Abstract
Coagulation abnormalities are a rare but critical complication associated with plasma cell diseases. We herein present a case of multiple myeloma (MM) with complicated coagulopathy. Initially, the patient showed severe bleeding tendency due to concomitant acquired hemophilia A and acquired von Willebrand syndrome. Interestingly, the patient also exhibited hyperactivation of factor IX. During treatment for MM, the bleeding complications were ameliorated; however, the patient had central retinal vein occlusion. All of the coagulation abnormalities were completely resolved after the complete remission of MM. This case suggests that MM patients may have concomitant risks for both bleeding and thromboembolic complications.
Keywords: multiple myeloma, acquired von Willebrand disease, acquired hemophilia A
Introduction
Coagulation system abnormalities represent an important complication in patients with plasma cell disorders (1, 2). Approximately 6-7% of patients with monoclonal gammopathy of uncertain significance (MGUS) and approximately 10% of patients with primary amyloidosis have venous thromboembolisms (3). The administration of immunomodulatory drugs such as thalidomide, lenalidomide or pomalidomide for multiple myeloma (MM) further increase the risk of thrombosis (3). In contrast, although the incidence is low, patients with plasma cell diseases also suffer from bleeding tendencies (1, 2). Various pathological mechanisms, including vascular injury caused by hyperviscosity syndrome, decreased platelet production, and impaired platelet function due to paraproteins, are involved in hemorrhagic complications associated with MM. In addition, a series of case reports demonstrated that acquired hemophilia A (AHA) and acquired von Willebrand syndrome (AVWS) can cause severe bleeding in MM patients (4). In certain cases, treatment for MM and suppression of paraprotein production ameliorated concomitant coagulopathy (5, 6), suggesting that paraproteins play a central role in the abnormal coagulation of MM patients. We herein present a case of MM complicated by a coagulation abnormality that resolved after allo-hematopoietic stem cell transplantation (allo-HCT), which was performed to treat MM.
Case Report
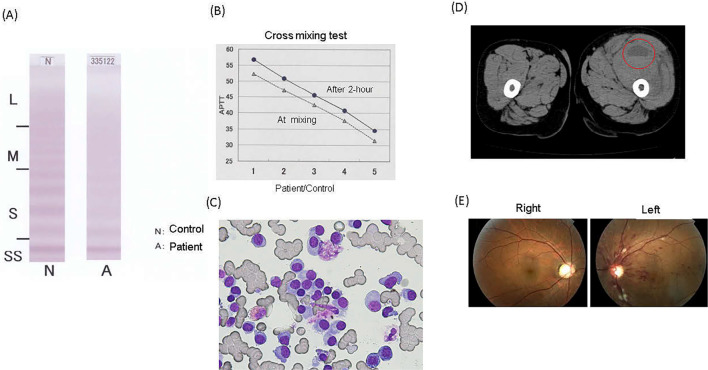
A 52-year-old Japanese man was referred to our hospital with continuous oral bleeding. He exhibited oozing bleeding that had continued for approximately one month. The patient had had no prior episodes of bleeding and no family history of bleeding or hematological disorders. The patient's platelet count, prothrombin time (PT), and concentrations of fibrinogen, D-dimer and fibrinogen-fibrin degradation products (FDP) were normal; however, his activated partial thromboplastin time (APTT) was prolonged (51.4 seconds), and his von Willebrand factor (vWF) ristocetin cofactor activity (vWF:Rco) was decreased (16%). We also confirmed a decrease in his vWF antigen levels (vWF:Ag). The patient was negative for lupus anti-coagulants. A multimer analysis revealed that the quantity of high-molecular-weight multimers was decreased (Figure A). Based on these findings, we diagnosed the patient with AVWS. Although the patient's factor VIII activity was also decreased to 25%, no inhibitor of factor VIII was detected at this time. A cross-mixing test showed an inhibitor pattern (Figure B). We also noticed marked elevation of factor IX activity. Other blood tests revealed normal white blood cell counts and anemia (hemoglobin 8.8 g/dL). Serum biochemistry tests revealed increased serum levels of IgA (4,020 mg/dL), decreased serum levels of IgG (122 mg/dL) and IgM (18 mg/dL), and