Abstract
The number of patients with methotrexate-associated lymphoproliferative disorder (MTX-LPD) is increasing. We describe a case of MTX-LPD of the stomach. After treatment with methotrexate for rheumatoid arthritis, the patient developed left cervical lymphadenopathy and an ulcerative lesion in the stomach, which was presumed to be a mucosa-associated lymphoid tissue (MALT) lymphoma. However, we suspected MTX-LPD, based on the clinical course and the positivity of in situ hybridization for the detection of the Epstein-Barr encoding region. After the cessation of MTX, the left cervical lymphadenopathy and the gastric lesion disappeared. This is first report of gastric MTX-LPD that was presumed to be MALT lymphoma.
Keywords: methotrexate, lymphoproliferative disorder, rheumatoid arthritis, MALT lymphoma
Introduction
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory arthritis in adults (1). Methotrexate (MTX) has become the initial drug of choice for treating most patients with RA (2). Both the usage and dosage of MTX have increased consistently (3). Lymphoproliferative disorders (LPDs) occasionally develop in individuals with immunodeficiency disorders, such as primary immune disorders, human immunodeficiency virus infection, or iatrogenic immunosuppression following solid organ or bone marrow allograft transplantation, or iatrogenic immunosuppression in association with the administration of MTX (4). Iatrogenic immunodeficiency-associated LPD is categorized as an immunodeficiency-associated LPD in the Revised 4th version of World Health Organization classification of lymphoid neoplasms (5).
The population with RA is increasing in Japan; this can be attributed to the increasing of aging population (6). Along with this change, the number of patients with MTX-LPD is increasing (7). The most common presenting symptom of MTX-LPD is superficial lymphadenopathy (33.3%), followed by the presence of extranodal masses (27.0%) and-much less frequently-abdominal or bone pain, fever, cough, and thrombocytopenia (4).
