Highlights
-
•
Enzymes are highly advantageous for consistent retting of flax aiming at composite applications.
-
•
Pectinase enzymes play a major role in releasing fibers from the bast.
-
•
Compromise between properties of enzymatically extracted fibers and composite performance.
-
•
Recovery of enzymes important in view of overall process economy.
Keywords: Flax fibers, Enzymatic extraction, Composites
Abstract
Enzymes are highly advantageous compared to dew retting to reach fibers of high and consistent quality. However, no unambiguous insights have been retained from the research, i.e. lacking a clear directive of which enzyme activities are strictly needed. Methods for evaluating enzymatic retting should be standardized, with characterization of chemical, morphological and mechanical properties and analysis of the ease of extraction. Moreover, evaluation should not only be focused on the microscopic level of the fiber but the performance of the resulting composite materials should be assessed as well. The review also covers research challenges for introducing enzymatic treatment in large scale production as well as inherent limitations and economic aspects. Besides their high selectivity and environmentally-friendly processing conditions, applying enzymes may also result in a less severe mechanical post-treatment implying less fiber damage. Moreover, recycling of enzymes and utilization of byproducts may increase the economic feasibility of the process.
1. Introduction
Fibers from flax (Linum usitatissimum) have a long history in the textile industry all over the world. Linen was one of the primary fibers for Europe during the years 1200 to 1700 and was used extensively for clothing [1]. The use of linen however dates back to 8000 BCE, where Egyptians used linen fabrics to mummify their nobility [2]. During recent years, flax and other natural plant-derived fibers such as hemp and bamboo have gained renewed interest. Due to their low density and high specific mechanical properties, bio-fibers can offer a worthy alternative to glass fibers as reinforcement for composites [[3], [4], [5]]. Not only their good mechanical properties but also their high availability, non-abrasiveness and cost-efficiency are a few other reasons that make fibers appealing for the use in composite materials [[6], [7], [8], [9]]. Unlike glass fibers, natural fibers are biodegradable and have a positive impact on CO2 emissions [8,10,11]. Furthermore, natural fibers are less damaging for health. All these benefits ensure an increase in the market potential of natural fibers for composite applications. Nevertheless, some problems still need to be resolved. Poor compatibility between fiber and matrix, problems related to moisture absorption and thermal stability pose some challenges for the near future in order to create robust high quality fibers.
Biochemically speaking, plant-based natural fibers consist mainly of cellulose, hemicellulose, lignin and pectin. The intrinsically high mechanical properties of natural fibers arise from the high amount of crystalline cellulose, oriented parallel to the fiber direction. For use in composites, the fibers need to be extracted from the plant, cleaned and aligned which can be realized through retting followed by a mechanical extraction procedure [5,6,12]. Traditional flax extractions start with a water or dew retting process. In case of water retting, harvested flax is submerged in running water or soaked in large tanks filled with water [13]. Micro-organisms present in water or plants will degrade pectins and hemicelluloses [13]. By water retting, fibers of good quality are obtained but high costs, water pollution and smell originating from fermentation products has made this method less attractive for large scale applications [[14], [15], [16]]. In dew retting, harvested flax is spread on the field in thin layers to promote faster drying and retting, which is carried out by fungi present in soil and on plants [13,14]. The retting process lasts several weeks, during which agricultural fields stay occupied. The quality of dew retted fibers is generally lower compared to water retted fibers, but the costs and water pollution are significantly reduced while a higher fiber yield is reached. Unfortunately, the efficiency of dew retting depends on geographical conditions. Regions with appropriate moisture and temperature conditions are essential for good microbial growth [13,14]. The dependency on weather according to regions, results in a variability in chemical [17] and mechanical properties of flax fibers and thus in inconsistency of the fiber quality, requiring the flax industry to mix the fibers to smooth out these variations.
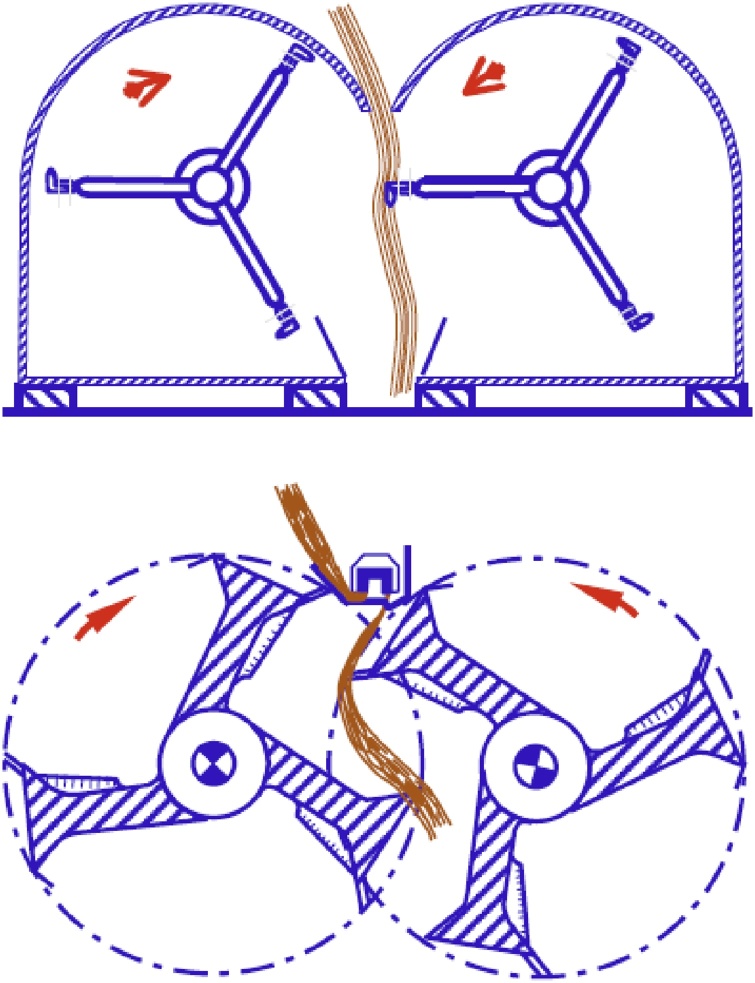
During mechanical extraction, the plant is subjected to a breaking process, followed by a series of cleaning steps (e.g. scutching (Fig. 1) and hackling). Scutching results in the removal of shives after the breaking step. Hackling refines and alignes the fibers by combing the bundles. During these extraction steps, fibers are not only cleaned and refined, but also aligned. The lengthy extraction and refining processes induce fiber damage and significantly increase fiber cost to levels higher than for E-glass fibers, jeopardizing the applicability in composites [12].
Fig. 1.
Examples of scutching turbines [12]. Reprinted with permission from Bos [12].
To improve the general applicability of natural fibers in composite materials, it is necessary to rethink the fiber extraction process aimed at composite applications, by minimizing variability and fiber damage. The development of a sustainable combined enzymatic and mechanical extraction approach could provide a solution to these problems. Enzymatic processes are characterized by their high selectivity, specificity and mild process conditions, while no chemicals are required. The main objective of this review consists therefore of providing general insights in the potential of enzymatic extraction methods for applying natural fibers in composites, while mapping potential hurdles still to overcome e.g. related to the evaluation of the fibers and composites strengthened with the extracted fibers but also towards the application of the extraction process on large scale and overall economy. Before describing the current status about enzymatic extraction of flax fibers, the structure and chemical composition of flax and enzyme mechanisms are discussed.
2. Structure and chemical composition of flax
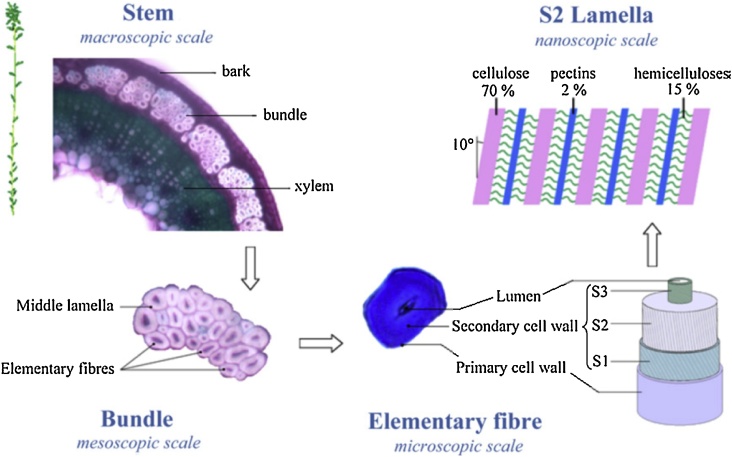
Natural fibers can be extracted from different parts of the plant, such as leaves, stems (bast fibers), fruits and seeds. Fig. 2 gives an illustration of the cross section of flax at different scales. Flax fibers are obtained from the bast and occur in bundles [5]. The fiber bundles consist of different elementary fibers which are held together in a network of lignin, hemicellulose and pectin [5,18]. Cell walls of elementary fibers exist of strongly oriented cellulose micro fibrils (in fact nano fibrils) with a high degree of polymerisation (6000–10000) and crystallinity (55–75 %) [[18], [19], [20], [21]]. The high cellulose content of flax fibers, containing amounts of 65 to 85%, combined with a good orientation of the micro fibrils, leads to the high mechanical properties of flax fibers.
Fig. 2.
Cross-section of flax at different scales [113]. Reprinted from Charlet et al. [113], copyright 2010, with permission from Elsevier.
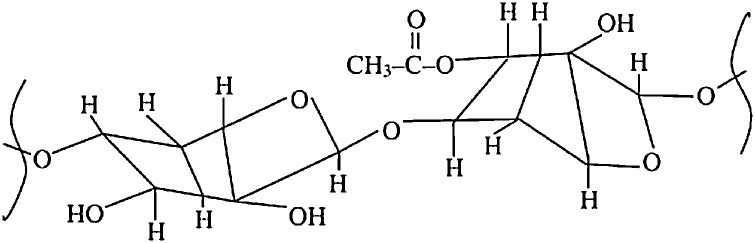
Cellulose is an unbranched homopolysaccharide composed of d-glucose units coupled with β-1,4 bonds (illustrated in Fig. 3) [21]. Each unit contains three hydroxyl groups, of utmost importance for hydrogen bond formation. These in their turn play an important role in the formation of the crystalline structure and determine the physical properties of cellulose [12]. The cellulose fibrils are embedded in a matrix of hemicellulose and lignin [5,9,21]. Next to crystalline cellulose, amorphous cellulose is also present in the surrounding matrix of the fibers.
Fig. 3.
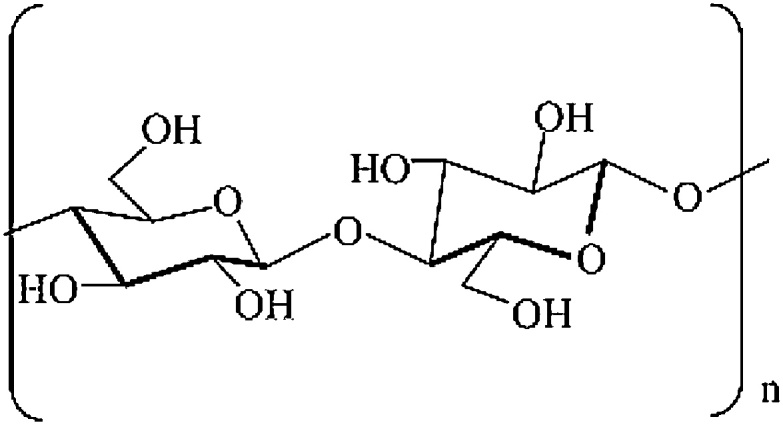
Cellulose structure [114]. Reprinted from Kabir et al. [114], copyright 2012, with permission from Elsevier.
Hemicellulose (Fig. 4) consists of polysaccharides composed of a combination of furanose and pyranose sugars like glucose, mannose, xylose, galactose, rhamnose and arabinose. In contrast to crystalline cellulose, hemicellulose is a branched polysaccharide exhibiting different side chains, resulting in a non-crystalline structure. Most common hemicellulose polysaccharides in plant species are xyloglucan and to a lesser extent arabinoxylan [22]. Hemicellulose forms the supporting matrix for the cellulose micro fibrils and is highly hydrophilic [21].
Fig. 4.
Hemicellulose structure [114]. Reprinted from Kabir et al. [114], copyright 2012, with permission from Elsevier.
Lignin, on the other hand, is a complex hydrophobic three-dimensional aromatic network which consists of p-hydroxyphenyl propane units and is illustrated in Fig. 5. Lignin has an amorphous structure and provides rigidity to the plant.
Fig. 5.
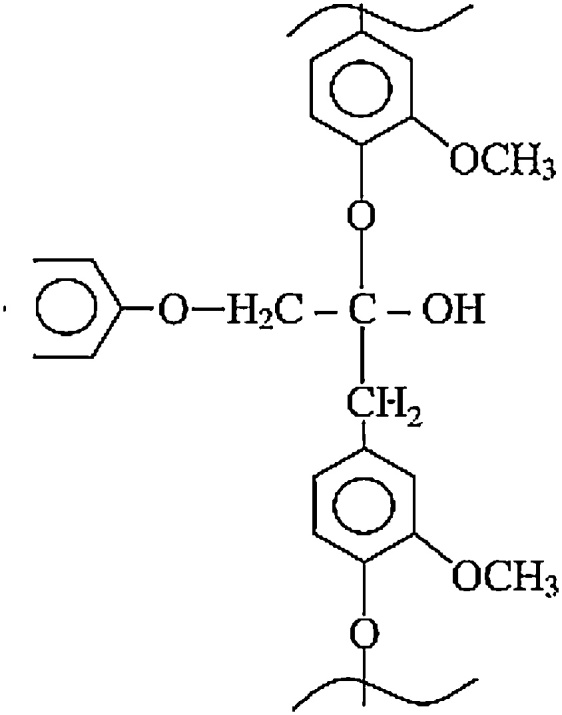
Lignin structure [114]. Reprinted from Kabir et al. [114], copyright 2012, with permission from Elsevier.