increased free κ immunoglobulin light chain (κ-chain/λ-chain ratio, 4.09). An immunofixation test using serum and urine revealed the existence of IgA-κ-type monoclonal protein. A bone marrow examination revealed increased plasma cells (58%) (Figure C). These plasma cells expressed CD38, CD138, CD56 and MCP-1 but not CD19 or CD49e. Based on these findings, the patient was diagnosed with MM according to the International Myeloma Working Group (IMWG) criteria (7). There were no evidence of solid tumors or auto-immune disease. We began treatment with bortezomib and dexamethasone (BD). After 1 cycle of BD, the patient experienced swelling and pain in his left thigh and was admitted to our hospital. A computed tomography (CT) scan revealed intramuscular bleeding in his left thigh (Figure D). Laboratory tests revealed that the prothrombin time international normalized ratio (PT-INR, 1.54) and APTT (69.7 seconds) were prolonged, and the IgA titer was elevated (5,230 mg/dL) relative to the levels at the time of the diagnosis. The patient's factor VIII activity had also worsened (17%), and an inhibitor of factor VIII was detected, leading to a diagnosis of AHA. To treat AHA, recombinant activated factor VIIa was administered, and the patient's bleeding tendency was ameliorated. In addition, we changed the chemotherapeutic regimen from BD to cyclophosphamide, bortezomib and dexamethasone (CyBorD). After the initiation of treatment with the CyBorD regimen, the patient's vWF: Rco activity recovered to the normal range (98%), and his factor VIII activity also increased to 77%, whereas his factor IX activity remained enhanced. At 53 days after starting the CyBorD regimen, the patient suddenly experienced poor vision in his left eye. Based on the retinal findings (Figure E), he was diagnosed with central retinal vein occlusion (CRVO). We continued treatment with CyBorD; however, the patient exhibited progressive disease. Thus, we began treatment with bortezomib, thalidomide and dexamethasone (VTD) together with low-dose aspirin as prophylaxis against thromboembolism, as recommended by guidelines. Despite treatment with VTD, the patient's IgA level continued to gradually increase. Conversely, the activities of both vWF and factor VIII decreased again, and the patient exhibited macrohematuria. After this episode, the patient was treated with lenalidomide and dexamethasone followed by autologous hematopoietic stem cell transplantation (auto-HCT) with high-dose melphalan. However, M-protein remained present and the level gradually increased. Thus, we performed allogeneic hematopoietic stem cell transplantation (allo-HCT). After allo-HCT, he achieved stringent complete remission (sCR), which led to the complete resolution of his coagulation abnormalities. His vWF and factor VIII activities recovered to normal levels, the inhibitor of factor VIII disappeared, and his factor IX activity decreased to within the normal range. The patient's laboratory data are summarized in Table.
Figure.
(A) The multimer analysis of plasma vWF using an agarose gel. (B) The cross-mixing test showed an inhibitor pattern. The column numbers show the ratio of patient’s plasma to normal plasma. 1, 100 to 0; 2, 75 to 25; 3, 50 to 50; 4, 25 to 75; and 5, 0 to 100. (C) Bone marrow aspiration smears. An increased number of plasma cells was observed. (D) Computed tomography. The red circle indicates intramuscular hemorrhage. (E) Photograph of the left fundus. Dilation of branches of the central retinal vein with extensive hemorrhaging and edema are observed. vWF: von Willebrand factor
Table.
The Coagulation Data of the Patients at Each Time Point.
