Abstract
Objective
To evaluate irreversible electroporation (IRE) for locally advanced pancreatic cancer (LAPC).
Methods
This study was approved by our local review board. Eight patients with histologically proven LAPC ≤5 cm were prospectively enrolled to undergo ultrasound-guided IRE. The primary endpoint was complications within 90 days. Secondary outcomes were the overall survival (OS) and time to local progression. Safety was assessed using Common Terminology Criteria for Adverse Events Version 4.0.
Results
All patients were treated successfully. The median procedure time was 150 min. The median largest tumor diameter was 29.5 mm (20.0-48.0 mm) in the pancreatic head (n=5) and body (n=3). Open (n=4) and percutaneous (n=4) approaches were used. No patients died within 90 days after IRE. There were 5 minor complications in 3 patients and 4 major complications in 3 patients. The incidence rates of major complications did not differ significantly between the approaches. The median time to local progression after IRE was 12.0 months, and the median OS was 17.5 months from IRE and 24.0 months from the diagnosis, with no significant differences between the approaches.
Conclusions
Percutaneous and open IRE may be acceptable for patients with LAPC (despite some major adverse events) and may represent a useful new therapeutic option.
Keywords: IRE, irreversible electroporation, locally advanced pancreatic cancer, safety
Introduction
Irreversible electroporation (IRE) is a relatively new minimally invasive image-guided technique for the interventional oncologic treatment of soft tissue tumors. The application of ultrashort high-voltage electrical pulses leads to an irreversible increase in the permeability of the cell membrane, which can lead to cell death (1).
Unlike thermal ablation techniques, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, which induce tissue necrosis by the deposition of high or low thermal energy, IRE induces tissue necrosis with no (or only minimal) thermal energy deposition. It has therefore been employed worldwide for the treatment of locally advanced pancreatic cancer, particularly because it can preserve heat-sensitive structures located near the tumor, such as nerves, bile ducts, vessels, and the gastrointestinal tract (2).
For the above reasons, IRE has been employed in actual clinical practice in a number of developed countries, such as the United States, European countries, Taiwan, Hong Kong, and Australia. However, IRE has not yet gained widespread acceptance in Japan due to device lag, even though initial published reports have suggested that IRE is an attractive alternative treatment option for tumors in a variety of organs, including the liver, pancreas, kidney, and prostate (3-6).
In this report, the first eight consecutive IRE patients treated at a single center for locally advanced pancreatic cancer are presented. The primary objective of this study was to assess the safety of the technique (including both the percutaneous and open approaches), and the secondary outcomes were the survival and recurrence. To our knowledge, this is the first study to investigate the clinical application of IRE therapy in patients with pancreatic cancer in Japan.
Materials and Methods
IRE has not yet been approved for insurance coverage in Japan, and this study was therefore conducted as physician-led clinical research after approval was obtained from our local medical ethics committee. All patients gave their written informed consent. The study was designed and conducted in accordance with the principles of the Declaration of Helsinki.
Trial protocol and inclusion and exclusion criteria
The present study was designed as a prospective trial. The primary objective of the study was to assess the safety of the technique (including both percutaneous and open approaches), and the secondary outcomes were the survival and recurrence. The inclusion criteria for the study were as follows: adult patients with histologically proven locally advanced pancreatic cancer with a maximum axial diameter of 5 cm, as defined in the National Comprehensive Cancer Network staging system for pancreatic cancer (version 1); previous chemotherapy or radiation therapy allowed if treatment was completed 4 weeks or more before IRE; American Society of Anesthesiologists performance status 1-2; and an acceptable bone marrow, liver, and renal function [specifically, hemoglobin level ≥8.0 g/dL, neutrophil count ≥1,500/mm3, platelet count ≥50×109/L, total bilirubin level ≤1.5×upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase levels ≤2.5×ULN, serum creatinine level ≤1.5×ULN, and international normalized ratio <1.5]. The exclusion criteria were patients with a metallic biliary wall stent, patients with cardiac arrhythmias or a pacemaker, and patients with epilepsy.