However, MTX-LPD of the stomach is uncommon (8). We herein report a case of MTX-LPD of the stomach.
Case Report
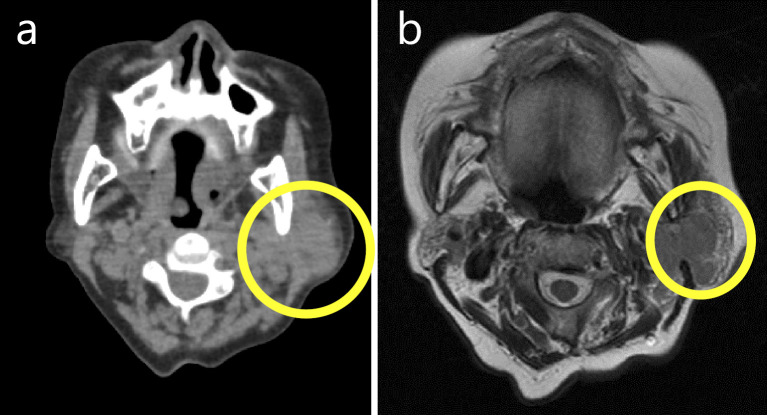
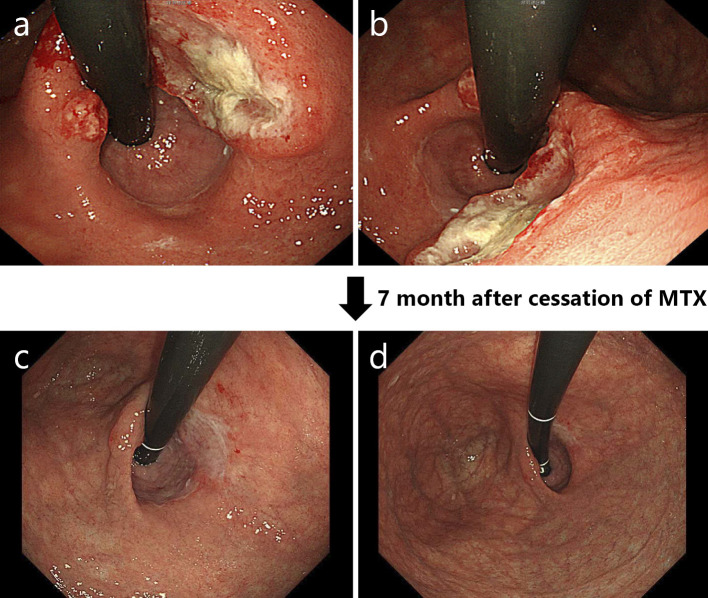
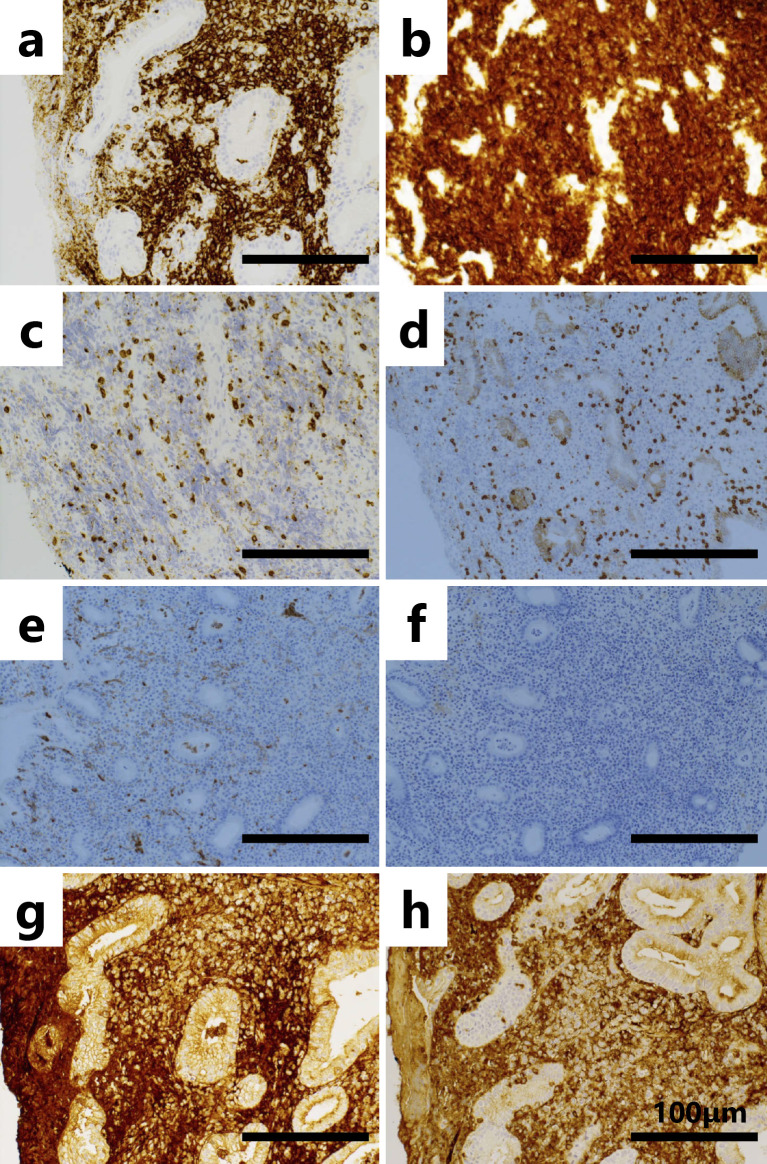
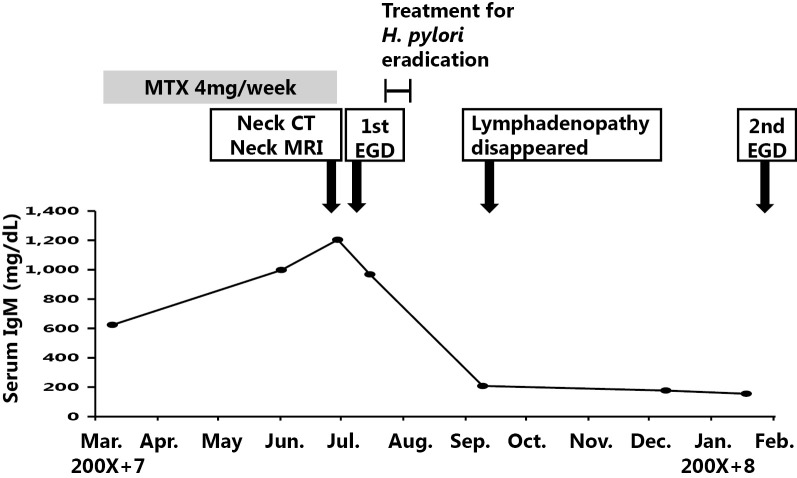
The patient was an 83-year-old woman who had been undergoing medical treatment for RA (4 mg/week of MTX) since 200X and who had been followed up at the Department of Rheumatology at our hospital. She was concurrently diagnosed with nonsteroidal anti-inflammatory drug-induced gastric ulcer at the Department of Gastroenterology and Hepatology. Observation with yearly esophagogastroduodenoscopy (EGD) was subsequently initiated. In June 200X+7, she complained of left cervical lymphadenopathy without other symptoms (e.g., morning stiffness, fever, weight loss, or night sweats). A physical examination revealed left cervical lymphadenopathy of approximately 3×3 cm in diameter. Computed tomography and magnetic resonance imaging scans of the body and neck were immediately taken and revealed left cervical and supraclavicular lymphadenopathy (Fig. 1a and b). Laboratory tests revealed a normal blood cell count, elevated immunoglobulin (Ig)M (1,204 mg/dL; reference range, 60-250 mg/dL), and an elevated soluble interleukin-2 receptor level (652 U/mL; reference range, 145-519 U/mL). Based on these findings, MTX-LPD was suspected and treatment with MTX was discontinued in June. In July 200X+7, follow-up EGD for the examination of the gastric ulcer revealed an ulcerative lesion at the lesser curvature of the stomach (Fig. 2a and b). Biopsy specimens of the ulcerative lesion showed lymphoid cell infiltration into the lamina propria (Fig. 3a-c) In these specimens, CD20-positive (Fig. 4a) and CD79α-positive (Fig. 4b) B lymphocytes were predominant, while CD3-positive T lymphocytes (Fig. 4c) and CD5-positive T lymphocytes (Fig. 4d) were less predominant. Moreover, the specimens were CD 10- and CD 21-negative (Fig. 4e and f). The κ/λ ratio of the B lymphocytes showed no dissociation (Fig. 4g and h). The biopsy specimen was not examined to evaluate t(11;18)/API2-MALT1 translocation. Based on these results, a pathological diagnosis of mucosa-associated lymphoid tissue (MALT) lymphoma was made. In the same month, we prescribed vonoprazan [20 mg, twice daily (b.i.d.)], clarithromycin (400 mg, b.i.d.), and amoxicillin (750 mg, b.i.d.) for 7 days to treat gastric MALT lymphoma because a positive urea breath test (19.3%, standard range 0-2.4%) result indicated Helicobacter pylori infection (9, 10). However, on the basis of the patient's clinical history, MTX-LPD was considered as one of the differential diagnoses. Using a biopsy specimen obtained during the first EGD procedure, in situ hybridization was retrospectively performed to detect the Epstein-Barr encoding region (EBER). The result was positive, indicating Epstein-Barr virus (EBV) infection (Fig. 5). In September 200X+7, 3 months after the discontinuation of MTX treatment, a physical examination revealed that the left cervical lymphadenopathy had disappeared. At the same time, the increased serum level of IgM had decreased to within the reference range (Fig. 6).
Figure 1.
Cervical non-contrast computed tomography (a) and cervical non-contrast T2-weighted magnetic resonance imaging (b) scans revealing left cervical lymphadenopathy. The area of lymphadenopathy is circled.
Figure 2.
In July 200X+9, an ulcerative lesion was found in the lesser curvature of the stomach by esophagogastroduodenoscopy (a, b). Seven months after the cessation of methotrexate, scarring of the previous ulcerative lesion was confirmed by esophagogastroduodenoscopy (c, d).
