Summary
Background
Pfs25H-EPA, a protein-protein conjugate Plasmodium falciparum transmission-blocking vaccine, is safe and induces functional antibodies in malaria-naive individuals. In the first malaria transmission-blocking vaccine field trial, we assessed Pfs25H-EPA/Alhydrogel® safety and functional immunogenicity in Malian adults.
Methods
After dose-escalation, we conducted a double-blind, randomised, comparator-controlled trial (ClinicalTrials.gov number NCT01867463) in Bancoumana, Mali. One hundred 18–45-year-old healthy adults were assigned (1:1) by block randomisation to 47µg Pfs25 or comparator vaccine. Primary outcome was safety and tolerability for all vaccinees. Sample size was calculated for transmission-blocking activity, measured by standard membrane-feeding assay and direct skin feeds after 3 or 4 vaccinations.
Findings
Pfs25H vaccinees reported more solicited AEs (137 vs 86, p=0·022; Fishers exact) and related AEs (191 vs 126, p=0·034), but other AEs did not differ between Pfs25H and comparator groups (792 vs. 683). Pfs25 antibody titres increased with each dose to peak geometric mean 422·3 EU (95%CI 290–615) post-dose 4, then declined relatively rapidly (half-life 42 days) versus EPA titres (59 days) (median ratio day 600/peak 0·18 vs 0·29, respectively, p=<0·001, paired Wilcoxon’s test). Serum transmission-reducing activity was greater for Pfs25H than comparator post-dose 4 (p<0·001, paired Wilcoxon’s test) but not 3 (p=0·09), similar to that seen in malaria-naïve US vaccinees. Repeated direct skin feeds, wherein mosquitoes fed directly on participants, were well-tolerated but did not differ between Pfs25H and comparator vaccinees post-dose 4 (p=1, conditional exact), yielding 5/41 and 4/38 individuals (respectively) with at least one positive DSF.
Interpretation
Pfs25H-EPA/Alhydrogel® was well-tolerated and induced significant serum activity by membrane feeding assay, but not by direct skin feed thus failing to provide actual transmission blocking activity. This activity required four doses and titres declined rapidly; alternative antigens or combinations should be assessed to improve activity.
Introduction
Among human malaria parasite species, Plasmodium falciparum (Pf) is deadliest. Global malaria deaths decreased by over a third in the past decade as control measures scaled up, but nevertheless 730,000 malaria deaths occurred in 2015.1 Policy-makers and researchers have increasingly focused on malaria eradication as the only sustainable solution. Eradication will require effective interventions to interrupt transmission,2 and vaccines have been key for smallpox and polio programs.
Malaria transmission-blocking vaccines (TBV) are based on the insight that human antibodies can attack parasites in the mosquito.3,4 Whole gamete vaccination of animals induced antibodies against mosquito sexual stage but not blood stage parasites;3 hence, TBV can block onward transmission without directly reducing host infection. Monoclonal antibodies generated by whole organism vaccination were used to identify candidate TBV antigens. These include gamete surface proteins P230 and P48/45 first expressed by gametocytes in human blood,5 and zygote surface proteins P25 and P28 expressed post-fertilization in the mosquito.6,7 These antigens are multi-domain cysteine-rich proteins and generally difficult to produce as properly folded recombinant protein. P. falciparum P25 (Pfs25) antigen was the first expressed as recombinant protein8 and has subsequently remained the leading TBV candidate.
Vaccines based on Pfs25 or its Plasmodium vivax orthologue Pvs25 failed to advance clinically owing to poor immunogenicity9,10 or excessive reactogenicity thought related to adjuvant formulations.11 We previously reported that conjugation to immunogenic carriers enhances TBV antibody titre and duration in animals.12,13 Recombinant Pichia-expressed, His-tagged Pfs25 conjugated to recombinant EPA and formulated in Alhydrogel® (Pfs25H-EPA/Alhydrogel®) induced functional antiserum in malaria-naïve US volunteers that reduced Pf transmission to mosquitoes in a laboratory assay.14 In that trial, antibody titre and avidity increased progressively from second to fourth dose, and titre correlated with serum functional activity after final dose.
Here, we report the first TBV trial in a malaria-exposed target population. We show that Pfs25H-EPA/Alhydrogel® is safe and induces functional antibody in Malian adults that reduces parasite transmission to Anopheles stephensi mosquitoes in a laboratory assay.
Methods
Study design and participants
This double-blind, randomised, comparator-controlled trial in healthy 18–45 year olds was conducted from May 2013 to Mar 2015 in and around Bancoumana, Mali, a rural village of approximately 10,000 inhabitants 60 km southwest of Bamako. Malaria is hyperendemic with highly seasonal transmission from June to December (figure S1).
Healthy 18-45-year-old men or non-pregnant, non-breastfeeding women who consented were eligible to enroll if they were available for the trial duration, known village residents, and willing to undergo mosquito direct skin feeding (DSF) assays. Women of child-bearing potential were required to use reliable contraception throughout vaccination. Individuals were excluded for abnormal laboratories (including HIV, hepatitis B, hepatitis C tests), previous malaria vaccine, or recent immunosuppressive drugs, vaccines, or blood products. Inclusion and exclusion criteria are listed in the appendix (pp6–7).
We conducted the trial in accordance with Good Clinical Practice guidelines and institutional procedures and guidelines. Each village provided community permission and all participants provided informed consent. The study was approved by the Mali ethics review board (Faculté de Médecine de Pharmacie et d’OdontoStomatologie, Bamako), U.S. National Institute of Allergy and Infectious Diseases (NIAID, National Institutes of Health [NIH], Bethesda, MD) institutional review board, and Mali national regulatory authority, and was conducted under FDA IND 14781.
Randomisation and masking
For safety, the trial progressed stepwise with two phases: pilot safety cohort, then main cohort staggered as two vaccination groups (appendix p14–15). In both cohorts, subjects were block-randomised (1:1) to study vaccine (pilot: Pfs25H-EPA/Alhydrogel® 16µg; main: Pfs25H-EPA/Alhydrogel® 47µg) or comparator (Euvax B for vaccinations #1, 2, 3; Menactra® for vaccination #4; appendix p12). Group assignments were masked to participants and investigators, with randomisation codes in sealed envelopes held by site pharmacist. Products for injection were covered by opaque tape and labeled with study identification number. Group assignments were unmasked at final study visit, four months after second vaccination in pilot safety cohort (to promote completion of hepatitis B vaccination series), and six months after fourth vaccination in main cohort. After unblinding, all Pfs25H vaccinees were offered Euvax B and Menactra®.
Procedures
Pfs25H-EPA/Alhydrogel® vaccine consists of Pichia pastoris-expressed hexa-His-tagged recombinant Pfs25 (Pfs25H; Walter Reed Army Institute of Research Pilot Bioproduction Facility [WRAIR PBF], Silver Spring, Maryland) conjugated to an E. coli-expressed recombinant Pseudomonas aeruginosa ExoProtein A (EPA; WRAIR PBF) and adjuvanted with Alhydrogel® (Brenntag, Denmark). Each vial contained 78µg/mL conjugated Pfs25H, 93µg/mL conjugated EPA, and 1600µg/mL Alhydrogel® in 0·8mL volume. For pilot safety cohort (n=10), participants received 0·2mL (Pfs25H, 16µg) at days 0 and 56; for main cohort (n=50), participants received 0·6mL injections (Pfs25H, 47µg) at days 0, 56, 112, and 480. Licensed comparator vaccine Euvax B (1·0mL recombinant hepatitis B vaccine; LG Life Sciences, Jeonbuk-do, South Korea) was given at days 0 and 56 in the pilot safety cohort (n=10) and at days 0, 56, and 112 in the main cohort (n=50); and Menactra® (0·5mL meningococcal polysaccharide vaccine for Neisseria meningitidis serogroups A, C, Y, and W-135; Sanofi Pasteur Inc., Swiftwater, Pennsylvania) was given at study day 480 in the main cohort (n=50). Local pediatricians completed vaccinations in deltoid muscles of alternating arms; study clinicians performed follow up and adverse event (AE) assessment. Participants were considered enrolled upon first vaccination.
