Abstract
Rituximab (RTX) has become a therapeutic option for inducing remission of anti-neutrophil cytoplasmic autoantibody-associated vasculitis (AAV). However, the optimum dosage of RTX to induce remission of AAV and reduce adverse events, such as infection, remains unclear. We herein report an elderly and renally impaired patient with alveolar hemorrhaging due to refractory AAV who was successfully treated with single infusion of RTX. Single infusion of RTX may be a therapeutic option in refractory AAV patients who are vulnerable to infections.
Keywords: alveolar hemorrhaging, anti-neutrophil cytoplasmic autoantibody-associated vasculitis, cyclophosphamide, elderly, rituximab
Introduction
Alveolar hemorrhaging (AH) of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) progresses rapidly to respiratory failure and is associated with the short- and long-term mortality (1). Although a combination of glucocorticoids plus either cyclophosphamide (CY) or rituximab (RTX) is recommended for the induction of remission in AAV (2, 3), it is sometimes difficult to choose the optimum therapy for elderly, renally insufficient or compromised patients. Single administration of RTX for AAV patients has been proposed to reduce the risk of infection (4); however, whether or not it is effective in elderly patients with renal insufficiency, who may be more vulnerable to infections than most, is unclear.
We herein report an elderly and renally insufficient patient with AH due to refractory AAV who was successfully treated with single infusion of RTX.
Case Report
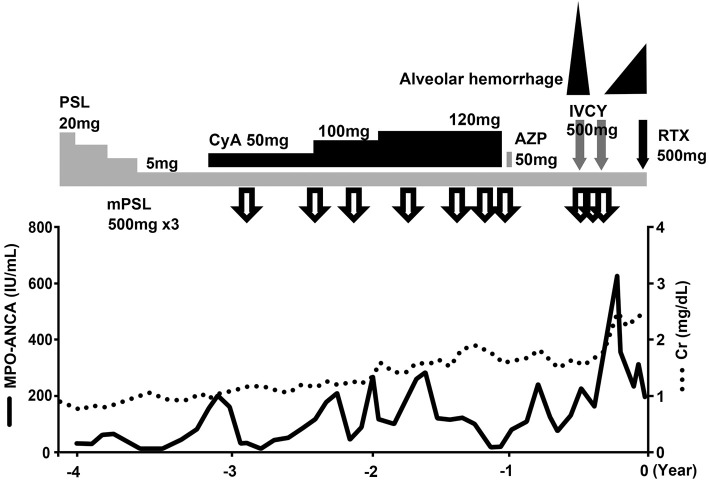
An 80-year-old woman with chronic lower respiratory tract infection and a history of hepatitis B infection visited her physician due to worsening leg edema and hemosputum. Five years earlier, she had been diagnosed with myeloperoxidase-antineutrophil (MPO)-ANCA-related necrotizing glomerulonephritis by a renal biopsy and treated with steroid pulse therapy followed by 20 mg of prednisolone (PSL) per day orally. The amount of PSL was reduced to 5 mg per day due to complications of steroid psychosis. She took steroid pulse therapies whenever her AAV was exacerbated, as steroid psychosis did not occur with the steroid pulse therapy. Immunosuppressive agents, such as cyclosporine or azathioprine, could not be administered due to the side effect of pancytopenia. Therefore, she continued to receive 5 mg of PSL per day for AAV (Fig. 1).
Figure 1.
Clinical course. The time point of rituximab administration is indicated as 0 years. Whenever the patient’s ANCA-associated glomerulonephritis was exacerbated, steroid pulse therapies with the concomitant administration of cyclosporine or azathioprine were performed. However, her renal function gradually worsened. Three months before the administration of rituximab, hemoptysis was observed. We therefore administered intravenous cyclophosphamide twice, but alveolar hemorrhaging occurred again. ANCA: anti-neutrophil cytoplasmic autoantibody, AZP: azathioprine, CyA: cyclosporine, IVCY: intravenous cyclophosphamide, MPO: myeloperoxidase, mPSL: methyl prednisolone, PSL: prednisolone, RTX: rituximab
Three months before admission, she experienced rapidly progressive renal dysfunction and hemosputum, with AH revealed by chest computed tomography. Single intravenous infusion of cyclophosphamide (IVCY) and steroid pulse therapy were effective for her AH, and she later required treatment with IVCY and steroid pulse therapy again due to the recurrence of AH.
