Abstract
Objective
The mechanisms underlying the intrarenal renin-angiotensin system (RAS) activation depend on the conditions of kidney diseases. In angiotensin II (AngII) infusion models, the circulating AngII is filtered into the renal tubular lumens, activating intrarenal RAS. However, in the chronic kidney disease (CKD) models, plasma angiotensinogen (AGT) is filtered into the tubular lumens because of glomerular injury, activating intrarenal RAS. The intrarenal dopamine system activation reduces intrarenal AGT expression and suppresses the intrarenal RAS activity in AngII infusion models. However, the relationship between the intrarenal dopamine system and intrarenal RAS has not been elucidated. Therefore, this study was conducted to determine that relationship in CKD patients.
Methods
We recruited 46 CKD patients (age: 51.1±20.0 years; 16 men; causes of CKD: chronic glomerulonephritis, 34; diabetic nephropathy, 2; nephrosclerosis, 4; and others, 6) not undergoing dialysis or taking RAS blockers. The urinary dopamine (U-DOPA) level, an indicator of intrarenal dopamine activity, and the urinary AGT (U-AGT) level, a surrogate marker of intrarenal RAS activity, were measured.
Results
As the CKD stages progressed, the U-DOPA levels decreased while the U-AGT levels increased. The U-DOPA levels were significantly and negatively correlated with the U-AGT levels but significantly and positively correlated with the estimated glomerular filtration rate (eGFR). A multiple regression analysis revealed that the U-DOPA levels were associated with the U-AGT levels after adjusting for age, sex, body mass index, and blood pressure (β=-0.38, p=0.045). However, no correlation was observed when eGFR was also adjusted (β=-0.17, p=0.29).
Conclusion
The negative correlation between the intrarenal dopamine system and intrarenal RAS in CKD patients may be affected by the renal function.
Keywords: intrarenal dopamine system, kidney, renin-angiotensin system, urinary angiotensinogen
Introduction
The critical role of the circulating renin-angiotensin system (RAS) in the arterial pressure and sodium homeostasis regulation has been widely known for many years (1). However, the focus on the role of the RAS in the pathophysiology of hypertension and organ injury has shifted to focus on role of the local RAS in specific tissues (2). Since all RAS components are present in the kidney, it has been shown that intrarenal RAS activation has a critical role in the pathophysiology of renal damage and blood pressure elevation, regardless of the circulating RAS, in some animal models and patients with chronic kidney disease (CKD) and hypertension (1, 3-8). Angiotensinogen (AGT) is the only known substrate for renin, the rate-limiting enzyme in the RAS. AGT levels influence the RAS activation since they are close to the Michaelis-Menten constant for renin (9, 10). Furthermore, urinary AGT (U-AGT) is reported to be a useful biomarker that reflects the intrarenal RAS activity and CKD severity (11-14).
The mechanisms of intrarenal RAS activation depend on the conditions of kidney diseases. In the angiotensin II (AngII) infusion models, AngII, not AGT, is filtered into the renal tubules because of its small molecular composition, thereby activating the intrarenal RAS (12). In contrast, in CKD animal models, AGT that is filtered through the damaged glomeruli limits intrarenal RAS activity, as reported by Matsusaka et al. (15).
Dopamine as a neurotransmitter performs various important functions in the central nervous system. However, it is also an important endogenous modulator of the kidney function. The intrarenal dopamine system is a local independent natriuretic system necessary to maintain fluid and electrolyte balance, blood pressure levels, and renal redox steady state (16). Intrarenal dopamine is synthesized in proximal tubular cells independently of the nerve activity. Dopamine excreted into the urine is almost exclusively derived from intrarenally formed dopamine (17, 18). Furthermore, urinary dopamine (U-DOPA) is reported to be an indicator that reflects the intrarenal dopamine system activity (19).