Participants were monitored 30 minutes post-vaccination for AEs, then assessed for safety on days 1, 3, 7, 14, and 28, and monthly thereafter until unblinding. Study clinical personnel were always available for unscheduled visits. Solicited local AEs were recorded for 14 days and systemic AEs for 28 days after vaccinations (table S1). Unsolicited AEs including symptomatic malaria, serious AEs (SAE), and new onset chronic illnesses were recorded throughout the study. SAEs included death, life-threatening event, inpatient hospitalization, persistent or significant incapacity, congenital anomaly, or medically important event. Protocol-specified laboratories, including complete blood count with differential, creatinine, alanine aminotransferase, and urinalysis were completed before and days 3 and 14 after vaccination. AE grading was based on US FDA guidelines for vaccine clinical trials15 and adapted to local normal reference ranges (table S2, S3).
Blood smears (BS) were prepared before each vaccination, at least monthly post vaccination, or when clinically indicated. Starting two weeks after third and fourth vaccinations, BS were prepared at every DSF visit. Symptomatic malaria was defined as asexual parasitaemia with axillary temperature of at least 37·5°C, clinical signs and symptoms of malaria, or both. Artemether/lumefantrine was provided for symptomatic malaria; in accordance with Malian Government guidelines, asymptomatic parasitaemia was not treated. BS were examined by trained technicians and standard procedures.
Using published methods,14 Pfs25 and EPA antibodies were measured by ELISA on day of vaccination, 14 days post-vaccination, and periodically post-dose 3 and 4. For both anti-Pfs25 and anti-EPA antibodies, half-life was calculated using the following equation: Nt = N0 (1/2)^(t/t1/2), where N0 is the starting titer (day 494), Nt is the ending titer (day 600), and t is time in days. Antibody avidity was measured by modified ELISA using 6M urea during washing; avidity index was the ratio of optical density (OD) value with urea over the OD without urea in TBS/Tween washing buffer.
Functional activity was measured by the standard membrane feeding assay (SMFA) on sera using published methods.14 Transmission-reducing activity (TRA) was defined as (mean oocyst count of the assay control – mean oocyst count of the test sample)/mean oocyst count of the assay control x 100. Day zero Transmission-reducing activity (Day 0 TRA) is defined as (mean oocyst count of day zero of the test sample – mean oocyst count of the test sample)/mean oocyst count of day zero of the test sample x 100. Transmission-blocking activity (TBA) was defined as (mean prevalence in assay control – mean prevalence in the test sample)/mean prevalence in the assay control x 100.
As another secondary objective, DSF were conducted weekly for six weeks on BS-positive (asexual or sexual) main cohort participants starting 14 days after third vaccination (Sep to Nov 2013), and then on all main cohort participants starting 14 days after fourth (Sep to Nov 2014). Briefly, two mesh-covered cups with up to 30 pre-starved lab-adapted female A. coluzzi mosquitoes were placed on the participant’s calf by trained staff for 15–20 mins. Participants were then offered topical antihistamines and/or topical antipruritics and followed actively for 24–48 hours for any AEs. Bloodfed mosquitoes were transported back to Bamako, stored in secure insectary, and dissected a week later for oocyst counts. Further DSF details are in appendix pp9–10.
The study protocol described Direct Membrane Feeding Assays (DMFA) as a secondary outcome for measuring transmission-blocking activity, whereby fresh blood drawn from study participants is fed to mosquitoes through a membrane. However, our pilot studies indicated that DSF was equally sensitive to DMFA and logistically simpler, hence we used DSF as the sole measure of functional activity in the field.
We assessed potential co-infections in main cohort before fourth vaccination, using previously published methods.
Hemoglobinopathy was not exclusionary unless clinically relevant. Hemoglobin typing was completed retrospectively.
Outcomes
Study primary objective was to assess safety, tolerability, and reactogenicity of repeated immunisation of increasing doses (16µg or 47µg) of Pfs25H-EPA/Alhydrogel®. This was assessed by the occurrence and severity of local and systemic AEs occurring within 14 (local) or 28 (systemic) days after each vaccination and occurrence of SAEs. The secondary outcome measure was immunogenicity on day 14 post-vaccination in the per-protocol population confirmed by the presence of antibodies against Pfs25 measured by ELISA IgG and antibody functionality assessed by SMFA and by DSF.
Statistical analysis
All participants who received at least one dose of vaccine were included in safety analyses, including the pilot safety cohort. Safety signals were investigated by the proportion of subjects and the count reports overall, and for a given AE of a specific grade and relationship to vaccination. We used Fisher’s exact tests to compare proportions and Wilcoxon Mann Whitney tests to compare AE counts.
We compared immunogenicity between groups by Wilcoxon Mann Whitney test at specific time points, and by linear generalized estimating equations (GEE) and linear mixed effects models (LMER) conditional on subject over all time points. The R packages gee16, lme417, and lmerTest18 were used for these repeated measures analyses, respectively. We compared seroconversion rates using conditional exact test for given time points; R package “exact2×2”. ELISA titers are described using geometric means (Prism v7, GraphPad Software, San Diego, CA).
Functional activity was assessed by two endpoints, SMFA and DSF. SMFA results were compared using Wilcoxon Mann-Whitney for each time point separately and their association with longitudinal ELISA values and EC50 were investigated using GEE. The DSF results were compared using many different analysis methods, including logistic and count mixed effects models at the mosquito and DSF level including random intercepts for DSF and subject, respectively. Logistic and count GEE models were also fit at mosquito and DSF levels. Both the GEE and mixed-effects models were run over all subjects, as well as in the subgroup of subjects with detectable gametocytes at the time of DSF. As well, the proportion of subjects with at least one positive DSF following vaccination 4 was compared via conditional exact test. No adjustment for multiple comparisons was made. For further details, see appendix pp12–13.
Sample size calculations, described in detail in appendix pp11–12, were based on the primary safety endpoint for both the pilot and main cohort, but also took into account the secondary functional activity objectives for the main cohort.
The study was monitored for safety by an independent Data and Safety Monitoring Board (DSMB) and a local medical monitor. This trial is registered at ClinicalTrials.gov, number NCT01867463.
Results
Between May 15 and Jun 16, 2013, 230 individuals were screened, of whom 20 were randomised (1:1) in pilot safety cohort to receive 16µg Pfs25H-EPA/Alhydrogel® (n=10) or comparator (n=10). First vaccinations in pilot safety cohort (figure 1) were given in May 2013, second vaccinations in Jun 2013, and scheduled unblinding occurred in Nov 2013. Ten individuals who received comparator and 9 who received 16µg Pfs25H completed two vaccinations and were followed until end of study. One subject who received 16µg Pfs25H did not meet eligibility criteria to continue following receipt of vaccination #1 due to slightly elevated creatinine at baseline (Grade 1).
Figure 1. Trial profile.
