Letter to the Editor
Platelet factor 4 (PF4)/heparin and protamine/heparin (PRT/heparin) complexes elicit antigen-specific antibodies in 25–50% of heparin-exposed patients in certain clinical settings (Lee et al 2013; Bauer et al 1997). In recent studies (Khandelwal et al, 2016), we showed that antibody response to protein/heparin complexes may, in part, be derived from their complement-activating properties. These studies, performed with flow-based assays, showed that PF4/heparin ultra-large complexes (ULCs) added to blood of healthy donors, or generated in situ in patients receiving heparin, activate complement in a heparin-dependent manner leading to selective binding of antigen to B-cells via complement receptor 2/ CD21. These studies also showed that complement activation by PF4/heparin complexes occurs in plasma and not on the cell-surface.
Flow-based techniques, however, are cumbersome, costly and require technical expertise. To circumvent these limitations, we have developed a robust and simple two-stage functional capture immunoassay for detecting complement activation by PF4/heparin complexes. In this communication, we show that an antigen-C3 capture immunoassay can be used for studying mechanisms of immune activation, examining complement activating effects of variant heparins and investigating the therapeutic potential of complement inhibitors.
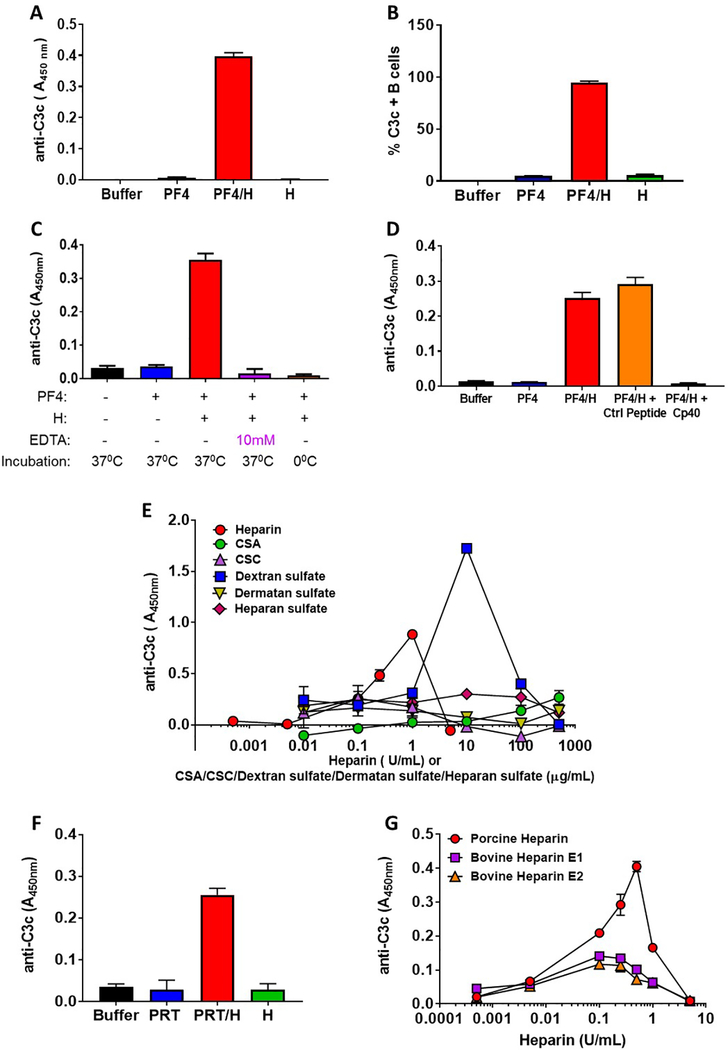
In this assay, plasma is first incubated with PF4/heparin complexes (25 μg/ml and 0.25 U/mL respectively; formed at a PF4:heparin molar ratio (PHR) of 6.6), or equivalent amounts of PF4 alone, heparin alone or buffer. After a one hour incubation complement-fixed antigen is captured by KKO, a PF4/heparin specific monoclonal antibody and complement fragments containing C3 are detected using a biotinylated anti-C3c antibody (Please refer to supplemental data section for detailed methods). As shown in Fig. 1A, this assay detects the activation of complement as indicated by bound C3c to captured PF4/heparin complexes, but not when plasma is incubated with equivalent amounts of PF4 alone, heparin alone or buffer. The degree of complement activation by PF4/heparin complexes seen in this assay relative to PF4 or heparin alone is comparable to findings using flow cytometry endpoints (Khandelwal, et al 2016) (Fig. 1B). In data not shown, we demonstrate that a polyclonal rabbit anti-PF4 antibody recognizing both PF4 and PF4/heparin complexes could substitute for KKO in the immunoassay, albeit with a higher background. To demonstrate requirements for an intact complement pathway, we inhibited complement by using EDTA, ice or the cyclic peptide complement inhibitor Cp40 (Zhang, et al 2015) and examined PF4/heparin-induced complement activation. As shown in Figs. 1C and 1D, C3 generation by PF4/heparin complexes is abrogated if complement is inhibited by EDTA, ice (0°C temperature) or the C3/C3b inhibitor, Cp40.
