Abstract
A 66-year-old man presented with subacute sensorimotor neuropathy in association with small cell lung cancer. Tests for the anti-ganglioside antibody GM1-IgM were positive. Chemotherapy and intravenous immunoglobulin treatment led to a slight improvement in neurological symptoms. Four additional cases of neuropathy accompanied by anti-ganglioside antibody and lung cancer have been reported. The most commonly reported pattern was subacute sensorimotor neuropathy. Patients died from cancer progression after 5 to 18 months. There is evidence that anti-ganglioside antibody inhibits tumor progression, prolonging the patient survival. However, severe neurological disturbance may offset the survival benefit of anti-ganglioside antibody in patients with paraneoplastic neurological syndrome.
Keywords: small cell, lung cancer, ganglioside, paraneoplastic, neuropathy, Guillain-Barré syndrome
Introduction
Paraneoplastic neurological syndromes (PNSs) are neurological disorders caused by the remote effects of a neoplasm rather than by a tumor or metastasis (1). PNSs are subclassified as classical or non-classical (2). Of syndromes affecting the peripheral nervous system, subacute sensory neuropathy and chronic gastrointestinal pseudo-obstruction are considered classical, whereas subacute sensorimotor neuropathy is non-classical syndrome.
In cancer cells, gangliosides are involved in several biological functions, including cell growth, invasion and angiogenesis (3, 4). Several types of gangliosides have been detected with high frequency in small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) samples (5, 6). Although anti-ganglioside antibodies are rarely detected in lung cancer patients, the incidence of detection increases under certain conditions. Of 29 patients with peripheral neuropathy and cancer without onconeural antibodies, 9 (31%) tested positive for anti-ganglioside antibodies (mainly anti-GM1 IgM) (7). On the recommendation of an international panel of neurologists, anti-ganglioside antibodies are not classified as onconeural antibodies (8).
Because the clinical features of neuropathies with anti-ganglioside antibody underlying lung cancer remain unknown, the accumulation of such cases is important to improve our understanding of the role of this antibody on PNS development. We herein report a case of subacute sensorimotor neuropathy with anti-ganglioside GM1 antibody concomitant with SCLC. In addition, we review the literature to assess the clinical features of PNSs with anti-ganglioside antibody associated with lung cancer.
Case Report
A 66-year-old man presented with progressive muscle weakness and numbness in the distal extremities without any proceeding infectious episode. His symptoms progressed and eventually resulted in difficulty walking within two months, at which time he was admitted to our hospital. His history was unremarkable, and he had received no medications. He had a history of smoking 2 packs of cigarettes per day for 46 years. He was mentally alert but exhibited motor disturbance (muscle weakness and atrophy), sensory disturbances (touch and pain), and urinary and fecal incontinence. He was unable to maintain a sitting position. Diminished deep tendon reflexes and abnormal reflexes (Babinski and Chaddock) were observed in the lower extremities.
Brain magnetic resonance imaging (MRI) results were normal. No swelling or enhancement in the spinal cord, cauda equine, or nerve roots was observed on cervical to lumbar spine MRI. Cerebrospinal fluid (CSF) exhibited a cell count of 19/μL (all mononuclear cells), a protein level of 116 mg/dL, a glucose level of 67 mg/dL, and negative cytology (lymphocytes only). An electrophysiological examination demonstrated slightly decreased motor nerve conduction velocities and an extended duration and decreased amplitude of compound muscle action potentials (CMAPs) (Table 1). Sensory action potentials were not evoked. The F waves of ulnar and tibial nerves were prolonged (31.9 and 58.0 ms, respectively). Thus, the neurological pattern was consistent with subacute sensorimotor neuropathy, which is not consistent with Guillain-Barré syndrome (GBS).
Table 1.
Nerve Conduction Findings.
| Nerve | Nerve conduction velocity (m/s) |
Compound muscle action potential | |
|---|---|---|---|
| Duration (ms) | Amplitude (mV) | ||
| Median nerve | 48.2 | 7.68 | 6.50 |
| Ulner nerve | 47.8 | 7.44 | 5.64 |
| Tibial nerve | 39.1 | 4.95 | 2.77 |
Sensory action potentials were not evoked.