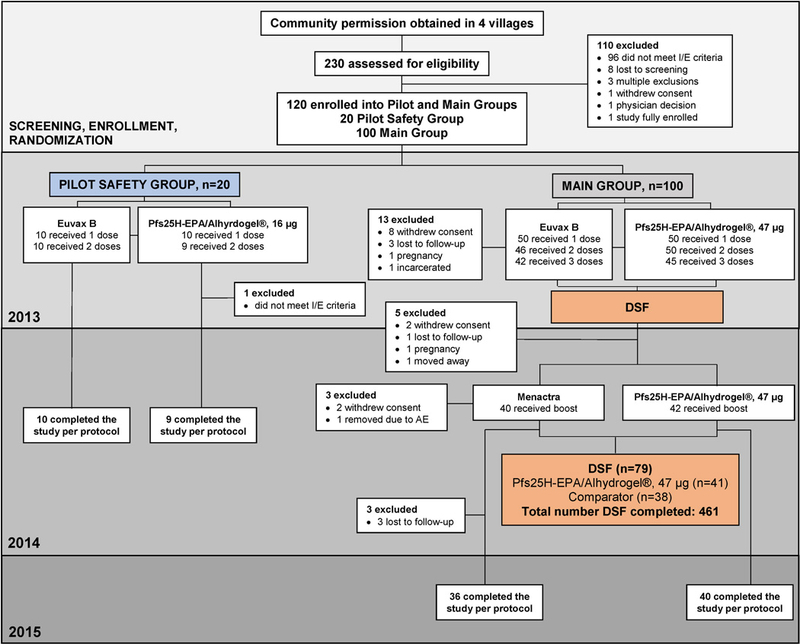
Study completion was defined as staying in the study until the end of the trial (Study Day 660).
100 participants enrolled in the main cohort (figure 1) to receive 47µg of Pfs25H-EPA/Alhydrogel® (n=50) or comparator (n=50) in two randomised sets, staggered by one week for safety. All randomised participants received at least one vaccination and were eligible for safety analyses. In main cohort, 42 comparators received three doses and 40 received booster doses the following year; 45 vaccinees received three doses Pfs25H and 42 received booster doses the following year. Of those who received booster doses, 38 comparators and 41 Pfs25H vaccinees completed DSF evaluation, while 36 comparators and 40 vaccinees completed end-of-study visit (figure 1).
Most enrolled participants were men (72·5%) from Bancoumana village (47%) with hemoglobin AA (72·9%), mean age 34·4 years (SD ±7·7) and mean weight 65·6kg (SD ±10·2) (table 1).
Table 1.
Characteristics of subjects vaccinated.†
| Pilot Safety Cohort | Main Cohort | Total (n=120) |
|||
|---|---|---|---|---|---|
| Pfs25H, 16µg (n=10) |
Comparator (n=10) |
Pfs25H, 47µg (n=50) |
Comparator (n=50) |
||
| Gender | |||||
| Male | 7 (70%) | 5 (50%) | 34 (68%) | 41 (82%) | 87 (72·5%) |
| Female | 3 (30%) | 5 (50%) | 16 (32%) | 9 (18%) | 33 (27·5%) |
| Age – years | |||||
| Mean (SD) | 39·8 (4·7) | 34·7 (7·9) | 33·4 (8·1) | 34·2 (7·4) | 34·4 (7·7) |
| Range | 32–44 | 18–44 | 18–44 | 19–44 | 18–44 |
| Weight – kg | |||||
| Mean (SD) | 65·2 (15·3) | 66·0 (11·7) | 64·6 (9·8) | 66·7 (9·2) | 65·6 (10·2) |
| Range | 47–97 | 52–90 | 45–87 | 51–89 | 45–97 |
| Village | |||||
| Bancoumana | 10 (100%) | 10 (100%) | 18 (36%) | 18 (36%) | 56 (47%) |
| Samako | 0 (0%) | 0 (0%) | 18 (36%) | 14 (28%) | 32 (27%) |
| Kolle | 0 (0%) | 0 (0%) | 2 (4%) | 3 (6%) | 5 (4%) |
| Siranikoro | 0 (0%) | 0 (0%) | 3 (6%) | 2 (4%) | 5 (4 %) |
| Djiguidala | 0 (0%) | 0 (0%) | 6 (12%) | 6 (12%) | 12 (10%) |
| Gonsolo | 0 (0%) | 0 (0%) | 2 (4%) | 3 (6%) | 5 (4%) |
| Missira | 0 (0%) | 0 (0%) | 1 (2%) | 4 (8%) | 5 (4%) |
| Co-infections‡ | |||||
| S. haematobium | -- | -- | 0 (0%) | 0 (0%) | 0 (0%) |
| Helminth | -- | -- | 2 (4·9%) | 4 (10·5%) | 6 (7·6%) |
| Protozoa | -- | -- | 6 (14·6%) | 6 (15·8%) | 12 (15·2%) |
| Haemoglobinopathies┼ | |||||
| Hb AA | 9 (100%) | 5 (50%) | 37 (74%) | 35 (71·4%) | 86 (72·9%) |
| Hb AS | 0 (0%) | 2 (20%) | 10 (20%) | 9 (18·4%) | 21 (17·8%) |
| Hb SC | 0 (0%) | 0 (0%) | 0 (0%) | 2 (4·1%) | 2 (1·7%) |
| Hb SC | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hb CC | 0 (0%) | 1 (10%) | 0 (0%) | 0 (0%) | 1 (0·8%) |
| Hb AC | 0 (0%) | 2 (20%) | 3 (6%) | 3 (6·1%) | 8 (6·8%) |
Data are number (%), unless otherwise specified.
Co-infections were not measured at baseline. They were assessed prior to Vaccination #4 in August/September 2014. 82 participants in the main cohort only (43 Pfs25H vaccine; 39 comparator) were evaluated for urinary schistosomiasis. 79 participants in the main cohort only (41 Pfs25H vaccine, 38 comparator). One participant in the comparator group was positive for both helminth and protozoa and is counted once in each category.
Hemoglobin typing was completed on 19 pilot safety cohort participants (9 Pfs25H vaccine; 10 comparator) and 99 main cohort participants (50 Pfs25H vaccine; 49 comparator)
Participants who received 16µg or 47µg Pfs25H had a good safety and tolerability profile, with most AEs being Grade 1 or 2 (table S4). Total AEs, local reactogenicity, laboratory abnormalities (including neutropenia), or unsolicited AEs did not differ significantly between Pfs25H and comparator groups, based on number of unique individuals having at least one vaccination (table 2; table S4-S7). Pfs25H vaccinees (16µg and 47µg combined) reported more solicited systemic AEs (p=0·022; Fishers exact) and related AEs (p=0·034; Fishers exact); these were also significantly different when comparing the count and rate (count/time at risk) per subject (table 2; table S4-S6). These differences between groups did not increase with increasing number of doses (table 2; table S4-S6).
Table 2. Frequency of adverse events and serious adverse events following vaccination according to vaccine dose, cohort, and relatedness.†.
