Abstract
Background:
The veracity of the association between antibiotic use and hemolytic uremic syndrome (HUS) caused by Escherichia coli O157:H7 has been a topic of debate. We postulated that criteria used to define HUS affect this association.
Methods:
We reviewed 471 hospitalized E. coli O157:H7 cases reported in Washington State, 2005–2014, to determine HUS status by various case definitions and antibiotic treatment. We used age-adjusted logistic regression models to estimate the effect of treatment on HUS status according to four common, but heterogeneous, definitions: the Council of State and Territorial Epidemiologists (CSTE) definition, hematology-focused and age-focused definitions from the literature, and hospital diagnosis.
Results:
Inter-annual variation in antibiotic use was high, but no meaningful change in antibiotic use was observed over this ten-year period. Thirteen percent of cases <18 years-old received antibiotics, compared to 54% of cases ≥18 years-old. The CSTE, hematology-focused, agefocused, and hospital diagnosis definitions identified 149, 57, 74, and 89 cases of HUS, respectively. The association between antibiotic treatment and HUS varied by definition: CSTE odds ratio (OR) 1.57 [95% confidence interval (CI) 0.98, 2.55]; hematology-focused OR 1.73 (95% CI 0.83, 3.54); age-focused OR 2.29 (95% CI 1.20, 4.39); and hospital diagnosis OR 1.94 (95% CI 1.01, 3.72).
Conclusions:
Each definition yielded an estimate of the association in the direction of increased risk of HUS with antibiotics. While the range of OR point estimates was relatively small, confidence intervals for two HUS definitions crossed the null and two did not, potentially altering the inference an investigator makes. Discrepant reports of the association between antibiotic use and HUS in the literature might be due in part to the choice of HUS definition, and a consistent definition of HUS should be adopted for research and public health purposes.
Keywords: E. coli O157:H7, hemolytic uremic syndrome, Shiga toxin-producing Escherichia coli, antibiotics
INTRODUCTION
In patients with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli (STEC1) infections, hemolytic uremic syndrome (HUS) is the overarching outcome of concern. Characterized by hemolytic anemia, thrombocytopenia, and renal injury, HUS often necessitates renal replacement therapy (Garg et al., 2003; Karch et al., 2005) and has 3–5% case fatality (Gould et al., 2009; Mody et al., 2015; Scheiring et al., 2008). HUS incidence is highest among children <5 years old (Centers for Disease Control and Prevention, 2016; Crim et al., 2015; Crim et al., 2014; Marder et al., 2017).
In the first communication attributing HUS to STEC, Karmali et al. (1983) noted that many patients had received antibiotics before renal failure ensued. A 2016 meta-analysis of the topic identified 17 subsequent studies directly assessing this potential association and showing both protection and harm (Freedman et al., 2016). Limited to studies with least risk of bias, the meta-analysis estimated a 2.24-greater odds of HUS among E. coli O157:H7 cases treated with antibiotics compared to those left untreated (Freedman et al., 2016).
Despite this analysis, questions remain about the effect of antibiotics on HUS progression (Mody and Griffin, 2016). There are multiple potential sources of bias in studies of the association, such as confounding by indication and mixture of the effects of several classes of antibiotics. Misclassification of the outcome, HUS, can also introduce bias. Studies vary in the definition of HUS used, and in the rigor with which they validate HUS status among their cases according to the chosen definition. This variation extends to public health practice, as well.
To determine how commonly used definitions of HUS affect estimates of the association between antibiotic use and HUS, we validated the HUS status of over 400 hospitalized cases of E. coli O157:H7 infection in Washington State according to four common definitions. We also examined temporal trends in antibiotic treatment in this cohort over a ten-year period.
METHODS
Record abstraction
We retrospectively reviewed all hospitalized, culture-confirmed E. coli O157:H7 cases reported to the Washington State Department of Health through passive surveillance between 2005 and 2014. Note that some children in the current analysis also contributed data to concurrent studies (Denno et al., 2012; Hickey et al., 2011; Melamed et al., 2017; Qin et al., 2015; Wong et al., 2012). We attempted to obtain the chart from each hospital listed on the Department of Health case report form, and demographics, disease course, laboratory tests, and antibiotic treatment were abstracted. Cases spending at least one midnight in an inpatient medical facility were considered to have been hospitalized. Hospitals were classified as children’s hospitals based on membership in the Children’s Hospital Association (https://www.childrenshospitals.org/). The data abstraction form was piloted on five children and seven adults, including one with HUS, and revised before initiating the full review.