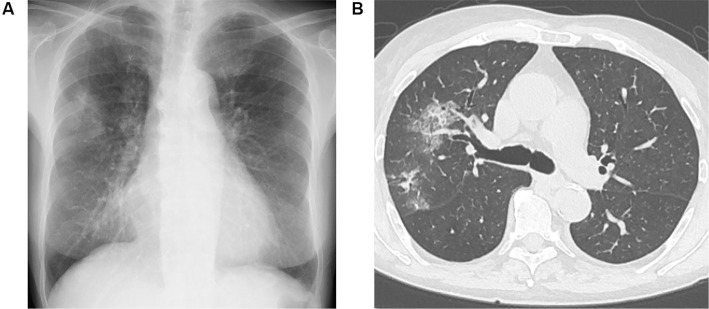
She was thus admitted to our hospital because of the recurrence of uncontrollable AH. On admission, her height was 156 cm, body weight 48 kg and body surface area 1.45 m2. Her body temperature was 36.6°C, heart rate 88 bpm and blood pressure 162/77 mmHg. Her percutaneous oxygen saturation was 99% on atmospheric air. Her breath sounds were diminished in the left lower lung field. She had facial and leg edema. The laboratory values at the time of the administration of RTX were as follows (Table): white blood cells count, 6,370/μL; hemoglobin, 8.5 g/dL; hematocrit, 29.3%; platelets, 212,000/μL; blood urea nitrogen, 65.4 mg/dL, serum creatinine level, 3.39 mg/dL; C-reactive protein, 0.77 mg/dL and IgG level, 628.0 mg/dL. A urinalysis showed protein 3+ and blood 3+ with sediment that contained 30 to 49 red blood cells per high-power field; the protein-to-creatinine ratio in the urine was 7.60 g/gCr. The MPO-ANCA levels were elevated at 274 IU/mL, and proteinase-3-ANCA (PR3-ANCA) and anti-glomerular basement membrane (GBM) antibody were negative. Bilateral multiple patchy and ground glass opacities were seen on chest X-ray and computed tomography images (Fig. 2A and B).
Table.
Laboratory Tests at the Time of Rituximab Administration.
| <Complete blood cell counts> | <Chemical analysis> | <Urinalysis> | ||||||
| WBC | 6,370 | /µL | Total protein | 5.6 | g/dL | Protein | 3+ | |
| Neutrophils | 80.9 | % | Albumin | 3.5 | g/dL | Occult blood | 3+ | |
| Lymphocytes | 6.0 | % | Blood urea nitrogen | 65.4 | mg/dL | RBCs | 30-49 | /HPF |
| Monocytes | 10.8 | % | Creatinine | 3.39 | mg/dL | Protein | 7.60 | g/gCr |
| Eosinophils | 2.0 | % | eGFR | 10.7 | mL/min/1.73 m2 | epithelium casts | 10-19 | /WF |
| Hb | 8.5 | g/dL | AST | 18 | IU/L | granular casts | 5-9 | /HPF |
| MCV | 89.3 | fL | ALT | 19 | IU/L | RBC-cast | 1-4 | /WF |
| Platelets | 212,000 | /µL | Na | 138 | mmol/L | |||
| <Serological test> | K | 5.5 | mmol/L | |||||
| IgG | 628.0 | mg/dL | Cl | 106 | mmol/L | |||
| MPO-ANCA | 274 | IU/mL | Ca | 8.0 | mg/dL | |||
| PR3-ANCA | negative | P | 4.7 | mg/dL | ||||
| Anti-GBM antibody | negative | CRP | 0.77 | mg/dL | ||||
| HBs antigen | 0.00 | IU/mL | ||||||
| HBs antibody | >1,000.0 | mIU/mL | ||||||
| HBc antibody | 8.21 | S/CO | ||||||
| HBV-DNA | negative | |||||||
MCV: mean corpuscular volume, MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibodies, PR3-ANCA: proteinase-3-anti-neutrophil cytoplasmic antibodies, GBM: glomerular basement membrane, HBs: hepatitis B surface, HBc: hepatitis B core, S/CO: Sample RLU/cut-off, HBV: hepatitis B virus, eGFR: estimated glomerular filtration rate, AST: asparatate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, RBCs: red blood cells
Figure 2.