Participants were actively monitored on days 1, 3, 7, 14, and 28 post vaccination and then monthly during the long-term safety follow-up until unblinding. Medically qualified study personnel were available at all times for unscheduled visits. Solicited AEs includes local AEs which were recorded for 14 days and systemic AEs which were recorded for 28 days after each vaccination. Unsolicited AEs, including symptomatic malaria, serious AEs, and new onset of chronic illness were recorded throughout the study. Protocol-specified laboratory assessments were completed before vaccination and days 3 and 14 after vaccination and reported in this table if ≤28 days from vaccination. Blood smears (BS) were prepared before each vaccination, at least monthly post vaccination, or when clinically indicated. Starting 2 weeks after third and fourth vaccination, BS were prepared at every DSF visit. Symptomatic malaria was defined as asexual parasitaemia with axillary temperature of at least 37·5°C, clinical signs and symptoms of malaria, or both. Blood smear positive defined as at least 1 P. falciparum asexual parasite seen on blood smear. Gametocyte positive defined as at least 1 gametocyte seen by 1 of 2 readers on blood smear.
| |
Pilot Safety Cohort | Main Cohort | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfs25H, 16µg | Comparator | Pfs25H, 47µg | Comparator | Pfs25H | Comparator | |||||||
| Total No. |
No. Individuals (%) |
Total No. |
No. Individuals (%) |
Total No. |
No. Individuals (%) |
Total No. |
No. Individuals (%) |
Total No. |
No. Individuals (%) |
Total No. |
No. Individuals (%) |
|
| Any AE | ||||||||||||
| Total | 72 | 10 (100%) | 89 | 10 (100%) | 857**** | 50 (100%) | 680**** | 49 (98%) | 929 | 60 (100%) | 769 | 59 (98%) |
| Post Dose #1 | 23 | 9 (90%) | 16 | 8 (80%) | 135 | 45 (90%) | 110 | 45 (90%) | 158** | 54 (90%) | 126** | 53 (88%) |
| Post Dose #2 | 49 | 9 (100%) | 73 | 10 (100%) | 152 | 46 (92%) | 121 | 39 (85%) | 201 | 55 (93%) | 194 | 49 (88%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 367 | 45 (100%) | 288 | 42 (100%) | 367 | 45 (100%) | 288 | 42 (100%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 203 | 42 (100%) | 161 | 40 (100%) | 203 | 42 (100%) | 161 | 40 (100%) |
| Related to vaccine | 19 | 8 (80%) | 10 | 5 (50%) | 172**** | 48 (96%) | 116**** | 42 (84%) | 191**** | 56 (93%)* | 126**** | 47 (78%)* |
| Solicited | 6 | 4 (40%) | 8 | 4 (40%) | 131**** | 46 (92%)* | 78**** | 33 (66%)* | 137**** | 50 (83%)* | 86**** | 37 (62%)* |
| Unsolicited | 66 | 10 (100%) | 81 | 10 (100%) | 726 | 50 (100%) | 602 | 49 (98%) | 792 | 60 (100%) | 683 | 59 (98%) |
| Total SAE | 0 | 0 (0%) | 0 | 0 (0%) | 1 | 1 (2%) | 2 | 2 (4%) | 1 | 1 (2%) | 2 | 2 (3%) |
|
SAE Related to
vaccine |
0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) |
| Local Reactogenicity | ||||||||||||
| Total | 5 | 3 (30%) | 7 | 4 (40%) | 87**** | 39 (78%) | 55**** | 31 (62%) | 92**** | 42 (70%) | 62**** | 35 (58%) |
| Post Dose #1 | 3 | 2 (20%) | 3 | 2 (20%) | 23 | 21 (42%) | 15 | 15 (30%) | 26 | 23 (38%) | 18 | 17 (28%) |
| Post Dose #2 | 2 | 2 (22%) | 4 | 3 (30%) | 29** | 26 (52%)* | 14** | 13 (28%)* | 31** | 28 (47%) | 18** | 16 (29%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 17 | 17 (38%) | 20 | 20 (48%) | 17 | 17 (38%) | 20 | 20 (48%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 18** | 18 (43%)* | 6** | 6 (15%)* | 18** | 18 (43%)* | 6** | 6 (15%)* |
| Solicited Systemic AEs | ||||||||||||
| Total | 1 | 1 (10%) | 1 | 1 (10%) | 44**** | 27 (54%)* | 23**** | 14 (28%)* | 45**** | 28 (47%)* | 24**** | 15 (25%)* |
| Post Dose #1 | 1 | 1 (10%) | 0 | 0 (0%) | 12 | 10 (20%) | 10 | 9 (18%) | 13 | 11 (18%) | 10 | 9 (15%) |
| Post Dose #2 | 0 | 0 (0%) | 1 | 1 (10%) | 11 | 9 (18%) | 6 | 4 (9%) | 11 | 9 (15%) | 7 | 5 (9%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 13** | 10 (22%) | 3** | 3 (7%) | 13** | 10 (22%) | 3** | 3 (7%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 8 | 8 (19%) | 4 | 4 (10%) | 8 | 8 (19%) | 4 | 4 (10%) |
| Laboratory Abnormalities | ||||||||||||
| Total | 14** | 7 (70%) | 3** | 2 (20%) | 113 | 33 (66%) | 83 | 32 (64%) | 127 | 40 (67%) | 86 | 34 (57%) |
| Post Dose #1 | 8** | 5 (50%)* | 0** | 0 (0%)* | 23 | 19 (38%) | 21 | 14 (28%) | 31 | 24 (40%) | 21 | 14 (23%) |
| Post Dose #2 | 6 | 5 (56%) | 3 | 2 (20%) | 30 | 19 (38%) | 23 | 17 (37%) | 36 | 24 (41%) | 26 | 19 (34%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 30 | 20 (44%) | 16 | 13 (31%) | 30 | 20 (44%) | 16 | 13 (31%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 30 | 18 (43%) | 23 | 16 (40%) | 30 | 18 (43%) | 23 | 16 (40%) |
| Neutropenia | 9 | 6 (60%) | 2 | 2 (20%) | 63 | 25 (50%) | 40 | 20 (40%) | 72**** | 31 (52%) | 42**** | 22 (37%) |
| Grade 1 | 7**** | 5 (50%)* | 0**** | 0 (0%)* | 40 | 24 (48%) | 34 | 20 (40%) | 47 | 29 (48%) | 34 | 20 (33%) |
| Grade 2 | 2 | 2 (20%) | 2 | 2 (20%) | 23**** | 13 (26%) | 6**** | 5 (10%) | 25 | 15 (25%) | 8 | 7 (12%) |
| Malaria | ||||||||||||
|
Symptomatic
Malaria |
11 | 6 (60%) | 9 | 6 (60%) | 116** | 44 (88%) | 84** | 40 (80%) | 127** | 50 (83%) | 93** | 46 (77%) |
| Post Dose #1 | 1 | 1 (10%) | 1 | 1 (10%) | 11 | 11 (22%) | 5 | 5 (10%) | 12 | 12 (20%) | 6 | 6 (10%) |
| Post Dose #2 | 10 | 6 (67%) | 8 | 6 (60%) | 18 | 16 (32%) | 13 | 13 (28%) | 28 | 22 (37%) | 21 | 19 (34%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 51 | 34 (76%) | 36 | 26 (62%) | 51 | 34 (76%) | 36 | 26 (62%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 36 | 32 (76%) | 30 | 27 (68%) | 36 | 32 (76%) | 30 | 27 (68%) |
|
Blood Smear
Positive |
19 | 9 (90%) | 30 | 7 (70%) | 308 | 48 (96%) | 254 | 42 (84%) | 327 | 57 (95%)* | 284 | 49 (82%)* |
| Post Dose #1 | 2 | 1 (10%) | 2 | 1 (10%) | 18 | 16 (32%) | 10 | 9 (18%) | 20 | 17 (28%) | 12 | 10 (17%) |
| Post Dose #2 | 17 | 8 (89%) | 28 | 6 (60%) | 45 | 30 (60%) | 40 | 27 (59%) | 62 | 38 (64%) | 68 | 33 (59%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 152 | 43 (96%)* | 126 | 33 (79%)* | 152 | 43 (96%)* | 126 | 33 (79%)* |
| Post Dose #4 | N/A | N/A | N/A | N/A | 93 | 41 (98%)* | 78 | 32 (80%)* | 93 | 41 (98%)* | 78 | 32 (80%)* |
|
Gametocyte
positive |
2 | 2 (20%) | 9 | 5 (50%) | 51 | 28 (56%) | 48 | 19 (38%) | 53 | 30 (50%) | 57 | 24 (40%) |
| Post Dose #1 | 0 | 0 (0%) | 0 | 0 (0%) | 3 | 3 (6%) | 6 | 6 (12%) | 3 | 3 (5%) | 6 | 6 (10%) |
| Post Dose #2 | 2 | 2 (22%) | 9 | 5 (50%) | 6 | 6 (12%) | 3 | 3 (7%) | 8 | 8 (14%) | 12 | 8 (14%) |
| Post Dose #3 | N/A | N/A | N/A | N/A | 27 | 20 (44%) | 23 | 11 (26%) | 27 | 20 (44%) | 23 | 11 (26%) |
| Post Dose #4 | N/A | N/A | N/A | N/A | 15 | 10 (24%) | 16 | 10 (25%) | 15 | 10 (24%) | 16 | 10 (25%) |
Data are number (%), unless otherwise specified.