Serological findings were negative for anti-nuclear, anti-SS-A, and anti-SS-B antibodies, and the thyroid function was normal. Tests for anti-onconeural antibodies against Hu, Yo, Ri, CV2, Ma2, and Amp were negative (Mayo Clinic Laboratories, Rochester, USA). Among anti-ganglioside antibodies, GM1-IgM was positive, while all others (GM2, GD1a, GD1b, GT1b, GQ1b) were negative (Kindai University, Osaka, Japan). Chest computed tomography revealed a small nodule in the right upper lung lobe (Figure A) and right hilar; the mediastinal and right subclavicular lymph nodes were also recognized (Figure B). Needle aspiration from the lymph node revealed small cell cancer. Distant metastasis was recognized in lumbar vertebra, without compression to the spine. The final diagnosis was SCLC [extensive disease, cT1N3M1b (OSS), stage IV] accompanied by possible PNS.
Figure.
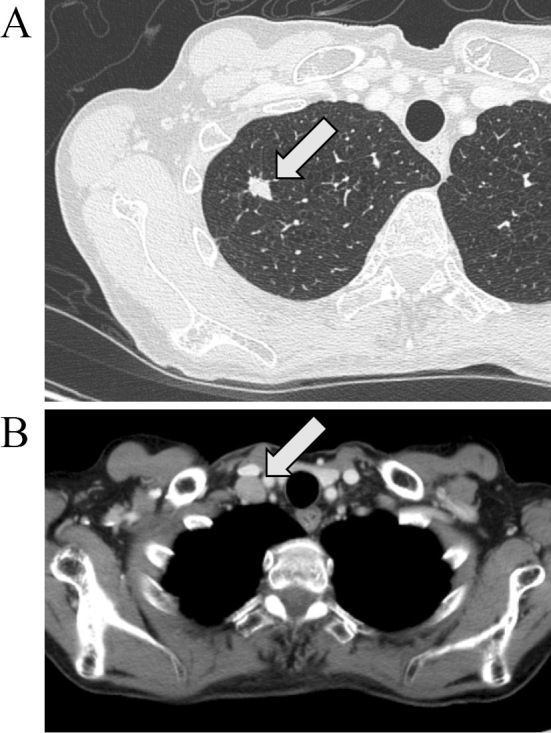
CT scans of a patient with subacute sensorimotor neuropathy accompanied by small cell lung cancer. (A) Primary lesion in the right upper lobe. (B) Enlarged subclavicular lymph node.
The patient received four cycles of chemotherapy with carboplatin and etoposide as well as two cycles of intravenous immunoglobulin (IVIg, 15 g each). Although chemotherapy elicited a partial response, the improvement in neurological symptoms was minimal, and the patient remained bedridden. Eight months later, progression of the disease was evidenced by bone metastasis. The patient received no additional treatment for cancer and neuropathy due to his generally poor condition. He ultimately died of cancer progression 18 months after receiving the diagnosis of cancer.
Discussion
To assess the clinical features of PNS with anti-ganglioside antibodies associated with lung cancer, we searched the literature using PubMed (https://www.ncbi.nlm.nih.gov/pubmed). We also evaluated some additional references from the retrieved articles. To our knowledge, five cases of PNS with anti-ganglioside antibodies associated with lung cancer, including the present case, have been reported (7, 9-11), as summarized in Table 2. Histology revealed that the lung cancer was SCLC in three patients and adenocarcinoma in one. All cases were peripheral polyneuropathies. Importantly, symptoms of neuropathy occurred prior to the cancer diagnosis in all described cases. The neuronal disturbances were sensorimotor in two cases and pure motor in two. Three of five patients showed subacute progression, while one exhibited chronic progression, and one showed acute progression. The characteristics of the CSF are described in four cases: three exhibited normal cellularity, and two showed normal protein levels. In all cases, anti-GM1 antibodies were detected, and two of them were IgM. Anti-GD1a was also detected in one case. Regarding treatment, corticosteroids elicited no response in two of two cases. IVIg elicited some response in two of four cases, and chemotherapy targeting the cancer resulted in temporary improvement. However, the patient prognosis was poor; all died of cancer progression between 5 and 18 months of the diagnosis.