In the interest of patient safety, we employed a stepwise registration procedure. Specifically, only 2 patients were registered initially, and subsequent patients were registered 2 at a time, with the study to be terminated immediately if the adverse event rate (≥CTCAE grade 4) was more than 25% during a 1-month period.
Patients
Between January 2015 and June 2016, eight patients with a histological diagnosis of pancreatic adenocarcinoma were selected to undergo IRE. All patients were discussed at a multidisciplinary conference including surgeons, radiologists, and interventional gastroenterologists. Treatment plans were formulated based on a consensus and reassessed prospectively based on image findings and clinical considerations. Patients were also allocated to one of two approaches: the percutaneous approach or the open approach. This decision was made at the multidisciplinary conference based on the tumor location and visibility on ultrasound (US). The demographic data of the patients are shown in Table 1.
Table 1.
Patient Characteristics.
| Characteristics | All: #1, 2, 3, 4, 5, 6, 7, 8 |
Percutaneous: #2, 3, 4, 5 |
Open: #1, 6, 7, 8 |
p values |
|---|---|---|---|---|
| Number of patients | 8 | 4 | 4 | n.s. |
| Male gender: n (%) | 5 (62.5%) | 3 (75.0%) | 2 (50%) | n.s. |
| Median age: years (IQR) | 64 (59.5-70.3) | 62.5 (58.8-64) | 68.5 (60.5-73.5) | |
| Location: n (%) | ||||
| Head | 5 (62.5%) | 2 (50.0%) | 3 (75.0%) | n.s. |
| Neck/body | 3 (37.5%) | 2 (50.0%) | 1 (25.0%) | n.s. |
| Median tumor size: mm (IQR) | 29.5 (26.0-42.5) | 30.5 (26-42.5) | 29.5 (22.3-43.5) | n.s. |
| Chemotherapy before IRE: n (%) | ||||
| Gemcitabine | 2 (25.0%) | 0 (0%) | 2 (50.0%) | n.s. |
| FOLFIRINOX | 2 (25.0%) | 1 (25.0%) | 1 (25.0%) | n.s. |
| Gemcitabine+nab-paclitaxel | 4 (50.0%) | 3 (75.0%) | 1 (25.0%) | n.s. |
| Radiation therapy before IRE: n (%) | ||||
| Intensity-modulated | 3 (37.5%) | 2 (50.0%) | 1 (25.0%) | n.s. |
| Proton | 1 (12.5%) | 1 (25.0%) | 0 (0%) | n.s. |
| Median IRE procedure duration: min (IQR) | 150 (137.25-184.25) | 145.5 (112.5-165.75) | 168 (139.5-205.5) | n.s. |
| Median probes: n (range) | 4 (3-4) | 4 (3-4) | 3.5 (3-4) | n.s. |
| Median probe exposure: mm (range) | 15 (10-15) | 12.5 (10-15) | 15 (10-15) | n.s. |
| Median hospital stay: days (range) | 15 (12-26) | 14 (12-15) | 21 (14-26) | n.s. |
| Complications: n (%) | ||||
| Within 30 days (NCI-CTCAE) | ||||
| Grade 1-2 | 2 (25.0%) | 2 (25.0%) | 0 (0%) | n.s. |
| Grade 3-4 | 2 (25.0%) | 0 (0%) | 2 (25.0%) | n.s. |
| Within 120 days (NCI-CTCAE) | ||||
| Grade 1-2 | 2 (25.0%) | 1 (12.5%) | 1 (12.5%) | n.s. |
| Grade 3-4 | 2 (25.0%) | 1 (12.5%) | 1 (12.5%) | n.s. |
Adverse events were graded using NCI-CTCAE version 4.0.
#: patient number, IQR: interquartile range, FOLFIRINOX: fluorouracil, leucovorin, irinotecan, oxaliplatin, NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events
Anesthesia
Standard hemodynamic monitoring was employed in all patients. General anesthesia was induced by a general anesthesiologist with propofol, fentanyl, and rocuronium. Propofol and remifentanil were used for maintenance. To prevent pulse-induced arrhythmias, an Accusync electrocardiogram (ECG)-gating device (Accusync 72; Accusync Medical Research Corporation, Milford, USA) was connected to a five-lead ECG to synchronize pulse delivery within the refractory period of the heart. Immediately before the start of IRE delivery, complete muscle relaxation was induced with rocuronium to prevent generalized muscle contractions. A defibrillator pad was placed on the patient’s chest as a precautionary measure.
