reporter clone was selected and further targeted for MYF5 alleles. Finally, the correct integration of the transgenes in PAX7-tdTomato/MYF5-EGFP double reporter clones was verified in single cell-derived clones using sequencing and Southern blot (Figure S1). Reporter clones were further characterized by karyotyping and teratoma assay to ensure normal karyotype and pluripotency potential.
Validation of reporter activity using dCas9-VP160 activators
To validate reporter activity, artificial transcriptional activation was done by transfection of a set of sgRNA-dCas9 activators for PAX7 and MYF5 as described (Wu et al., 2016a, 2016b). The reporter hESC clone was plated as single cell/low density in Matrigel coated plates in IMDIM medium supplemented with ITS. Following 2 days of differentiation, the cells were transfected using validated dCas9-VP160 activators for PAX7 and MYF5 (Wu et al., 2016a, 2016b). Reporter activity was then analyzed using direct fluorescent microscopy and flow cytometry. tdTomato and EGFP positive cells were sorted and stained for the PAX7 or MYF5 using immuno-fluorescence (Figure S1) to confirm the reporter activities.
Muscle Histology and quantification
Muscle samples were harvested and embedded in OCT compound (n = 6 mice per experimental group). Samples were the frozen using liquid nitrogen-cooled isopenthane and used for histology. Muscle sections or cells were fixed using 4% PFA/PBS and after blocking, used for IHC as described (Darabi et al., 2012). Primary antibodies included mouse monoclonal anti-Pax7 (MAB1675, R&D and PAX7, DSHB), rabbit anti-Myf5 (SCBT), mouse anti-MYH1 (MF20, DSHB), mouse anti-human dystrophin (MABT827, Millipore), rabbit anti-human lamin A/C (ab108595, Abcam) and mouse anti-human CD10 (312202, Biolegends). For secondary antibodies, appropriate Alexa Fluor antibodies (Invitrogen) were used. Engraftment quantification was done by serial sectioning of the muscle alongside its longitudinal axis (with 100-micron intervals) to allow evaluation of cell migration from injection site. Number of human cells (hLamin A/C), human dystrophin-positive fibers and human cells expressing PAX7 were counted using low magnification images (10x) at 100-micron intervals for each muscle.
Surface marker profiling
Reporter cells were analyzed at days 4 and 10, which represent expression of PAX7-tdTomato and MYF5-EGFP respectively. Screening was performed using BD Lyoplate Human Cell Surface Marker Screening Panel (BD Biosciences), which contains 242 purified monoclonal antibodies to cell surface markers. The cells were harvested with Accutase, and after PBS wash were resuspended in flow cytometry staining buffer. Cells were then plated in 96 well plates (5 × 105 cells/well) and incubated with primary antibodies on ice for 30 min. Cells were then spun down and after another PBS wash incubated with appropriate secondary anti-bodies on ice for 30 min. After final wash with PBS, the cells were resuspended in 200ml of flow cytometry buffer containing EDTA and DAPI (to exclude dead cells) and analyzed using BD LSR Fortessa X-20 High Performance Multicolor Cell Analyzer, which provided High Throughput Sampling (HTS) from 96 well plates.
RNA-Seq
hESCs were analyzed at 5 differentiation time-points (Day0, Day4, Day10-sorted for MYF5 EGFP positive or negative- and day20 myotubes after myogenic differentiation of MYF5 EGFP positive fraction). For each time-point, 3 independent biological replicates were harvested for further analysis. 1 3 106 cells were harvested / sorted at each time-point and RNA extracted using QIAGEN miRNeasy RNA isolation kit (217004, QIAGEN) according to manufacturer’s protocol. Each RNA sample was converted to cDNA using oligo-dT primers and was further converted into indexed sequencing libraries using Illumina kit. Indexed libraries were subsequently pooled together and then sequenced on an Illumina HiSeq 4000 for a 50 cycle single end run. To compare transcriptome profile of PAX7/MYF5 double reporter hESC derived myogenic cells to human skeletal myoblasts, we enlisted ENCODE polyA RNA-Seq data of the human skeletal muscle myoblasts from the Wold lab (GEO: GSM958744).