Table 2.
Reported Cases of Neuropathy with Anti-ganglioside Antibody Associated with Lung Cancer.
| Case | Reference | Age, Sex | Histology | Progression | Neuropathy | Consistent with GBS | CSF, cell number, protein (mg/dL) | Antibodies | Treatment (improvement of neuropathy) | Prognosis, months from onset of cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 62, F | Small | Subacute | Sensorimotor | No | 5, 131 | GM1-IgM | Steroid (No), Chemotherapy (No), IVIg (Yes) | Died, 11 |
| 2 | 10 | 73, M | Adeno | Chronic | Motor, Axonal | No | Normal, Normal | IgG1 and IgG3 to GD1a and GM1 | Steroid & IVIg (No) | Died, 6 |
| 3 | 7 | 84, M | ND | Subacute | Axonal | No | ND | GM1 | ND | ND |
| 4 | 11 | 67, M | Small | Acute | Motor | Yes | 0, 25 | GM1-IgG | IVIg (No), chemotherapy (Temporary) | Died, 5 |
| 5 | Present case | 66, M | Small | Subacute | Sensorimotor, Urinary & fecal incontinence | No | 19, 116 | GM1-IgM | Chemotherapy & IVIg (Minimal) | Died, 18 |
GBS: Guillain-Barré syndrome, CSF: cerebrospinal fluid, IVIg: intravenous immunoglobulin, ND: not described
GBS is an immune-mediated polyneuropathy that is usually preceded by an infection, such as Campylobacter jejuni enteritis. GBS usually results in acute sensorimotor or motor neuropathy, and anti-ganglioside antibodies are detected in more than half of affected patients (12). An animal model of axonal GBS has been induced by sensitization with GM1 ganglioside (13). Thus, the role of ganglioside and anti-ganglioside antibody in the pathogenesis of GBS has been established. GBS is a non-classical PNS (2). In a large epidemiologic study, 9 out of 435 patients with GBS developed cancer in the 6 months preceding or following the GBS onset. Based on the expected number of malignancies, the calculated odds ratio of GBS incidence was 2.4, suggesting a possible correlation between some cases of GBS and cancer (14). To our knowledge, 12 cases with GBS or GB-like syndromes associated with lung cancer have been reported (11, 15-25). Interestingly, anti-ganglioside antibody was detected in only one case (11). Instead, anti-Hu antibody was detected in two cases (19, 24), and one presented with anti-CASPR2 antibody (22). In contrast, the present study revealed that three of five patients with anti-ganglioside antibody showed subacute progression (Table 1), while only one patient showed acute progression consistent with GBS (11). Anti-ganglioside antibodies were also detected in 5 of 41 healthy controls, albeit at a lower frequency than in patients with neuropathy and cancer (7). Antibodies against Fucosyl-GM1 were detected in healthy controls and at a similar frequency in SCLC patients (26). These findings suggest that anti-ganglioside antibodies might not be the cause of cancer-associated neuropathy.