IRE procedures
Three gastroenterologists, each with more than 10 years of experience in image-guided intervention, performed all interventions using a commercially available IRE system (NanoKnifeⓇ; AngioDynamics, Latham, USA). All interventions were performed using either the percutaneous approach or the open surgical approach with the assistance of surgeons using a dedicated US system (Aplio™ 500; Toshiba Medical Systems Corporation, Otawara, Japan) equipped with a 3.75-MHz convex transducer (PVT-375BT; Toshiba Medical Systems Corporation) or with a 7.0-MHz convex transducer (PVT-745BTH; Toshiba Medical Systems Corporation). To ensure the precise placement and stable positioning of the IRE electrodes, CT/US fusion (Smart Fusion; Toshiba Medical Systems Corporation) and a needle-tracking system (Smart Navigation; Toshiba Medical Systems Corporation) were used, both employing a magnetic field. Because this was a pilot study and the accuracy of these software functions in IRE therapy had not yet been established, the physicians were free to accept or reject the navigation information provided by the software program.
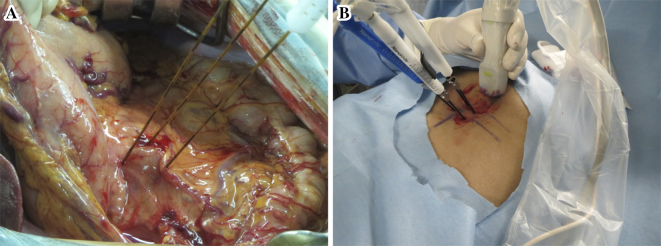
The number and configuration of the electrodes to be used for IRE were determined based on the size and shape of the tumor, ideally including a 0.5-cm tumor-free margin. Two or more insulated 15-cm IRE electrodes with an exposure length of 10-15 mm were placed within and around the tumor (Fig. 1). All electrodes were placed as parallel as possible to one another to help ensure homogeneous energy delivery.
Figure 1.
Overview of irreversible electroporation (IRE) for pancreatic cancer. A: open IRE, B: percutaneous IRE.
Electroporation was performed between all electrode pairs that were separated from each other by less than 2.4 cm, including diagonal ablations. First, 10 tentative pulses of 1,500 V/cm with a duration of 90 μs were delivered via each electrode pair, after which the delivered current was verified. The target current was in the range of 20-50 A, and in order to avoid over- or under-current, the voltage settings were manually adjusted in response to pending over- or under-current. Subsequently, in the initial 2 patients, 1 cycle of 90 pulses was delivered via each electrode pair, and in the remaining 6 patients, 3 cycles of 30 pulses were delivered sequentially via each electrode pair. The reason for this modification was that we experienced one patient (the initial case of this study) who developed interstitial edematous pancreatitis (CT grade 3) one day after IRE. Based on this severe adverse event, we modified our IRE procedures to improve the safety.
For larger tumors, the electrodes were repositioned or pulled back to ablate the remaining part of the tumor. Technical success of ablation was defined as the ability to successfully deliver all planned pulses (at least 90) in accordance with the size and dimensions of the lesion as well as to ensure that the current (which was monitored by tracking the actual delivered current) showed a change of at least 5 A from that of the initial 10 pulses delivered. If this was not achieved, another 30-90 pulses were applied until the criteria were met.
Follow-up protocol
The follow-up protocol included clinical and laboratory assessment and contrast-enhanced CT at one month and then at three-month intervals (Fig. 2). Early post-IRE scanning was performed to identify early complications of this new technique, such as venous thrombosis, but not to evaluate the treatment efficacy. The development of new low-density lesions in the region treated by IRE was considered evidence of local progression. Similarly, suspicious low-density lesions in the liver or lung were considered evidence of distant metastasis. Peritoneal recurrence was defined as the presence of suspicious nodules in the peritoneum or omentum, or the presence of newly identified ascites. No patients were lost to follow-up.