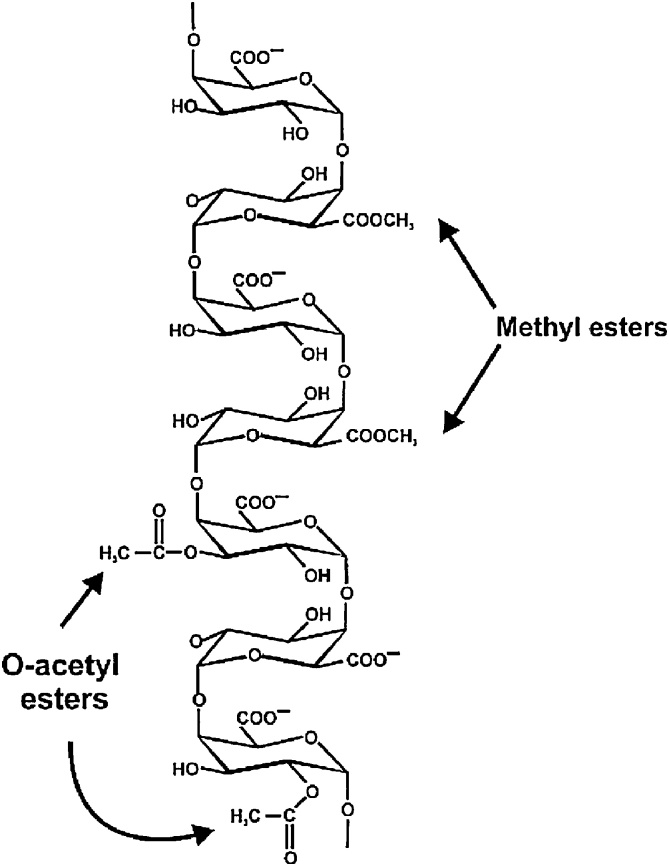
Besides cellulose, hemicellulose and lignin, pectins are also present in natural fibers [9,21]. Pectin is a hydrophilic polysaccharide with a complex branched structure and is composed of α-1,4 bonded d-galacturonic acids, sometimes coupled with l-rhamnose through β-1,2 bonds [18,23], known as rhamnogalacturonan I (RG I). Homogalacturonan, xylogalacturonan and rhamnogalacturonan II (more complex with 11 different sugar residues) are present as well in the cell wall [22]. Homogalacturonan with illustration of methylesters and O-acetyl esters is presented in Fig. 6. Pectin provides flexibility to the plant [21]. Inside the elementary fiber, amorphous cellulose, hemicellulose and pectin form the matrix between the crystalline micro fibrils; while on the surface of the elementary fiber, pectin and lignin are of primary importance.
Fig. 6.
Illustration homogalacturonan [118]. Reprinted from Ridley et al. [118], copyright 2001, with permission from Elsevier.
The chemical composition of flax has been described by several authors. It should however be mentioned that in the literature it is not always specified whether technical or elementary fibers were analyzed and whether they result from green or retted flax. Table 1 gives an overview of the chemical composition data found in literature. These data are supplemented with results from our KU Leuven Ghent Technology campus lab facilities. The gravimetrical method outlined by Bledzki et al. [24] and based on TAPPI T264 and NREL procedures [[25], [26], [27]], was used for characterization of green fibers (Amina), harvested by Verhalle in Belgium in 2015. This gravimetrical method is able to determine the content of cellulose, hemicellulose, lignin and extractives of dry flax fiber material. Pectin on the other hand is determined with m-hydroxydiphenyl reaction with galacturonic acid results in a pink chromogen, measured spectrophotometrically at 520 nm [[28], [29], [30]].
Table 1.
Chemical composition of flax fibers in literature and experimental data (in percent dry weight).
| Cellulose | Hemi-cellulose | Lignin | Pectin | Moisture | Wax | Extractives | References |
|---|---|---|---|---|---|---|---|
| 64.1 ± 3.1 | 16.1 ± 3.0 | 4.1 ± 0.4 | – | 8.1 ± 0.2 | – | – | [31] |
| 64.1 | 16.7 | 2.0 | 1.8 | 10 | 1.5 | – | [32] |
| 74 | 12 | 5 - 6 | – | – | – | 7 - 8 | [24] |
| 71 | 18.6 – 20.6 | 2.2 | – | – | 1.5 | – | [33] |
| 71 | 18.6 | 2.2 | 2.3 | 10 | 1.7 | – | [19] |
| 60 - 70 | 17 | 2 - 3 | 10 | – | 2 | – | [5] |
| 65 - 85 | 10 - 18 | 1 - 4 | 1 - 3 | – | – | – | [18] |
| 65 | 16 | 2.5 | – | – | – | – | [34] |
| 62 - 72 | 18.6 - 20.6 | 2 - 5 | 2.3 | 8 - 12 | 1.5 – 1.7 | – | [35] |
| 72.4 | 9.5 | 2.9 | 3.5 | – | – | 8.8 | [119] |
| 60 - 81 | 14 – 18.6 | 2 - 3 | 1.8 – 2.3 | – | – | – | [36] |
| 62.8 | 17.1 | 2.8 | 4.2 | – | 1.4 | – | [2] |
| 71.2 | 18.5 | 2.2 | 2 | – | 1.6 | 4.3 | [37] |
| 63.8 ± 1.7 | 13.3 ± 1.0 | 4.9 ± 1.2 | 6.1 ± 0.4 | 6.0 ± 0.3 | – | 12.0 ± 0.9 | This study |
Table 1 shows a cellulose content ranging from 60 to 85 w/w % for flax fibers. The hemicellulose content varies from 9.5 to 20.6 w/w %, while the lignin content is limited to 1–6 w/w % and pectin concentration ranges from 1 to 10 w/w %. A cellulose content of 63.8 ± 1.7 w/w % was determined for Amina green fibers in our lab facilities. Hemicellulose content amounted to 13.3 ± 1.0 w/w % while lignin had a weight percentage of 4.9 ± 1.2 w/w %. Pectin was present with a content of 6.1 ± 0.4 w/w %. The experimental data obtained fit well within the specified ranges available in literature.
The chemical composition of flax or flax fibers can also be determined by measuring the individual monosaccharides after hydrolysis of the polymers, using Gas Chromatography (GC). The prederivatisation step consists of a conversion of the monosaccharides to alditol acetates with sodium borohydride and acetic anhydride [38]. Akin et al. [39] determined the glucose content of unretted and dew retted Ariane flax fiber with the GC method. The glucose content of unretted fibers was 43.40 ± 1.83 w/w %, while in case of dew retted fiber, the content amounted to 64.95 ± 3.88 w/w % [39]. Retting leads thus to an increase in glucose (cellulose) content and hence to a purification of the cellulose fibers.
Characterization of the Amina green fibers in our lab facilities with Gas Chromatography resulted in a glucose content of 54.8 ± 1.2 w/w %. Comparison of the data from Akin et al. [39] with the experimental results show that the Amina fibers appear to have a higher glucose and thus cellulose content. A cellulose content of 43.4 w/w % is rather low and indicates a low purity of the fiber. The Gas Chromatography method results in lower fiber cellulose contents compared with the experimental data from the gravimetrical method (Table 1) for the same fiber sample. The method used for characterization of the chemical content and the associated discrepancy between two methods should be taken into careful consideration when comparing data from literature.
Knowledge about the fiber composition is crucial when impregnating fibers in composite materials, to understand which polymers are responsible for contributing or impairing the interfacial interaction between fiber and matrix but also to evaluate the extraction process. Degradation of specific polymers like hemicellulose and pectin can lead to a more efficient extraction of the fiber from the stem and can alter the fiber-matrix interface. Hence, enzymes are very valuable for specific and selective degradation of the aforementioned polysaccharides. Next paragraph will provide the necessary insights into the state-of-the-art knowledge about enzymatic treatment of flax fibers and the potential hurdles still to overcome.
3. Enzyme mechanisms and enzymatic treatments
3.1. Enzyme mechanisms
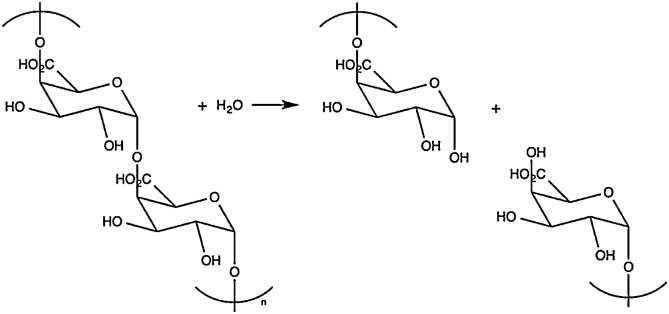
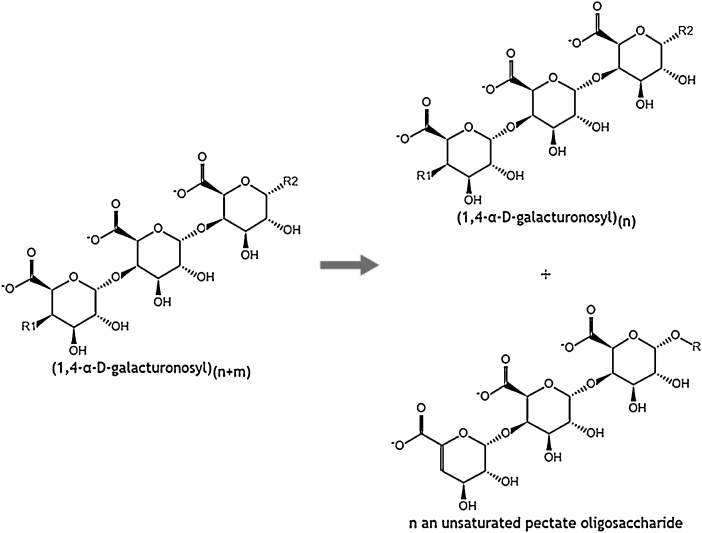
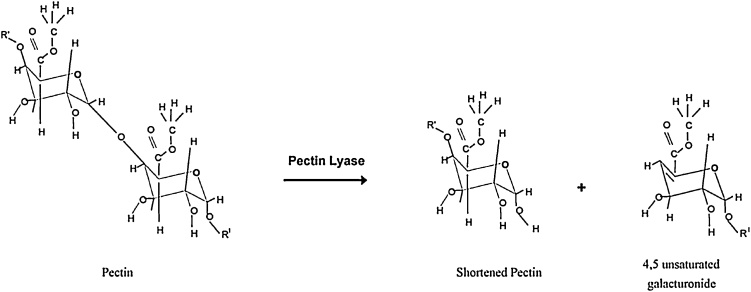
The main enzymes playing an essential role in the enzymatic extraction of natural fibers are pectinases, hemicellulases (mainly xylanases), laccases and cellulases [40]. Pectinases are used to hydrolyze pectin, resulting in the separation of fibers and non-fiber components [14,41]. Pectinmethylesterase (EC 3.1.1.11) removes the methyl groups to give access to the depolymerizing enzymes like polygalacturonase or pectate lyase. Polygalacturonase (EC 3.2.1.15) randomly hydrolyses α-1,4 galactosiduronic bonds in homogalacturonans (see Fig. 7) [42], while pectate lyase (EC 4.2.2.2) results in eliminative cleavage to give oligosaccharides with 4-deoxy-α-d-galact-4-enuronosyl groups at their non-reducing ends and α-d-glucuronic acid (Fig. 8) [43]. Deesterification of methyl groups is not necessary in case of pectin lyase (EC 4.2.2.10), an enzyme preferring high methyl esterified pectin as substrate (illustrated in Fig. 9).
Fig. 7.
Enzyme mechanism of endopolygalacturonase on homogalacturonan [42].
Fig. 8.
Enzyme mechanism of pectate lyase on pectate [115].
Fig. 9.
Enzyme mechanism of pectin lyase [120]. Reprinted from Yadav et al. [120], copyright 2009, with permission from Elsevier.
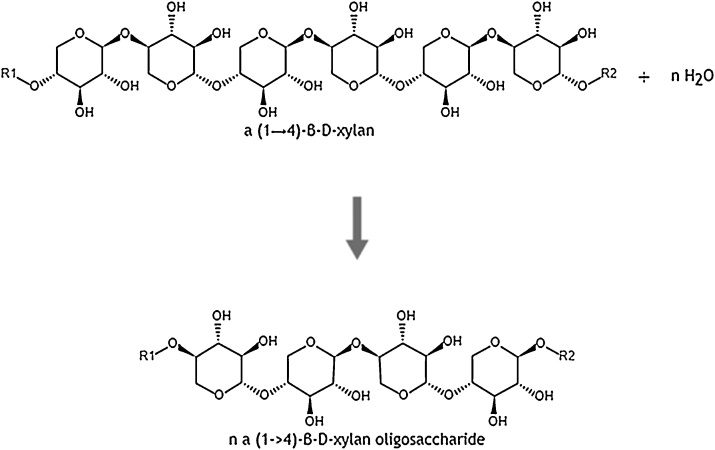
Xylanases on the other hand break down the hemicellulosic material around and in the fiber bundles by hydrolyzing the β-1,4-bonds in xylan chains, which causes a weaker structure [31]. The most important enzymes for degrading arabinoxylan are endo-1,4-β-xylanase and β-xylosidase. Endo-1,4-β-xylanase (EC 3.2.1.8) attacks the xylan backbone arbitrarily which forms xylo-oligosaccharides (Fig. 10). In contrast, β-xylosidase (EC 3.2.1.37) yields xylose by removing the terminal monosaccharide at the non-reducing end of the oligosaccharides (Fig. 11).
Fig. 10.
Enzyme mechanism of endoxylanase [116].
Fig. 11.
Enzyme mechanism of β-xylosidase [117].
Cellulases are responsible for degradation of cellulose polymers, which can occur in two structural forms. The majority of cellulose forms a crystalline structure wherein cellulases act upon the irregularities in the fibers (e.g. kinkbands), resulting in loss of fiber strength [14]. Cellulases hydrolyze the β-1,4-glucosidic bonds in the cellulose polymer. The activity of cellulases should not be too extensive in order to preserve the fiber strength. However, another structural form of cellulose present in the matrix is amorphous cellulose, of which the degradation may improve the extractability and lessen the moisture absorption of the fiber. Determination of the crystalline index of flax fiber resulted in 85% crystallinity and thus a percentage of 15% amorphous cellulose [44,45]. Cellulases degrade amorphous cellulose in the matrix before degrading crystalline cellulose, indicating the potential of the use of well-chosen amounts of cellulase in an enzyme formulation for extraction [46]. Another enzyme that can affect the extraction process of natural fibers is laccase, which is able to degrade the lignin structure in the matrix.
Integrating enzymatic extraction of fibers in the current processing of flax poses a major challenge for the near future. In the past, a lot of enzymes have been tested. However, the enzymatic extraction process is not yet implemented in industry. Hence, creating a comprehensive review of the state-of-the-art of enzymatic extraction of fibers is essential in order to provide an overview of recent developments and remaining problems still to overcome and to understand the role of the different enzymes in view of obtaining the desired fiber properties. Furthermore, insights will be given on up-scaling and cost related aspects.
3.2. Enzymatic treatments
3.2.1. Commercially available enzyme mixtures
Enzyme mixtures already developed for the enzymatic extraction of flax fibers are Flaxzyme, Ultrazym (both from Novozymes, Denmark) and SP 249 (Novozymes, USA), which are all pectinase-rich mixtures [14,[47], [48], [49], [50]]. Viscozyme L (Novozymes, USA) is a pectinase-rich multi-enzyme mixture, also containing cellulase, β-glucanase, hemicellulase, xylanase and arabinase and is similar to Flaxzyme. Novozymes (USA) also developed BioPrep 3000 L, which is a commercial pectate lyase derived from genetically modified Bacillus species [14]. Another enzyme, Lyvelin (Lyven, France), contains an endopolygalacturonase from Aspergillus niger. Other investigated enzymes originating from micro-organisms are PGase I (a polygalacturonase from Aspergillus niger), PGase II (a polygalacturonase from Rhizopus species) and EPG (an experimental endopolygalacturonase from Rhizopus oryzae) [14,51,52,23].