This review was conducted to enhance surveillance activities and deemed exempt by the Washington State Institutional Review Board.
HUS definitions
We considered four primary definitions of HUS (Appendix, Box A.1): Council of State and Territorial Epidemiologists (CSTE), hematology-focused, age-focused, and hospital diagnosis. The 1996 CSTE confirmed and probable criteria for postdiarrheal HUS (reaffirmed 2009) are used to report HUS cases to CDC (Council of State and Territorial Epidemiologists, 2009). The definition requires: anemia (Harriet Lane Service (Johns Hopkins Hospital), 2009); hematuria, proteinuria, serum creatinine concentration ≥1.0 mg/dL for children <13 years-old and ≥1.5 mg/dL for ≥13-year-olds, or 50% increase in serum creatinine concentration from baseline; HUS following diarrhea; and evidence of microangiopathic changes or HUS onset within 21 days of diarrhea onset (Council of State and Territorial Epidemiologists, 2009). The hematology-focused definition, which has been most frequently employed in studies using FoodNet data (Mody et al., 2015; Mody et al., 2012; Ong et al., 2012), is similar to the CSTE definition but includes thrombocytopenia, does not accept hematuria or proteinuria as sufficient evidence of renal injury, and requires microangiopathic changes on blood smear. The agefocused definition has been used in HUS case series from the Pacific Northwest (Ake et al., 2005; Klein et al., 2002; Wong et al., 2000; Wong et al., 2012), elsewhere in North America (Freedman et al., 2017), and Europe (Bielaszewska et al., 2006; Bielaszewska et al., 2007). It requires, all on the same day, hematocrit <30%, platelet count <150,000/mm3, and serum creatinine concentration above the upper limit of normal for age (Meites, 1989). Diagnosis of HUS in the discharge note or charge codes was included as the fourth definition (Appendix, Box A.1).
Antibiotic Use
Non-topical antibiotics of any class recorded in the chart as administered before or during hospitalization were included, but only if these agents were given one or more days before a reference date. For cases designated as HUS by any of the four definitions, the reference date was the day criteria for HUS were met. For cases who met no definition for HUS, the reference date was the date of the lowest observed hematocrit value, or if the hematocrit was not measured, the lowest circulating platelet concentration value. The first day of illness was considered to be the first day of diarrhea.
Antibiotic use was compared across strata of pertinent demographic and clinical variables. For age <18 vs. ≥18 years-old, the proportion and asymptotic 95% confidence intervals (CI) of hospitalized E. coli O157:H7 cases receiving antibiotics were calculated, and annual proportions were graphed.
Statistical Analysis
We considered several variables in our causal model for the association between antibiotic use and HUS (Appendix, Figure A.1). Because inclusion in this study was conditioned on hospitalization, adjustment for age and symptoms were considered necessary to control for confounding. However, because almost all cases experienced bloody diarrhea, there was insufficient variation to include this particular variable in the model. In the presence of bloody diarrhea, we believed that vomiting would not drive decisions to use these agents. Therefore, the primary analysis was not conditioned on vomiting. Logistic regression was used to estimate the association between HUS status, as determined by each of the four definitions, and antibiotic use, adjusted for age (continuous). The antibiotic use coefficient was exponentiated to obtain the odds ratio (OR), and the 95% CI was calculated.
We examined the frequency of administration of individual antibiotic classes. For the three most commonly administered classes (nitroimidazoles, fluoroquinolones, and β-lactams), we repeated the primary analysis. Exposed cases were those who received an antibiotic of the given class prior to their reference date. Unexposed cases were those who received no antibiotics prior to their reference date.
We conducted three sensitivity analyses. First, for cases whose hospital charts indicated use of an antibiotic but without indication if treatment was commenced before HUS onset or the case’s reference date, we imputed values that would, in turn, minimize and maximize the observed association according to each definition. Second, we repeated the regression models including only cases with bloody diarrhea to remove potential confounding through bloody diarrhea. Third, we repeated the regression models excluding cases who received antibiotics prior to hospitalization to remove this potential source of selection bias.
We used R (R Core Team, 2017) for all analyses.
RESULTS
Of 1160 culture-confirmed E. coli O157:H7 cases in Washington State during the study period, 471 (41%) were hospitalized. No hospital was specified on case report forms for 18 cases, and records for 20 additional cases could not be located at the hospital entered in the State database. We could not determine antibiotic use or the day of antibiotic initiation for 28 (6.5%) of the 433 remaining cases. Hence, primary analyses were limited to the 405 cases with complete antibiotic use information.