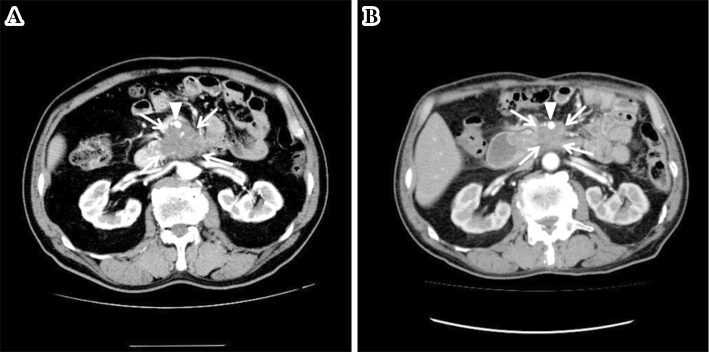
Figure 2.
Representative arterial phase contrast-enhanced CT images of a patient with locally advanced pancreatic cancer located in the head of the pancreas and treated with IRE. A: The pre-IRE pancreatic tumor (arrows) shows central hypoenhancement with slight peripheral enhancement. It also encases the superior mesenteric artery by more than 180° (arrowhead). B: The post-IRE (at 1 year) pancreatic tumor (arrows) is markedly smaller than at the baseline. It still encases the superior mesenteric artery by more than 180° (arrowhead).
Statistical analyses
Data were collected prospectively. Morbidity and mortality were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 4.0). The overall survival of patients with pancreatic cancer was calculated as months from the date of IRE to death or the last follow-up visit, and the progression-free survival was calculated from the date of IRE to the appearance of imaging evidence of recurrence. Recurrence was defined as the radiographic appearance of a new local or distant lesion. Continuous variables, which were reported as the median (range) except when the interquartile range was specified, were compared using the Wilcoxon signed-rank test for variables with a non-normal distribution. All statistical analyses were performed with a computer software package (JMPⓇ version 11; SAS Tokyo, Japan).
Results
The IRE procedures were technically successful in all eight patients, which means that all tumors were treated according to the protocol.
Safety and complications
Systemic chemotherapy was re-initiated in all of the patients within three months. The regimen of chemotherapy varied among the patients. No patients died within 90 days after the procedure. Overall, 5 of the 8 patients experienced 10 adverse events (Table 2) within 120 days after the procedure. There was no significant difference in the complications between the percutaneous and open procedures. Two patients (#4 and #8) experienced one complication, two patients (#2 and #5) experienced two complications, and one patient (#1) experienced four complications (Table 2).
Table 2.
IRE-related Adverse Events.
| Adverse Events (NCI-CTCAE) | Grade 1-2 | Grade 3 | Grade 4 | Treatment |
|---|---|---|---|---|
| Infection | ||||
| Pancreatitis | 1A(#4) | 1A(#1) | Antibiotics, transfusion | |
| Abscess | 1B(#1) | Antibiotics | ||
| Vascular | ||||
| Pseudoaneurysm rupture | 2 (#1B, 2B) | Arterial embolization | ||
| SMV thrombus | 1B(#1) | Anticoagulant therapy | ||
| Gastrointestinal | ||||
| Abdominal pain | 1A(#5) | Analgesics | ||
| Duodenal edema → Vomiting | 1A(#8) | Antiemetics, gastric drainage | ||
| Nausea | 1A(#5) | Antiemetics | ||
| Total | 5 | 2 | 2 |
A: complication occurred within 30 days after IRE, B: complication occurred 31 days or more after IRE.
Adverse events were graded using NCI-CTCAE version 4.0.