Dopamine's action on the D1-like receptors in the kidney increases sodium excretion in the renal tubules and antagonizes the action of enhancing sodium reabsorption due to the intrarenal RAS activation (20). Furthermore, dopamine decreases the mRNA and protein expression of AGT in the proximal renal tubules and AT1R action and suppresses the renin activity in AngII infusion models (21-23). Through these actions, the intrarenal dopamine system is considered to suppress the intrarenal RAS activity in AngII infusion models.
The mechanisms underlying the intrarenal RAS activation differ between AngII infusion models and CKD models. The relationships between dopamine and the RAS in the kidney may differ, and whether or not the intrarenal dopamine system suppresses the intrarenal RAS activity in CKD models has not been indicated. Thus, this study was conducted to clarify the mutual relationship between the intrarenal dopamine system and intrarenal RAS in CKD patients.
Materials and Methods
Subjects with and without CKD
This study was approved by the ethics committee of Hamamatsu University School of Medicine (No. 14-273) and carried out in accordance with the guidelines set by the Declaration of Helsinki. Subjects with and without CKD were involved in this study, and written informed consent was provided. A total of 19 volunteers without CKD (non-CKD individuals) and 46 CKD patients admitted to our hospital from February 2012 to June 2014 were recruited. Patients undergoing dialysis (CKD stage 5D) or taking RAS blockers [i.e., AngII receptor blockers, angiotensin-converting enzyme (ACE) inhibitors, mineralocorticoid receptor blockers, direct renin inhibitors] and those whose CKD was not caused by glomerular diseases (e.g., tubulointerstitial nephritis) or had an unknown cause were excluded. Because antihypertensive drugs other than RAS blockers might affect the intrarenal dopamine system and intrarenal RAS, 12 patients who were taking antihypertensive drugs were excluded (calcium channel blockers, 9; alpha-blocker, 1; beta-blockers, 4; thiazide diuretic, 1) and subgroup analyses were performed in 34 patients who were not taking any antihypertensive drugs.
Study protocols
Ambulatory blood pressure monitoring (ABPM) using an automatic device (TM-2431; A and D, Tokyo, Japan) was carried out for 24 hours with 30-min intervals, and blood samples were collected at 6:00 AM at the end of the ABPM, after the non-CKD individuals and CKD patients had rested in the supine position for at least 15 minutes. Urine samples were also obtained on the same day the ABPM was done. The blood samples were centrifuged at 3,000 rpm at 4℃ for 10 minutes, while the urine samples were centrifuged at 1,500 rpm at 4℃ for 5 minutes. Both sets of samples were stored at -80℃ until assays were performed.
Measurements
The non-CKD individuals' and CKD patients' clinical data such as age, sex, height, weight, and body mass index (BMI) were collected at the time of admission. The serum creatinine (sCr), urinary creatinine, albumin, and protein concentrations were measured in the clinical laboratory of the Hamamatsu University School of Medicine. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese eGFR equation (24). The plasma renin activity (PRA) and plasma AngII concentrations for the circulating RAS were determined by radioimmunoassay (RIA) (SRL, Tokyo, Japan), while the U-AGT concentrations were measured by an enzyme-linked immunosorbent assay (ELISA) from 24-h collected urine samples, as previously described (25, 26). In addition, U-DOPA concentrations indicating intrarenal dopamine activity (17-19) were measured by high-performance liquid chromatography (HPLC) (SRL) from 24-h urine samples.
Statistical analyses
Results are reported as the mean±standard deviation (SD). Because the PRA and urinary excretion levels of albumin, protein, dopamine, and AGT did not conform to a normal distribution, a logarithmic transformation was applied. Differences in the daily U-DOPA and U-AGT excretion levels among non-CKD and each CKD stage were examined using the Tukey's honest significant difference test, while correlations between the daily U-DOPA excretion levels and other parameters were evaluated using the Pearson's product-moment correlation coefficient. Furthermore, in a subgroup analysis, the correlations between the daily U-DOPA excretion levels and other parameters were evaluated using the Pearson's product-moment correlation coefficient in CKD patients who were not taking any antihypertensive drugs.