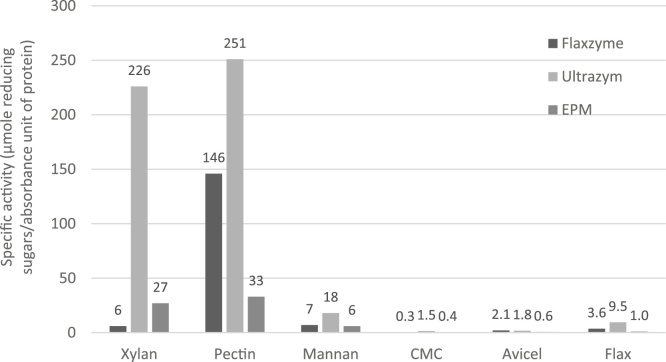
In 1997, Akin et al. investigated the effect of Flaxzyme (no longer available), Ultrazym (both Novo Nordisk, Denmark) and an enriched pectinase mixture (EPM) (Genencor International, USA) on the degradation of flax. For determination of enzyme activity, following substrates were applied: oat spelt xylan, citrus pectin, locust bean gum mannan, carboxymethyl cellulose (CMC), Avicel cellulose (microcrystalline cellulose) and ground flax. The largest enzyme activity was observed for the Ultrazym enzyme mixture (Fig. 12).
Fig. 12.
Enzyme activities of Flaxzyme, Ultrazym and EPM (Adapted from Akin et al. [52]).
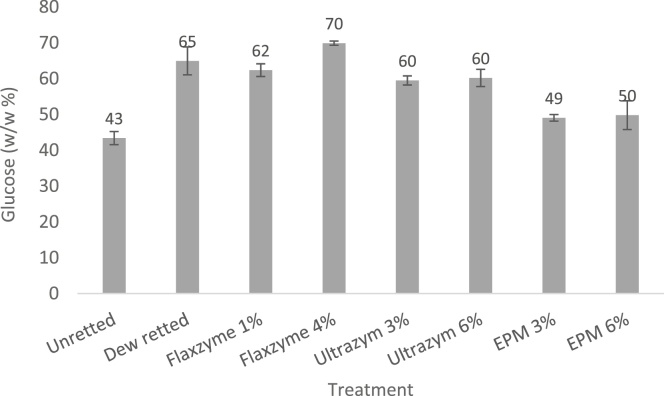
Fig. 12 shows that Ultrazym exhibits a higher activity for all substrates, with exception of Avicel [52]. Enzymatic treatments on flax stems were effectuated with 1 and 4 v/v % Flaxzyme, 3 and 6 w/v % Ultrazym and 3 and 6 v/v % EPM in 0.1 M acetate buffer (pH 5.0) during 24 h at 40 °C, with a liquid to fiber ratio (v/w) of 13:1. Glucose content of enzymatically treated flax fibers determined by Gas-Liquid Chromatography are illustrated in Fig. 13.
Fig. 13.
Glucose content in Ariane flax fiber before and after retting or enzymatic extraction (Adapted from Akin et al. [52]).
All enzymatically treated fibers as well as dew retted fibers exhibited an elevated glucose content, hence a purification of the fiber [52]. Treatment with Flaxzyme 4% resulted in the highest glucose content of 699.0 ± 5.7 mg/g. Results after enzymatic extraction showed that Flaxzyme treatment appeared to be more effective in separating fibers from the core [52]. The restricted extraction with EPM can be confirmed by the lower enzyme activities present (Fig. 12). Ultrazym on the other hand, showed the highest enzyme activities but was not able to result in a glucose level as high as the dew retted fiber. A possible explanation for the limited effect of Ultrazym is because the many different enzyme activities result in products that could start inhibiting each other. A clear insight in the individual role of each enzyme is essential, as well as the possible synergistic effects when combined. Also not unimportant is the ratio in which xylanases are present relative to pectinases. When equally dosed, enzyme mixtures tend to deliver inadequate retted fibers. However if pectinase enzymes are dosed with at least 10 times the xylanase concentration, retting will occur more smoothly. Commercially available enzyme mixtures specifically developed for retting of flax like Viscozyme or Flaxzyme, exhibit a xylanase fraction much smaller than the pectinase fraction. Nevertheless, enzymatic extractions can not be evaluated based on cellulose content alone. Next to chemical properties, morphological properties and mechanical characterization of fiber and resulting composite material are also important factors. Another parameter that should be taken into account is the amount of long fibers resulting after enzymatic extraction.
3.2.2. Use of chelator
A careful selection of enzymes and their dosages is crucial in order to obtain an efficient extraction process. Adding chelators to the enzyme formulation may also have a distinct effect due to their complexation characteristics with calcium. Since calcium is highly present in the epidermis, adding chelators can play an essential role in an improved removal of the epidermis and cuticle, which leads to easier degradation of the plant cell walls and thus a higher retting efficiency [[53], [54], [55]]. The use of a chelator could hence limit the necessary amount of enzyme [55]. In many studies, the retting efficiency is evaluated based on a visual grade system, the Fried Test, ranging from 0 to 3 or the Expanded Fried Test, ranging from 0 to 5 or 6, when going from no separation to a full separation [41,51,[55], [56], [57]]. However, Adamsen et al. [53,56] remarked that the removal of cuticle, related to quality, and the separation of bundles, related to fineness, are important characteristics that are not evaluated by the Fried Test. Hence, conclusions should not be based on Fried Test results alone [53] and other parameters of mechanical testing should also be taken into account in order to properly evaluate the treatment.
Adamsen et al. [3,56]) tested the effect of different concentrations of chelators, i.e. ethylenediaminetetraacetic acid (EDTA), oxalic acid, trisodium phosphate, sodium tripolyphosphate and sodium gluconate, on retting efficiency under alkaline conditions. Flax treatment with EDTA and sodium tripolyphosphate appeared to result in the highest retting efficiency based on Fried Test scores of 3.0 ± 0.0 and 2.8 ± 0.5, respectively. Adamsen et al. [56] also described that compared to EDTA, oxalic acid showed a higher affinity for calcium, but oxalate and calcium form an insoluble complex on flax fibers, which could lead to problems in the extraction process. EDTA appeared thus to be more effective than oxalic acid [56].
EDTA (15 mmole), sodium oxalate (30 mmole) and sodium citrate (30 mmole) were subsequently tested in combination with Flaxzyme (0.36%) at pH 5 and were evaluated with a Fried Test. Based on Fried test results, EDTA as chelator led to the highest increase in retting efficiency [55]. Flaxzyme was furthermore tested at varying concentrations and formulations: 0.3% Flaxzyme, 0.3% Flaxzyme + 50 mmole EDTA, 0.05% Flaxzyme + 50 mmole EDTA and 3.0% Flaxzyme [58], at 40 °C and pH 5 during 4 h. Fineness was measured by evaluating the micronaire (Mic) value of the fibers, originally used for the assessment of fineness and maturity of cotton fibers in textile industry, and is an indicator of air permeability [59]. Montalvo Jr. [59] found a direct relationship between micronaire and the product of fineness and maturity. Simplified, a low micronaire value matches a fine fiber. Fiber strength was measured with elongation by Stelometer and expressed in g/tex units, as force per unit density (tex is g per 1000 m). The unity g/tex is called tenacity as well and the higher the value, the stronger the fiber. Treatment with 0.05% Flaxzyme and 50 mmole EDTA resulted in fibers as fine (5.2 ± 0.1 Mic) as and stronger (18.2 ± 2.0 g/tex) as the fibers resulting from the treatment with 0.3% Flaxzyme (5.3 ± 0.1 Mic and 13.3 ± 1.7 g/tex) [58]. Addition of chelator can definitely lead to an improved formulation for extraction and is able to limit the required enzyme concentration.
Akin et al. [60] tested various concentrations of Viscozyme L (0.3 or 0.05 v/v %) in combination with EDTA (at 25 or 50 mM, pH 5.0), while using the Spray Enzymatic Retting (SER) method [61]. Crimped flax stems were incubated at 40 °C with the enzyme formulations during 24 h and results showed Viscozyme 0.05% in combination with 50 mM EDTA delivered the most optimal fibers. Lowering the concentration of the chelator and limiting the use of additional chemicals plays an important role in case of purification of enzyme solutions or cost reduction. This is why the addition of 25 mM EDTA was considered sufficient in order to create a beneficial formulation for retting flax [60]. Later research showed that with 18 mM EDTA similar results were observed as with 25 mM EDTA [62].
Akin et al. [62] tested varying enzyme concentrations of Viscozyme L (from 0.05 to 0.3%) with lower chelator levels of Mayoquest 200 (0.4 to 1.8%). Mayoquest 200 is a commercial product from Callaway Chemical Co. (Georgia) containing about 36 to 38% sodium EDTA with about 40% total dissolved salts (sodium hydroxide and sodium nitrilotriacetate) [56]. The chelator was used at levels of 0.4% (3.65 mM EDTA), 0.7% (7.3 mM) and 1.8% (18.25 mM). Mechanically disrupted flax stems were soaked with the enzyme formulation during 2 min and incubated during 24 h at 40 °C [62]. Fiber strength and fiber fineness were determined (results in Table 2) and results show that fiber strength decreased with increasing enzyme level, independent of chelator level. Fine fiber yield on the other hand was higher when more chelator was applied [62]. Fineness knows a minimal improvement at higher chelator level and higher enzyme level [62]. Depending on the final application and keeping the cost factor in mind, treatment with 0.05% Viscozyme and 1.8% Mayoquest 200 (18.25 mM EDTA) can be favoured as optimal treatment.
Table 2.
Overview of treatments in literature, with conditions and resulting fiber fineness and fiber strength [51,62,23].
| Reference | Formulation | Conditions | Fineness (Mic) | Strength (g/tex) | |
|---|---|---|---|---|---|
| [58] | A | 0.05 % Flaxzyme + 50 mmole EDTA | 40 °C, pH 5.0, 4 h | 5.2 ± 0.1 | 17.35 |
| B | 0.30% Flaxzyme + 50 mmole EDTA | 40 °C, pH 5.0, 4 h | 4.85 | 11.7 | |
| C | 0.30 % Flaxzyme | 40 °C, pH 5.0, 4 h | 5.2 | 12.85 | |
| D | 3.0 % Flaxzyme | 40 °C, pH 5.0, 4 h | 4.5 | 5.2 | |
| [60] | E | 0.30 % Vz + 50 mM EDTA | 40 °C, pH 5.0, 24 h | 3.9 ± 0.1 | 13.0 ± 1.3 |
| F | 0.30 % Vz + 25 mM EDTA | 40 °C, pH 5.0, 24 h | 4.6 ± 0.1 | 15.8 ± 1.8 | |
| G | 0.05 % Vz + 50 mM EDTA | 40 °C, pH 5.0, 24 h | 5.8 ± 0.1 | 24.0 ± 2.0 | |
| H | 0.05 % Vz + 25 mM EDTA | 40 °C, pH 5.0, 24 h | 5.7 ± 0.1 | 20.9 ± 1.3 | |
| [62] | 0 % Vz + 0 % Mayoquest | 40 °C, pH 5.0, 24 h | 8.0 ± 0.0 | 26.9 ± 0.8 | |
| 0.05 % Vz + 0.4 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.7 ± 0.1 | 24.0 ± 1.4 | ||
| 0.05 % Vz + 0.7 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.9 ± 0.0 | 23.9 ± 5.5 | ||
| 0.05 % Vz + 1.8 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.7 ± 0.1 | 24.6 ± 2.3 | ||
| 0.1 % Vz + 0.4 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.6 ± 0.1 | 20.3 ± 2.5 | ||
| 0.1 % Vz + 0.7 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.6 ± 0.1 | 17.9 ± 2.3 | ||
| 0.1 % Vz + 1.8 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.1 ± 0.0 | 20.3 ± 1.8 | ||
| 0.2 % Vz + 0.4 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.4 ± 0.1 | 18.1 ± 0.6 | ||
| 0.2 % Vz + 0.7 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.0 ± 0.5 | 17.6 ± 0.0 | ||
| 0.2 % Vz + 1.8 % Mayoquest | 40 °C, pH 5.0, 24 h | 6.9 ± 0.4 | 17.7 ± 1.4 | ||
| 0.3 % Vz + 0.4 % Mayoquest | 40 °C, pH 5.0, 24 h | 7.5 ± 0.7 | 15.3 ± 0.5 | ||
| 0.3 % Vz + 0.7 % Mayoquest | 40 °C, pH 5.0, 24 h | 6.9 ± 0.1 | 18.1 ± 1.3 | ||
| 0.3 % Vz + 1.8 % Mayoquest | 40 °C, pH 5.0, 24 h | 6.9 ± 0.0 | 19.5 ± 0.7 | ||
| [23] | I | PGase + 18 mM EDTA | 40 °C, pH 5.0, 20 h | 6.8 ± 0.7 | 40.6 ± 1.9 |
| J | 0.05 % Viscozyme + 18 mM EDTA | 40 °C, pH 5.0, 20 h | 7.1 ± 0.6 | 32.2 ± 2.2 | |
| [51] | K | EPG + 50 mmole oxalic acid | 40 °C, pH 6.0, 24 h | – | 23 ± 6 |
| L | SP249 + 50 mmole oxalic acid | 40 °C, pH 5.0, 24 h | – | 42 ± 11 | |
| M | EPG + 50 mmole EDTA | 40 °C, pH 6.0, 24 h | 7.7 | 41 ± 5.2 | |
| N | SP249 + 50 mmole EDTA | 40 °C, pH 5.0, 24 h | 7.5 | 40 ± 5.1 |
I: Polygalacturonase enzyme was obtained from Aspergillus niger and was used at an activity equal to 0.05% Viscozyme [23] K:.Treatment with EPG from Rhizopus oryzae was performed with an activity of 18.1 U/ml at a small scale (12 stems). L: SP249 treatment was performed with an equal activity as treatment G, on a small scale (12 stems). M: Treatment with EPG from Rhizopus oryzae was performed with an activity of 18.1 U/ml at a pilot scale of 50 g flax stems. N: SP249 treatment was performed at an equal activity as treatment I, at a pilot scale of 50 g flax stems. [51].
3.2.3. Polygalacturonase
Henriksson et al. [48] investigated the effect of enzymes originating from Rhizomucor pusillus and observed a similar retting efficiency on flax stems for the culture filtrate of R. pusillus in comparison with Flaxzyme (incubation in 50 mM sodium acetate buffer, pH 5.0, during 22 h at 50 °C). The resulting enzyme solution had a major pectinase activity, low pectin methylesterase activity and no pectin lyase activity. Cellulase activity was almost absent and no xylanase activity was observed in the filtrate [48]. This led to the conclusion that hemicellulases and cellulases may not be necessarily required to perform retting of flax [48]. The ignorance about which specific pectinases are required for efficient enzymatic retting was further investigated. Within this context, Zhang et al. [41] tested a purified Aspergillus niger polygalacturonase (Megazyme) and seven other commercial enzyme mixtures (Rohapect PL, PTE, DA6L, MA PLUS, D5L special and MPE (all from Röhm, Finland) and Pectinex (Novo Nordisk, Denmark)). Flax stems were treated in 50 mM ammonium acetate buffer containing 10 mM EDTA (pH 5.0) during 6.5 h at 27 °C. Except the A. niger PGase was incubated for 22 h at 40 °C in 50 mM ammonium acetate buffer (pH 4) with 6 mM EDTA. An Expanded Fried Test led to the conclusion that the only activity that was essential to perform retting was the degradation of low esterified pectin [41]. Zhang et al. [41] hypothesized that “degradation of the smooth regions (i.e. non-methylated polygalacturonic acid) in the middle lamella pectin is the most important step in enzymatic retting”. Earlier work from Sharma [50] already indicated that pectinase is indeed essential in the enzymatic extraction of flax fibers.