Figure 1:
(A) Antigen-C3 capture immunoassay detects complement activation by PF4/heparin complexes in plasma. Plasma from a healthy donor was incubated with buffer or antigen (PF4, 25μg/mL ± heparin 0.25 U/mL) or heparin alone (0.25 U/mL) and binding of C3c was determined by ELISA as described in supplemental methods. The bar graph shows the anti-C3c absorbance in different incubation conditions. (B) Flow-based method for complement activation by PF4/heparin complexes. Whole blood from a healthy donor was incubated with buffer or antigen (PF4, 25μg/mL ± heparin, 0.25 U/mL) or heparin alone (0.25 U/mL) and the binding of C3c to the B cells was determined by flow cytometry. The bar graph shows the % of C3c-positive B cells in different incubation conditions (C) Antigen-C3c immunoassay is sensitive to complement inhibition. Plasma from a healthy donor was incubated with buffer or antigen (PF4, 25 μg/mL ± heparin 0.25 U/mL) in presence or absence of 10 mM EDTA for 60 minutes at 37° C or 0°C and the binding of C3c to the complexes was determined by ELISA with a KKO coated plate. The bar graph shows the anti-C3c absorbance in different conditions. (D) Peptide C3 inhibitor Cp40 inhibits complement activation by PF4/heparin complexes. Plasma from a healthy donor was incubated with buffer or antigen (PF4, 25μg/mL ± heparin 0.25 U/mL) in the presence or absence of 5 μM Cp40 or Control (Ctrl) peptide and binding of C3c was determined by ELISA with a KKO coated plate. The bar graph shows the anti-C3c absorbance in different incubation conditions. (E) Complement activation by PF4-UFH and PF4/GAGs. Plasma from a healthy donor was incubated with fixed does of PF4 (25 μg/mL) and varying doses of UFH (0.0005–5.0 U/mL) or GAGs (0.01–500 μg/mL) and the binding of C3c to the PF4/heparin or PF4/GAGs complexes was determined by ELISA as described in supplemental methods. The graph shows the anti C3c absorbance at different concentrations of UFH or different GAGs. (F) Detection of complement activation by PRT/heparin complexes. Plasma from a healthy donor was incubated with buffer or PRT ( 100 μg/mL ) ± heparin (10 U/mL) or heparin alone (10 U/mL) and binding of C3c was determined by ELISA on an anti-PRT/heparin antibody (ADA) coated plate. The bar graph shows the anti C3c absorbance in different incubation conditions. (G) Comparison of complement activation by porcine and bovine (E1 and E2) UFH. Plasma from a healthy donor was incubated with fixed does of PF4 (25 μg/mL) and varying doses of porcine or bovine UFH (0.0005– 5 U/mL) and the binding of C3c to the PF4/heparin complexes was determined by ELISA with a KKO coated plate. The graph shows the anti C3c binding at different concentrations of porcine/bovine UFH.