(A) Chest X-ray and (B) representative axial image of the chest computed tomography before the administration of rituximab. A chest X-ray film showed the cardiothoracic ratio as 58%, and bilateral multiple patchy and ground glass opacities in the upper field of the lung. A chest computed tomographic image showed alveolar infiltrates of S2 and S3 of right lung.
Intravenous infusion of 500 mg of RTX was used for her refractory AAV. B-cell depletion was confirmed, as the proportion of CD19-positive cells was 0.14% after 2 weeks. Her AH caused by AAV was ameliorated after RTX therapy. Three weeks later, she developed bacterial pneumonia, which was successfully treated with antibiotics. After two months, maintenance hemodialysis was needed because of end-stage kidney failure, though her AH had not relapsed. After 6 months, the B-cell depletion was sustained, with the proportion of CD19-positive cells at 0.48%. The level of MPO-ANCA was 69.8 IU/L under treatment with 5 mg of PSL. She has been stable as an outpatient with maintenance hemodialysis.
Discussion
We experienced a case of refractory AH that was successfully treated with single infusion of RTX in an elderly and renally insufficient patient with AAV. Refractory AAV often needs strong immunosuppressive treatment, which might sometimes induce critical infectious complications and lethal results, especially in elderly patients. Regarding the benefits versus the harm of such therapy, single RTX administration might be a safer and more effective treatment than strong immunosuppressive agents for refractory AAV in elderly patients with renal insufficiency.
In elderly patients with AAV, Timlin et al. reported that RTX therapy was effective for remission induction (5). For the induction of remission of AAV, it is common to administer RTX at a dose of 375 mg/m2 every week for 4 consecutive weeks or 1,000 mg twice every 2 weeks (6). Regarding the number of administrations and dosage of RTX, single infusion of RTX was effective not only at a dose of 375 mg/m2 but also at a dose of 500 mg/body for remission induction of AAV (4, 7).
Our case was complicated with many risks of infection, such as the history of hepatitis B virus infection, chronic lower respiratory tract infection, and sustained administration of steroids. The efficacy of RTX at 500 mg, the dosage that was decided in order to reduce the risk of infections, was investigated and compared with that of azathioprine as maintenance treatment of AAV patients; RTX showed a better outcome than azathioprine (8). The risk of infection with RTX may be lower with a single infusion of RTX than at the normal dose (7, 9). In our case, B-cell depletion was achieved with a single dose of RTX at 500 mg and was maintained for six months. However, despite the single infusion of RTX, our patient became complicated with bacterial pneumonia, which was cured with antibiotic treatment due to its mild severity. No HBV reactivation was observed, as the titer of HBV-DNA continued to be negative for six months after RTX administration. No clinical data comparing the rate of refractory AAV with single infusion of RTX and four weekly infusions of RTX have been reported. One clinical study reported that, among 14 patients who achieved complete remission with single infusion of RTX, 4 (29%) had relapsed as of 24 months (4). Another clinical study reported that, among 76 patients who achieved complete remission with 4 weekly infusions of RTX, 24 (32%) had relapsed as of 18 months (10). Given these findings, there may be no marked difference in the relapse rate between single infusion of RTX and four weekly infusions of RTX. There was also considered to be no marked difference in the B cell depletion duration between single infusion of RTX and four weekly infusions of RTX.