Data processing
RNA-Seq reads were mapped to the human transcriptome (GENCODE release 19) and human genome (hg19) using TopHat (v2.1.1) default setting without allowing novel splicing junctions. The TopHat default setting allows up to two mismatches (Trapnell et al., 2009). Only uniquely mapped reads were used for subsequent analyses. To quantify expression level for each gene, the number of sequencing reads aligned to each gene for each sample were tabulated using the featureCounts function from the Subread pack-age (1.5.2) (Liao et al., 2014). Before normalizing the data, genes not sufficiently quantified in our dataset were removed i.e., for a gene to be included in the downstream analyses, we required at least one sequencing read aligned to the gene in more than nine samples (out of 15). After filtering out genes not sufficiently quantified, the gene level count data were transformed to log2 Counts per Million (CPM) and TMM normalized using the edgeR package (Robinson et al., 2010).
Principal components analysis
Singular value decomposition was performed on centered and scaled RNA-Seq count data using the prcomp function in the stats package of R environment for statistical computing.
Differential expression analysis
Differential expression tests were computed with limma (Ritchie et al., 2015; Smyth, 2004) using R Bioconductor package. For testing differences in transcript level between differentiation time points, log2 transformed, TMM normalized RNA-Seq data was fitted to a fixed effects model (differentiation effect). For each gene, the differentiation coefficient (effectively log2 ratio between time points) is tested against the null hypothesis that the coefficient is equal to zero using empirical Bayes moderated t-statistics. To account for multiple testing, nominal p values were adjusted using Benjamini-Hochberg procedure to acquire estimates of false discovery rate.
Gene ontology enrichment analysis
To identify enriched pathways or gene ontology (GO) terms among differentially expressed genes between MYF5-positive and MYF5-negative cells, the GOseq package (version 1.28.0) was used (Young et al., 2010). To account for potential biases introduced by gene length, the built-in gene length database was used to compute a probability weighting function. The default Wallenius method and built-in Gene Ontology database was then used to test for GO term enrichment. Separate analyses were performed for each of the 3 following GO categories: Biological Processes, Cellular Components, and Molecular Functions. For each category, genes that have no GO term association were excluded. Separate enrichment analyses were performed for genes that are upregulated in MYF5-positive cells, and genes that are downregulated in MYF5-positive cells (i.e., at 5% FDR, relative to MYF5-negative cells).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical details of each experiment can be found in figure legends. Differences between samples/groups were assessed by using the unpaired two-tailed Student’s t test. p values < 0.05 were considered significant.
Supplementary Material
Highlights.
A PAX7/MYF5 reporter hESC line allows prospective isolation of myogenic progenitors
HTS for surface markers identifies CD10 and CD24 as potential purification markers
Pathway screen allows defining a short-term myogenic differentiation protocol
Engraftment (new myofibers/satellite cells) confirms myogenic potential of the cells
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the NIH under award numbers 1R01AR068293 and 1R21AR071583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors also would like to thank Dr. Michael Kyba for generous sharing of the NSG-mdx4Cv mice.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact Dr. Radbod Darabi (Radbod.Darabi@uth.tmc.edu).
DATA AND SOFTWARE AVAILABILITY
The accession number for the RNA-seq data reported in this paper is GEO: GSE121154.