Figure 3.
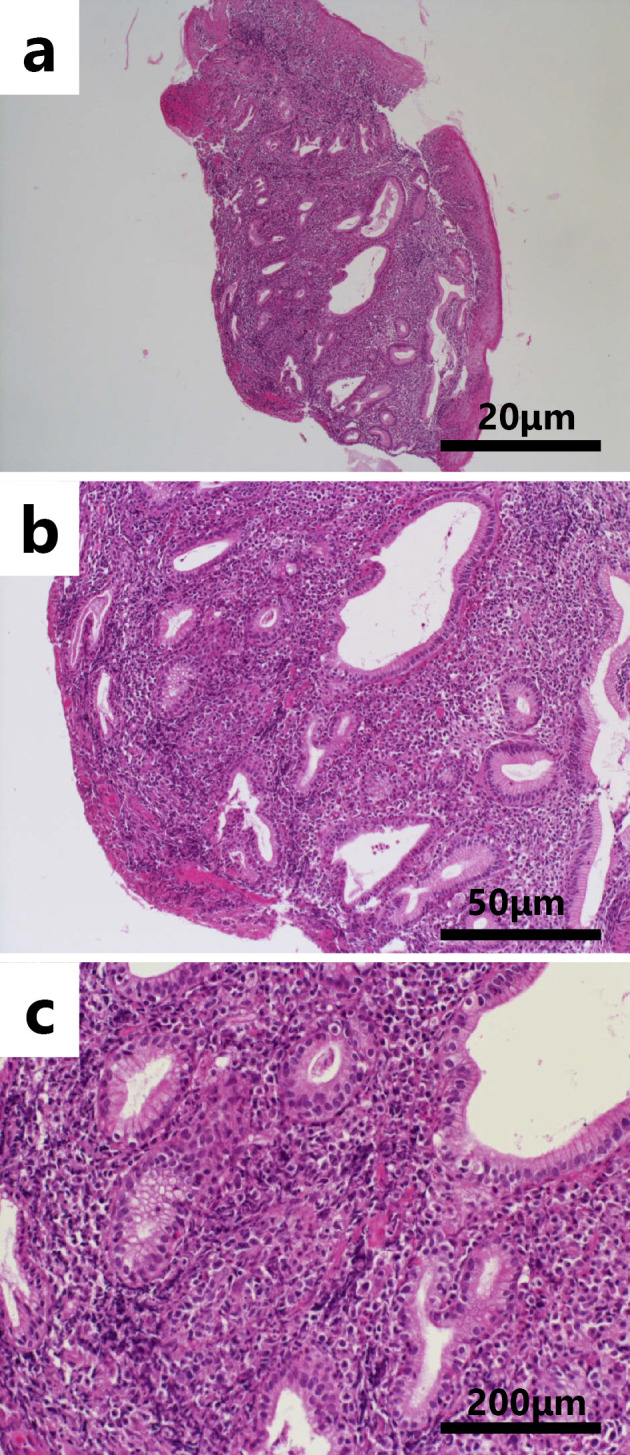
Lymphoid cell infiltration in the lamina propria with Hematoxylin and Eosin staining in a low-power field (a: ×40), b middle-power field (b: ×100) and high-power field (c: ×400). Scale bar indicates 20 μm (a), 50 μm (b), and 200 μm (c), respectively.
Figure 4.
Immunohistochemistry of the biopsy specimen obtained from the ulcerated lesion. CD20 (a), CD79α (b), CD3 (c), CD5 (d), CD10 (e), CD21 (f),κ light chain (g) and λ light chain staining (h). All scale bars indicate 100 μm. The magnification of these images is ×200.
Figure 5.