Subsequently, Evans et al. [23] characterized fibers treated with polygalacturonases of Aspergillus niger (PGase), Rhizopus spp. (PGase I and PGase II) and a polygalacturonase containing mixture Viscozyme L. Enzymes were dosed with the same level as endopolygalacturonase activity present in 0.05 v/v % Viscozyme L, containing 18 mM EDTA. Enzymatic treatments on crimped flax stems were effectuated during 20 h at 40 °C and pH 5.0. The highest fine fiber yields and strongest fibers were obtained by A. niger PGase (6.8 ± 0.7 Mic and 40.6 ± 1.9 g/tex) and Viscozyme L (7.1 ± 0.6 Mic and 32.2 ± 2.2 g/tex) [23]. Polygalacturonase enzymes again appear to be essential for the extraction of flax fibers from the stem [23], which confirms the hypothesis of Zhang et al. [41].
In another research published in 2002, Akin, Slomczynski, et al. [51], tested the retting of flax stems with a purified endopolygalacturonase (EPG) from Rhizopus oryzae, which was obtained earlier from dew retted flax. Treatment on small scale with 18.1 U/ml EPG in 50 mmole oxalic acid in morpholinethane sulphonic acid (MES) buffer (pH 6.0) was compared with SP 249 treatment (Novozymes) with an equal activity (0.05 v/v % + 50 mmole oxalic acid in 50 mmole acetate buffer, pH 5.0). The fibers retted with SP 249 on a small scale were significantly stronger than the fibers after treatment with EPG (42 ± 11 g/tex vs. 23 ± 6 g/tex) [51]. However, a larger scale test using an EPG formulation containing EDTA as chelator instead of oxalic acid resulted in fibers with strength (41 ± 5.2 g/tex) and fineness (7.7 Mic) properties similar to those retted with the commercial enzyme mixture SP 249 (40 ± 5.1 g/tex and 7.5 Mic) [51]. The difference in fiber strength after EPG treatment on small and large scale tests (23 ± 6 g/tex vs. 41 ± 5.2 g/tex) is noteworthy. The question raises if this difference is caused by the usage of EDTA at large scale instead of oxalic acid at small scale, which was already linked with possible problems due to the formation of insoluble complexes, depending on pH. The article, however, does not give any explanation for this dissimilarity between both EPG treatments. The potential of polygalacturonase is again confirmed, although EDTA as chelator plays a major role.
An overview of the discussed treatments, their conditions, fiber fineness and strength is illustrated in Table 2.
Table 2 enables to make a comparison between different researches and shows a remarkably broad range of fiber strength through the different studies. Taking into account both fiber fineness and fiber strength, Viscozyme 0.05% treatments (with addition of EDTA or Mayoquest) [60,62] and polygalacturonase treatments [23,51] can be considered as the treatments with the most potential.
Treatments H and J in Table 2 are both treatments with 0.05% Viscozyme and 18 to 25 mM EDTA, where a resulting fiber strength is obtained of 32,2 ± 2,2 and 20,9 ± 1,3 g/tex, respectively. These treatments also slightly differ in duration, but it is questionable that an increase of incubation time of 4 h is responsible for such a decrease in fiber strength. Another plausible explanation of the strength differences could be appointed to the inherent fiber strength depending on the cultivar of flax. However, in this case, both researches use the same ‘Ariane’ cultivar from South Carolina as fiber flax sample [23,60], raising even more questions about the divergent fiber strengths. A comparable treatment with 0.05% Viscozyme and 1.8% Mayoquest [62] (18.25 mM EDTA) resulted in fibers with a strength of 24.6 ± 2,3 g/tex and was also performed on the same flax cultivar. In this case, higher fiber widths were observed even though the incubation time was 24 h. The results might lead to the conclusion that comparable treatments carried out on the same flax cultivar can show more deviating results than expected. Question arises if differences in strengths are related to the processing itself or possible heterogeneity of flax samples.
3.2.4. Pectate lyase
Akin et al. [14] investigated an alkaline pectate lyase (BioPrep 3000 L) and made a comparative study with Viscozyme L and Lyvelin (an endopolygalacturonase). Flax was incubated at 50 °C (40 °C for Viscozyme) during 24 h after 2 min of soaking in enzyme formulations (control was soaked in water for 2 min). Data from treatments with PGase I (from Aspergillus niger) and PGase II (from Rhizopus spp.) (pH 5.0) were obtained from Evans et al. [23]. Other enzymatic treatments were effectuated at a non-defined higher pH, with the exception of Viscozyme (pH 5.0). The enzymatic treatments were evaluated based on fine fiber yield and fiber properties like strength and fineness (results in Table 3).
Table 3.
Yield and properties of flax enzymatically retted with different enzyme formulations [14]. Adapted from [14]. Copyright 2004. Reprinted by permission from Taylor & Francis Ltd.
| Treatment | Fine fiber yield (%) | Strength (g/tex) | Fineness (Mic) |
|---|---|---|---|
| Control | 13.5 ± 2.3 c | 43.6 ± 4.4 a | 8.0 ± 0.0 a |
| Viscozyme (0.05 %)a | 19.5 ± 3.4 ab | 33.3 ± 2.5 c | 7.1 ± 0.6 bc |
| Lyvelin (0.05%)a | 10.4 ± 0.7 c | 29.4 ± 3.0 d | 6.6 ± 0.2 c |
| Lyvelin (0.1%)a | 12.7 ± 1.1 c | 24.5 ± 1.0 e | 5.7 ± 0.2 d |
| BioPrep (0.05%)a | 17.1 ± 0.7 b | 41.0 ± 0.8 ab | 8.0 ± 0.0 a |
| BioPrep (0.01%)a | 13.4 ± 1.4 c | 39.9 ± 1.3 ab | 8.0 ± 0.0 a |
| BioPrep (0.05%) + STPPb | 18.1 ± 1.6 b | 39.7 ± 0.1 b | 7.7 ± 0.5 ab |
| PGase I (A. niger)a, c | 22.7 ± 2.5 a | 40.6 ± 1.9 ab | 6.8 ± 0.7 c |
| PGase II (Rhizopus spp.)a, c | 13.4 ± 2.8 c | 23.4 ± 1.5 e | 7.2 ± 0.3 bc |
a,b,c,d,e: values within columns with different letters differ at P < 0.05.
Mayoquest 200 was used to provide 20 mM EDTA.
Sodium tripolyphosphate (100 mM).
Data adapted from Evans et al. [23].
Results from Table 3 show that the finest fibers were obtained after treatment with Lyvelin, but the strength and fine fiber yield were much lower compared to the other enzymatic treatments [14]. BioPrep treatments resulted in almost no damage in fiber strength compared to the control. Fineness on the other hand did not improve in comparison with the other resulting fibers [14]. Treatment with PGase I resulted in the highest fine fiber yield and a strength as high as that of BioPrep treated fibers. Fineness was also slightly improved after PGase I treatment. In comparison with PGase I, PGase II contains more cellulase which leads to a larger reduction in fiber strength [14,23]. As well as PGase II, Viscozyme treatment, an enzyme mixture also containing cellulase activity, results in a higher decrease in strength compared to BioPrep and PGase I treatment. These results show that it should be considered very carefully whether the presence of cellulase in an enzyme formulation is beneficial. Again a big discrepancy in fiber strength and fine fiber yield is noticed between Viscozyme treatment (0.05%, 20 mM EDTA) from Table 3 and the comparable Viscozyme treatment (0.05%, 18.25 mM EDTA) from Table 2, even though similar conditions were used. Compared to the control treatment, enzymatic treatments result in improved fineness but no enhanced strength. Other research however found that enzymatic retted flax fibers were coarser but stronger compared to water retted flax fibers [63]. Treatment with PGase I from A. niger looks most promising with the highest fine fiber yield obtained and high mechanical strength. Pectate lyase (BioPrep 3000 L) also seems very promising.
The extraction at alkaline pH with BioPrep was also investigated by Akin et al. [54]. The commercial product BioPrep 3000 L containing alkaline pectate lyase (PaL) was originating from a genetically modified Bacillus lichiniformis [54]. The optimal concentration of pectate lyase in combination with EDTA from Mayoquest 200 was investigated, based on fine fiber yield and strength of retted fibers. Optimal conditions were pH 9 and 55 °C for PaL and pH 5 and 40 °C for Viscozyme L. Total duration time was 24 h. All PaL treatments resulted in less finer fibers with higher strength compared to fibers treated with 0.05 v/v % Viscozyme ( + 18 mM EDTA) (27.4 ± 6.0 g/tex and 3.9 ± 0.3 Mic). The results showed that it is not recommended to combine pectate lyase enzyme and chelator, which possibly induces some inactivation of PaL [54]. Treatment with EDTA results in removing the cuticle, possibly induced by the elimination of calcium bridges [54]. The literature showed that treatments with polygalacturonase or pectate lyase [14,54] can lead to better retted fibers when compared with Viscozyme treatments. It can imply that cellulase is not required in the retting enzyme mixture and that single pectinases can be successful in retting as such.
Pectate lyase was also studied by Alix et al. [64]. Alix et al. [64] investigated green Hermes flax from 2004 (H4V), dew retted flax (H4R) and flax treated with polygalacturonase (PG) from Lyvelin (Lyven) (H4V-PG, pH 5.0) and with pectate lyase (PaL) from Peclyve (Lyven) (H4V-PaL, pH 8.0). Unfortunately, no information was given about the incubation time of the enzymatic treatment or the applied temperature. Technical fibers were evaluated by determining failure strength (σf), failure strain (εf) and Young’s modulus (E) (see results in Table 4).
Table 4.
Tensile properties of technical fibers: green fibers (H4V), dew-retted fibers (H4R), fibers treated with polygalacturonase (H4V-PG) and fibers treated with pectate lyase (H4V-PaL) with σf = failure strength, εf = failure strain, E = Young’s modulus and Ø = diameter value [64]. Reprinted from [64], copyright 2012, with permission from Elsevier.
| H4V | H4R | H4V-PG | H4V-PaL | |
|---|---|---|---|---|
| σf (MPa) | 305 ± 120 | 310 ± 120 | 325 ± 115 | 470 ± 165 |
| εf (%) | 1.3 ± 0.4 | 1.1 ± 0.4 | 1.9 ± 0.9 | 1.4 ± 0.5 |
| E (GPa) | 31 ± 12 | 32 ± 12 | 22 ± 12 | 37 ± 15 |
| Ø (μm) | 135 ± 33 | 85 ± 20 | 95 ± 32 | 76 ± 16 |
Results in Table 4 show that flax treated with pectate lyase (pH 8.0) (H4V-PaL) presented the highest failure strength with a significant difference with the other flax samples, despite the large standard deviations [64]. H4V-PaL treatment also resulted in the finest fiber. Alix et al. [64] concluded that because of the good mechanical properties, “enough pectins remained in the fiber cell-junctions to transfer tensile stress to the elementary fibers within the divided bundle”. The lower failure strength and E-modulus for H4V-PG could be explained by extra glucanase activity in the Lyvelin enzyme mixture [64]. Almost no difference is observed between green retted (H4V) and dew retted fibers (H4R), except for a reduction in diameter value. No increase in strength or Young’s modulus is present, while normally an improvement should be notable. These results however are overall low results. The E-modulus of flax fibers should be in the range of 50–70 GPa and also strength results are very low. Unfortunately, there is no benchmark value but it could be worthwhile evaluating the test method, which could be the cause for the low strength.
3.2.5. Possible synergism between pectinases and other enzymes
Previously discussed studies show the potential of pectinase enzymes, more specifically of polygalacturonase and pectate lyase. Some enzymes with no promising effect on an individual basis can lead however to synergism when combined with other enzymes. Hence, it is essential to test individual enzymes and strategic combinations of different pure activities.
Akin et al. [51] investigated the combination of several enzymes with purified endopolygalacturonase (EPG) from Rhizopus oryzae and evaluated the effect on the basis of an Expanded Fried Test. Treatments were effectuated with 18.1 U/ml EPG in 50 mmole oxalic acid in morpholinethane sulfonic acid (MES) buffer (pH 6.0). The effect of adding pectin methyl esterase, pectin lyase, xylanase or endoglucanase to EPG was tested at an enzyme:EPG ratio of 1:30 U with flax segments incubated at 40 °C for 24 h. Adding pectin methyl esterase and pectin lyase, neither alone nor together, appeared to have an effect for improving retting efficiency based on the Expanded Fried Test [51]. Addition of xylanase or endoglucanase did not result in a more efficient enzymatic treatment either [14,51]. However, it should be noted that Fried Test Scores do not give a complete characterization of the properties and potential of the fibers. Other properties like for example fiber fineness and fiber strength should also be determined to properly evaluate the enzymatic treatments.
The final purpose of enzymatic treatments can differ between studies. Enzymatic treatments can be carried out on whole flax stem segments, in view of releasing fibers from the woody core to find an alternative extraction process for dew retting. In some studies, however, enzymatic treatments are performed on fibers already extracted, either manually or mechanically, in order to investigate the modification of certain properties of the fiber, for example the hydrophilicity, moisture absorption or thermal stability.
The company Inotex (Czech Republic) is working on enzymatic formulations for retting of flax fibers. Foulk et al. [57] investigated two enzyme mixtures from Inotex, Texazym BFE and Texazym DLG, along with Multifect Pectinase FE and Multifect Xylanase (both from Genencor International, New York). All enzyme mixtures were tested on manually extracted fibers during 24 h at their optimal conditions. Experiments at optimal conditions of the enzymes showed that based on the Fried Test, Texazym BFE resulted in a good retting efficiency in the presence of 18 mM EDTA. Optimal retting conditions of Texazym BFE were with a concentration of 2%, 18 mM EDTA and incubation at 60 °C, pH 7.5 during 24 h [57]. Decreasing temperature led to a decline in the retting efficiency of Texazym BFE. In case of Multifect Pectinase FE, best retting results were achieved with 0.2% enzyme, 18 mM EDTA at 45 °C (pH 3.9), whereas in the absence of the chelator, the enzyme was less effective [57] illustrating again the necessity of adding chelator to the enzyme formulation. Multifect Pectinase FE was also tested in combination with Multifect Xylanase. However, no improvement in retting degree was observed [57]. The usage of BioPrep (0.1%) in combination with EDTA (18 mM) as enzyme formulation (at pH 8.0 and 9.0) resulted in a good retting efficiency, strong fibers, high yields and the best cost ratios [57]. Hence, the alkaline pectate lyase from BioPrep emerges as another important pectinase enzyme for the enzymatic extraction of flax fibers. The results are however in contrast with earlier discussed results stipulating that using EDTA in combination with pectate lyase is not recommended.
3.2.6. Effect of enzymes on morphological properties
The mechanical properties of enzymatically treated fibers are also an essential aspect for evaluation and selection of the desired enzyme activities. Not to neglect are the chemical and morphological properties of the flax fibers, before and after enzymatic treatment. Changes in for example cellulose content can give a representative image of the purification degree of the fiber. George et al. [31] tested various enzymes from Novozymes (Table 5) and investigated the effect on the chemical and morphological properties of flax and hemp fibers.