| At diagnosis | At intramuscular bleeding | At CRVO | After allo-HCT | Reference range | |
|---|---|---|---|---|---|
| IgA, mg/dL | 4,020 | 6,060 | 4,260 | 133 | 93-393 |
| APTT, seconds | 51.4 | 67.8 | 50.9 | 31.6 | 27.0-39.5 |
| Factor-II, % | 78 | 56 | 66 | 100 | 75-135 |
| Factor-V, % | 75 | 60 | 70 | 145 | 70-135 |
| Factor-VII, % | 59 | 50 | 66 | 100 | 75-140 |
| Factor-VIII, % | 25 | 17 | 27 | 95 | 60-150 |
| Factor-IX, % | ≥200 | ≥200 | ≥200 | 126 | 70-130 |
| Factor-X, % | 76 | 53 | 63 | 106 | 70-130 |
| Factor-XI, % | 45 | 24 | 39 | 95 | 75-145 |
| Factor-XII, % | 40 | 23 | 34 | 71 | 50-150 |
| Factor-XIII, % | 81 | 55 | 71 | 109 | 70-140 |
| vWF:Rco, % | 16 | 11 | 29 | 63 | 60-170 |
| vWF:Ag, % | 25 | 28 | 54 | 88 | 50-155 |
| F-VIII inhibitor, Bethesda | 0 | 1 | 0 | 0 | 0 |
| F-IX inhibitor, Bethesda | 0 | 0 | 0 | 0 | 0 |
CRVO: central retinal vein occlusion, allo-HCT: allogeneic hematopoietic cell transplantation, APTT: activated partial thromboplastin time, vWF: von Willebrand factor, vWF:Rco: von Willebrand factor ristocetin cofactor activity, vWF:Ag: antigen levels of von Willebrand factor, F-VIII: factor-VIII, F-IX: factor-IX
Discussion
B-cell malignancies associated with the production of abnormal immunoglobulin occasionally lead to the development of coagulation system impairment (1, 2). Elevation of serum factor VIII or vWF or acquired protein C resistance can cause thromboembolic complications. In contrast, auto-antibodies against factor VIII induce severe hemorrhage, a condition known as AHA. Additionally, plasma cell diseases are sometimes associated with acquired von Willebrand syndrome (AVWS), which leads to bleeding complications (4). Although the pathogeneses of the coagulopathies observed in plasma cell disorders are complicated, paraproteins play central roles. Several findings support this notion. Paraproteins can act as neutralizing antibodies against multiple coagulation factors. Furthermore, in certain cases, coagulation abnormalities resolve after the treatment of the underlying plasma cell disease (5, 6). In the present case, both hemorrhagic and thrombotic complications were observed during treatment for the underlying MM. Initially, the patient exhibited mucocutaneous bleeding associated with AVWS as well as increased factor IX activity. The initial treatment failed to control the MM, and the patient experienced intramuscular bleeding together with the emergence of auto-antibodies against factor VIII. Intensive treatment for MM was accompanied by a relative improvement of both AVWS and AHA; however, the increased activation of factor IX did not improve and he suffered from CRVO. CRVO is known to be associated with MM. In this context, CRVO mainly occurs due to hyperviscosity syndrome (8). In addition, various coagulation disorders can also trigger this complication (9). It is well accepted that elevated factor IX activity is a risk factor for thrombosis (10). Thus, we hypothesized that the marked factor IX activation observed in the present case might have at least partially caused the patient's CRVO. Interestingly, Wootla et al. reported that auto-antibodies against factor IX enhanced factor IX activity (11). Although we do not have definitive evidence, the aforementioned notions suggest that the paraproteins in the present patient might have been involved in the activation of factor IX. The finding that the factor IX activity returned to normal after allo-HCT supports this hypothesis.
Our present observations suggest that the paraproteins observed in plasma cell diseases could have multiple functions that may include causing both hemorrhagic and thromboembolic complications. Physicians should be aware that paraproteins are associated with the risk of both bleeding and thromboembolism.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Eby CS. Bleeding and thrombosis risks in plasma cell dyscrasias. Hematology Am Soc Hematol Educ Program 2007: 158-164, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Eby C. Pathogenesis and management of bleeding and thrombosis in plasma cell dyscrasias. Br J Haematol 145: 151-163, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Auwerda JJA, Sonneveld P, de Maat MPM, Leebeek FWG. Prothrombotic coagulation abnormalities in patients with paraprotein-producing B-cell disorders. Clin Lymphoma Myeloma 7: 462-466, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Dicke C, Schneppenheim S, Holstein K, et al. Distinct mechanisms account for acquired von Willebrand syndrome in plasma cell dyscrasias. Ann Hematol 95: 945-957, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Katagiri S, Akahane D, Amano K, Ohyashiki K. Long-term remission of acquired von Willebrand syndrome associated with multiple myeloma using bortezomib and dexamethasone therapy. Haemophilia 22: e557-e559, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Innao V, Allegra A, Morreale R, Russo S, Musolino C. Disappearance of acquired hemophilia A after complete remission in a multiple myeloma patient. Turk J Hematol 34: 184-185, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15: e538-e548, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Borgman CJ. Concomitant multiple myeloma spectrum diagnosis in a central retinal vein occlusion: a case report and review. Clin Exp Optom 99: 309-312, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Napal JJ, Neila S, Pérez-Montes R, Sierra I, Ruiz S, Hernández JL. The role of coagulation disorders in patients with retinal vein occlusion. QJM 109: 97-102, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Lowe GDO. Factor IX and thrombosis. Br J Haematol 115: 507-513, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Wootla B, Christophe OD, Mahendra A, et al. Proteolytic antibodies activate factor IX in patients with acquired hemophilia. Blood 117: 2257, 2011. [DOI] [PubMed] [Google Scholar]