Multiple linear regression analyses were used to identify the relationships between U-DOPA and U-AGT excretion levels. Age, sex, and BMI were selected as independent variables since these parameters are commonly used when performing multiple linear regression analyses. Furthermore, the blood pressure (BP) and eGFR were also adjusted for because the impairment of the renal dopamine system is associated with the development of hypertension (16), and the BP is significantly and positively correlated with the U-AGT excretion (25), while the eGFR is associated with the U-DOPA excretion levels.
A p value <0.05 was considered statistically significant. Statistical analyses were done using the Statistical Package for the Social Sciences (SPSS) software program (Version 23, IBM, Armonk, USA).
Results
Characteristics of non-CKD individuals and CKD patients
A total of 19 non-CKD individuals and 46 CKD patients were recruited. The causes of CKD were as follows: chronic glomerulonephritis, 34; diabetic nephropathy, 2; nephrosclerosis, 4; and others, 6. The mean age of the non-CKD individuals was 36.5±15.4 years, and the mean age of the CKD patients was 51.1±20.0 years. Eleven of the non-CKD individuals and 16 of the CKD patients were men. The mean log daily U-DOPA and U-AGT excretion levels were 2.99±0.28 μg/day and 0.81±0.47 μg/day in the non-CKD individuals and 2.74±0.34 μg/day and 2.10±0.85 μg/day in the CKD patients, respectively (Table 1).
Table 1.
Characteristics of Non-CKD Individuals and CKD Patients.
| Non-CKD individuals | CKD patients | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 36.5 | ± | 15.4 | 51.1 | ± | 20.0 | <0.01 | |||
| Sex, male/female | 11/8 | 16/30 | 0.09 | |||||||
| BMI, kg/m2 | 21.3 | ± | 2.4 | 21.4 | ± | 3.7 | 0.90 | |||
| SBP, mmHg | 118.5 | ± | 14.7 | 124 | ± | 18.5 | 0.29 | |||
| DBP, mmHg | 72.0 | ± | 9.0 | 72.2 | ± | 10.0 | 0.94 | |||
| MBP, mmHg | 87.3 | ± | 10.6 | 88.9 | ± | 11.6 | 0.61 | |||
| Pulse rate, /min | 70.9 | ± | 8.3 | 66.1 | ± | 8.3 | 0.36 | |||
| sCr, mg/dL | 0.74 | ± | 0.11 | 2.30 | ± | 3.25 | <0.01 | |||
| eGFR, mL/min/1.73 m2 | 90.5 | ± | 21.5 | 48.2 | ± | 28.3 | <0.01 | |||
| CKD stage | 1:5, 2:13, 3:16, 4:5, 5:7 |
|||||||||
| PRA, ng/mL/h | 1.24 | ± | 0.92 | 2.07 | ± | 2.27 | 0.039 | |||
| Plasma AngII, pg/mL | 9.11 | ± | 5.2 | 12.5 | ± | 11.2 | 0.10 | |||
| Log (U-DOPA/day, μg/day) | 2.99 | ± | 0.28 | 2.74 | ± | 0.34 | <0.01 | |||
| Log (U-AGT/day, μg/day) | 0.81 | ± | 0.47 | 2.10 | ± | 0.85 | <0.01 | |||
| Log (U-Alb/day, mg/day) | 0.70 | ± | 0.19 | 2.63 | ± | 0.61 | <0.01 | |||
| Log (U-Pro/day, mg/day) | 1.52 | ± | 0.15 | 2.92 | ± | 0.50 | <0.01 | |||