Table 5.
Tested enzymes with their optimum pH and temperature with corresponding activity [31]. Reprinted from [31], copyright 2014, with permission from Elsevier.
| Enzyme | Optimum conditions |
Activity | |
|---|---|---|---|
| pH | Temperature (°C) | ||
| Xylanase | 7 | 70 | 1000 AXU/g |
| Xylanase (10% cellulase) | 6 | 50 | 2500 FXU-S/g |
| Polygalacturonase | 4 | 45 | 3800 PGNU/ml |
| Laccase | 7 | 50 | 1000 LAMU/g |
Treatments were effectuated on mechanically extracted fibers at optimal pH and optimal temperature of the given enzyme. Concentrations of 2, 6 and 10 w/v % enzyme were used with a 50:1 liquid to fiber ratio, while treatment lasted 90 min (with continuous shaking at 80 rpm) [31]. Table 6 shows the results of the chemical characterization of the untreated and treated hemp and flax fibers, i.e. the cellulose, hemicellulose, lignin and pectin content.
Table 6.
Chemical characterization of the treated hemp and flax fiber samples (adjusted data adapted from [65]). Reprinted by permission from [65], Springer Nature, copyright 2016.
| Fiber | System | Component (% dry weight) |
% Mass loss after treatment | |||||
|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Pectin | Protein | Ash | |||
| Hemp | Untreated | 67.77 | 12.14 | 7.24 | 3.95 | 6.32 | 2.31 | – |
| Xylanase | 70.07 | 8.23 | 7.34 | 1.51 | 9.72 | 3.12 | 4.12 | |
| Xylanase + cellulase | 64.29 | 13.21 | 7.25 | 2.36 | 9.42 | 3.47 | 10.58 | |
| Laccase | 70.42 | 11.36 | 7.12 | 1.49 | 7.36 | 2.25 | 3.22 | |
| Polygalacturonase | 75.12 | 6.98 | 5.55 | 0.53 | 8.09 | 3.72 | 8.17 | |
| Flax | Untreated | 64.08 | 16.10 | 4.14 | 7.18 | 6.18 | 1.53 | – |
| Xylanase | 69.40 | 12.41 | 3.85 | 3.40 | 8.16 | 2.78 | 8.31 | |
| Xylanase + cellulase | 68.07 | 10.85 | 5.22 | 2.99 | 10.13 | 2.74 | 19.44 | |
| Laccase | 70.04 | 14.83 | 1.61 | 3.53 | 7.76 | 2.23 | 9.91 | |
| Polygalacturonase | 80.26 | 3.34 | 1.87 | 1.36 | 9.92 | 3.25 | 23.21 | |
The results in Table 6 show a significant decrease in cellulose content after treatment with the xylanase + cellulase mixture, in case of hemp fibers [31,65]. The clear reduction in cellulose content confirms the possible damage cellulase enzymes can cause to the fiber and hence their strength. All other treatments on hemp and flax fibers result in an increase of cellulose content, hence a purification of the fiber. Treatment with xylanase and polygalacturonase resulted in a significant reduction in hemicellulose and pectin content. In case of flax fibers, lignin content declines after laccase treatment, but also polygalacturonase treatments results in degradation of the lignin network. George et al. [65] attributes the increase in protein and ash content to enzymes entrapped in the fiber network after treatment. The highest mass losses after treatment are observed for xylanase + cellulase treatments and polygalacturonase treatment on flax fibers. Morphological changes were observed by performing Scanning Electron Microscope (SEM) analysis. Results of the untreated and treated hemp fibers are illustrated in Fig. 14.
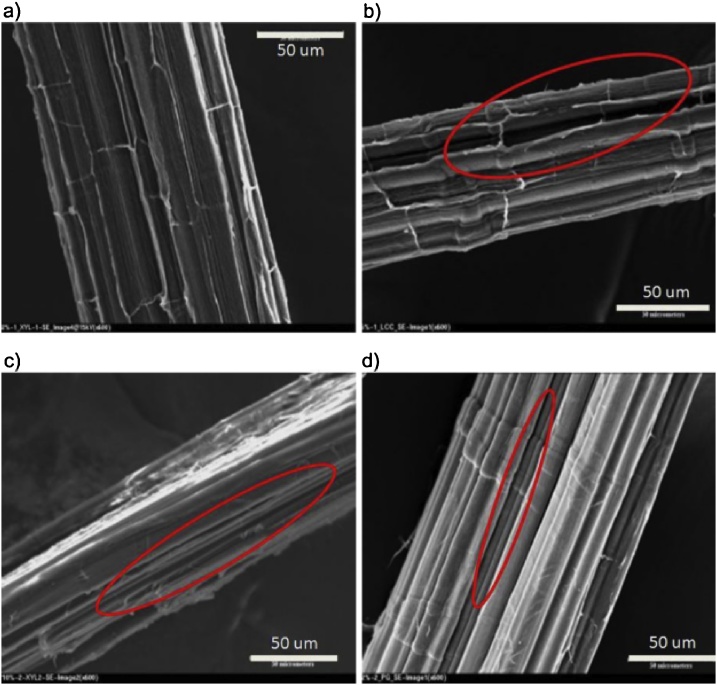
Fig. 14.
SEM images of hemp: (a) untreated, (b) laccase, (c) xylanase + cellulase and (d) polygalacturonase. The circles illustrate the defibrillation after treatment with enzymes [31]. Reprinted from George et al. [31], copyright 2014, with permission from Elsevier.
Fig. 14 represents the influence of the laccase (b), xylanase + cellulase (c) and polygalacturonase (d) treatment compared with the untreated hemp fiber (a). Enzymatic treatments clearly resulted in a defibrillation of the technical fibers and a removal of the impurities on the fiber surface. According to George et al. [31], “lignin removal exposes the fibers, xylanase degrades hemicellulose and forms cracks on the fiber surface while polygalacturonase removes material from the fiber surface”. The research shows that not only pectinases can contribute to the separation of fibers. Other enzymes like xylanase and laccase can play a significant role and can lead to synergism when applied together with pectinases. Hence, it is imperative to determine and evaluate chemical, morphological as well as mechanical properties after critically selected combinations of enzyme activities. It is important to note that enzymatic treatments have been effectuated on flax fibers already mechanically processed before treatment. Treatment on fibers instead of flax could imply an easier accessibility of the fiber for the enzyme to the surrounding matrix and impedes to make conclusions about the extractability of the fiber after enzymatic treatment of whole flax stems.
3.2.7. Pretreatments
Although the choice of enzymes in formulations is essential for extraction of flax fibers, pretreatments can also positively affect the extraction process. Akin et al. [66] tested the influence of pre-soaking flax in water but no unambiguous conclusion could be made. Henriksson et al. [55] and Foulk et al. [67] studied the effect of mechanical disrupting flax stems through fluted rollers (called crimping) and succeeded to improve absorption of enzyme formulations by the flax stems. The accessibility of enzymes in hemp fibers could also be considerably improved after a hydrothermal pre-treatment at 100 kPa and 121 °C, due to less parenchyma cell residues on the fiber surface and more void spaces between the fibers tin the stem structure [68]. Adding different treatment steps however requires the acquisition of adapted devices and can complicate and extend the entire extraction process. A comparative assessment should thus be carried out to see whether or not certain pretreatments have to be introduced into the extraction process of flax fibers.
3.2.8. Conclusion
The potential of pectinase enzymes has been illustrated, more specifically of polygalacturonase and pectate lyase. However, sometimes undefined side activities were present in the enzyme mixtures. Testing pure enzymes leads to insight into which activities are essential for the loosening, separation and extraction of flax fibers. Consequently, some enzymes show no promising effect on an individual basis but can lead however to synergism when combined with other enzymes. Therefore, it is essential to test individual enzymes and also strategic combinations of different pure activities. Enzymatic treatments are evaluated by characterization of chemical, morphological and mechanical properties, for example cellulose content, fiber fineness and fiber strength. In order to select the optimal treatment, a consensus needs to be reached concerning the most significant mechanical properties. This decision is mainly depending on the future application of the treated fibers. In case of textile applications for apparel, the yield and fineness of fibers can be predominant compared to the strength of these fibers. With textiles, the strength of the yarns, which is determined by the twist during the spinning process, is more important than the strength of the fiber itself. As reinforcement for composite applications on the other hand, the strength of the individual fiber is crucial for the resulting strength of the composite materials. Yield is also important because this factor influences the final cost, while fineness of the fibers may become important for a good interface between fiber and matrix. Furthermore, a coarse fiber can have lower transversal properties due to internal failure. Hence, assessment of the effect of enzymatic treatments on the performance of the resulting composite materials by standardized protocols is another essential aspect in the evaluation of enzymatic treatments.
4. Composite performance
The effect of enzymatic treatments on fiber properties, chemical, mechanical as well as morphological, are important to comprehend but the influence on the performance of the final application, namely composite materials based on the enzymatically treated natural fibers, should definitely be taken into account. To assess the performance of the composite materials reinforced with enzymatically treated natural fibers, characterization of interfacial strength and transversal and longitudinal properties is needed. As will become clear in next paragraphs, the evaluation of the different properties is not always standardized, making comparison of data less straightforward.
4.1. Parameters affecting composite performance
Composite performance is affected by fiber quality, matrix selection, interface strength, fiber dispersion, fiber orientation, manufacturing and porosity [69]. First of all, fiber quality includes fineness, elongation at break, tensile stiffness and strength and purity of the fiber [70,71]. Properties of the chosen matrix are also defining composite characteristics. Polypropylene (PP), polyethylene (PE), polystyrene (PS) and polyvinyl chloride (PVC) are potential thermoplastic matrices. However, thermoset matrices like polyester, epoxy, phenol formaldehyde and vinyl esters are more frequently used for natural fiber reinforced composites [33,72]. Epoxy excels due to good adhesion and mechanical properties, low moisture content, limited cure shrinkage and processing ease [33]. Research into alternatives for the petrochemical based matrices recently led to the introduction of bio-based matrices like polylactic acid (PLA), polyhydroxybutyrate (PHB) and starch [33,72,73]. Interface strength is extremely important for transferring load between matrix and fibers and can be realized through mechanical interlocking, electrostatic, chemical and inter-diffusion bonding [69]. Incompatibility between hydrophobic matrices and hydrophilic natural fibers is one of the biggest challenges to overcome. Fiber dispersion is dependent on compatibility. Proper moistening of the fiber during impregnation will result in good fiber dispersion. Fiber volume fraction is another predominant factor. Strength and stiffness of a composite material increases with increasing fiber volume fraction and strength is optimal at 40–55 w/w % [69]. Subsequently, the orientation of the fiber in the composite material is also important since fibers have anisotropic properties [11]. Fibers give high stiffness and strength to the composite material in the orientation of their alignment [74].
Depending on the final application of the composite, the applied load will determine whether to construct a unidirectional composite, a woven or a non-woven material. Hence, the composite production itself is another essential step in the realization of a high performing composite. The formation of porosities is a known problem occurring when poor impregnation during composite processing takes place [75]. According to Madsen et al. [76] four types of porosity exist in natural fiber reinforced thermoset composites: fiber porosity in airfilled cavities in the fibers, interface porosity due to poor fiber matrix compatibility, impregnation porosity inside fiber bundles caused by poor impregnation, and matrix porosity arising by gas bubbles present in the resin during impregnation. Some porosity phenomena can be limited by careful consideration of the processing method for composite production.
4.2. Effect of enzymatic treatment on composite performance
The effect of enzymatic treatments on composite performance is investigated by only a very few research groups although it is essential to evaluate the behavior of enzymatically treated fibers in the final application. Both the effect of enzymes on the fibers itself as well as on final composites should be investigated. Hence, a more structured research approach is necessary.
The effect of water, EDTA, enzyme treatment with Pectinex AR and a combination of the latter two was investigated by Stuart et al. [77]. Pectinex AR from Novo Nordisk contained 747 U/ml xylanase, 1170 U/ml polygalacturonase and 91 U/ml cellulase [77]. Enzyme treatment was effectuated on flax fibers with 0.5 v/v % Pectinex AR at 40 °C and pH 4.5 during 2 h. Treatment with EDTA was performed with 17 mM EDTA (pH 11.0) at 60 °C for 3 h. Combination of the latter two was effectuated in two ways. A first combination consisted of an enzyme formulation containing 17 mM EDTA (pH 4.5) containing 0.5 v/v % Pectinex AR and treatment was carried out during 3 h at 40 °C. Another treatment was completed by performing the EDTA treatment (pH 11.0), followed by washing, drying and the individual enzyme treatment (pH 4.5). Individual fibers showed no significant difference in Young’s modulus but did show a decrease in tensile strength compared to the untreated fibers [77]. During these tests however, a gauge length of 25 mm was used while fibers have lengths of 5 to 50 mm, with an average of about 25 mm. When the fibers are shorter than the gauge length, the tensile strength will be determined by the adhesion between the elementary fibers. Almost every enzymatic treatment will lower this adhesion, resulting in decreased fiber strength even though fibers do not break. Hence, when the gauge length is high, there is more chance of measuring the fiber-fiber interface instead of the tensile strength of the fiber [78]. The characterization of tensile strength of flax fibers however is still very challenging, leading to a large variation in tensile properties found in literature. Tensile properties of fibers are scattered due to different methods of determination and the absence of a uniform cross-sectional area, next to the natural variation each flax sample has [79]. Not only tensile properties are scattered, other properties of natural fibers are also highly variable. This distribution in properties can be ascribed to differences in the fiber cultivar itself (composition and structure), influence of different testing methods and testing of fibers on different scales (fiber bundles or elementary fibers) [80].
In a composite material, applied load between elementary fibers is transferred through the matrix. The strength of the composite will be determined by the tensile strength of the elementary fibers till break. The composite strength is dependent on the adhesion as well, but it is definitely not the only factor. Next to the tensile strength of the elementary fiber and the adhesion between fiber and matrix, the accessibility of the fiber for the matrix is also a determinative factor. Cleaner and finer fibers are hence more accessible for the matrix, thus improving the composite strength. Stuart et al. [77] used a wet forming technique to produce reinforcement mats with 2 mm fiber segments. Three fiber mats were then used to make a composite with epoxy resin as matrix, covered with glass plates at top and bottom. When looking at the tensile properties of the resultant non-woven laminate composites, the untreated fiber composite appeared to have the lowest tensile strength while the other composites exhibited an improvement, especially the composite with EDTA treated fibers, assuming an enhanced adhesion between fiber and matrix [77]. Compared to these EDTA treated fibers, other treatments resulted in a lower tensile strength of the composite, even though an improvement was still observed compared to the composite with untreated fiber. Even though fibers after enzyme and combined treatments were cleaner and more separated, the mechanical properties of the composites with enzyme and combined EDTA and enzyme treated fibers were inferior and could be explained by the degrading cellulase side-activity, attacking the cellulose structure and thus impairing strength [77].