#: patient number, NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events, DIC: disseminated intravascular coagulation, SMV: superior mesenteric vein
Two grade 4 complications occurred. One patient (#2) was readmitted for hematemesis and epigastric pain with hemodynamic instability 112 days after IRE. Emergency gastrointestinal endoscopy showed a part of a tumor with coagulated blood exposed in the intestinal lumen near the ligament of Treitz. We then performed abdominal angiography, which showed bleeding from a pseudoaneurysm into the intestine. This was managed by embolization with NBCA-lipiodol (1:2.5). The other patient (#1) was readmitted for bloody stool 107 days after IRE. Contrast-enhanced CT showed a pseudoaneurysm in the first jejunal artery. This was also managed by embolization with NBCA-lipiodol (1:3).
Two grade 3 complications occurred in two patients. One patient (#1) developed interstitial edematous pancreatitis (CT grade 3) one day after IRE. This patient also developed an abscess with an internal fistula requiring noninvasive management. The other patient (#8) experienced vomiting due to duodenal edema one day after IRE, which required temporary nasogastric drainage and placement of a nasojejunal feeding tube.
With regard to hematological and biochemical examinations, after intervention, the serum amylase levels were elevated in two patients (#1 and #4), both of whom showed signs of pancreatitis (Fig. 3). No remarkable changes were observed in other laboratory values.
Figure 3.
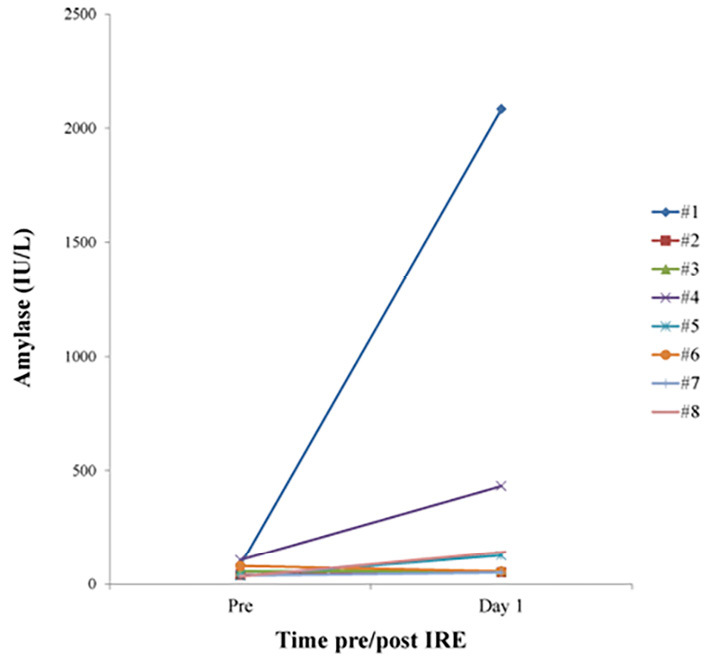
Serum amylase levels pre- and post-IRE.
Survival analyses
After a median follow-up period of 17.5 months [interquartile range (IQR): 9.0-20.5 months], the median OS from the diagnosis was 24.0 months (IQR: 17.5-24.8 months), and the median OS from IRE was 17.5 months (IQR: 9.0-20.5 months). The median time to local progression after IRE was 12.0 months (IQR: 8.5-15.3 months). The median time to overall progression after IRE was 5.0 months (IQR: 2.5-9.8 months) (Table 3).
Table 3.
Oncological Data for Each Patient.