Antibiotics were administered to 133 (33%) hospitalized E. coli O157:H7 cases prior to the reference date: 42 received one, 74 received two, and 14 received three or more antibiotics. We could not determine the identity of the antibiotics received for three cases; all three were 2 years-old, one had HUS according to all definitions, and one had HUS according to the CSTE definition and hospital diagnosis. Antibiotic use varied across age strata (Table 1), with 13% (95% CI 9.3%, 19%) of children <18 years-old receiving antibiotics compared to 54% (95% CI 47%, 61%) of adults ≥18 years-old. The age-specific proportion of antibiotic-treated cases fluctuated year-to-year with no discernible pattern (Figure 1). Antibiotic use across strata of other important demographic and clinical variables, including bloody diarrhea and vomiting, was similar. Approximately 16% of all infected patients developed anuria, and 10% underwent dialysis (Table 1). Among the 211 children <18 years-old, 119 were hospitalized initially or subsequently at a designated children’s hospital, of whom 17 (14%; 95% CI 9.1%, 22%) received antibiotics. Eleven (12%; 95% CI 6.8%, 20%) of the 92 children hospitalized at general care hospitals received antibiotics (Appendix).
Table 1.
Clinical and demographic characteristics by antibiotic treatment
| Variable | No Antibiotics | Antibiotics |
|---|---|---|
| Total | 272 | 133 |
| Age (years), n (p) | ||
| 0 to <5 | 88 (0.32) | 12 (0.09) |
| 5 to <10 | 62 (0.23) | 4 (0.03) |
| 10 to <18 | 33 (0.12) | 12 (0.09) |
| 18 to <60 | 57 (0.21) | 58 (0.44) |
| ≥60 | 32 (0.12) | 47 (0.35) |
| Sex, n (p) | ||
| Female | 156 (0.57) | 78 (0.59) |
| Male | 116 (0.43) | 55 (0.41) |
| Day * of specimen collection, median (IQR) | 3 (2, 4) | 3 (2, 4.75) |
| Blood in stool, n (p) | 259 (0.97) | 126 (0.95) |
| Vomiting, n (p) | 166 (0.64) | 79 (0.65) |
| Days hospitalized, median (IQR) | 3 (2, 6) | 4 (2, 6) |
| Urine output **, sn (p) | ||
| Anuria | 25 (0.17) | 8 (0.15) |
| <0.5 ml/kg/hr | 47 (0.33) | 21 (0.39) |
| Underwent dialysis, n (p) | 29 (0.11) | 11 (0.09) |
| Type of hospital (cases <18 years-old), n (p) | ||
| Children’s hospitals | 102 (0.56) | 17 (0.61) |
| General hospitals | 79 (0.44) | 11 (0.39) |
Day refers to the number of days since diarrhea onset [18]
Urine output was documented for only 200 cases
Abbreviations: IQR, interquartile range; n, number; p, proportion
Figure 1. Annualized antibiotic use in hospitalized E. coli O157:H7 cases reported in Washington State, 2005–2014.
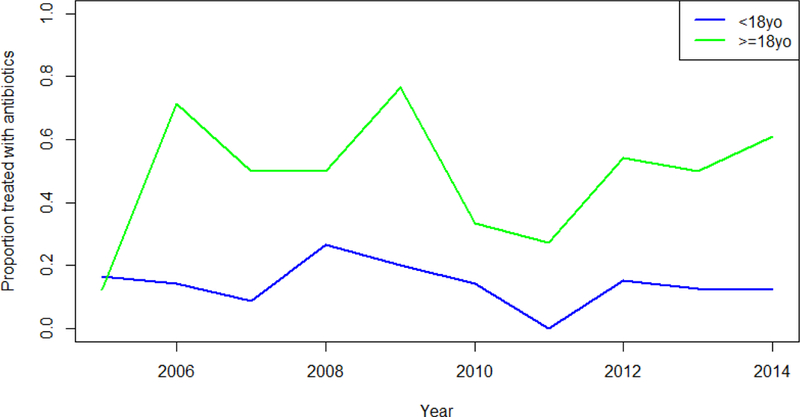
The proportion of E. coli O157:H7 cases receiving antibiotics was greater in adults than children.