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean blood pressure, sCr: serum creatinine, eGFR: estimated glomerular filtration rate, CKD: chronic kidney disease, PRA: plasma renin activity, AngII: angiotensin II, U-DOPA: urinary dopamine, U-AGT: urinary angiotensinogen, U-Alb: urinary albumin, U-Pro: urinary protein
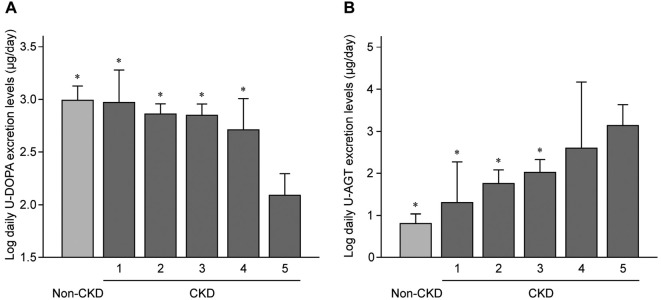
In Fig. 1A, where the daily U-DOPA excretion levels for non-CKD and each CKD stage were presented, the U-DOPA excretion levels in patients with CKD stage 5 were significantly lower than those in non-CKD individuals and patients with other CKD stages, revealing that the U-DOPA excretion levels tended to decrease as the CKD stage progressed. Conversely, as shown in Fig. 1B, the U-AGT excretion levels in patients with CKD stage 5 were significantly higher than those in non-CKD individuals and patients with CKD stages 1, 2, and 3, showing that the U-AGT excretion levels tended to increase as CKD stages progressed.
Figure 1.
A: A comparison of the urinary dopamine (U-DOPA) excretion levels among non-CKD individuals and each CKD stage. B: A comparison of the urinary angiotensinogen (U-AGT) excretion levels among non-CKD individuals and each CKD stage. *p<0.01 vs. CKD stage 5. CKD: chronic kidney disease
Correlations between the U-DOPA excretion levels and other parameters
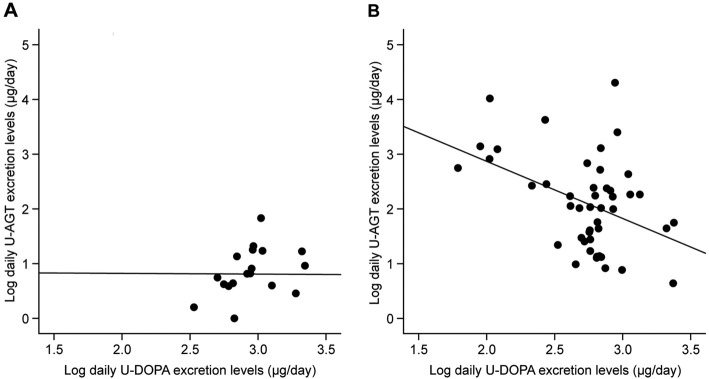
The relationships between the daily U-DOPA excretion levels and the clinical parameters were examined. The U-DOPA levels were not correlated with the U-AGT levels in the non-CKD individuals (r=-0.0078, p=0.98)(Fig. 2A). In contrast, a significant negative relationship was observed between the daily U-DOPA and U-AGT excretion levels in the CKD patients (r=-0.42, p<0.01)(Fig. 2B). Furthermore, the daily U-DOPA excretion levels had a significant and positive correlation with the eGFR (r=0.64, p<0.01) and a significant and negative correlation with the age (r=-0.29, p=0.049) in the CKD patients. The systolic BP (SBP) was most significantly correlated with the U-DOPA excretion levels (r=-0.33, p=0.025), and the mean BP (MBP) tended to correlate with the U-DOPA excretion levels (r=-0.28, p=0.058). In contrast, no marked relationships were found between the diastolic BP (DBP) and the U-DOPA excretion levels (r=-0.19, p=0.22) (Table 2). The same tendencies were observed between the U-DOPA excretion levels and the daytime or nighttime BP (data not shown). Similar to the results in all patients, the U-DOPA excretion levels showed a significant and negative correlation with the U-AGT excretion levels (r=-0.37, p=0.030) and a significant positive correlation with the eGFR (r=0.51, p<0.01) in the 34 CKD patients who were not taking any antihypertensive drugs.
Figure 2.