AH was observed in 15.4% of AAV patients in Japan, and the 1- and 5-year survival rates in AAV patients with AH were 56.2% and 41.5%, respectively (1). AAV patients with AH often have renal dysfunction. One of the advantages of RTX is the lack of any need to adjust the dosage due to renal dysfunction. The risks of leukocytopenia and infection were lower in the RTX group than in the CY group in AAV patients with severe renal dysfunction (11, 12). Pulmonary lesions, including AH, were shown to be the largest predictive factor for relapse and mortality in dialysis-dependent AAV patients (13). AH in AAV patients complicated with kidney failure like the present case is a critical issue, and its control is a problem directly related to the survival prognosis. As such, prompt and safe treatment is required.
Since our patient had been treated with CY twice before the administration of RTX, CY instead of RTX might have induced remission of AH. However, AH recurred shortly after the first treatment with CY and did not improve after the second treatment with CY. Thus, it was unlikely that CY was ultimately effective in the present case. The BSR/BHPR guideline and EULAR guideline showed that refractory AAV should be treated with RTX rather than CY (2, 3), and previous reports have also described RTX as useful for treating refractory AAV (6, 14).
In conclusion, single administration of RTX might be an option for elderly AAV patients with renal dysfunction and a high risk of infection. We expect that the accumulation of more cases will clarify the long-term efficacy and safety of single infusion of RTX therapy for AAV.
Author's disclosure of potential Conflicts of Interest (COI).
Masaaki Ito: Honoraria, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Bayer Yakuhin and Takeda Pharmaceutical; Research funding, SANWA KAGAKU KENKYUSHO, Daiichi Sankyo, Bristol-Myers Squibb, Bayer Yakuhin, Nippon Shinyaku, Takeda Pharmaceutical, MSD, Otsuka Pharmaceutical, Astellas Pharma, Shionogi and Biotronik Japan.
References
- 1. Hirayama K, Kobayashi M, Usui J, et al. Pulmonary involvements of anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis in Japan. Nephrol Dial Transplant 30(Suppl): i83-i93, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Ntatsaki E, Carruthers D, Chakravarty K, et al. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology (Oxford) 53: 2306-2309, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 75: 1583-1594, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Turner-Stokes T, Sandhu E, Pepper RJ, et al. Induction treatment of ANCA-associated vasculitis with a single dose of rituximab. Rheumatology (Oxford) 53: 1395-1403, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Timlin H, Lee SM, Manno RL, Seo P, Geetha D. Rituximab for remission induction in elderly patients with ANCA-associated vasculitis. Semin Arthritis Rheum 45: 67-69, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones RB, Ferraro AJ, Chaudhry AN, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 60: 2156-2168, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Saito A, Takeuchi Y, Kagaya S, et al. Remission induction therapy with rituximab for microscopic polyangiitis: a feasibility study. Tohoku J Exp Med 242: 53-62, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371: 1771-1780, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221-232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 396: 417-427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah S, Hruskova Z, Segelmark M, et al. Treatment of severe renal disease in ANCA positive and negative small vessel vasculitis with rituximab. Am J Nephrol 41: 296-301, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Pepper RJ, Chanouzas D, Tarzi R, et al. Intravenous cyclophosphamide and plasmapheresis in dialysis-dependent ANCA-associated vasculitis. Clin J Am Soc Nephrol 8: 219-224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasegawa M, Hattori K, Sugiyama S, et al. A retrospective study on the outcomes of MPO-ANCA-associated vasculitis in dialysis-dependent patients. Mod Rheumatol 26: 110-114, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Guerry MJ, Brogan P, Bruce IN, et al. Recommendations for the use of rituximab in anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology(Oxford) 51: 634-643, 2011. [DOI] [PubMed] [Google Scholar]