N/A = not applicable.
Significant by Fishers exact (p≤0.05)
Significant by Wilcoxon rank sum only for count for each individual (p≤0.05). Counts between vaccinations done only by count Wilcoxon rank sum test.
Significant by Wilcoxon rank sum only for rate (count/time at risk) for each individual (p≤0.05).
Significant by Wilcoxon rank sum for both count and rate (p≤0.05).
In the pooled Pfs25H cohort (16µg and 47µg), the most common solicited AEs included injection site-related events (n=42/60, 70%; comparators n=35/60, 58%) and headache (n=18/60, 30%; comparators n=13/60, 22%); majority were Grade 1 or 2, except one Grade 3 injection site pain in a comparator participant (table 2; table S4). Among injection site-related events, injection site pain (n=41/60, 68%; comparators n=34/60, 58%) was most common. The number of injection site-related events per participant was similar between groups, and did not consistently increase with successive vaccine doses; however, by overall count and rate there were more reported local reactogenicity events, including injection site pain, in Pfs25H 47µg vaccinees (table S5).
The most common unsolicited AEs included symptomatic malaria, cold, and rhinitis. In total, 26 Grade 3 AEs were reported (Pfs25H, n=12; comparator, n=14), all deemed not related or unlikely related to vaccine except for one episode of injection site pain in a comparator subject. Three SAEs (Pfs25H: spontaneous abortion; comparator: snake bite, trauma) were reported, all deemed unrelated to vaccination; two subjects were excluded from further vaccination. No participants were removed from study due to a related AE of any severity.
The study halted once for multiple Grade 2 neutropenias, reviewed by DSMB and Sponsor, and study restarted. Upon unblinding, neither the number of unique subjects reporting neutropenia nor the severity of neutropenia differed significantly between Pfs25H vaccinees and comparators. However, Pfs25H vaccinees in the main cohort had a higher overall count and rate of neutropenias than comparators, including Grade 2 neutropenias (table 2; table S7). Further safety analyses are in the appendix (tables S4-S7).
A significantly larger number of symptomatic malaria episodes occurred per Pfs25H vaccinee versus comparator over the entire study (Pfs25H: 127 occurrences; comparator: 93 occurrences; p=0·036; Wilcoxon rank sum for count), but this lost significance after accounting for time at risk (p=0·082; Wilcoxon rank sum for rate) (table 2). Nor were Pfs25H vaccinees and comparators different in number of symptomatic malaria episodes after any one vaccination, nor with increasing vaccine dosage, nor on a unique individual basis (table 2). Similarly, vaccinees and comparators did not differ in frequency or rate of blood smear-positive or gametocyte-positive events, except after dose 3 and 4, when the number of unique Pfs25H, 47µg vaccinees with at least one positive blood smear was higher (table 2; table S8).
No Pfs25 antibody titres were detected in Pfs25H vaccinees prior to vaccination, nor in the comparator groups at any time point (figure 2). 5/9 participants in the safety pilot cohort who received two doses of Pfs25H, 16µg developed a detectable response (table S9). In the main cohort, antibody titres to Pfs25 were detected in only 1/50 (2%) individual after the first dose, 27/45 (60%) after the second dose, and 39/44 (88·6%) after the third dose (figure 2). Titres decreased to undetectable levels in all vaccinees by dose 4 (one year after dose 3). However, a clear anamnestic response developed following fourth dose, when highest titres were achieved (geometric mean 422·3, 95% CI 290–615), which correlated with post-dose 3 titres (figure S2). Pfs25 titres were detected in all but one Pfs25H vaccinee (40/41; 97·6%) in main cohort after dose 4. Antibody titres against EPA were detected after the first dose (29/49; 59%), and significantly increased with each dose (figure 2) as seen previously in U.S. vaccinees.14 Peak geometric mean antibody titres according to treatment doses are available at all evaluable time points in the appendix (table S10). Anti-Pfs25 titres were positively associated with anti-EPA over all time points, but decayed more rapidly than did anti-EPA titres after fourth dose (T1/2 = 42 days versus 59 days, respectively), again as seen in U.S. vaccinees.14 For Pfs25 antibody titres and EPA, the median ratio at day 600/peak was 0·18 vs 0·29, respectively (p=<0·001, paired Wilcoxon’s test).
Figure 2. Anti-Pfs25 and anti-EPA IgG ELISA titres.
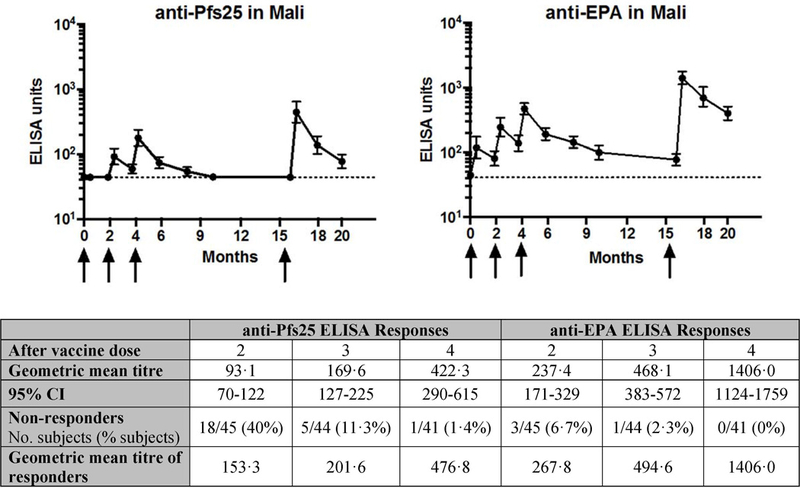
Arrows indicate the day of vaccination. Only data from main cohort who received 47µg Pfs25H-EPA/Alhydrogel® vaccination presented. Note all comparator participants were below the level of detection for anti-Pfs25 responses at all timepoints. Closed circles represent geometric mean antibody level and black bars 95% confidence intervals (CI). Non-responders are defined as those subjects who did not have an ELISA unit > 44 EU.
Functional activity by SMFA, which includes transmission-reducing activity (TRA; reduction in the number of oocysts per mosquito) and transmission-blocking activity (TBA; reduction in the proportion of mosquitoes infected), was measured on sera from all available participants in main cohort at baseline and 14 days following third and fourth vaccination. At baseline, a few participants had significant TRA (table S10). Average TRA by SMFA was not significantly different in vaccinees versus comparators following third dose by Wilcoxon test (vaccinated and comparator medians are 41·4% and 27·8%; p=0·09) but was significantly higher in the vaccinated group after fourth dose (p<0·001) (figure 3A–B, table S9–10). TBA was not different between vaccinees and comparators (figure 4A), but SMFA TBA results are known to be highly variable and dependent upon infection intensity in control mosquitoes.19,20
Figure 3. Serum transmission reducing activity after Pfs25H-EPA/Alhydrogel® dose 4.