Antibiotic Use and HUS
Among the 405 cases with antibiotic use data, sufficient data existed to allocate HUS status for 403, 405, 404, and 397 patients using the CSTE, hematology-focused, age-focused, and hospital diagnosis definitions, respectively. The CSTE, hematology-focused, age-focused, and hospital diagnosis definitions identified 149, 57, 74, and 89 cases of HUS, respectively, corresponding to HUS rates among hospitalized patients of 37%, 14%, 18%, and 22%, respectively. After adjusting for age, the age-focused definition yielded the strongest estimate of association, OR 2.29 (95% CI 1.20, 4.39), followed by hospital diagnosis (OR 1.94; 95% CI 1.01, 3.72), the hematology-focused definition (OR 1.73; 95% CI 0.83, 3.54); and the CSTE definition (OR 1.57; 95% CI 0.98, 2.55) (Table 2).
Table 2.
Association between antibiotic treatment and HUS among reported E. coli O157:H7 cases for four common definitions of HUS
| Definition | HUS | No Antibiotics | Antibiotics | OR* (95% CI) |
|---|---|---|---|---|
| Age-focused | Yes | 51 | 23 | 2.29 (1.20, 4.39) |
| No | 220 | 110 | ||
| Hospital diagnosis | Yes | 66 | 23 | 1.94 (1.01, 3.72) |
| No | 202 | 106 | ||
| Hematology-focused | Yes | 42 | 15 | 1.73 (0.83, 3.54) |
| No | 230 | 118 | ||
| CSTE | Yes | 96 | 53 | 1.57 (0.98, 2.55) |
| No | 175 | 79 |
Adjusted for age (continuous)
Abbreviations: CI, confidence interval; CSTE, Council of State and Territorial Epidemiologists; HUS; hemolytic uremic syndrome; OR, odds ratio
The most commonly used antibiotic classes were nitroimidazoles (n=93), fluoroquinolones (n=75), and β-lactams (n=41) (Table 3). Stratifying by individual antibiotic classes produced estimates in a similar range, from OR 1.26 to 2.87, to the analysis of all antibiotics, with wide confidence intervals. However, the pattern of association was not identical across definitions of HUS. All definitions except CSTE estimated the largest OR for β-lactam antibiotics. Using the age-focused definition, β-lactam antibiotics were associated with a 2.87 greater odds of HUS than cases receiving no antibiotics (95% CI 1.10, 7.35).
Table 3.
Frequency of specific antibiotic classes and class-specific associations with HUS, as determined by four common definitions
| Antibiotic Class | N | Age-focused | Hospital Diagnosis | Hematology-focused | CSTE | ||||
|---|---|---|---|---|---|---|---|---|---|
| HUS | OR (95% CI) | HUS | OR (95% CI) | HUS | OR (95% CI) | HUS | OR (95% CI) | ||
| Nitroimidazoles | 93 | 11 | 1.93 (0.80, 4.58) | 10 | 1.54 (0.62, 3.72) | 8 | 1.94 (0.71, 5.10) | 37 | 1.63 (0.93, 2.86) |
| Fluoroquinolones | 75 | 7 | 1.69 (0.56, 4.84) | 7 | 1.49 (0.50, 4.19) | 5 | 1.71 (0.47, 5.72) | 26 | 1.38 (0.73, 2.60) |
| β-Lactams | 41 | 9 | 2.87 (1.10, 7.35) | 8 | 1.87 (0.68, 4.91) | 6 | 2.01 (0.67, 5.46) | 14 | 1.26 (0.59, 2.59) |
| Macrolides | 10 | 2 | DNC | 2 | DNC | 1 | DNC | 3 | DNC |
| Glycopeptides | 7 | 0 | DNC | 0 | DNC | 0 | DNC | 4 | DNC |
| TMP-SMX | 3 | 3 | DNC | 3 | DNC | 2 | DNC | 3 | DNC |
| Aminoglycosides | 2 | 2 | DNC | 2 | DNC | 2 | DNC | 2 | DNC |
| Tetracyclines | 1 | 0 | DNC | 0 | DNC | 0 | DNC | 0 | DNC |
All associations were adjusted for age (continuous). The unexposed group for all analyses was composed of individuals with no antibiotic use prior to the reference date (n=272). HUS cases by definition were: 51, age-focused definition (1 missing HUS status); 66, hospital diagnosis (4 missing); 42, hematology-focused definition (0 missing); and 96, CSTE (1 missing). Note that column totals do not sum to the values in Table 2, because 88 of the 133 cases receiving antibiotics received >1 antibiotic.