The relationship between the daily urinary dopamine (U-DOPA) and angiotensinogen (U-AGT) excretion levels. A: Non-CKD individuals, B: CKD patients. CKD: chronic kidney disease
Table 2.
Relationships between Daily Urinary Dopamine (U-DOPA) Excretion and Clinical Parameters in Chronic Kidney Disease (CKD) Patients.
| r | p | |
|---|---|---|
| Age, years | -0.29 | 0.049 |
| BMI, kg/m2 | -0.25 | 0.090 |
| SBP, mmHg | -0.33 | 0.025 |
| DBP, mmHg | -0.19 | 0.22 |
| MBP, mmHg | -0.28 | 0.058 |
| eGFR, mL/min/1.73 m2 | 0.64 | <0.01 |
| Plasma AngII, pg/mL | -0.11 | 0.47 |
| Log (U-Alb/day, mg/day) | -0.12 | 0.43 |
| Log (U-Pro/day, mg/day) | -0.16 | 0.29 |
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean blood pressure, eGFR: estimated glomerular filtration rate, AngII: angiotensin II, U-Alb: urinary albumin, U-Pro: urinary protein
A multiple linear regression analysis of the U-DOPA and U-AGT excretion levels in CKD patients
A multiple linear regression analysis was used to identify relationships between the U-DOPA and U-AGT excretion levels in CKD patients. The association between the U-DOPA and U-AGT excretion levels was maintained when the age, sex, BMI, and DBP or MBP as independent variables were modified (DBP: β=-0.38, p<0.01 and MBP: β=-0.38, p=0.045). A similar association was shown when the age, sex, BMI, and SBP, as independent variables, were modified (β=-0.36, p=0.064). However, no association at all was observed between the U-DOPA and U-AGT excretion levels when the eGFR was included for adjustment with these parameters (SBP: β=-0.18, p=0.28, DBP: β=-0.035, p=0.80 and MBP: β=-0.17, p=0.29) (Table 3).
Table 3.
Multiple Linear Regression Analyses of Daily Urinary Dopamine (U-DOPA) Excretion Levels with Regard to Age, Sex, Body Mass Index (BMI), Blood Pressure (BP), Estimated Glomerular Filtration Rate (eGFR), and Daily Urinary Angiotensinogen (U-AGT) Excretion Levels in Chronic Kidney Disease (CKD) Patients.
| SBP | DBP | MBP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r=0.49 | p=0.042 | r=0.69 | p<0.01 | r=0.43 | p<0.01 | r=0.69 | p<0.01 | r=0.50 | p=0.040 | r=0.69 | p<0.01 | |||
| β | p | β | p | β | p | β | p | β | p | β | p | |||
| Age, years | -0.17 | 0.30 | 0.10 | 0.50 | -0.20 | 0.11 | 0.18 | 0.19 | -0.17 | 0.27 | 0.18 | 0.23 | ||
| Sex | -0.035 | 0.83 | -0.022 | 0.87 | -0.019 | 0.88 | -0.040 | 0.70 | -0.041 | 0.79 | 0.015 | 0.91 | ||
| BMI, kg/m2 | -0.20 | 0.17 | -0.083 | 0.50 | -0.19 | 0.11 | -0.097 | 0.36 | -0.21 | 0.16 | -0.13 | 0.32 | ||
| BP, mmHg | 0.035 | 0.88 | 0.32 | 0.12 | -0.070 | 0.59 | 0.12 | 0.31 | 0.066 | 0.73 | 0.28 | 0.11 | ||
| eGFR, mL/min/1.73 m2 | 0.80 | <0.01 | 0.79 | <0.01 | 0.79 | <0.01 | ||||||||
| Log (U-AGT/day, μg/day) | -0.36 | 0.064 | -0.18 | 0.28 | -0.38 | <0.01 | -0.035 | 0.80 | -0.38 | 0.045 | -0.17 | 0.29 | ||
BMI: body mass index, BP: blood pressure, SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean blood pressure, eGFR: estimated glomerular filtration rate, U-AGT: urinary angiotensinogen
Discussion
In this study, a negative correlation was observed between the U-DOPA and U-AGT excretion levels in CKD patients. The correlation was maintained even with adjustments for age, sex, BMI, and BP. However, no correlation between the U-DOPA and U-AGT excretion levels was observed when the eGFR was added for adjustment as well. This result indicates that the negative relationship between intrarenal dopamine system activity and intrarenal RAS activity may be merely affected by the renal function in CKD patients.