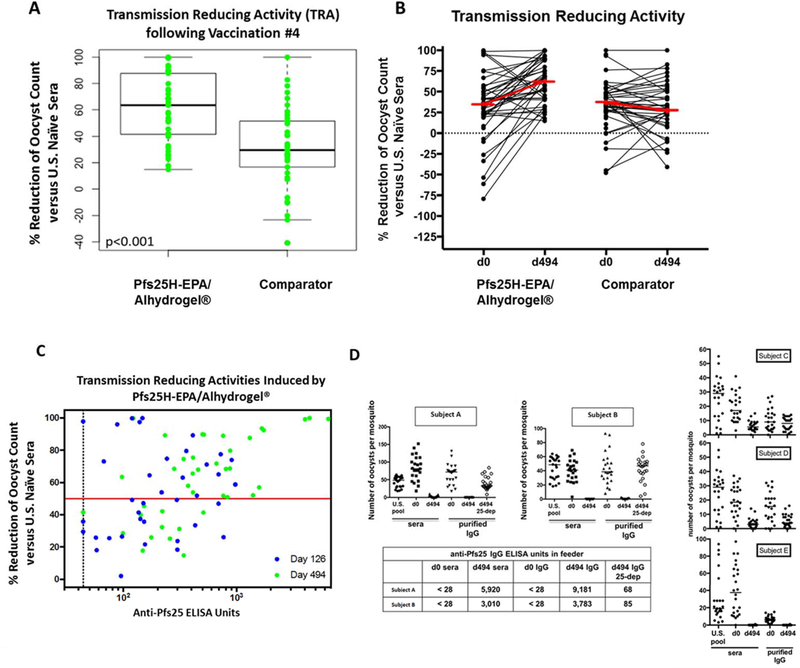
Standard membrane feeding assay (SMFA) performed on sera from pre-vaccination and 14 days post-dose 4 shows (figures 3A and3B) increase in serum functional activity in Pfs25H-EPA/Alhydrogel® versus comparator vaccinees and (figure 3C) non-linear association of antibody titers with serum activity (p-value 0.01 GEE). Figure 3D confirms in participants with the highest anti-Pfs25 ELISA titres and corresponding high functional activity by SMFA, that the functional responses are Pfs25-dependent. For figure 3A and 3B, each data point represents the average of two results in the SMFA in an individual subject with the thick black line (3A) or thick red line (3B) indicating the median. For figure 3C, the thick red line indicates the threshold representing significant biological activity (50% transmission reducing activity), and the dotted vertical black line indicates 44 ELISA units as the limit of detection of the assay. The blue and green dots correspond to the data points on Study Day 126: 14 days post Vaccination #3; Study Day 494: 14 days post Vaccination #4. For figure 3D, SMFA of post-dose 4 sera and IgG from five (Subjects A-E) Pfs25-EPA/Alhydrogel®, 47µg vaccinees. Two individuals with relatively high antibody titres show high functional activity for serum as well as the purified IgG fraction which can be ablated by depletion of Pfs25-specific IgG of two vaccinees (Subjects A–B) on an antigen-affinity column. Comparator = Euvax B (for vaccinations #1, 2, 3) and Menactra® (for vaccination #4).
Figure 4. Serum transmission blocking activity and direct skin feed summary after Pfs25H-EPA/Alhydrogel® dose 4.
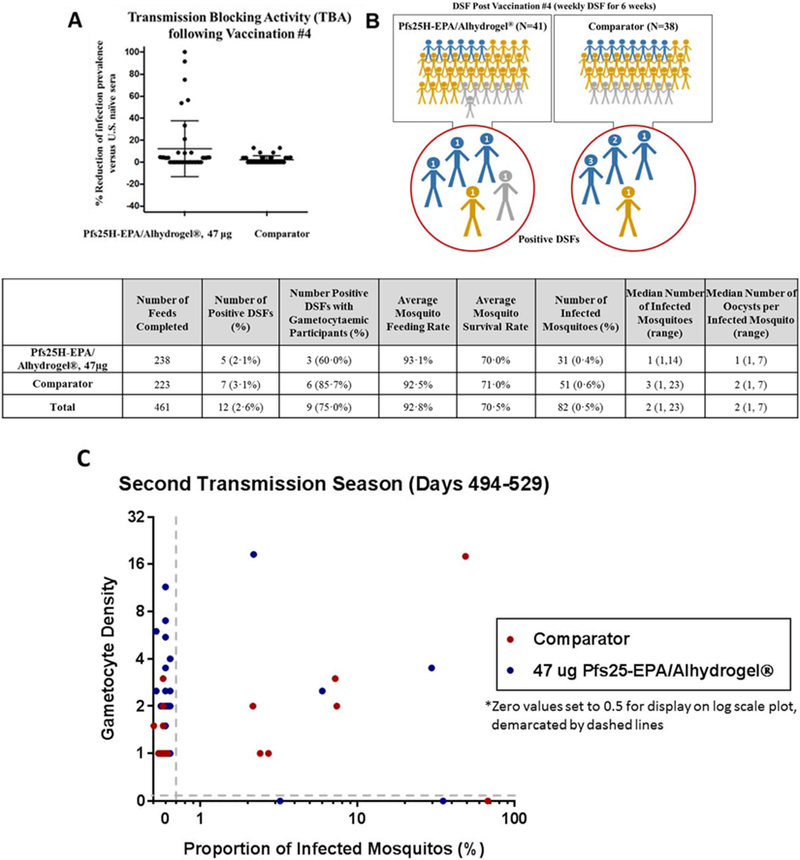
Standard membrane feeding assay (SMFA) performed on sera from 14 days post-dose 4 (Day 494) shows (figure 4A) a subset of participants who received Pfs25H-EPA/Alhydrogel® but none who received comparator vaccine displayed activity that reduced infection prevalence by more than 20%. Direct skin feed (DSF) assays show (figure 4B) that a small number of subjects who received vaccine (N=5) or comparator (N=4) transmitted parasites to colony-raised mosquitoes at one or more timepoints. Each study subject is also presented as having had any gametocytaemia (blue figures), any parasitaemia (gold figures), or no parasitaemia (gray figures) throughout the DSF period. For subjects who yielded a positive DSF, the number of positive DSF during the season of follow-up is indicated as a numeric value on the figure’s head. DSF outcomes by group, indicating number and percentage of positive DSF, as well as the median (range) of infected mosquitoes for positive DSFs, and the median (range) number of oocysts per infected mosquito are presented in the table. The number (percentage) of participants with gametocytaemia at the time of positive DSF is also indicated. Figure 4C shows the relationship between gametocyte density (gametocytes per 1000 WBC) in the study participants and proportion of infected mosquitoes (number of oocyst infected mosquitoes/number of dissected mosquitoes x100) resulting from DSF assays performed each week for 6 weeks post-dose 4 in the second season, stratified by test and comparator vaccine groups. Most but not all DSF with at least one oocyst infected mosquito resulted from assays on study participants with detectable gametocytes, and conversely many study participants with detectable gametocytemia failed to transmit parasites to mosquitoes (19 events for Pfs25H; 11 events for comparator). DSF where values for both gametocyte density and proportion mosquitoes infected were zero (214 for Pfs25H; 205 for comparator vaccine) are not displayed.