The observation that Cp40 (Fig. 1D) significantly inhibits PF4/heparin triggered complement activation suggests a potential therapeutic role for these inhibitors in prevention of anti-PF4/heparing antibodies that trigger heparin induced thrombocytopenia (HIT) This strategy may be particularly effective for patients undergoing CPB, many of who develop anti-PF4/heparin antibodies after a one time exposure (Bauer, et al 1997).
Other applications of the antigen- C3 capture immunoassay include investigations of pathogenic mechanisms in HIT. Recent studies have shown that autoimmune HIT (Warkentin, et al 2014) arises from host reactivity to PF4/glycosaminoglycans (GAGs) complexes. To determine if PF4/GAG complexes are comparable to PF4/heparin complexes in eliciting complement activation, we used a fixed concentration of PF4 and variable doses of GAGs in the antigen-C3 immunoassay. As previously shown, we noted a bell-shaped response for complement activation using PF4/UFH complexes, with maximal C3c detection occurring at PF4:heparin molar ratios (PHRs) of 1.6. Not surprisingly, complexes of PF4 and dextran sulfate, a highly sulfated bacterial-derived glucan, showed robust complement activation (PHR 0.64). However, we noted minimal complement activation by PF4/GAG complexes. Addition of chondroitin sulfate A or C (CSA, CSC), dermatan sulfate, and/or heparan sulfate over a wide-range of concentrations to plasma containing PF4 did not generate C3c fragments (Fig. 1E). These findings are unlikely explained by lack of KKO binding to PF4/GAG complexes, as prior studies show adequate recognition of PF4/GAGs by KKO (Arepally, et al 2000). These striking differences in complement activation by PF4/heparin versus PF4/GAG complexes, are perhaps responsible for the relatively high incidence of anti-PF4/heparin antibody formation after heparin exposure (Bauer, et al 1997) as compared to the rare occurrence of autoimmune HIT (Warkentin, et al 2014).
To demonstrate the versatility of this assay for investigating complement activation by other heparin-binding proteins, we examined complement activation by PRT. Using a newly described monoclonal anti-PRT/heparin antibody (ADA), (Lee, et al 2017), we show that PRT/heparin ULCs, like PF4/heparin ULCs, activate complement, whereas PRT alone or heparin alone do not (Fig. 1F). These findings support previous observations from the 1970’s and 1980’s of shared properties of complement activation by a variety of polycationic/polyanionic compounds(Fiedel, et al 1976) and likely account for their in vivo immunogenicity (Chudasama, et al 2010, Lee, et al 2013) as well.
Other animal sources of heparin, such as bovine and ovine heparin, are being clinically developed due to concerns over vulnerability of the porcine heparin supply chain as the sole source of pharmaceutical heparin (Monakhova, et al 2018). As the antigen-C3 immunoassay can readily assess the complement-activating properties of variant heparins, we compared two bovine heparins (E1 and E2) with commercial porcine heparin. Using a fixed dose of PF4 (25 μg/mL) and equipotent concentrations of porcine or bovine UFHs (0.0005–5 U/mL), we showed differences in the complement activating profiles of the two bovine heparins relative to porcine heparin (Fig. 1G). Whereas low concentrations of porcine and bovine heparins were similar with respect to complement activation (concentrations 0.0005–0.005 U/mL), porcine heparin at concentrations of 0.1–1 U/mL, appeared to have stronger complement- activating effects. While additional in vivo studies are needed to correlate complement activation with immunogenicity, this assay is nonetheless useful for comparative investigations of biosimilar heparins.