It has been reported that patients with onconeural antibodies generally have better survival rates than those without onconeural antibodies, particularly in cases with anti-Hu antibodies (27, 28). Although the early detection of lung cancer has the potential to dramatically prolong the survival, some symptoms caused by PNSs, such as disturbed consciousness, may limit appropriate standard therapies for cancer, which may result in a reduced survival time (28). In the present case, chemotherapy was not reinitiated upon cancer relapse due to the poor general condition of the patient, which was attributed to PNS. Additional case studies are needed to determine how anti-ganglioside antibodies affect the prognosis of lung cancer. However, cumulative evidence shows that anti-ganglioside antibody inhibits tumor progression. The addition of monoclonal anti-GD2 antibodies resulted in the marked growth suppression and apoptosis of GD2-expressing SCLC cell lines (3). Similarly, anti-GM2 antibodies inhibited metastases of GM2-expressing SCLC cells and prolonged the survival of SCID mice (29). NSCLC patients with a high N-glycolylneuraminic acid (NeuGc)-containing ganglioside expression had a low overall survival rate (6). Furthermore, racotumomab-alum, an anti-idiotype vaccine targeting the NeuGcGM3 tumor-associated ganglioside, prolonged the survival in NSCLC patients (30). Although the role of anti-ganglioside antibodies in PNS pathogenesis remains unclear, they may inhibit the tumor progression and contribute to the long-term survival of patients with PNS. Severe neurological symptoms often worsen the overall condition and nutritional status of the patient and can induce secondary infections, thereby limiting cancer therapy. These conditions may offset the benefit of anti-ganglioside antibodies in the PNS patient survival.
In the present case, abnormal reflexes (Babinski and Chaddock) were positive. Although no abnormal findings in the brain and spine were detected by MRI, it is possible that anti-GM1 antibody affected the pyramidal tract, as in a previously reported case of GBS (31).
In conclusion, subacute sensorimotor neuropathy but not GBS is commonly characterized by the presence of anti-ganglioside antibodies and lung cancer. Additional studies are needed to clarify the role of anti-ganglioside antibodies in the PNS pathogenesis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Prof. Susumu Kusunoki and his colleagues (Kindai University, Faculty of Medicine, Osaka, Japan) for the measurement of anti-ganglioside antibodies.
References
- 1. Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 5: 197-223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2: 22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida S, Fukumoto S, Kawaguchi H, Sato S, Ueda R, Furukawa K. Ganglioside G(D2) in small cell lung cancer cell lines: enhancement of cell proliferation and mediation of apoptosis. Cancer Res 61: 4244-4252, 2001. [PubMed] [Google Scholar]
- 4. Liu Y, Wondimu A, Yan S, Bobb D, Ladisch S. Tumor gangliosides accelerate murine tumor angiogenesis. Angiogenesis 17: 563-571, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuentes R, Allman R, Mason MD. Ganglioside expression in lung cancer cell lines. Lung Cancer 18: 21-33, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi N, Chiba H, Kuronuma K, et al. Detection of N-glycolyated gangliosides in non-small-cell lung cancer using GMR8 monoclonal antibody. Cancer Sci 104: 43-47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antoine JC, Camdessanché JP, Ferraud K, Caudie C. Antiganglioside antibodies in paraneoplastic peripheral neuropathies. J Neurol Neurosurg Psychiatry 75: 1765-1767, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75: 1135-1140, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itou T, Enomoto S, Makita Y, et al. A patient of sensorimotor neuropathy with small cell lung carcinoma and anti-GM1 antibody. Rinsho Shinkeigaku (Clin Neurol) 42: 878-880, 2002(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10. De Toni L, Marconi S, Nardelli E, et al. Gangliosides act as onconeural antigens in paraneoplastic neuropathies. J Neuroimmunol 156: 178-187, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Watanuki S, Kinoshita K, Oda A, Kobayashi H, Satoh H, Tokuda Y. Occam's Razor or Hickam's dictum: a paraneoplastic or coincidental occurrence of lung cancer and Guillain-Barré syndrome. Intern Med 53: 1569-1573, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Naik GS, Meena AK, Reddy BAK, Mridula RK, Jabeen SA, Borgohain R. Anti-ganglioside antibodies profile in Guillain-Barré syndrome: Correlation with clinical features, electrophysiological pattern, and outcome. Neurol India 65: 1001-1005, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Yuki N, Yamada M, Koga M, et al. Animal model of axonal Guillain-Barré syndrome induced by sensitization with GM1 ganglioside. Ann Neurol 49: 712-720, 2001. [PubMed] [Google Scholar]