REFERENCES
- Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree CL, Nash N, Lowe DA, Perlingeiro RC, and Kyba M (2013). A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell trans-plantation. Stem Cells 31, 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, and Studer L (2007). Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med 13, 642–648. [DOI] [PubMed] [Google Scholar]
- Bene´ MC (2005). Immunophenotyping of acute leukaemias. Immunol. Lett 98, 9–21. [DOI] [PubMed] [Google Scholar]
- Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, and Olwin BB (2014). p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med 20, 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchin B, Chen J, and Barberi T (2013). Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Reports 1, 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, Buck-ingham M, and Cossu G (2006). The Wnt/beta-catenin pathway regulates Glimediated Myf5 expression during somitogenesis. Development 133, 3723–3732. [DOI] [PubMed] [Google Scholar]
- Buckingham M (1996). Skeletal muscle development and the role of the myogenic regulatory factors. Biochem. Soc. Trans 24, 506–509. [DOI] [PubMed] [Google Scholar]
- Buckingham M (2006). Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev 16, 525–532. [DOI] [PubMed] [Google Scholar]
- Buckingham M (2017). Gene regulatory networks and cell lineages that underlie the formation of skeletal muscle. Proc. Natl. Acad. Sci. USA 114, 5830–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, and Rigby PW (2014). Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 28, 225–238. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, and Relaix F (2003). The formation of skeletal muscle: from somite to limb. J. Anat 202, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Deasy BM, Pollett J, and Huard J (2005). Cell therapy for muscle regeneration and repair. Phys. Med. Rehabil. Clin. N. Am 16, 889–907, viii. [DOI] [PubMed] [Google Scholar]
- Caron L, Kher D, Lee KL, McKernan R, Dumevska B, Hidalgo A, Li J, Yang H, Main H, Ferri G, et al. (2016). A Human Pluripotent Stem Cell Model of Facioscapulohumeral Muscular Dystrophy-Affected Skeletal Muscles. Stem Cells Transl. Med 5, 1145–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bous-son F, Zidouni Y, Mursch C, Moncuquet P, et al. (2015). Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol 33, 962–969. [DOI] [PubMed] [Google Scholar]
- Chalamalasetty RB, Dunty WC Jr., Biris KK, Ajima R, Iacovino M, Beisaw A, Feigenbaum L, Chapman DL, Yoon JK, Kyba M, and Yamaguchi TP (2011). The Wnt3a/b-catenin target gene Mesogenin1 controls the segmentation clock by activating a Notch signalling program. Nat. Commun 2, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Colgan TD, Walton KL, Gregorevic P, and Harrison CA (2016). The TGF-β Signalling Network in Muscle Development, Adaptation and Disease. Adv. Exp. Med. Biol 900, 97–131. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lim H, Estrellas K, Mula J, Cohen TV, Zhang Y, Donnelly CJ, Richard JP, Kim YJ, Kim H, et al. (2016). Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myo-blasts Derived using a Human iPSC-Based Model. Cell Rep 15, 2301–2312. [DOI] [PubMed] [Google Scholar]
- Connelly JC, Skidgel RA, Schulz WW, Johnson AR, and Erdos EG (1985). Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc. Natl. Acad. Sci. USA 82, 8737–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, and Perlingeiro RC (2008). Lineage-specific reprogramming as a strategy for cell therapy. Cell Cycle 7, 1732–1737. [DOI] [PubMed] [Google Scholar]
- Darabi R, and Perlingeiro RC (2016). Derivation of Skeletal Myogenic Precursors from Human Pluripotent Stem Cells Using Conditional Expression of PAX7. Methods Mol. Biol 1357, 423–439. [DOI] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, and Perlingeiro RC (2008). Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat. Med 14, 134–143. [DOI] [PubMed] [Google Scholar]
- Darabi R, Pan W, Bosnakovski D, Baik J, Kyba M, and Perlingeiro RC (2011). Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev 7, 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, and Per-lingeiro RC (2012). Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Angelis L, Zhao J, Andreucci JJ, Olson EN, Cossu G, and McDermott JC (2005). Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev. Biol 283, 171–179. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperlein HH, Vichev K, Heidrich FM, and Kurth T (2007). BMP-4 and Noggin signaling modulate dorsal fin and somite development in the axolotl trunk. Dev. Dyn 236, 2464–2474. [DOI] [PubMed] [Google Scholar]