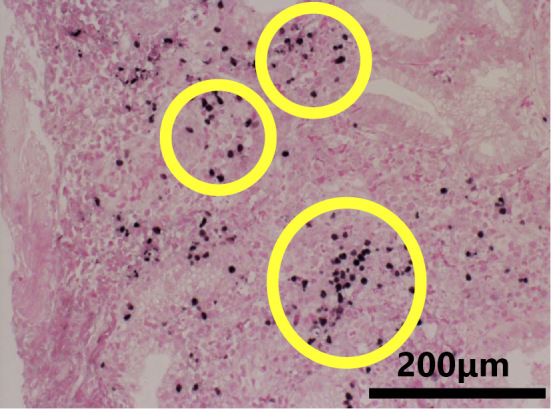
In situ hybridization to detect the the Epstein-Barr encoding region (EBER) indicated the Epstein-Barr virus in infiltrating lymphocytes. EBER-positive cells are circled. The scale bar indicates 200 μm. The magnification of this image is ×400.
Figure 6.
The patient’s clinical course.
In November 200X+7, at 4 months after treatment for H. pylori infection, H. pylori eradication was confirmed by a negative H. pylori stool antigen test result. In February 200X+8, at 7 months after the first EGD procedure, a second EGD procedure revealed scarring at the previous site of the ulcerative lesion (Fig. 2c and d). According to a previous report, after the eradication of H. pylori in patients with MALT lymphoma, remission from lymphoma was achieved within a median time of 5 months (11). This was in line with our case. However, a case of EBV-positive gastric MALToma has been reported (12), the rate of EBV infection in gastric MALToma has previously been reported to be low (13). Conversely, the rate of EBV infection in gastric MTX-LPD has also been reported to be high (14). Based on the patient's entire clinical course and previous reports, which were grounds for the diagnosis of MTX-LPD, we finally diagnosed the patient with MTX-LPD and not MALT lymphoma.
Discussion
In our case, without additional information about the patient's clinical course and EBER positivity the presumed pathological diagnosis would have been MALT lymphoma. MTX-LPD can exhibit any morphology. Although the sub-classification of MTX-LPD has not been established, diffuse large B cell lymphoma (DLBCL) is the most common subtype of MTX-LPD. Other types of B cell lymphoma include Follicular lymphoma, MALT lymphoma, Burkitt lymphoma, and sometimes even peripheral T-cell lymphoma or Hodgkin lymphoma (14).
EBV infection is an important factor for the clinical course and development of MTX-LPD. Among cases of MTX-LPD, the rate of EBV positivity in patients who showed spontaneous regression was significantly higher than that in patients without regression (15). Moreover, EBV positivity can be considered a favorable prognostic factor for spontaneous regression. The spontaneous regression of MTX-LPD and EBV infection in our case were in line with the findings of a previous report (4). MTX can activate the release of infectious EBV from latently infected cells. In this manner, EBV persistence and reactivation may underlie the pathogenesis of LPD (15). Since our patient was EBER-positive, EBV re-activation might have occurred. Furthermore, in our case, in addition to remission in the left cervical lymph nodes and the stomach, the cessation of MTX led to the normalization of the serum level of IgM. A complication of type 2 cryoglobulinemia has been reported in a patient with MTX-LPD (16). In the near future, invasive tests such as EGD will be difficult to perform in our patient because of her advanced age. In such cases, the serum level of IgM may be a good non-invasive marker of disease progression.
In this case, the improvement of the cervical lesion and the normalization of the serum IgM level were achieved at 3 months after cessation of MTX (2 months after treatment for H. pylori infection). These effects are not reported to have resulted from H. pylori eradication. H. pylori eradication was considered to have played a minimal role in the clinical course of this case and it was considered that MTX-LPD would have developed, even in the absence of H. pylori infection.
We searched PubMed for the key words “MTX,” “LPD,” and “stomach” to find reports on gastric MTX-LPD. We found two reports from Japan. In one case, a gastric lesion presumed to be gastric cancer was reported (7). In the other case, an extra-gastric lesion in the mediastinum and chest wall was reported, which was presumed to be DLBCL (8). Thus, our report is the first case of MTX-LPD that was presumed to be MALT lymphoma.