Abbreviations: CI, confidence interval; CSTE, Council of State and Territorial Epidemiologists; DNC, did not calculate due to insufficient sample size of exposed; HUS, hemolytic uremic syndrome; OR, odds ratio; TMP-SMX, trimethoprim-sulfamethoxazole
In sensitivity analyses, imputing the values of cases whose charts reported antibiotic use but did not indicate if use was before the reference date produced the same trend among definitions (Appendix, Table A.1). The association was strongest using the age-focused definition, followed by hospital diagnosis, and the hematology-focused and CSTE definitions. Restricting the analysis to cases with bloody diarrhea yielded results similar to those in the primary analysis (Appendix, Table A.2). Specifically, the point estimates for all except the CSTE definition increased modestly (e.g. from 1.94 to 2.09 for hospital diagnosis). Using the CSTE definition decreased the OR from 1.57 to 1.50. Restricting the analysis to cases who did not receive antibiotics prior to hospitalization, point estimates were attenuated, with the largest difference observed for hospital diagnosis, from 1.94 to 1.58 (Appendix, Table A.2).
DISCUSSION
We found that the strength of the association between antibiotic treatment and HUS varies with the definition of HUS applied, though there is substantial overlap among confidence intervals. Notably, each definition yielded an effect estimate in the direction of increased HUS risk after having received antibiotics. The variation in effect sizes was modest, and all effect sizes were within clinically meaningful ranges. However, 95% confidence intervals for the CSTE and hematology-focused definitions did not exclude 1.0. The greatest estimate, using the agefocused definition, was 2.29, corresponding to a 129% increase in HUS risk when antibiotics are given. In other words, assuming an HUS prevalence of 15–18% typical among children infected with E. coli O157:H7 (Karch et al., 2005; Klein et al., 2002), for every 6–7 cases given antibiotics, one excess HUS case will result. This number needed to treat to cause harm (i.e. HUS) slightly exceeds that reported by Wong, et al (2012).
Overall, infected adults received antibiotics more frequently than did infected children. This striking difference could reflect that most E. coli O157:H7 and HUS research has focused on pediatric populations, which is appropriate given the much greater incidence of E. coli O157:H7 infections and HUS in this age group. Interestingly, there were inter-annual fluctuations in antibiotic use in both age groups. Our study also provides an opportunity to examine secular changes in antibiotic selection relative to United States cohorts from the 1990s and early 2000s. For example, in an earlier study of 259 children under age 10 infected with E. coli O157:H7, trimethoprim-sulfamethoxazole was used in 9 cases (25% of those receiving antibiotics) and metronidazole was used in only 3 (Wong et al., 2012). A study of E. coli O157:H7 cases <20 years-old reported sulfonamide use in 16% of controls and nitroimidazoles in only 2% (Smith et al., 2012). Trimethoprim-sulfamethoxazole, the only sulfonamidecontaining antibiotic we documented in this cohort, was administered to only 2 of the 32 antibiotic-receiving cases <20 years-old and to none of those under age 10. Conversely, nitroimidazoles (primarily metronidazole) were administered to 16 cases <20-years-old and 4 of the 13 antibiotic-receiving cases <10 years-old.
For all antibiotic classes, risk estimates were in the direction of an increased likelihood of developing HUS after receipt of antibiotics. While confidence intervals were wide, barring a definitive conclusion that specific classes are harmful, we found no suggestion of a protective effect for any class. The strongest effect was observed for β-lactam antibiotics using the agefocused definition (OR 2.87), similar to the odds ratio of 4.3 for β-lactams reported by Smith et al. (2012). Studies of the Shiga toxin-producing and enteroaggregative O104:H4 outbreak strain have renewed interest in azithromycin as a potential treatment (Nitschke et al., 2012). Though macrolides (100% azithromycin) were not used frequently in our study, 1 to 3 of the 10 cases receiving them progressed to HUS, depending on definition. The 2 cases with the greatest definition consensus received azithromycin alone. Wong et al. (2012) reported a frequency of 25% HUS among children receiving azithromycin, and Smith et al. (2012) identified azithromycin use in 5% of HUS cases vs. 2% of controls, though in no single study was the association statistically significant. Nonetheless, current data fail to exonerate azithromycin as a risk factor for HUS in E. coli O157:H7 infections, and we caution against its use pending further data.
Despite the broad range of effect estimates in the literature, we found consistency between reported effect estimates and those found in our study. Using a definition of HUS most similar to the age-specific definition, Wong et al. estimated an OR of 3.6 (Wong et al., 2012). The OR estimate from the age-focused definition (OR=2.29) was closest to that reported in a recent meta-analysis among studies with low risk of bias (OR=2.24) (Freedman et al., 2016). This definition requires specific criteria for hematocrit, platelets, and serum creatinine, with the latter based on normal upper limit for age. This set of criteria likely excludes some cases with mild disease, but it is sensitive to the sometimes small absolute changes in serum creatinine seen in very young children with kidney injury.