- Erdos EG, and Skidgel RA (1988). Human neutral endopeptidase 24.11 (NEP, enkephalinase); function, distribution and release. Adv. Exp. Med. Biol 240, 13–21. [DOI] [PubMed] [Google Scholar]
- Erdos EG, and Skidgel RA (1989). Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 3, 145–151. [PubMed] [Google Scholar]
- Evans WJ (2010). Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr 91, 1123S–1127S. [DOI] [PubMed] [Google Scholar]
- Fang X, Zheng P, Tang J, and Liu Y (2010). CD24: from A to Z. Cell. Mol. Immunol 7, 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam DT, Menon V, Bretz NP, and Pruszak J (2017). The CD24 surface antigen in neural development and disease. Neurobiol. Dis 99, 133–144. [DOI] [PubMed] [Google Scholar]
- Hagos EG, and Dougan ST (2007). Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev. Biol 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MR, Hiserodt J, Paras K, Fujiwara W, Eskin A, Jan M, Xi H, Young CS, Evseenko D, Nelson SF, et al. (2018). ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol 20, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y, Suk S, Shih YR, Seo T, Du B, Xie Y, Li Z, and Varghese S (2014). WNT3A promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment. Sci. Rep 4, 5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nagata N, Kurokawa H, and Yamanaka S (2014). iPS cells: a game changer for future medicine. EMBO J 33, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wen Y, Kuroda K, Hannon K, Rudnicki MA, and Kuang S (2014). Notch signaling deficiency underlies age-dependent depletion of satel-lite cells in muscular dystrophy. Dis. Model. Mech 7, 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, and Bursac N (2013). Engineering skeletal muscle repair. Curr. Opin. Biotechnol 24, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren A, Tamir Y, and Bengal E (2006). The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol 252, 224–230. [DOI] [PubMed] [Google Scholar]
- Kim J, Magli A, Chan SSK, Oliveira VKP, Wu J, Darabi R, Kyba M, and Perlingeiro RCR (2017). Expansion and Purification Are Critical for the Therapeutic Application of Pluripotent Stem Cell-Derived Myogenic Progenitors. Stem Cell Reports 9, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Madonna R (2012). Human-induced pluripotent stem cells: in quest of clinical applications. Mol. Biotechnol 52, 193–203. [DOI] [PubMed] [Google Scholar]
- Magli A, Incitti T, Kiley J, Swanson SA, Darabi R, Rinaldi F, Selvaraj S, Yamamoto A, Tolar J, Yuan C, et al. (2017). PAX7 Targets, CD54, Integrin a9b1, and SDC2, Allow Isolation of Human ESC/iPSC-Derived Myogenic Progenitors. Cell Rep 19, 2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguer-Satta V, Besanc¸on R, and Bachelard-Cascales E (2011). Concise review: neutral endopeptidase (CD10): a multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells 29, 389–396. [DOI] [PubMed] [Google Scholar]
- Martin BL, and Kimelman D (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D, Singh S, and Narayan G (2016). Role of B cell development marker CD10 in cancer progression and prognosis. Mol. Biol. Int 2016, 4328697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol 27, 743–745. [DOI] [PubMed] [Google Scholar]
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, and Miura K (2013). Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res 112, 523–533. [DOI] [PubMed] [Google Scholar]
- Poncet C, Frances V, Gristina R, Scheiner C, Pellissier JF, and Figarella-Branger D (1996). CD24, a glycosylphosphatidylinositol-anchored molecules is transiently expressed during the development of human central nervous system and is a marker of human neural cell lineage tumors. Acta Neuropathol 91, 400–408. [DOI] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, and Rudnicki MA (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med 20, 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R, Maroto M, and Lassar AB (1998). Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev 12, 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bio-conductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, and Munoz-Canoves P (2011). Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr. Top. Dev. Biol 96, 167–201. [DOI] [PubMed] [Google Scholar]