In general, anti-Pfs25 titres correlated with functional activity (figure 3C). To confirm that activity was mediated by vaccine-specific antibodies, SMFA was performed using IgG purified from select individuals (n=5, figure 3D) with high titres (> 1,000 units) and high TBA. For two individuals with highest titres, and sufficient yield of purified IgG, Pfs25-specific IgG was then depleted on affinity columns, which resulted in substantial or complete loss of activity measured in SMFA (figure 3D, subjects A and B). Thus, functional activity in vaccinees was contained in the IgG fraction directed against Pfs25. To determine if increased activity observed post-dose 4 was associated with quality of antibody, antibody avidity to Pfs25 was measured in a modified ELISA using a chaotropic agent (figure 5). Using a mixed effects statistical model of the log of the avidity index, relative avidity to Pfs25 differed significantly between day 70 (14 days post dose 2) and day 126 (14 days post dose 3; p=0·026) as well as between day 70 and day 494 (14 days post dose 4; p<0·001) (figure S3–4). Therefore, increased activity was associated with increased antibody avidity.
Figure 5. Avidity index in sera after subsequent vaccinations.

Avidity testing was performed on sera from 14 days post-dose 2 (Study Day 70), post-dose 3 (Study Day 126), and post-dose 4 (Study Day 494).
Using a linear GEE model for the log of the oocyst count ratio from the SMFAs and using the square root of the ELISA values on sera from 14 days post dose 3 and dose 4, we found that anti-Pfs25 levels are associated with SMFA values (p=0·01; figure 3C). Using this same model, which was previously published for EC50 calculations, but using Day 0 TRA as control given pre-existing activity in Malian adults, the estimated EC50 of serum anti-Pfs25 level is 49μg/mL (95% CI, 22·5 – 78·2 μg/mL; GEE method), similar to what was seen in the earlier US phase 1 study (EC50 = 57·2 μg/ml, 95% CI 44·7 – 76·8 μg/mL).14 Using their day zero TRA as control (“Day 0 TRA”), the mean TRA at day 126 for Pfs25 vaccinees was 1·60% (T-based 95% CI, −12·78 – 15·99); at day 494, the mean TRA was 26·64% (T-based 95% CI, 5·85 – 47·43); and for days 126 and 494 combined, the mean TRA was 13·53% (robust 95% CI, 0·73 – 26·32).
627 DSFs were completed during the entire study. Following vaccination #3, DSFs were piloted in parasite carriers only and at various timepoints. Starting 14 days following the fourth dose (booster dose), 79 participants (41 Pfs25H vaccinees, 38 comparators) completed 461 DSF (figure 1); two comparators did not undergo DSF due to unrelated AE (trauma) and to consent withdrawal, respectively; one Pfs25H vaccinee also withdrew consent prior to DSFs. Among DSF performed following vaccination 4, there were 12 positive DSFs, including 5 in Pfs25H vaccinees and 7 in comparators (figure 4C) for a DSF positivity rate of 2.6%. Nine unique individuals (5 Pfs25H, 4 comparators) yielded positive DSF, for a subject level transmission rate of 11·4%, for having infected at least one mosquito (figure 4B).
Among the 14 unique individuals who were gametocytaemic at the time of a DSF, 6 (42·9%; 3 Pfs25H, 3 comparator) infected at least one mosquito in their concurrent DSF. A conditional exact test of the ratio of the proportion of subjects with positive DSF among those with concurrent detectable gametocytaemia suggests there is no difference in the proportion of infective participants between Pfs25H and comparator based on DSF (p=1). We also found no significant difference between the proportion of infective participants by DSF compared to those on Pfs25H and comparator among subjects with concurrent detectable gametocytes (p=1; conditional exact test). Among the many other statistical methods and outcomes investigated based on DSF, there were no significant differences found between the vaccinated and comparator subjects in average oocyst count, mosquito infection rate, DSF positivity rate or subjects infecting at least one mosquito (figure 4B).
Discussion
Transmission-blocking vaccines have been envisioned since the 1970s as an interventional concept to support malaria elimination efforts. Transmission to the mosquito represents a parasite bottleneck, with usually less than five oocysts per mosquito, whereas an infected human can harbor as many as 1013 asexual stage parasites in the blood stream. However, earlier attempts to develop a transmission-blocking vaccine foundered on poor immunogenicity9,10 or reactogenicity related to the adjuvant-formulated products.11 We recently showed that chemical conjugation to the carrier protein ExoProtein A (EPA) yields a Pfs25 particle immunogen21 that was safe and immunogenic when formulated with the aluminum adjuvant Alhydrogel® and administered to malaria-naïve U.S. volunteers.14 We report here the first trial of a transmission-blocking vaccine in the field, showing that the recombinant protein-protein conjugate vaccine Pfs25-EPA/Alhydrogel is safe and induces functional serum activity in Malian adults similar to the activity induced in US adults.14
Although the mean antibody titre in Malians was about half that observed in U.S. vaccinees,14 the pattern of the response was similar between the two studies. Malians showed no pre-existing responses to Pfs25, and generally no responses after first dose, consistent with post-fertilization expression in the mosquito.22 Antibody titres increased progressively with each dose, including the final boost one year later. The reason for the modestly lower antibody titres in Malians is unknown to us. Individuals with HIV or viral hepatitis infections were excluded. Responses to other vaccines are also lower in resource-poor tropical communities, and malnutrition and co-infections including parasitic infections can suppress vaccine responses.23 Of note, our trial cohort was largely healthy young adults, and helminth infections were uncommon, presumably a beneficial result of the periodic mass de-worming campaigns. Malaria itself can suppress vaccine responses,24 and we are currently assessing antimalarial treatment prior to vaccinations in Mali.
Despite modestly lower antibody titres in Malian versus U.S. vaccinees, post-vaccination serum functional activity appeared similar. In both trials, 27% of vaccinees attained serum functional activity after fourth vaccination that reduced oocyst burden in mosquitoes by >80% by SMFA. The relationship between SMFA and in vivo measurements of transmission-blocking activity has not been studied in humans. Data modeling suggests that serum reduction of oocyst numbers by SMFA (ie, transmission-reducing activity) correlates with a reduced prevalence of infected mosquitoes (ie, transmission-blocking activity) when oocyst numbers are low in the control mosquitoes receiving non-immune serum25; notably, oocyst counts are typically low (<5 per midgut) in wild-caught mosquitoes.26 Notably, serum activity in U.S. vaccinees was largely lost by 8 weeks post dose 4 when Pfs25 titres had decreased by more than half, 14 hence we expect a similar decline in activity with titres seen here.
Malian adults displayed pre-existing functional activity that reduced parasite transmission measured in SMFA to variable degrees, with a few individuals demonstrating high activity that persisted over time. Naturally acquired activity has been associated with antibody against known pre-fertilization malaria antigens such as Pfs230.26 While this pre-existing activity likely persisted in vaccinees throughout the trial (as it did in comparators), we did not observe a strong correlation between functional activity measured at baseline and at post-vaccination 4, whereas Pfs25 titres were highly correlated with activity. Further, we showed that high serum activity in Pfs25H vaccinees was mediated by Pfs25-specific antibodies. Thus, we conclude that the functional serum activity we measured post-dose 4 in most vaccinees was largely related to immunization.