L-3, 4-dihydroxyphenylalanine (L-DOPA), with a molecular weight of 197, is freely filtered through the glomeruli and degraded by aromatic L-amino acid decarboxylase (AADC) in the proximal tubules, resulting in dopamine production and release into the urine. Thereafter, dopamine is metabolized and inactivated by catechol-O-methyl-transferase (COMT) and monoamine oxidase (MAO). It works as not only a neurotransmitter but also a regulator of body fluid volume and blood pressure. In addition, intrarenal dopamine suppresses the intrarenal RAS activation in AngII infusion models (21, 27). Zhang et al. showed that mice with selective proximal tubule AADC deletion had decreased dopamine levels in the kidney and urinary dopamine excretion levels, as well as decreased natriuresis and diuresis in response to L-DOPA, and they also developed salt-sensitive hypertension. In addition, these mice had an increased expression of renin and AngII type 1b receptor and a decreased expression of AngII type 2 receptor and Mas in response to chronic AngII infusion (28). Furthermore, Yang et al. reported that chronic AngII infusion increased the renal expression of both COMT and MAO, reduced the intrarenal dopamine levels, and caused albuminuria and tubulointerstitial damage in wild-type mice, while the renal damage was ameliorated in COMT knockout mice (21). Likewise, Choi et al. revealed that the AngII receptor blocker increased intrarenal dopamine and improved the renal damage induced by AngII infusion and concluded that the AT1R-mediated intrarenal RAS activation caused renal damage by intrarenal dopamine reduction (27).
In contrast to previous reports (20-23, 27, 28), this study demonstrated that a negative correlation between the U-DOPA and U-AGT excretion levels was not observed when adjustments were made for the renal function. The reason for the discrepancy between these studies is unclear. However, two possibilities may be considered.
First, the interaction between the intrarenal dopamine system and intrarenal RAS differed between AngII infusion models and CKD models because of the different mechanisms of intrarenal RAS activation. As mentioned earlier, the mechanism underlying the intrarenal RAS activation was considered to be as follows: AngII is filtered through the glomerulus because of its small molecular composition and internalized via AT1R in the proximal tubules. Thereafter, AngII exerts positive feedback action on the intrarenal AGT expression in the proximal tubules. Finally, the increased intrarenal AGT production results in an increase in the U-AGT excretion, enhancing distal AngII formation (14, 29). In contrast, Matsusaka et al. recently demonstrated that AGT in the kidney did not increase in liver-specific AGT knockout mice but did increase in kidney-specific AGT knockout mice using models of inducible podocyte injury; they thus concluded that intrarenal RAS activation originates from filtered liver-derived AGT in CKD models, where plasma AGT is filtered into the tubular lumens because of glomerular injury (15). Thus, differences in the activation system of intrarenal RAS may influence differences in the activation of the intrarenal dopamine system. Although it is very difficult to clarify the contribution of different methods of intrarenal RAS activation to the intrarenal dopamine system activation, we plan to conduct experiments using a CKD model mouse with liver AGT specifically knocked out.
Second, the degrees of tubulointerstitial damage differ between AngII infusion models and CKD models. Because renal damage, including proximal tubular damage, is generally less serious in the AngII infusion model, intrarenal dopamine production is decreased by the suppression of AADC and an increase in COMT or MAO due to the augmentation of intrarenal RAS. However, given the significant positive relationships between the renal function and tubular atrophy or interstitial fibrosis (30), the expression of AADC is decreased due to extensive proximal tubular damage, and the intrarenal dopamine production is decreased according to the progression of renal dysfunction in CKD patients (including all CKD stages). We investigated the correlation between the U-DOPA and urinary α1-microglobulin, which is a surrogate marker of tubular injury, and found a significant negative correlation between these factors (r=-0.36, p=0.027, data not shown). These results are therefore considered to support our hypothesis.