MTX is commonly used in the treatment of RA. According to a previous report, the mean duration of MTX treatment and mean cumulative dose of the development of MTX-LPD were 5.2 years (range, 1.4-13 years) and 2,200 mg (range 500-5,200 mg), respectively (17). Our patient was treated with MTX (4 mg per week) for 7 years. This duration and dose were in line with those in a previous report. Complete remission generally occurs within 4 weeks after the cessation of MTX and appears to persist for an average of 15 months. If no remission is achieved-even after the withdrawal of MTX-treatment for malignant lymphoma, additional treatment such as chemotherapy, should be considered (18).
Approximately 60% of patients who are EBER-positive can achieve remission after the withdrawal of MTX (10). According to Ichikawa et al., recurrence and/or residual disease were observed in 46% of patients with MTX-LPD who achieved complete or partial remission after the cessation of MTX (15). Thus, our patient should be strictly followed up to detect recurrence and/or residual disease.
In conclusion, we experienced a case of MTX-LPD that was presumed to be MALT lymphoma. MTX-LPD can manifest as any pathological state and may occur in any extranodal organ. When a gastric lesion is encountered during MTX treatment, physicians should consider MTX-LPD as a differential diagnosis.
Author's disclosure of potential Conflicts of Interest (COI).
Tatsuhiro Masaoka: Honoraria, Takeda Pharmaceutical.
Financial Support
This study was supported by Keio Gijuku Academic Development Funds (T.M.).
References
- 1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58: 15-25, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology (Oxford) 51(Suppl): vi28-vi36, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Yamanaka H, Inoue E, Tanaka E, et al. Influence of methotrexate dose on its efficacy and safety in rheumatoid arthritis patients: evidence based on the variety of prescribing approaches among practicing Japanese rheumatologists in a single institute-based large observational cohort (IORRA). Mod Rheumatol 17: 98-105, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 34: 322-331, 2007. [PubMed] [Google Scholar]
- 5. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. WHO Press, Geneva, 2017: 462-464. [Google Scholar]
- 6. Kato E, Sawada T, Tahara K, et al. The age at onset of rheumatoid arthritis is increasing in Japan: a nationwide database study. Int J Rheum Dis 20: 839-845, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Satoh K, Yoshida N, Imaizumi K, et al. Reversible methotrexate-associated lymphoproliferative disorder resembling advanced gastric cancer in a patient with rheumatoid arthritis. Am J Med Sci 338: 334-335, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda K, Nakamura T, Kinoshita T, et al. Methotrexate-related lymphoproliferative disorder of the stomach in a patient with rheumatoid arthritis: a case of disease regression after methotrexate cessation. Clin J Gastroenterol 9: 17-21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr 60: 208-210, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65: 1439-1446, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol 8: 105-110, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Oka K, Shinonaga M, Nagayama R, et al. Coexistence of primary pulmonary Hodgkin lymphoma and gastric MALT lymphoma associated with Epstein-Barr virus infection: a case report. Pathol Int 60: 520-523, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Chan WY, Chan EK, Chow JH. Epstein-Barr virus-associated gastric lymphomas are distinct from mucosa-associated lymphoid tissue-type lymphomas: genetic abnormalities of p53 gene. Diagn Mol Pathol 10: 153-160, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Hasserjian RP, Chen S, Perkins SL, et al. Immunomodulator agent-related lymphoproliferative disorders. Mod Pathol 22: 1532-1540, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 91: 20-28, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Matsui Y, Miura Y, Sugino N, Kaneko H, Watanabe M, Tsudo M. Methotrexate-associated lymphoplasmacytic lymphoma complicated with type 2 cryoglobulinemia. Int J Hematol 93: 253-256, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Mariette X, Cazals-Hatem D, Warszawki J, et al. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood 99: 3909-3915, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Saito S, Kaneko Y, Yamaoka K, Tokuhira M, Takeuchi T. Distinct patterns of lymphocyte count transition in lymphoproliferative disorder in patients with rheumatoid arthritis treated with methotrexate. Rheumatology (Oxford) 56: 940-946, 2017. [DOI] [PubMed] [Google Scholar]