- 14. Vigliani MC, Magistrello M, Polo P, Mutani R, Chiò A; Piemonte and Valle d'Aosta Register for Guillain-Barré Syndrome Risk of cancer in patients with Guillain-Barré syndrome (GBS). A population-based study. J Neurol 251: 321-326, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Defanti CA, Brambilla A, Erli LC, Tredici G. Acute polyradiculoneuritis (Guillain-Barré) associated with the syndrome of inappropriate ADH secretion (Schwartz-Bartter): initial symptom of pulmonary neoplasia. Riv Neurobiol 30: 303-308, 1984(in Italian). [PubMed] [Google Scholar]
- 16. Klingon GH. The Guillain-Barré syndrome associated with cancer. Cancer 18: 157-163, 1965. [DOI] [PubMed] [Google Scholar]
- 17. Cicero G, Fulfaro F, Caraceni A, et al. A case of Guillain-Barré syndrome in a patient with non small cell lung cancer treated with chemotherapy. J Chemother 18: 325-327, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Nokura K, Nagamatsu M, Inagaki T, et al. Acute motor and sensory neuronopathy associated with small-cell lung cancer: a clinicopathological study. Neuropathology 26: 329-337, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Eimil M, Benito-León J. Guillain-Barré-like syndrome heralding small-cell lung cancer. Eur J Neurol 14: e15-e16, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Naveed S, Okoli K, Hollingsworth J, Kasmani R. Guillain-Barré syndrome as a paraneoplastic manifestation of small-cell carcinoma of lung. South Med J 103: 156-158, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Ferrufino E, Camarasa A, Chiner E. Guillain-Barre syndrome as an initial manifestation of small cell lung carcinoma. Arch Bronconeumol 47: 107-108, 2011(in Spanish). [DOI] [PubMed] [Google Scholar]
- 22. Tüzün E, Kinay D, Hacohen Y, Aysal F, Vincent A. Guillain-Barré-like syndrome associated with lung adenocarcinoma and CASPR2 antibodies. Muscle Nerve 48: 836-837, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Jung I, Gurzu S, Balasa R, et al. A coin-like peripheral small cell lung carcinoma associated with acute paraneoplastic axonal Guillain-Barré-like syndrome. Medicine (Baltimore) 94: e910, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakurai T, Wakida K, Kimura A, Inuzuka T, Nishida H. Anti-Hu antibody-positive paraneoplastic limbic encephalitis with acute motor sensory neuropathy resembling Guillain-Barré syndrome: a case study. Rinsho Shinkeigaku (Clin Neurol) 55: 921-925, 2015(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 25. Kim MH, Hwang MS, Park YK, et al. Paraneoplastic Guillain-Barré Syndrome in Small Cell Lung Cancer. Case Rep Oncol 8: 295-300, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adler G, Pacuszka T, Lewartowska A, Rowinska E, Oblakowski P, Panasiewicz M. Small cell lung cancer is not associated with the presence of anti-fucosyl-GM1 ganglioside autoantibodies reactive in immunoenzymatic test. Lung Cancer 34: 383-385, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Graus F, Dalmou J, Reñé R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 15: 2866-2872, 1997. [DOI] [PubMed] [Google Scholar]
- 28. Kanaji N. Prognosis of patients with paraneoplastic syndromes associated with lung cancer. Austin J Pulm Respir Med 1: id1006, 2014. [Google Scholar]
- 29. Yamada T, Bando H, Takeuchi S, et al. Genetically engineered humanized anti-ganglioside GM2 antibody against multiple organ metastasis produced by GM2-expressing small-cell lung cancer cells. Cancer Sci 102: 2157-2163, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Alfonso S, Valdés-Zayas A, Santiesteban ER, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 20: 3660-3671, 2014. [DOI] [PubMed] [Google Scholar]
- 31. Tomita M, Watanabe H, Morozumi S, et al. Pyramidal tract involvement in Guillain-Barré syndrome associated with anti-GM1 antibody. J Neurol Neurosurg Psychiatry 81: 583-585, 2010. [DOI] [PubMed] [Google Scholar]