In summary, we show the utility of a simple and rapid functional immunoassay for investigating complement-activating effects of PF4/heparin complexes. In addition to understanding fundamental mechanisms related to the immune pathogenesis of HIT, this assay will be helpful for facilitating studies of complement therapeutics and biologic characterization of generic and/or novel heparins under development.
Supplementary Material
Acknowledgements:
Supported by the National Institutes of Health P01 HL110860 (GMA) and AI030040 (JDL)
Footnotes
Conflict of Interest:
GMA has an awarded patent for KKO (US Application NO 60/143,536).
Competing interest statement
J.D. Lambris is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogs such as Cp40/AMY-101), inventor of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which are developed by Amyndas Pharmaceuticals. J.D. Lambris is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (i.e., 4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives such as APL-2).
The other authors declare no competing interest.
Addendum:
Conception and design: S. Khandelwal, G. Arepally
Provision of study materials: G. Arepally, S. Khandelwal, G. Lee, J Liu, D. Keire, C. Sommers, J Lambris, E. Reis
Collection and assembly of data: S. Khandelwal, A Johnson, J. Ravi
Data analysis and interpretation: G. Arepally, S. Khandelwal, G. Lee, J Liu, D. Keire, C. Sommers, A. Johnson, J. Ravi, J. Lambris, E. Reis
Manuscript writing: G. Arepally, S. Khandelwal, G. Lee
Final approval of manuscript: G. Arepally, S. Khandelwal, G. Lee, A. Johnson, J Liu, D. Keire, C. Sommers, J. Ravi, J. Lambris, E. Reis
FDA Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Presented in part at the 58th Annual meeting of American Society of Hematology at San Diego, CA, 2016.
CITING LITERATURE
- 1.Arepally GM, Kamei S, Park KS, Kamei K, Li ZQ, Liu W, Siegel DL, Kisiel W, Cines DB & Poncz M (2000) Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood, 95, 1533–1540. [PubMed] [Google Scholar]
- 2.Bauer TL, Arepally G, Konkle BA, Mestichelli B, Shapiro SS, Cines DB, Poncz M, McNulty S, Amiral J, Hauck WW, Edie RN & Mannion JD (1997) Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation, 95, 1242–1246. [DOI] [PubMed] [Google Scholar]
- 3.Chudasama SL, Espinasse B, Hwang F, Qi R, Joglekar M, Afonina G, Wiesner MR, Welsby IJ, Ortel TL & Arepally GM (2010) Heparin modifies the immunogenicity of positively charged proteins. Blood, 116, 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiedel BA, Rent R, Myhrman R & Gewurz H (1976) Complement activation by interaction of polyanions and polycations. Immunology, 30, 161–169. [PMC free article] [PubMed] [Google Scholar]
- 5.Khandelwal S, Lee GM, Hester CG, Poncz M, McKenzie S, Sachais BS, Rauova L, Kelsoe G, Cines DB, Frank M & Arepally GM (2016) The antigenic complex in HIT binds to B-cells via complement and complement receptor 2 (CD21). Blood, 128, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GM, Joglekar M, Kuchibhatla M, Khandelwal S, Qi R, Rauova L & Arepally GM (2017) Serologic characterization of anti-protamine/heparin and anti-PF4/heparin antibodies. Blood Advances, 1, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GM, Welsby IJ, Phillips-Bute B, Ortel TL & Arepally GM (2013) High incidence of antibodies to protamine and protamine/heparin complexes in patients undergoing cardiopulmonary bypass. Blood, 121, 2828–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monakhova YB, Diehl BWK & Fareed J (2018) Authentication of animal origin of heparin and low molecular weight heparin including ovine, porcine and bovine species using 1D NMR spectroscopy and chemometric tools. J Pharm Biomed Anal, 149, 114–119. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE, Basciano PA, Knopman J & Bernstein RA (2014) Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood, 123, 3651–3654. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Shao D, Ricklin D, Hilkin BM, Nester CM, Lambris JD & Smith RJ (2015) Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology, 220, 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.