- Sheeba CJ, Andrade RP, and Palmeirim I (2016). Mechanisms of vertebrate embryo segmentation: Common themes in trunk and limb development. Semin. Cell Dev. Biol 49, 125–134. [DOI] [PubMed] [Google Scholar]
- Shelton M, Metz J, Liu J, Carpenedo RL, Demers SP, Stanford WL, and Skerjanc IS (2014). Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports 3, 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton M, Kocharyan A, Liu J, Skerjanc IS, and Stanford WL (2016). Robust generation and expansion of skeletal muscle progenitors and myo-cytes from human pluripotent stem cells. Methods 101, 73–84. [DOI] [PubMed] [Google Scholar]
- Shi Y, Inoue H, Wu JC, and Yamanaka S (2017). Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov 16, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC (1999). TGF beta inhibitors. New and unexpected requirements in vertebrate development. Trends Genet 15, 3–5. [DOI] [PubMed] [Google Scholar]
- Smyth GK (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol 3, Article3. [DOI] [PubMed] [Google Scholar]
- Sohn RL, and Gussoni E (2004). Stem cell therapy for muscular dystrophy. Expert Opin. Biol. Ther 4, 1–9. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, and Buckingham M (2000). The birth of muscle progenitor cells in the mouse: spatiotemporal considerations. Curr. Top. Dev. Biol 48, 225–268. [DOI] [PubMed] [Google Scholar]
- Takahashi K, and Yamanaka S (2013). Induced pluripotent stem cells in medicine and biology. Development 140, 2457–2461. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Narita M, Yokura M, Ichisaka T, and Yamanaka S (2009). Human induced pluripotent stem cells on autologous feeders. PLoS ONE 4, e8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Zhao M, Xiang B, Chang C, and Lu Q (2016). CD24: from a Hematopoietic Differentiation Antigen to a Genetic Risk Factor for Multiple Autoimmune Diseases. Clin. Rev. Allergy Immunol 50, 70–83. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, Yamamoto T, Nish-ino T, Shoji E, Sehara-Fujisawa A, Manabe Y, et al. (2013). Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLoS ONE 8, e61540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, et al. (2012). Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med 4, 140ra89. [DOI] [PubMed] [Google Scholar]
- Tonegawa A, and Takahashi Y (1998). Somitogenesis controlled by Noggin. Dev. Biol 202, 172–182. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, and Salzberg SL (2009). TopHat: discovering splice junctions with RNA-seq. Bioinformatics 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, and Rudnicki MA (2011). Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol 13, 127–133. [DOI] [PubMed] [Google Scholar]
- Wen Y, Bi P, Liu W, Asakura A, Keller C, and Kuang S (2012). Consti-tutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol 32, 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, and Hogan BL (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9, 2105–2116. [DOI] [PubMed] [Google Scholar]
- Wu J, Hunt SD, Xue H, Liu Y, and Darabi R (2016a). Generation and Characterization of a MYF5 Reporter Human iPS Cell Line Using CRISPR/ Cas9 Mediated Homologous Recombination. Sci. Rep 6, 18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hunt SD, Xue H, Liu Y, and Darabi R (2016b). Generation and validation of PAX7 reporter lines from human iPS cells using CRISPR/Cas9 technology. Stem Cell Res. (Amst.) 16, 220–228. [DOI] [PubMed] [Google Scholar]
- Xi H, Fujiwara W, Gonzalez K, Jan M, Liebscher S, Van Handel B, Schenke-Layland K, and Pyle AD (2017). In Vivo Human Somito-genesis Guides Somite Development from hPSCs. Cell Rep 18, 1573– 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Tabebordbar M, Iovino S, Ciarlo C, Liu J, Castiglioni A, Price E, Liu M, Barton ER, Kahn CR, et al. (2013). A zebrafish embryo culture sys-tem defines factors that promote vertebrate myogenesis across species. Cell 155, 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, and Oshlack A (2010). Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, et al. (2008). Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111, 1933–1941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.