| # | OS from the diagnosis (months) | OS from IRE (months) | Local progression-free survival (months) | Overall progression-free survival (months) | Local recurrence | Distant lesion | Site of metastasis | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 27 | 27 | 9 | No | Yes | LN, lung | N/A |
| 2† | 15 | 4 | 4 | 4 | Yes | No | N/A | DIC |
| 3† | 25 | 17 | 13 | 6 | No | Yes | peritoneal | Debilitation due to pancreatic cancer |
| 4 | 24 | 21 | 10 | 10 | Yes | No | N/A | N/A |
| 5 | 24 | 19 | 8 | 2 | No | Yes | Liver | N/A |
| 6† | 17 | 7 | 12 | 1 | No | Yes | Liver | Debilitation due to pancreatic cancer |
| 7 | 24 | 18 | 16 | 4 | No | Yes | peritoneal | N/A |
| 8† | 19 | 15 | 12 | 12 | Yes | No | N/A | Debilitation due to pancreatic cancer |
| Median | 24.0 | 17.5 | 12.0 | 5.0 | N/A | N/A | N/A | N/A |
| IQR | 17.5-24.8 | 9.0-20.5 | 8.5-15.3 | 2.5-9.8 | N/A | M/A | N/A | N/A |
#: patient number, OS: overall survival, IQR: interquartile range, †: dead at the time of writing this manuscript, DIC: disseminated intravascular coagulation, LN: lymph node, NA: not applicable
Discussion
In this study, we prospectively analyzed eight patients with locally advanced pancreatic cancer who were randomly allocated to undergo percutaneous or open US-guided IRE. First, with regard to the safety, there were no treatment-related deaths, and the 90-day mortality rate was 0%. Serious (grade 3 or 4) adverse events occurred in 2 (25%) of the 8 patients. Thus, the main findings of our study indicate that both percutaneous and open IRE may be acceptable for the treatment of patients with locally advanced pancreatic cancer.
Although our series included only a small number of patients, these findings are comparable to the previously reported outcomes for both percutaneous and open pancreatic IRE. In a study involving percutaneous IRE reported by Narayanan et al. (7), the authors retrospectively analyzed 50 patients who had undergone percutaneous CT-guided IRE for unresectable locally advanced pancreatic cancer over a 5-year period. In their series, no treatment-related deaths were observed, and the 30-day mortality rate was 0%. Serious (grade 3 or 4) adverse events occurred in 10 (20%) of the 50 patients, with the most common being abdominal pain. No patients experienced major bleeding, and in six of the seven patients who developed pancreatitis, it was mild and self-limited. The rate of thrombosis in the treatment field did not exceed 6%, and thrombosis did not lead to any serious adverse events in any of the patients.
In a study involving open IRE reported by Martin et al. (8), an in situ IRE group of 150 patients with unresectable tumors underwent open IRE. In their study, there were no deaths within 30 days of the procedure, and the rate of serious (grade 3 or 4) adverse events was 18%. However, two recently published reports describing preliminary single-institution experience with percutaneous (9) and open (10) IRE observed substantially higher rates of morbidity and mortality.
Interestingly, both of these studies (9, 10) reported upper gastrointestinal bleeding with or without duodenal ulceration, which were the predominant morbid and fatal complications and were often refractory to treatment. If we consider the theoretical mechanism of action of IRE, the bowel and vascular walls should remain intact owing to the sparing of collagenous structures. However, gastrointestinal bleeding led to life-threatening complications. Although a causal relationship between IRE and the occurrence of gastrointestinal bleeding could not be established, this complication should be kept in mind for patients who undergo IRE near the intestinal walls.
In the present study, two patients experienced life-threatening gastrointestinal bleeding. Both of these events were due to the intraluminal rupture of a pseudoaneurysm into the gastrointestinal tract. Compared to the findings of previous studies (9, 10), the time of the onset in both of our patients was relatively late: one at 112 days after IRE (#2) and the other at 107 days after IRE (#1). As a result, a causal relationship between IRE and intraluminal rupture of the pseudoaneurysm could not be established. One possible reason is that one patient (#1) developed interstitial edematous pancreatitis (CT grade 3), leading to the formation of an abscess with an internal fistula, which may have led to pseudoaneurysm formation. However, in the other patient, the cause was unclear, which may be suggestive of IRE-induced vessel injury.
We experienced one patient (the initial case of this study) who developed interstitial edematous pancreatitis (CT grade 3) one day after IRE. Based on this severe adverse event, we modified our IRE procedures. Specifically, 10 tentative pulses and then 1 cycle of 90 pulses were delivered initially, followed by 10 tentative pulses and then 3 cycles of 30 pulses delivered sequentially. We cannot be sure that this modification affected the incidence of pancreatitis, but we experienced only one patient who developed minor pancreatitis. Further evaluations are still needed to determine which IRE procedure is superior in terms of minimizing the risk of pancreas.