The intrarenal RAS activation is increased according to the renal damage. As a result, a negative relationship between the intrarenal dopamine and intrarenal RAS is found in CKD patients. Because it is difficult to clarify which mechanism out of these two possibilities contributed to the relationship between intrarenal dopamine system activity and intrarenal RAS activity depending on renal function through this clinical research, it will be necessary to evaluate the expression of AADC, COMT, and MAO or to perform pharmacological intervention using RAS inhibitors or dopamine-stimulating drugs in CKD animal models. We have now begun an animal study. These experiments may explain why a negative correlation was not found between the U-DOPA and U-AGT levels when the renal function was adjusted for in CKD patients. In contrast to the CKD patients, the non-CKD subjects showed no correlation between the U-DOPA and U-AGT levels. We suspect that because the U-AGT levels were not augmented under normal renal function conditions and the U-DOPA levels were not suppressed, no negative relationship manifested between them.
Conclusion
This study revealed a negative correlation between the intrarenal dopamine system and the intrarenal RAS. However, the association was not observed after adjusting the renal function for the age, sex, BMI, and BP. The intrarenal dopamine system likely has a renoprotective effect that inhibits the intrarenal RAS activity, but this association may merely be affected by the renal function in CKD patients.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by a Grant-in-Aid for Scientific Research (17K09693 to Naro Ohashi) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251-287, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation 89: 493-498, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316-322, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res 40: 413-422, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Singh R, Singh AK, Leehey DJ. A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol 288: F1183-F1190, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Ohashi N, Katsurada A, Miyata K, et al. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 36: 750-755, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431-439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592-597, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res 5: 86-89, 1971. [DOI] [PubMed] [Google Scholar]
- 10. Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension 27: 465-475, 1996. [DOI] [PubMed] [Google Scholar]
- 11. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579-585, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakano D, Kobori H, Burford JL, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol 23: 1847-1856, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto T, Nakagawa T, Suzuki H, et al. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558-1565, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Kobori H, Navar LG. Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int Rev Thromb 6: 108-116, 2011. [PMC free article] [PubMed] [Google Scholar]
- 15. Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int 85: 1068-1077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, et al. Renal dopaminergic system: pathophysiological implications and clinical perspectives. World J Nephrol 4: 196-212, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee MR. Dopamine and the kidney: ten years on. Clin Sci (Lond) 84: 357-375, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Carey RM. Theodore cooper lecture: renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297-302, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Isobe-Sasaki Y, Fukuda M, Ogiyama Y, et al. Sodium balance, circadian BP rhythm, heart rate variability, and intrarenal renin-angiotensin-aldosterone and dopaminergic systems in acute phase of ARB therapy. Physiol Rep 5: pii: e13309, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: control of renal sodium excretion and blood pressure. Hypertension 61: 673-680, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol 302: F742-F749, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension 53: 564-570, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 97: 2745-2752, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Isobe S, Ohashi N, Fujikura T, et al. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 19: 231-239, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Ishigaki S, Ohashi N, Isobe S, et al. Impaired endogenous nighttime melatonin secretion relates to intrarenal renin-angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol 20: 878-884, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Choi MR, Correa AH, del Valle, Turco V, Garcia FA, Fernández BE. Angiotensin II regulates extraneuronal dopamine uptake in the kidney. Nephron Physiol 104: 136-143, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Zhang MZ, Yao B, Wang S, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845-2854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135-143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohashi N, Isobe S, Ishigaki S, et al. Plasma soluble (pro)renin receptor reflects renal damage. PLoS One 26: 0156165, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]