Saleem et al. [81] studied the effect of polygalacturonase treatment on the mechanical characteristics of reinforced thermoplastic composites and the influence of applying maleic anhydride as compatibilizer. Hemp fibers were treated with 8 v/v % SIHA-Panzym DF (a polygalacturonase from Novozymes) during 4 h at 35 °C and pH 4.5 with 10 mM EDTA. Pectinase treatment resulted in a reduction of the cross-sectional area and decreased tensile strength of the fibers [81]. However, when impregnating these dried fibers in polypropylene, longitudinal as well as transverse properties all increased slightly in the absence of compatibilizer but even more in its presence, due to the covalent and cohesive coupling of cellulose hydroxyl groups to the maleic anhydride grafted polypropylene matrix [81]. It can be hypothesized that the polymer is capable of reconnecting elements of fiber which were disconnected by the enzymatic treatment.
These results [77,81] confirm that the effect of enzymatic treatments should not be evaluated on the mechanical properties of the treated fiber alone but the mechanical characterization of the resultant composite materials should be included. An increased composite strength, even though the composite was impregnated with fibers with an inferior strength, was also observed by Hendrickx et al. [71] by comparing fiber properties with back-calculated properties from composite tests. No correlation was observed between technical fiber stiffness and composite stiffness, probably because the composite behavior is dominated by the elementary fibers instead of the technical fibers [71]. Fiber bundles have inferior properties compared to elementary fibers, due to shearing between fibers in a bundle [80]. So even though fiber properties are less promising, composite performance can be superior due to reconnection of disconnected elements of the technical fiber. This will be further enhanced by improved fiber matrix interface adhesion or improved accessibility of the fiber. This hypothesis was also confirmed by Acera Fernández et al. [75]. Shah et al. [82] investigated the significant differences observed between measured and back-calculated properties of natural fibers, resulting in the questioning of the validity of the rule-of-mixtures for natural fiber reinforced composites. The rule-of-mixture assumes a perfect fiber-matrix interface and uniform fiber properties [82]. According to Shah et al. [82], error in fiber cross-section area, strain range in which stiffness is determined, differences in elementary and fiber bundle properties and the gauge length at which fiber tests are carried out, all contribute to errors and significant differences between measured and back-calculated properties. This indicates the importance of the characterization of the mechanical properties of the composite material.
In 2011, Foulk et al. investigated the influence of the time of an enzymatic treatment on the composite performance. Four different retting degrees were used, namely no retting (0 h), minimal retting (10 h), moderate retting (22 h) and full retting (46 h). Treatments were performed on mechanically extracted flax fiber bundles with a bacterial pectate lyase at pH 8.5 and 42 °C [70]. Composite materials were produced with a vinyl ester resin, but the orientation of the fibers was not mentioned. To test the interfacial strength between the fiber and the matrix, pullout tests as well as Interlaminar Shear Strength (ILSS) tests were executed. Tensile tests were carried out in order to determine the tensile modulus and tensile strength of the composite material. Table 7 illustrates the results of the research of Foulk et al. [70].
Table 7.
Interfacial and tensile strengths of enzymatically treated flax fiber composites [70]. Reprinted with permission from [70].
| Enzyme exposure (h) | Fiber volume fraction (%) | Pullout (MPa) | Specific ILSS (MPa) | Specific tensile modulus GPa/(g/cm³) | Specific tensile strength MPa/(g/cm³) |
|---|---|---|---|---|---|
| 0 | 38 | 12.21 b | 9.45 b | 2.34 a | 54.66 b |
| 10 | 39 | 17.07 a | 11.32 a | 2.07 a | 48.54 b |
| 22 | 34 | 15.74 a | 9.57 b | 2.18 a | 71.46 a |
| 46 | 37 | 12.53 b | 10.36 b | 2.76 a | 70.91 a |
*Values followed by different letters within columns are significantly different at P ≤ 0.05.
First of all, based on the low tensile strengths it seems that the composites are probably impregnated with randomly orientated fibers, even though this information is not given in the article. There is also no information available about the dispersion quality of the fibers in the composite or the fiber length used to produce the composites. The used approach however is promising since pullout tests are done to determine interface strength; ILSS tests give information about the interface strength and the influence of the impregnation quality, i.e. if porosities are present, and tensile tests determine tensile strength, interface strength and impregnation quality. However, the tensile tests are mainly relevant for unidirectional composites.
Looking at the interfacial bonding test results, Foulk et al. [70] could not deduce a correlation between the enzyme retting degree and the strength of the bond between the fiber and the matrix, but observed that the enzyme exposure time of 10 h did result in an improvement of interfacial strength which declined when increasing the enzyme treatment time [70]. The authors assume that the improved interface bond strength is caused by the removal of impurities and waxes on the fiber surface, but no explanation was proposed for the reduction of interface strength for longer treatment times. A possible explanation could be that longer enzymatic treatments affect the integrity of the technical fiber through defibrillation, which could lead to complications due to internal failure of the fibers. The specific tensile modulus does not show any obvious trends, however the specific tensile strength augments significantly after 22 and 46 h of enzyme treatment. An inverse trend is thus observed compared to the interfacial tests. When relatively compared to each other, treatment with an exposure time of 22 h resulted in a composite material with good properties, considering the high specific tensile strength and the limited reduction in interfacial strength. This treatment time is in line with other studies, also performing treatments during 24 h [14,51,52,57,60,62,83].
George et al. [65] made composite materials with hemp and flax fibers treated with the enzymes from earlier research, see Table 5 (with exception of pectinmethylesterase). Mechanically processed fibers (100 g) were treated with 2 v/v % enzyme solution with a liquid:fiber ratio of 15:1, during 90 min at the specific optimal conditions of each enzyme (Table 5). Control samples were treated in buffer solutions. Composite material was prepared by pelletizing fiber samples with a Kahl pellet press to reinforce a polypropylene matrix [65]. Fig. 15 shows the mechanical properties of enzymatically treated hemp and flax fiber reinforced composites.
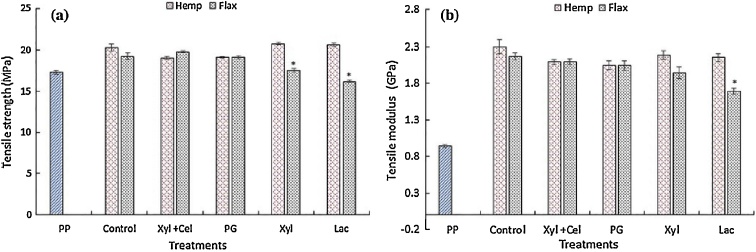
Fig. 15.
Mechanical properties (a) tensile strength and (b) tensile modulus of unreinforced polypropylene (PP), untreated hemp and flax fiber reinforced PP (control) and enzymatically treated hemp and flax fiber composites: xylanase + cellulase (Xyl + Cel), polygalacturonase (PG), xylanase (Xyl) and laccase (Lac). *Significantly different from control at P < 0.05 [65]. Reprinted by permission from George et al. [65], Springer Nature, copyright 2016.
Fig. 15a illustrates the tensile strength of the natural fiber reinforced PP composites in comparison with the untreated fiber reinforced composite (control) and shows no significant changes in tensile strength, with exception of Xyl and Lac-treated flax fiber reinforced PP (see *) [65]. George et al. [65] indicated that the decrease in tensile strength may be due to a reduced amount of lignin after treatment (see Table 6). They suggest that the removal of lignin and hemicellulose result in a weaker fiber structure. Likewise, no changes were observed in tensile modulus, except for the laccase-treated flax reinforced composite [65].
Tensile strength however is inherent to the fiber and cannot be endlessly improved by enzymatic treatments. Enzymatic treatments are able to degrade the surrounding polymeric matrix of the cellulosic fiber, resulting in a modification of the surface of the fiber and increased fineness, as well as possibly in a higher cellulose content. Surface modification can alter the adhesion between fiber and matrix and thus change the mechanical properties of the composite. Increasing cellulose content has the potential to increase composite stiffness and strength, certainly if weak polymeric substances in the fiber are replaced by a stiff and strong composite matrix (like epoxy). Increased surface roughness and fineness of the fiber and thus increased available surface area should result in more interlocking and improve the adhesion properties [71,75,84,85]. It can be concluded that fineness and purity of the fiber are important for the interfacial strength and consequently the composite performance. Improvement in composite performance should be investigated by studying longitudinal, as well as transversal and interfacial properties. When applying for example flexure loads, a combination of tensile, compression and shear loads are applied on the composite. Hence, transversal properties resulting from e.g. transverse three point bending tests should definitely be taken into account for evaluating the effect of enzymatic treatments. Evaluation of composites should thus consistently be based on longitudinal tensile tests with Impregnated Fiber Bundle Tests (IFBT) and characterization of transversal properties by performing transversal three point bending tests (which is also strongly influenced by impregnation quality and interface strength), in order to have a complete assessment of the composite. Additionally, pullout tests can be performed to obtain more information about the interface strength.
5. Moisture absorption and thermal stability
In order to increase the market potential of natural fiber-based composite materials (NFRC) in industry, the composite performance has to be equivalent to glass fiber reinforced composite materials (GFRC) and the production process cost cannot extensively exceed the cost of GFRC manufacturing. Drawbacks inherent to natural fibers like moisture absorption and thermal stability are important challenges to overcome in order to make NFRC as successful as GFRC. Moisture sensitivity of natural fibers is a major issue because water absorption lowers fiber stiffness, leading to internal stress due to swelling and shrinkage [11,86,87]. Research showed that tensile strength can increase with moisture content up to a relative humidity of 50–70 % [88], but decreases at higher relative humidity [80,89,90]. The water uptake of fibers has proven to be reduced by the dew retting process [91]. Enzymatic treatments can modify the fiber and make it more moisture resistant as well. By addressing the moisture absorption problem, the hydrophilicity of the fibers diminishes, leading to an improvement of the compatibility between fiber and hydrophobic matrices (particularly for thermoplastics).
In addition to enzymatic treatments, a higher moisture resistance or improvement of the interface compatibility between fiber and matrix can also be realized by chemical treatment. Treatment with alkali, silane treatment, acetylation and maleated coupling are examples of possible modification treatments [32,33,75]. Chemical treatment may result in degradation of lignin, wax and oils and disrupts the hydrogen bonding in the network structure, which increases the surface roughness [33]. Alkali treatment increases longitudinal and transverse properties of flax reinforced epoxy composites and is assumed to be due to the degradation of pectin [18,92,93]. Coupling agents like silane, acetic acid and maleic anhydride interact with the hydrophilic groups of the fiber, resulting in a more hydrophobic structure, which also leads to a decrease in moisture absorption. Alix et al. [94] investigated the effect of chemical treatments on water sorption of flax fibers. Fibers were esterified with maleic anhydride or treated with acetic anhydride, silane (γ-methacryloxypropyltrimethoxysilane) or styrene and were then characterized with Fourier Transform Infrared Spectroscopy (FTIR) and surface energy analysis [94]. Water sorption of the fiber was reduced after treatment with acetic anhydride and especially after styrene treatment. Styrene treatment also resulted in a reduced porosity of the fiber surface and increased the hydrophobic character of the surface without altering the mechanical properties [94]. Physical methods like corona and plasma treatment can also be applied to improve the interface compatibility by changing the surface energy of the cellulosic fibers [32,33]. However, chemical treatment requires chemical reagents making it less advantageous compared to a. biochemical process.
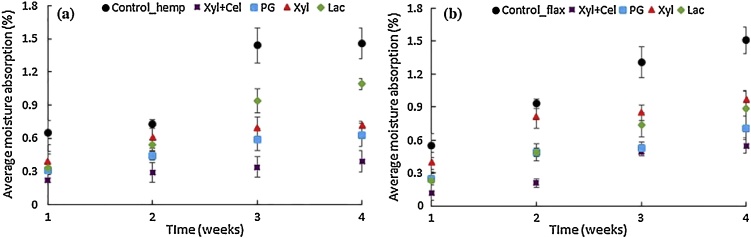
To increase the potential of natural fibers as reinforcement in composite materials, the challenge will be to inhibit moisture absorption as much as possible and to improve the thermal stability of the fiber, along with improving fiber matrix compatibility. Characterization of moisture absorption and thermal stability of fibers as well as their composites should therefore be evaluated. To date, the number of studies investigating composites impregnated with enzymatically treated fibers and moisture resistance and thermal stability of those fibers and/or composites is very limited. To our best knowledge, only one research group is investigating all these aspects, including chemical and morphological characterization [31,65].
To investigate the thermal stability of mechanically processed flax and hemp fibers treated with enzymes (see Table 5), thermogravimetric analysis (TGA) was carried out by George et al. [31]. Temperature was raised from 20 to 600 °C at a rate of 10 °C per minute under nitrogen atmosphere. The first degradation region ranges from 200 to 265 °C and comes from the removal of hemicellulose while the second region from 265 to 400 °C corresponds to the degradation of cellulose [31,95]. Flax fibers treated with xylanase + cellulase, xylanase and laccase exhibited lower thermal resistance compared to the control flax fibers. Flax fibers contain more hemicellulose than hemp fibers which led to a weaker structure after xylan degradation by xylanase containing treatments [31]. Laccase treatment also resulted in a weaker structure by degrading lignin in the fibril network, while pectinase treatment had no distinct effect on thermal stability [31]. It should however be noted that very minor differences were observed between the different results, taking into account standard deviations. Mapping the thermal stability of composites reinforced with these treated fibers will provide further insights.
In 2016, George et al. investigated the thermal stability and the moisture resistance of enzymatically treated fiber reinforced polypropylene composites. To measure thermal stability of the composite materials, thermogravimetric analysis (TGA) was performed. Results of the TGA are shown in Fig. 16 for hemp treated fibers and were similar for composites with flax treated fibers according to [65]. Fig. 16 illustrates two stages of degradation for most composites, due to different thermal stabilities of hemicellulose, lignin, pectin and cellulose [65]. Hemicellulose and pectin are components that degrade at lower temperature while cellulose and lignin are thermally more stable [31,65]. Composites impregnated with fibers treated with polygalacturonase and xylanase + cellulase were clearly thermally more stable than when impregnated with the other treated fibers, which is explained by removal of hemicellulose and pectin [65]. However, comparing these results with the chemical contents of the hemp fibers in Table 6, no removal of hemicellulose is observed after xylanase + cellulase treatment. Since xylanase treated fibers do not show an improved thermal stability as high as the xylanase + cellulase treated fibers, the improvement cannot be dedicated to a possible degradation of hemicellulose solely, even though hemicellulose content did decrease more after xylanase treatment. Degradation of amorphous cellulose by cellulase, resulting in mainly crystalline cellulose remaining, could be responsible for the increased stability. Polygalacturonase treatment did not show any improvement of the thermal stability of the fiber while the composite reinforced with these fibers exhibited an enhanced thermal stability. Hence, based on the thermal stability of enzymatically treated fibers, no unambiguous conclusion can be made concerning the thermal stability of the resultant composite material.
Fig. 16.
TGA thermographs of control, xylanase (Xyl), polygalacturonase (PG), xylanase + cellulase (Xyl + Cel) and laccase (Lac) treated hemp reinforced composites [65]. Reprinted by permission from George et al. [65], Springer Nature, copyright 2016.
Also water absorption of treated fibers and their resulting composite materials was tested by George et al. [31]. Fibers were characterized by contact angle measurement through tensiometry. A small contact angle (< 90°) corresponds to high wettability while large contact angles (> 90°) relate to low wettability [96]. All treated hemp fibers were characterized by a slightly smaller contact angle with water which means the surface of the fiber had a larger hydrophilicity [31]. According to the authors, each treatment resulted in a degradation of hemicellulose, pectin or lignin, which brought cellulose to the surface and thus revealed more hydroxyl and carboxyl groups [31]. Regarding this theory, this would have to be amorphous cellulose at the surface since crystalline cellulose behaves as a hydrophobic component due to the ordered structure. Then, however, there should have been a difference noticeable between treatment with and without cellulase activity. When studying the flax fibers, no changes in contact angle were observed after laccase and pectinase treatment, while after both xylanase treatments the contact angle showed a higher reduction than for hemp fibers [31].