With regard to the efficacy of IRE for locally advanced pancreatic cancer, Martin et al. (8) reported that their in situ group of patients with unresectable locally advanced pancreatic cancer had a median OS of 23.2 months from the time of the diagnosis and 18 months from the time of the procedure. Narayanan et al. (7) reported a median OS of 27 months from the time of the diagnosis and 14.2 months from the time of IRE. These results are comparable to our results. Although our series included only a small number of patients, we observed a median OS of 24 months from the time of the diagnosis and 17.5 months from the time of IRE. These results compare favorably with historical survival rates for this patient population (11, 12). Even for patients with resectable disease, the median survival period after resection has been reported to be 11-24 months in randomized studies of adjuvant chemotherapy.
Not only were the baseline characteristics of the patients in these three studies similar, but there were also some important similarities with regard to therapy before IRE. In the series of Martin et al. and the series of Narayanan et al., the median time from the diagnosis to IRE was 6.2 and 11.6 months, respectively. In our series, the median time from the diagnosis to IRE was 5.5 months. There is emerging evidence that a longer duration of induction chemotherapy is associated with a prolonged survival in patients with locally advanced pancreatic cancer, possibly due to the elimination of patients with rapid disease progression, which sets the stage for success of localized therapy (13, 14). Such a prolonged course of therapy before IRE may be as good as or better than other methods for selecting patients who would benefit from IRE after showing no evidence of disease progression during systemic therapy.
In addition, a previous study on radiofrequency ablation for locally advanced pancreatic cancer showed a higher incidence of early progression in patients who underwent radiofrequency ablation as initial treatment compared with those who underwent radiofrequency ablation as secondary treatment after neoadjuvant chemotherapy (15). This finding also emphasizes the importance of induction chemotherapy before IRE. Nonetheless, 5 (62.5%) of the 8 patients in our study developed distant metastases (lymph node, lung, liver, and peritoneum), reflecting the fact that pancreatic cancer remains a systemic disease. Earlier detection and better systemic therapies are urgently needed to support local control measures.
The present study has some serious limitations. First, the total number of treated patients was small. For the same reason, it may not necessarily be possible to extrapolate the results obtained for the overall survival in this study to a larger patient population. Second, all of the patients had undergone various types of treatment before IRE, and the timing of IRE also differed. In particular, previous radiotherapy may have affected the safety assessment. Finally, our patient group may have suffered from selection bias because patients who did not experience progression during systemic therapy, patients with a good performance status, patients with few comorbidities, and patients who often travelled significant distances to visit our hospital may have had a better than average clinical course due to a more favorable disease biology. This possibility cannot be ruled out given the lack of a control arm. To determine whether or not IRE improves the survival, a large-scale, prospective, randomized clinical trial is needed.
In conclusion, both percutaneous and open IRE may be acceptable for patients with locally advanced pancreatic cancer (although some major adverse events may occur) and may represent a new technological option in the treatment of this disease. Prospective randomized clinical trials will help clarify the role of IRE in the treatment of these patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 33: 223-231, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 215: 361-369, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat 12: 233-241, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 20: S443-S449, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 22: 611-621, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Valerio M, Dickinson L, Ali A, et al. A prospective development study investigating focal irreversible electroporation in men with localised prostate cancer: Nanoknife Electroporation Ablation Trial (NEAT). Contemp Clin Trials 39: 57-65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narayanan G, Hosein PJ, Beulaygue IC, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol 28: 342-348, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Martin RC 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 262: 486-494, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Scheffer HJ, Vroomen LG, de Jong MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology 282: 585-597, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Kluger MD, Epelboym I, Schrope BA, et al. Single-institution experience with irreversible electroporation for T4 pancreatic cancer: first 50 patients. Ann Surg Oncol 23: 1736-1743, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol 33: 1770-1778, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 44: 515-521, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Kadera BE, Sunjaya DB, Isacoff WH, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg 149: 145-153, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Khushman M, Dempsey N, Maldonado JC, et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 15: 667-673, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Girelli R, Frigerio I, Giardino A, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg 398: 63-69, 2013. [DOI] [PubMed] [Google Scholar]