Hospital diagnosis yielded similar results as the age-focused definition, varying primarily in its inclusion of somewhat milder cases, such as those with anemia and thrombocytopenia but without azotemia. Given the large number of facilities at which patients were hospitalized, this reassuring consistency suggests that hospital diagnosis could be used if specific laboratory values are not available (e.g. as in a retrospective study), with the caveat that some mild cases will likely be included.
The hematology-focused and CSTE definitions yielded lower ORs than reported in the recent meta-analysis (Freedman et al., 2016). Importantly, use of these definitions produced results that were not statistically significant, criteria often used to evaluate the veracity of an effect estimate. Similarly, using a definition of HUS most like the hematology-focused definition, Slutsker et al. (1998) found no association in their analysis of all cases, although no point estimate was provided for comparison and a subgroup analysis did identify increased risk.
There are systematic differences between the CSTE and hematology-focused definitions and the age-focused definition that may explain the variation in observed ORs. Use of criteria that do not require thrombocytopenia but do accept hematuria and/or proteinuria as evidence of kidney injury have been discouraged (Davis et al., 2013; Holtz et al., 2009). Indeed, the CSTE definition designated several patients as HUS cases even though anemia was the only criterion met. At the other extreme, the hematology-focused definition classified only 59 cases as HUS. This is in part due to its relatively insensitive criteria for serum creatinine concentration. For example, a 2-year-old child whose serum creatinine concentration increases from 0.4 mg/dL to 0.9 mg/dL would not be considered an HUS case under the hematology-focused definition despite meeting the definition of acute kidney injury (Kidney Disease Improving Global Outcomes, 2012). Additionally, the hematology-focused definition requires microangiopathic changes on smear, but this criterion was measured and documented variably across the 71 inpatient facilities that cared for patients in our cohort, suggesting that this definition may be problematic when using routinely-collected data. Although more stringent and excluding the majority of mild cases, it excluded several severe cases, including one requiring dialysis.
We encountered substantial variation in documentation, as well as in clinical testing for definition components, such as evidence of microangiopathic changes. Incomplete information on the timing of antibiotic initiation, particularly among cases who received antibiotics prior to hospitalization, resulted in missing data for antibiotic status for 31 cases. In sensitivity analysis, we found that some patterns of antibiotic use among those 31 cases might theoretically alter the strength of the association. However, the effect estimates did not change direction, and the variation across definitions was similar to that in the primary analysis. We also examined potential selection bias and confounding by indication and continued to find variation across definitions in all sensitivity analyses (Appendix).
Another limitation of our study was that we did not enter into analysis patients with postdiarrheal but culture-negative HUS. Based on studies from Washington State and elsewhere, many such patients are likely infected with E. coli O157:H7 (Mody et al., 2012; Tarr et al., 1990), and we cannot exclude the possibility that the inclusion of such patients in this analysis would have different risks associated with antibiotic use.
Sub-analysis of specific antibiotic classes offered limited power to detect effects due to the relatively small number of HUS cases after we removed cases treated with other antibiotics. Several years and/or large geographic areas must be combined to reach sample sizes adequate for sub-analyses, which should be a key consideration in future studies. With 10 years of data and over 400 E. coli O157:H7 cases, ours is the largest study of which we are aware to address this topic.
CONCLUSIONS
We demonstrate that outcome definition influences the strength of the observed association between antibiotic use and HUS. While the range of OR point estimates was relatively small, confidence intervals for two HUS definitions crossed the null and two did not. Given that most studies have relied on hypothesis testing with an alpha of 0.05 to judge the significance of their results, our findings show that definition can affect the ultimate inference made by a study, providing one potential explanation for divergent findings in the literature. A consistent case definition for HUS should be adopted to eliminate this potential source of variation in findings, thus providing better clarity for physicians referring to the published literature to make treatment decisions.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the work and participation of hospital personnel at the 71 facilities involved in this review, as well as the guidance and support of the Communicable Disease Epidemiology team at Washington State Department of Health. We thank Dr. Stephen Freedman for helpful comments on the manuscript.
FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health [F31AI126834]; the National Institute of Environmental Health Sciences, National Institutes of Health [T32ES015459]; and the Washington University Digestive Diseases Research Core Center [5P30 DK052574]. The funding bodies had no role in the design, data collection, analysis, or interpretation of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: CI, confidence interval; HUS, hemolytic uremic syndrome; OR, odds ratio; STEC, Shiga toxin-producing Escherichia coli
CONFLICT OF INTEREST
Declarations of interest: none.
REFERENCES
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI, 2005. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115, e673–680. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Friedrich AW, Aldick T, Schürk-Bulgrin R, Karch H, 2006. Shiga Toxin Activatable by Intestinal Mucus in Escherichia coli Isolated from Humans: Predictor for a Severe Clinical Outcome. Clin. Infect. Dis 43, 1160–1167. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Kock R, Friedrich AW, von Eiff C, Zimmerhackl LB, Karch H, Mellmann A, 2007. Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS One 2, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Summary of Notifiable Diseases, 2014. MMWR Morb. Mortal. Wkly. Rep 63, 1–154. [Google Scholar]
- Council of State and Territorial Epidemiologists, 2009. Public Health Reporting and National Notification for Hemolytic Uremic Syndrome (post-diarrheal), Atlanta, Georgia. [Google Scholar]
- Crim SM, Griffin PM, Tauxe R, Marder EP, Gilliss D, Cronquist AB, Cartter M, Tobin D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert B, Henao OL, 2015. Preliminary Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep 64, 495–498. [PMC free article] [PubMed] [Google Scholar]
- Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, Cartter M, Tobin-D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Lance S, Tauxe R, Henao OL, Centers for Disease Control and Prevention, 2014. Incidence and trends of infection with pathogens transmitted commonly through food-Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb. Mortal. Wkly. Rep 63, 328–332. [PMC free article] [PubMed] [Google Scholar]
- Davis TK, McKee R, Schnadower D, Tarr PI, 2013. Treatment of Shiga toxin-producing Escherichia coli infections. Infect. Dis. Clin. North Am 27, 577–597. [DOI] [PubMed] [Google Scholar]
- Denno DM, Shaikh N, Stapp JR, Qin X, Hutter CM, Hoffman V, Mooney JC, Wood KM, Stevens HJ, Jones R, Tarr PI, Klein EJ, 2012. Diarrhea etiology in a pediatric emergency department: a case control study. Clin. Infect. Dis 55, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SB, Eltorki M, Chui L, Xie J, Feng S, MacDonald J, Dixon A, Ali S, Louie M, Lee BE, Osterreicher L, Thull-Freedman J, 2017. Province-Wide Review of Pediatric Shiga Toxin-Producing Escherichia coli Case Management. J. Pediatr 180, 184–190 e181. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI, Alberta Provincial Pediatric Enteric Infection T, Nettel-Aguirre A, Chuck A, Lee B, Johnson D, Currie G, Talbot J, Jiang J, Dickinson J, Kellner J, MacDonald J, Svenson L, Chui L, Louie M, Lavoie M, Eltorki M, Vanderkooi O, Tellier R, Ali S, Drews S, Graham T, Pang XL, 2016. Shiga Toxin-Producing Escherichia coli Infection, Antibiotics, and Risk of Developing Hemolytic Uremic Syndrome: A Meta-analysis. Clin. Infect. Dis 62, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF, 2003. Long-term Renal Prognosis of DiarrheaAssociated Hemolytic Uremic Syndrome: A Systematic Review, Meta-analysis, and Metaregression. JAMA 290, 1360–1370. [DOI] [PubMed] [Google Scholar]
- Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, Griffin PM, 2009. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin. Infect. Dis 49, 1480–1485. [DOI] [PubMed] [Google Scholar]
- Harriet Lane Service (Johns Hopkins Hospital), 2009. The Harriet Lane Handbook: A Manual for Pediatric House Officers, 18th ed. Elsevier Mosby, Philadelphia, PA. [Google Scholar]
- Hickey CA, Beattie TJ, Cowieson J, Miyashita Y, Strife CF, Frem JC, Peterson JM, Butani L, Jones DP, Havens PL, Patel HP, Wong CS, Andreoli SP, Rothbaum RJ, Beck AM, Tarr PI, 2011. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch. Pediatr. Adolesc. Med 165, 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz LR, Neill MA, Tarr PI, 2009. Acute Bloody Diarrhea: A Medical Emergency for Patients of All Ages. Gastroenterology 136, 1887–1898. [DOI] [PubMed] [Google Scholar]