Testing water resistance of the resultant polypropylene composites (same composites as in Fig. 15) was carried out by immersing the composite samples in 100 ml distilled water at room temperature for 4 weeks and keeping track of weight changes [65]. Results of moisture absorption are presented in Fig. 17 and show that all enzymatic treatments resulted in less moisture absorption, with polygalacturonase and xylanase + cellulase treatments resulting in the highest moisture resistance [65].
Fig. 17.
Water absorption over 4 weeks for enzyme treated hemp (a) and flax (b) composites [65]. Reprinted by permission from George et al. [65], Springer Nature, copyright 2016.
Even though there was no improvement in contact angle of the treated hemp and flax fibers [31], when impregnated in polypropylene the composite materials feature a higher water resistance than in case of untreated hemp and flax fibers [65]. According to Espert et al. [97] moisture can penetrate into composite materials via three possible mechanisms: diffusion of water molecules inside the microgaps between polymer chains, capillary transport into the gaps and flaws at the interfaces between fibers and polymer and transport by microcracks in the matrix. Due to the enzymatic treatments, hemicellulose or pectin polymers degrades, thereby resolving and disconnecting the interface between elementary fibers. The disconnecting interface creates micro-cavities accessible for water molecules via the capillary mechanism, which explains the undesirable slightly increased water absorption of the enzymatically treated fibers. When impregnated in composites, these cavities can be filled with polymer, thereby impeding possible moisture absorption and thus leading to an enhanced moisture resistance of the composite material.
6. Upscaling and overall economy
Several aspects according to the characterization of chemical, morphological and mechanical properties on fiber level and as on composite level have been addressed in the review paper as well as challenges related to moisture absorption and thermal resistance. A complete mapping of fiber and composite properties is essential to gain the necessary insights in the potential of enzymatic extraction. Enzymes are highly beneficial due to their high specificity and selectivity; the enzymatic process can be easily controlled making the process not dependable on weather conditions while no chemicals are required resulting in less waste water. The enzyme cost however remains a major limitation.
Prior to introducing enzymatic treatments into the processing of flax in a large scale production, the enzymatic treatment needs to be scaled up. Naik et al. [98] investigated the design of a pilot scale flax retting process with Scourzyme (a pectate lyase). Upscaling was tested in a 14 l bath (560 g flax) with a liquid ratio of 1:25, at 60 °C and pH 8 during 9 h. The retting degree of the fibers after treatment was visually evaluated. Naik et al. [98] included also an economical evaluation and cost estimation of the retting process, based on chemical costs, investment costs, energy costs, man hours and waste water costs (Fig. 18).
Fig. 18.
Costs in €/kg flax per production in ton flax per year. Adapted from Naik et al. [98].
Fig. 18 illustrates that the two primary cost factors are waste water handling and chemicals. Approximately 87% of the chemical costs are enzyme costs. Hence, enzyme costs represents 34–37 % of the overall costs. Total cost reduction can be achieved by e.g. recovery and reuse of the enzymes.
Replacing the dew retting process with enzymatic extraction also reduces the rent for occupying fields for several weeks since flax plants will be harvested several weeks earlier. Harvesting flax plants with inferior and inconsistent quality due to bad weather conditions will be omitted, guaranteeing a bigger amount of high-quality fibers and thus guaranteeing yield and profit.
Valladares Juárez et al. [99] has built a 200 l pilot plant for producing fine flax fibers for textile applications. The process consisted of alkaline bathing of the fibers and treatment with a pectin lyase enzyme from Geobacillus thermoglucosidasius PB94 A. The pectinase enzyme is optimally active at 60 °C and pH 8.5. The high temperature of 60 °C is seen as an advantage because this temperature inhibits contamination of mesophilic cellulose degrading micro-organisms [99]. However, the high temperature is less economically attractive implying that a compromise needs to be made between final characteristics of fiber and in targeted application as well as process parameters of the enzymatic extraction process.
To design an economically feasible process, flax stems should be treated in a continuous system followed by a drying step and limited mechanical post-treatment. A limited mechanical extraction will result in less fiber damage and a higher fiber recovery which will have a positive impact on overall process economy. Recycling of enzymes and use of byproducts may further enhance the attractiveness of the process.
7. Future potential
As has been introduced already in the previous paragraph, cost of enzymes remains a major limitation for implementing enzymatic extraction of natural fibers on industrial scale. To make the enzymatic extraction process economically feasible, overall costs should be reduced. Recycling of enzymes is one of the many options to start lowering costs. Naik et al. [98] tested the reuse of enzymes for flax retting by recovering the liquid after treatment and volume was adjusted to the initial volume prior to the next treatment. Enzyme efficiency was evaluated with a Fried Test and microscopic visualization of the fibers. Recycling experiments showed that the enzyme Scourzyme L retained the same retting efficiency during three consecutive cycles. The possibility of recycling enzymes in the retting process implies an improvement of the cost efficiency of the process as it was demonstrated that enzyme cost represents approximately 35% of the overall cost [98]. In the aforementioned paper, the enzyme was not purified from the liquid remaining after the extraction process. Another approach could be to recover the enzyme(s) by ultrafiltration for subsequent enzymatic extraction cycles while the permeate containing byproducts such as saccharides can be further applied for energy generation. In this way, possible product inhibition will be omitted while meaningful use of byproducts will improve process feasibility.
Moreover, the price of commercially available enzymes is diminishing during recent years, In addition, a trend is noticed that focus of research on enzymatic retting has shifted towards the bioproduction of enzymes which can outperform commercial ones [98,[100], [101], [102], [103], [104], [105], [106]]. Anand et al. [100] produced a polygalacturonase enzyme from Aspergillus fumigatus MTCC 2584 and was then evaluated for its retting potential. Yinghua et al. [105] performed solid state fermentation with Aspergillus niger and used the crude enzyme for enzyme retting, so no further purification was necessary, leading to a reduction of the enzyme cost. Zhao et al. [106] produced enzymes from Bacillus licheniformis HDYM-04 with konjaku flour as inexpensive substrate. Another bioproduction to produce xylano-pectinolytic enzymes with Bacillus pumilus AJK has been published by Kaur et al. [103], using wheat bran and citrus peel as substrate. Pectate lyase and polygalacturonase were simultaneously produced by Bacillus tequilensis SV11-UV37 using solid state fermentation by Chiliveri et al. [101].
The effect of the enzymes from the aforementioned bioproductions on enzymatic extraction efficiency needs to be compared with commercially available enzymes. The use of highly purified enzymes can be less interesting due to the increased costs although research on enzyme efficiency should be first effectuated with pure enzymes to understand the role of each individual enzyme. Applying micro-organisms directly is another alternative for enzymatic retting. Liu et al. [104] compared field retting with fungal retting with Phlebia radiate Cel 26 on hemp fibers. Fungal retting however is not an option due to the long treatment time and the uncontrollability of the process.
After field retting, fibers are further cleaned and aligned by several mechanical post-treatment steps. One of the benefits of enzymatic retting – as has been already addressed in the previous paragraph - is that the enzymatic extraction step also has an effect on the further mechanical treatment. A less severe mechanical treatment will be necessary implying less fiber damage and a higher fiber yield. During mechanical extraction, shives are excreted as byproduct. Shives can be applied as stable bedding. In this way, also byproducts of flax can be applied usefully enlarging the potential of flax processing.
Applications of flax fibers are not restricted to textile industry and composite applications in aerospace or automotive; medical purposes are also arising. Because next to the endless benefits of good specific mechanical properties, low density, low acoustic and thermal conductivity, flax possesses antimicrobial activity as well. A lot of research has been done on the usage of flax in medical purposes, for example as wound dressing to heal wounds [[107], [108], [109], [110], [111], [112]]. Flax genomes have been genetically modified by introducing polyhydroxybutyrate (PHB) synthesis genes from Ralstonia eutropha, with the aim to improve biomechanical properties of the fiber [110]. The resulting fibers contain a higher amount of phenolics, which makes them more suitable for biomedical application and have proved to have antimicrobial activity in in vitro studies [110]. Even more, the side product of flax fiber production can be used as well for medical purposes. Flax shives were used for the extraction of phenolics and their effect was assessed on human fibroblasts. The flax shives extracts have proven to be applicable in wound healing process [107]. Aforementioned examples illustrate that flax not only has a high potential for composite materials but utilization in many other applications is becoming feasible.
8. Conclusions
The review addresses the effect of enzymatic extraction on natural fiber characteristics as well as on final composite properties including major challenges for future research. The biggest challenge is to produce a pure fiber while preserving the mechanical properties of the fiber and improving the mechanical properties of the resultant composite material. The review showed that polygalacturonase tends to be one of the most important enzymes to perform fiber extraction. Removal of pectin loosens the fibers which facilitates extraction. On the other hand, when impregnating these fibers, the presence of pectin can be beneficial for the quality of the interface by transferring applied loads between fiber and matrix and between fibers within the fiber bundle, addressing the complexity of the overall process.
Characterization of the chemical composition of the fiber as well as morphological and mechanical properties of fiber including also the characteristics of the final composite material are of primary importance. More research on composite performance of compounds impregnated with enzymatically treated fibers is absolutely required and essential to understand the specific role of the various enzymes. The review also clearly demonstrated the need for standardized evaluation methods of enzymatic treatments for critical comparison. To make the enzymatic extraction process economically attractive, problems related to moisture absorption and limited thermal stability should be resolved while overall costs should be limited. Recovery of enzymes and useful application of byproducts from flax and the enzymatic extraction process may contribute to reach this objective.
Declaration of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
This work was supported by Flanders Innovation & Entrepreneurship (VLAIO).
Contributor Information
Jana De Prez, Email: jana.deprez2@kuleuven.be.
Aart Willem Van Vuure, Email: aartwillem.vanvuure@kuleuven.be.
Jan Ivens, Email: jan.ivens@kuleuven.be.
Guido Aerts, Email: guido.aerts@kuleuven.be.
Ilse Van de Voorde, Email: ilse.vandevoorde@kuleuven.be.
References
- 1.Akin D.E. Linen most useful: perspectives on structure, chemistry, and enzymes for retting flax. ISRN Biotechnol. 2013;2013:23. doi: 10.5402/2013/186534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mather R.R., Wardman R.H. RSC Publishing; Cambridge: 2011. The Chemistry of Textile Fibers. [Google Scholar]
- 3.Kalia S. Springer; 2018. Lignocellulosic Composite Materials. [Google Scholar]
- 4.Lefeuvre A., Bourmaud A., Morvan C., Baley C. Tensile properties of elementary fibres of flax and glass: Analysis of reproducibility and scattering. Mater. Lett. 2014;130:289–291. [Google Scholar]
- 5.Sfiligoj Smole M., Hribernik S., Kleinschek K.S., Kreže T. Plant fibres for textile and technical applications. In: Grundas S., Stepniewski A., editors. Advances in Agrophysical Research. InTech; 2013. pp. 369–398. [Google Scholar]
- 6.Bourmaud A., Beaugrand J., Shah D.U., Placet V., Baley C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018;97:347–408. [Google Scholar]
- 7.De Prez J., Van Vuure A.W., Ivens J., Aerts G., Van de Voorde I. Evaluation of the extraction efficiency of enzymatically treated flax fibers. In: Fangueiro R., Rana S., editors. Advances in Natural Fibre Composites. Springer International Publishing; 2018. pp. 37–49. [Google Scholar]
- 8.Wambua P., Ivens J., Verpoest I. Natural fibres: can they replace glass in fibre reinforced plastics? Compos. Sci. Technol. 2003;63:1259–1264. [Google Scholar]
- 9.Yan L., Chouw N., Jayaraman K. Flax fibre and its composites - a review. Compos. Part B Eng. 2014;56:296–317. [Google Scholar]
- 10.Le Duigou A., Davies P., Baley C. Environmental impact analysis of the production of flax fibres to be used as composite material reinforcement. J. Biobased Mater. Bioenergy. 2011;5:153–165. [Google Scholar]
- 11.Van Vuure A.W., Ivens J., Verpoest I., Fuentes C., Osorio L., Trujillo E., Vo Hong N., Perremans D., Bensadoun F., Hendrickx K., Depuydt D. Natural fibre composites: reasearch highlights of the performance of bamboo, flax and hemp fibres. Fangueiro R., editor. 2nd International Conference on Natural Fibers. 2015:1–5. [Google Scholar]
- 12.Bos H. Technische Universiteit Eindhoven; the Netherlands: 2004. The Potential of Flax Fibres As Reinforcement for Composite Materials. PhD Thesis. [Google Scholar]
- 13.Speri M. Universita Degli Studi di Verona; Italy: 2011. Insights on Microbial and Biochemical Aspects of Retting for Bast Fiber Plant Processing in a Bioreactor. PhD Thesis. [Google Scholar]
- 14.Akin D.E., Henriksson G., Evans J.D., Adamsen A.P.S., Foulk J.A., Dodd R.B. Progress in enzyme-retting of flax. J. Nat. Fibers. 2004;1:21–47. [Google Scholar]
- 15.Morrison W.H., Archibald D.D., Sharma H.S.S., Akin D.E. Chemical and physical characterization of water- and dew-retted flax fibers. Ind. Crops Prod. 2000;12:39–46. [Google Scholar]