Pfs25-EPA/Alhydrogel has significant limitations. Four vaccine doses were required to achieve significant serum functional activity, both in the US14 and in Mali. Regimens that require fewer doses will be easier to implement, and we are exploring additional platforms such as more potent adjuvants for dose-sparing benefits. Furthermore, Pfs25 titres decreased rapidly after each post-dose peak, and the serum functional activity that we observed two weeks post-dose 4 had disappeared within eight weeks in the U.S trial.14 Pfs25 is a post-fertilization antigen, and therefore vaccine responses are not boosted during naturally occurring infections. The short period of activity is a serious limitation for any vaccine, and for practical purposes TBV activity should persist for at least a season of transmission. In mice, we have observed that more potent adjuvants, such as the liposomal adjuvant ALF-Q that incorporates QS21 and the TLR4 ligand GLA, can induce more durable antibody responses to Pfs25 vaccines,27 and we are now evaluating this in humans.
Finally, we have also developed a vaccine based on the pre-fertilization antigen Pfs230 to study either alone or in combination with Pfs25-EPA in human trials. Some naturally exposed individuals acquire antibodies against Pfs230 that correlate with serum functional activity,26 suggesting vaccine responses might be boosted during infection. Complement enhances Pfs230 antibody activity,28 and this effect may reduce the antibody titre required to block transmission. We also hypothesize that the combination of activities against pre- and post-fertilization antigens will exceed their individual activities, although evidence for this has not been conclusive in preclinical studies.
As TBVs advance in the field, new approaches to measure efficacy will be needed.29 A definitive efficacy trial for reducing malaria incidence may require numerous communities and hence be relatively large and expensive.30 As an interim endpoint, we explored DSF assays to measure vaccine activity, with colony-raised mosquitoes fed directly on participants.31 Weekly DSF was safe and well-tolerated, and yielded infected mosquitoes, although the rate of mosquito infection was low (~0.3%). We are currently expanding our DSF capacity to achieve sample sizes needed to confirm vaccine activity in future trials in Mali.
In conclusion, the Pfs25-EPA/Alhydrogel vaccine candidate was safe and immunogenic in Malian adults and induced significant serum activity after four doses that reduced parasite transmission to mosquitoes in a laboratory assay. This first field trial of a malaria TBV demonstrates functional immunogenicity in the target population despite lifetime exposure to Pf, however transmission-blocking activity was incomplete and Pfs25 antibody titres declined rapidly. Future studies should seek to increase and prolong functional antibody activity, possibly by combining Pfs25 with another antigen such as Pfs230, and to measure vaccine activity against naturally circulating infections.
Supplementary Material
Acknowledgments
Funding
US National Institute of Allergy and Infectious Diseases Intramural Research Program.
Role of the funding source
The study was funded by the Intramural Research Program of NIAID, NIH. NIAID scientists but not NIAID officials were involved in study design, study management, data collection, analysis, interpretation, and report writing. Principal investigators (IS, SAH) had full access to all study data and made the decision to submit for publication.
Footnotes
Conflicts of Interest
We declare that we have no conflicts of interest.
Contributor Information
Issaka Sagara, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Sara A. Healy, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Mahamadoun H Assadou, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Erin E. Gabriel, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Mamady Kone, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Kourane Sissoko, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Intimbeye Tembine, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Merepen A. Guindo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
M’Bouye Doucoure, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Karamoko Niaré, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Amagana Dolo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Kelly M. Rausch, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
David L. Narum, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
David L. Jones, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Nicholas J MacDonald, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Daming Zhu, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Rathy Mohan, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Olga Muratova, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Ibrahima Baber, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Mamadou B. Coulibaly, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Michael P. Fay, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Charles Anderson, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Yimin Wu, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Sekou F Traore, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Ogobara K. Doumbo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Science, Techniques and Technologies of Bamako, Mali.
Patrick E. Duffy, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
References
- 1.Mortality GBD, Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053): 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowdle WR. The principles of disease elimination and eradication. Bulletin of the World Health Organization 1998; 76 Suppl 2: 22–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 1976; 263(5572): 57–60. [DOI] [PubMed] [Google Scholar]
- 4.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 1976; 193(4258): 1150–1. [DOI] [PubMed] [Google Scholar]
- 5.Carter R, Kaushal DC. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. III. Changes in zygote surface proteins during transformation to mature ookinete. Molecular and biochemical parasitology 1984; 13(2): 235–41. [DOI] [PubMed] [Google Scholar]
- 6.Grotendorst CA, Kumar N, Carter R, Kaushal DC. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infection and immunity 1984; 45(3): 775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy PE, Pimenta P, Kaslow DC. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. The Journal of experimental medicine 1993; 177(2): 505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. The Journal of experimental medicine 1991; 174(5): 1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaslow DC. Transmission-blocking vaccines: uses and current status of development. International journal for parasitology 1997; 27(2): 183–9. [DOI] [PubMed] [Google Scholar]
- 10.Ockenhouse CF, Sun PF, Lanar DE, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. The Journal of infectious diseases 1998; 177(6): 1664–73. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PloS one 2008; 3(7): e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian F, Wu Y, Muratova O, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine 2007; 25(20): 3923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Przysiecki C, Flanagan E, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proceedings of the National Academy of Sciences of the United States of America 2006; 103(48): 18243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talaat KR, Ellis RD, Hurd J, et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PloS one 2016; 11(10): e0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials U.S. Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research; September 2007. [Google Scholar]
- 16.Carey V Generalized Estimation Equation Solver 2015. https://cran.r-project.org/web/packages/gee/gee.pdf (accessed August 1 2017).
- 17.Bates D, Machler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software; 2014. [Google Scholar]
- 18.Kuznetsova A, Brockhoff P, Christensen R. Tests in Linear Mixed Effects Models 2016. https://cran.opencpu.org/web/packages/lmerTest/lmerTest.pdf (accessed August 1 2017).
- 19.Miura K, Deng B, Tullo G, et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PloS one 2013; 8(3): e57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churcher TS, Blagborough AM, Delves M, et al. Measuring the blockade of malaria transmission--an analysis of the Standard Membrane Feeding Assay. International journal for parasitology 2012; 42(11): 1037–44. [DOI] [PubMed] [Google Scholar]
- 21.Shimp RL Jr., Rowe C, Reiter K, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013; 31(28): 2954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. The Journal of experimental medicine 1985; 162(5): 1460–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis 2009; 3(5): e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunnington AJ, Riley EM. Suppression of vaccine responses by malaria: insignificant or overlooked? Expert Rev Vaccines 2010; 9(4): 409–29. [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Swihart BJ, Deng B, et al. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 2016; 34(35): 4145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves PM, Carter R, Burkot TR, Quakyi IA, Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol 1988; 10(2): 209–18. [DOI] [PubMed] [Google Scholar]
- 27.Radtke AJ, Anderson CF, Riteau N, et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci Rep 2017; 7: 40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol 1987; 139(12): 4213–7. [PubMed] [Google Scholar]
- 29.Delrieu I, Leboulleux D, Ivinson K, Gessner BD, Malaria Transmission Blocking Vaccine Technical Consultation G. Design of a Phase III cluster randomized trial to assess the efficacy and safety of a malaria transmission blocking vaccine. Vaccine 2015; 33(13): 1518–26. [DOI] [PubMed] [Google Scholar]
- 30.White MT, Verity R, Churcher TS, Ghani AC. Vaccine approaches to malaria control and elimination: Insights from mathematical models. Vaccine 2015; 33(52): 7544–50. [DOI] [PubMed] [Google Scholar]
- 31.Brickley EB, Coulibaly M, Gabriel EE, et al. Utilizing direct skin feeding assays for development of vaccines that interrupt malaria transmission: A systematic review of methods and case study. Vaccine 2016; 34(48): 5863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