- Karch H, Tarr PI, Bielaszewska M, 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol 295, 405–418. [DOI] [PubMed] [Google Scholar]
- Karmali MA, Steele BT, Petric M, Lim C, 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1, 619–620. [DOI] [PubMed] [Google Scholar]
- Kidney Disease Improving Global Outcomes, 2012. Section 2: AKI Definition. Kidney Int Suppl (2011) 2, 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EJ, Stapp JR, Clausen CR, Boster DR, Wells JG, Qin X, Swerdlow DL, Tarr PI, 2002. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective pointof-care study. J. Pediatr 141, 172–177. [DOI] [PubMed] [Google Scholar]
- Marder EP, Cieslak PR, Cronquist AB, Dunn J, Lathrop S, Rabatsky-Ehr T, Ryan P, Smith K, Tobin D’Angelo M, Vugia DJ, Zansky S, Holt KG, Wolpert BJ, Lynch M, Tauxe R, Geissler AL, 2017. Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013– 2016. MMWR Morb. Mortal. Wkly. Rep 66, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites S, 1989. Pediatric Clinical Chemistry: Reference (Normal) Values, 3rd ed. AACC Press, Washington, D.C. [Google Scholar]
- Melamed R, Storch GA, Holtz LR, Klein EJ, Herrin B, Tarr PI, Denno DM, 2017. Case-Control Assessment of the Roles of Noroviruses, Human Bocaviruses 2, 3, and 4, and Novel Polyomaviruses and Astroviruses in Acute Childhood Diarrhea. J. Pediatr. Infect. Dis 6, e49–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody RK, Griffin PM, 2016. Editorial Commentary: Increasing Evidence That Certain Antibiotics Should Be Avoided for Shiga Toxin-Producing Escherichia coli Infections: More Data Needed. Clin. Infect. Dis 62, 1259–1261. [DOI] [PubMed] [Google Scholar]
- Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, Tobin-D’Angelo M, Smith G, Spina N, Hurd S, Lathrop S, Palmer A, Boothe E, Luna-Gierke RE, Hoekstra RM, 2015. Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J. Pediatr 166, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Mody RK, Luna-Gierke RE, Jones TF, Comstock N, Hurd S, Scheftel J, Lathrop S, Smith G, Palmer A, Strockbine N, Talkington D, Mahon BE, Hoekstra RM, Griffin PM, 2012. Infections in pediatric postdiarrheal hemolytic uremic syndrome: factors associated with identifying shiga toxin-producing Escherichia coli. Arch. Pediatr. Adolesc. Med 166, 902909. [DOI] [PubMed] [Google Scholar]
- Nitschke M, Sayk F, Hartel C, Roseland RT, Hauswaldt S, Steinhoff J, Fellermann K, Derad I, Wellhoner P, Buning J, Tiemer B, Katalinic A, Rupp J, Lehnert H, Solbach W, Knobloch JK, 2012. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 307, 1046–1052. [DOI] [PubMed] [Google Scholar]
- Ong KL, Apostal M, Comstock N, Hurd S, Webb TH, Mickelson S, Scheftel J, Smith G, Shiferaw B, Boothe E, Gould LH, 2012. Strategies for surveillance of pediatric hemolytic uremic syndrome: Foodborne Diseases Active Surveillance Network (FoodNet), 20002007. Clin. Infect. Dis 54 Suppl 5, S424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Klein EJ, Galanakis E, Thomas AA, Stapp JR, Rich S, Buccat AM, Tarr PI, 2015. Real-Time PCR Assay for Detection and Differentiation of Shiga Toxin-Producing Escherichia coli from Clinical Samples. J. Clin. Microbiol 53, 2148–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Scheiring J, Andreoli SP, Zimmerhackl LB, 2008. Treatment and outcome of Shiga-toxinassociated hemolytic uremic syndrome (HUS). Pediatr. Nephrol 23, 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsker L, Ries AA, Maloney K, Wells JG, Greene KD, Griffin PM, 1998. A nationwide case-control study of Escherichia coli O157:H7 infection in the United States. J. Infect. Dis 177, 962–966. [DOI] [PubMed] [Google Scholar]
- Smith KE, Wilker PR, Reiter PL, Hedican EB, Bender JB, Hedberg CW, 2012. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr. Infect. Dis. J 31, 37–41. [DOI] [PubMed] [Google Scholar]
- Tarr PI, Neill MA, Clausen CR, Watkins SL, Christie DL, Hickman RO, 1990. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J. Infect. Dis 162, 553–556. [DOI] [PubMed] [Google Scholar]
- Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI, 2000. The risk of the hemolyticuremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med 342, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI, 2012. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin. Infect. Dis 55, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