- 16.Paridah T., Amel A., Syeed O.A.S., Zakiah A. Retting process of some bast plant fibres and its effect on fibre quality: a review. BioResources. 2011;6:5260–5281. [Google Scholar]
- 17.Lefeuvre A., Baley C., Morvan C. Analysis of flax Fiber cell-Wall non-cellulosic polysaccharides under different weather conditions (Marylin variety) J. Nat. Fibers. 2017 [Google Scholar]
- 18.Van de Weyenberg I. KU Leuven; Belgium: 2005. Flax Fibres As a Reinforcement for Epoxy. PhD Thesis. [Google Scholar]
- 19.Bledzki A.K., Reihmane S., Gassan J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996;59:1329–1336. [Google Scholar]
- 20.Bourmaud A., Morvan C., Bouali A., Placet V., Perré P., Baley C. Relationships between micro-fibrillar angle, mechanical properties and biochemical composition of flax fibers. Ind. Crops Prod. 2013;44:343–351. [Google Scholar]
- 21.John M.J., Thomas S. Biofibres and biocomposites. Carbohydr. Polym. 2008;71:343–364. [Google Scholar]
- 22.Cosgrove D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 23.Evans J.D., Akin D.E., Foulk J.A. Flax-retting by polygalacturonase-containing enzyme mixtures and effects on fiber properties. J. Biotechnol. 2002;97:223–231. doi: 10.1016/s0168-1656(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 24.Bledzki A.K., Mamun A.A., Lucka-Gabor M., Gutowski V.S. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008;2:413–422. [Google Scholar]
- 25.Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J., Templeton D., Crocker D. NREL/TP-510-42618 Determination of structural carbohydrates and lignin in Biomass. NREL/MRI. 2012 [Google Scholar]
- 26.Sluiter A., Ruiz R., Scarlata C., Sluiter J., Templeton D. NREL/TP-510-42619 determination of extractives in biomass. NREL/MRI. 2008 [Google Scholar]
- 27.Technical Association of Pulp and Paper Industry . 2007. TAPPI T204 Solvent Extractives of Wood And Pulp., TAPPI Test Methods. [Google Scholar]
- 28.Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 29.Filisetti-Cozzi T.M.C.C., Carpita N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-z. [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Liu X., Zheng X., Tian Y. Extraction of pectin from flax fiber by chemical means. Int. J. Cloth. Sci. Technol. 2015;27:390–396. [Google Scholar]
- 31.George M., Mussone P.G., Bressler D.C. Surface and thermal characterization of natural fibres treated with enzymes. Ind. Crops Prod. 2014;53:365–373. [Google Scholar]
- 32.Bledzki A.K., Gassan J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999;24:221–274. [Google Scholar]
- 33.Faruk O., Bledzki A.K., Fink H.P., Sain M. Biocomposites reinforced with natural fibers: 2000-2010. Prog. Polym. Sci. 2012;37:1552–1596. [Google Scholar]
- 34.Troger F., Wegener G., Seemann C. Miscanthus and flax as raw material for reinforced particleboards. Ind. Crops Prod. 1998;8:113–121. [Google Scholar]
- 35.Dittenber D.B., Gangarao H.V.S. Critical review of recent publications on use of natural composites in infrastructure. Compos. Part A Appl. Sci. Manuf. 2012;43:1419–1429. [Google Scholar]
- 36.Biagiotti J., Puglia D., Kenny J.M. A review on natural fibre-based composites Part I: structure, processing and properties of vegetable fibres. J. Nat. Fibers. 2004;1:37–68. [Google Scholar]
- 37.Van Dam J.E.G. Common Fund For Commodities; 2002. Coir Processing Technologies: Improvement of Drying, Softening, Bleaching and Dyeing Coir fibre/yarn and Printing Coir Floor Coverings. Technical Paper No. 6. [Google Scholar]
- 38.Englyst H.N., Cummings J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1984;109:937–942. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- 39.Akin D.E., Gamble G.R., Morrison W.H., Rigsby L.L., Dodd R.B. Chemical and structural analysis of fibre and core tissues from flax. J. Sci. Food Agric. 1996;72:155–165. [Google Scholar]
- 40.Marek J., Antonov V., Bjelkova M., Smirous P., Fischer H. Enzymatic bioprocessing – new tool of extensive natural fibre source utilization. International Conference on Flax and Other Bast Plants. 2008:159–169. [Google Scholar]
- 41.Zhang J., Henriksson G., Johansson G. Polygalacturonase is the key component in enzymatic retting of flax. J. Biotechnol. 2000;81:85–89. doi: 10.1016/s0168-1656(00)00286-8. [DOI] [PubMed] [Google Scholar]
- 42.Brenda-Enzymes . 2018. Polygalacturonase [WWW Document]https://www.brenda-enzymes.org/enzyme.php?ecno=3.2.1.15 URL (accessed 8.1.18) [Google Scholar]
- 43.Brenda-Enzymes . 2018. Pectate Lyase [WWW Document]https://www.brenda-enzymes.org/enzyme.php?ecno=4.2.2.2 URL (Accessed 8 January 18) [Google Scholar]
- 44.Barneto A.G., Vila C., Ariza J., Vidal T. Thermogravimetric measurement of amorphous cellulose content in flax fibre and flax pulp. Cellulose. 2010;18:17–31. [Google Scholar]
- 45.Duchemin B., Thuault A., Vicente A., Rigaud B., Fernandez C., Eve S. Ultrastructure of cellulose crystallites in flax textile fibres. Cellulose. 2012;19:1837–1854. [Google Scholar]
- 46.Paloheimo M., Piironen J., Vehmaanperä J. Xylanases and cellulases as feed additives. In: Bedford M.R., Partridge G.G., editors. Enzymes in Farm Animal Nutrition. 2nd edition. CAB International; UK: 2010. p. 329. [Google Scholar]
- 47.Akin D.E. Flax - structure, chemistry, retting and processing. In: Müssig J., editor. Industrial Applications of Natural Fibres. Wiley; UK: 2010. pp. 89–108. [Google Scholar]
- 48.Henriksson G., Akin D.E., Slomczynski D., Eriksson K.-E.L. Production of highly efficient enzymes for flax retting by Rhizomucor pusillus. J. Biotechnol. 1999;68:115–123. [Google Scholar]
- 49.Himmelsbach D.S., Khalili S., Akin D.E. The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J. Sci. Food Agric. 2002;82:685–696. [Google Scholar]
- 50.Sharma H.S.S. Screening of polysaccharide-degrading enzymes for retting flax stem. Int. Biodeterior. Biodegrad. 1987;23:181–186. [Google Scholar]
- 51.Akin D.E., Slomczynski D., Rigsby L.L., Eriksson K.-E.L. Retting flax with endopolygalacturonase from Rhizopus oryzae. Text. Res. J. 2002;72:27–34. [Google Scholar]
- 52.Akin D.E., Morrison W.H., Gamble G.R., Rigsby L.L. Effect of retting enzymes on the structure and composition of flax cell walls. Text. Res. J. 1997;67:279–287. [Google Scholar]
- 53.Adamsen A.P.S., Akin D.E., Rigsby L.L. Chelating agents and enzyme retting of flax. Text. Res. J. 2002;72:296–302. [Google Scholar]
- 54.Akin D.E., Condon B., Sohn M., Foulk J.A., Dodd R.B., Rigsby L.L. Optimization for enzyme-retting of flax with pectate lyase. Ind. Crops Prod. 2007;25:136–146. [Google Scholar]
- 55.Henriksson G., Akin D.E., Rigsby L.L., Patel N., Eriksson K.-E.L. Influence of chelating agents and mechanical pretreatment on enzymatic retting of flax. Text. Res. J. 1997;67:829–836. [Google Scholar]
- 56.Adamsen A.P.S., Akin D.E., Rigsby L.L. Chemical retting of flax straw under alkaline conditions. Text. Res. J. 2002;72:789–794. [Google Scholar]
- 57.Foulk J.A., Akin D.E., Dodd R.B. Influence of pectinolytic enzymes on retting effectiveness and resultant fiber properties. BioResources. 2008;3:155–169. [Google Scholar]
- 58.Akin D.E., Rigsby L.L., Perkins W. Quality properties of flax fibers retted with enzymes. Text. Res. J. 1999;69:747–753. [Google Scholar]
- 59.Montalvo J.G., Jr Relationships between micronaire, fineness, and maturity. Part II. Exp. J. Cotton Sci. 2005;9:81–88. [Google Scholar]
- 60.Akin D.E., Foulk J.A., Dodd R.B., McAlister D.D. Enzyme-retting of flax and characterization of processed fibers. J. Biotechnol. 2001;89:193–203. doi: 10.1016/s0168-1656(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 61.Akin D.E., Dodd R.B., Perkins W., Henriksson G., Eriksson K.-E.L. Spray enzymatic retting: a new method for processing flax fibers. Text. Res. J. 2000;70:486–494. [Google Scholar]
- 62.Akin D.E., Foulk J.A., Dodd R.B. Influence on flax fibers of components in enzyme retting formulation. Text. Res. J. 2002;72:510–514. [Google Scholar]
- 63.Brunsek R., Tarbuk A., Butorac J. 8th Central European Conference on Fiber-Grade Polymers, Chemical Fibers and Special Textiles. 6. 2015. Bio-innovative flax retting. [Google Scholar]
- 64.Alix S., Lebrun L., Marais S., Philippe E., Bourmaud A., Baley C., Morvan C. Pectinase treatments on technical fibres of flax: effects on water sorption and mechanical properties. Carbohydr. Polym. 2012;87:177–185. doi: 10.1016/j.carbpol.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 65.George M., Mussone P.G., Alemaskin K., Chae M., Wolodko J., Bressler D.C. Enzymatically treated natural fibres as reinforcing agents for biocomposite material: mechanical, thermal, and moisture absorption characterization. J. Mater. Sci. 2016;51:2677–2686. [Google Scholar]
- 66.Akin D.E., Morrison W.H., Rigsby L.L., Evans J.D., Foulk J.A. Influence of water presoak on enzyme-retting of flax. Ind. Crops Prod. 2003;17:149–159. [Google Scholar]
- 67.Foulk J.A., Akin D.E., Dodd R.B. Processing techniques for improving enzyme-retting of flax. Ind. Crops Prod. 2001;13:239–248. [Google Scholar]
- 68.Liu M., Silva D.A.S., Fernando D., Meyer A.S., Madsen B., Daniel G., Thygesen A. Controlled retting of hemp fibres: effect of hydrothermal pre-treatment and enzymatic retting on the mechanical properties of unidirectional hemp/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2016;88:253–262. [Google Scholar]
- 69.Pickering K.L., Efendy M.G.A., Le T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2015;83:98–112. [Google Scholar]
- 70.Foulk J.A., Rho D., Alcock M.M., Ulven Ca., Huo S. Modifications caused by enzyme-retting and their effect on composite performance. Adv. Mater. Sci. Eng. Int. J. 2011;2011:1–9. [Google Scholar]
- 71.Hendrickx K., Romian R.D., Goedemé T., Van Vuure A.W., Ivens J. Rapid and effective methods for the screening of flax fibres for composite applications. Proceedings of the 20th International Conference on Composite Materials. 2015:1–9. [Google Scholar]
- 72.Mohanty A.K., Misra M., Drzal L.T. Sustainable Bio-composites from renewable resources: opportunities and challenges in the green materials world. J. Polym. Environ. 2002;10:19–26. [Google Scholar]
- 73.Gurunathan T., Mohanty S., Nayak S.K. A review of the recent developments in biocomposites based on natural gibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015;77:1–25. [Google Scholar]
- 74.Kaw A.K. 2nd ed. Taylor & Francis Group, LLC; 2006. Mechanics of Composite Materials. [Google Scholar]
- 75.Acera Fernández J., Le Moigne N., Caro-Bretelle A.S., El Hage R., Le Duc A., Lozachmeur M., Bono P., Bergeret A. Role of flax cell wall components on the microstructure and transverse mechanical behaviour of flax fabrics reinforced epoxy biocomposites. Ind. Crops Prod. 2016;85:93–180. [Google Scholar]
- 76.Madsen B., Thygesen A., Lilholt H. Plant fibre composites - porosity and volumetric interaction. Compos. Sci. Technol. 2007;67:1584–1600. [Google Scholar]
- 77.Stuart T., Liu Q., Hughes M., McCall R.D., Sharma H.S.S., Norton A. Structural biocomposites from flax - Part I: effect of bio-technical fibre modification on composite properties. Compos. Part A Appl. Sci. Manuf. 2006;37:393–404. [Google Scholar]
- 78.Charlet K., Béakou A. Mechanical properties of interfaces within a flax bundle – part I: experimental analysis. Int. J. Adhes. Adhes. 2011;31:875–881. [Google Scholar]
- 79.Haag K., Müssig J. Scatter in tensile properties of flax fibre bundles: influence of determination and calculation of the cross-sectional area. J. Mater. Sci. 2016;51 [Google Scholar]
- 80.Célino A., Fréour S., Jacquemin F., Casari P. The hygroscopic behavior of plant fibers: a review. Front. Chem. 2014:2. doi: 10.3389/fchem.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saleem Z., Rennebaum H., Pudel F., Grimm E. Treating bast fibres with pectinase improves mechanical characteristics of reinforced thermoplastic composites. Compos. Sci. Technol. 2008;68:471–476. [Google Scholar]
- 82.Shah D.U., Nag R.K., Clifford M.J. Why do we observe significant differences between measured and ‘back-calculated’ properties of natural fibres? Cellulose. 2016 [Google Scholar]
- 83.Nair G.R., Rho D., Yaylayan V., Raghavan V. Microwave assisted retting – a novel method of processing of flax stems. Biosyst. Eng. 2013;116:427–435. [Google Scholar]
- 84.Bismarck A., Aranberri-Askargorta I., Springer J., Lampke T., Wielage B., Stamboulis A., Shenderovich I., Limbach H.-H. Surface characterization of flax, hemp and cellulose fibers; surface properties and the water uptake behavior. Polym. Compos. 2002;23:872–894. [Google Scholar]
- 85.Bismarck A., Mohanty A.K., Aranberri-Askargorta I., Czapla S., Misra M., Hinrichsen G., Springer J. Surface characterization of natural fibers; surface properties and the water up-take behavior of modified sisal and coir fibers. Green Chem. 2001;3:100–107. [Google Scholar]
- 86.Célino A., Fréour S., Jacquemin F., Casari P. Characterization and modeling of the moisture diffusion behavior of natural fibers. J. Appl. Polym. Sci. 2013;130:297–306. [Google Scholar]
- 87.Célino A., Fréour S., Jacquemin F., Casari P. 2012. Study of the Diffusion Behavior of Natural Fibers; pp. 24–28. [Google Scholar]
- 88.Placet V., Cisse O., Boubakar M.L. Influence of environmental relative humidity on the tensile and rotational behaviour of hemp fibres. J. Mater. Sci. 2012;47:3435–3446. [Google Scholar]
- 89.Baley C. Analysis of the flax fibres tensile behaviour and analysis of the tensile stiffness increase. Compos. Part A Appl. Sci. Manuf. 2002;33:939–948. [Google Scholar]
- 90.Masseteau B., Michaud F., Irle M., Roy A., Alise G. An evaluation of the effects of moisture content on the modulus of elasticity of a unidirectional flax fiber composite. Compos. Part A Appl. Sci. Manuf. 2014;60:32–37. [Google Scholar]
- 91.Martin N., Mouret N., Davies P., Baley C. Influence of the degree of retting of flax fibers on the tensile properties of single fibers and short fiber/polypropylene composites. Ind. Crops Prod. 2013;49:755–767. [Google Scholar]
- 92.Van de Weyenberg I., Chi Truong T., Vangrimde B., Verpoest I. Improving the properties of UD flax fibre reinforced composites by applying an alkaline fibre treatment. Compos. Part A Appl. Sci. Manuf. 2006;37:1368–1376. [Google Scholar]
- 93.Van de Weyenberg I., Ivens J., De Coster A., Kino B., Baetens E., Verpoest I. Influence of processing and chemical treatment of flax fibres on their composites. Compos. Sci. Technol. 2003;63:1241–1246. [Google Scholar]
- 94.Alix S., Philippe E., Bessadok A., Lebrun L., Morvan C., Marais S. Effect of chemical treatments on water sorption and mechanical properties of flax fibres. Bioresour. Technol. 2009;100:4742–4749. doi: 10.1016/j.biortech.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 95.Yang H., Yan R., Chen H., Lee D.H., Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–1788. [Google Scholar]
- 96.Yuan Y., Lee T.R. Contact angle and wetting properties. In: Bracco G., Holst B., editors. Surface Science Techniques. Springer; 2013. p. 663. [Google Scholar]
- 97.Espert A., Vilaplana F., Karlsson S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004;35:1267–1276. [Google Scholar]
- 98.Naik N., Agrawal P., Gooijer H. 2014. Design of Sustainable Industrial Scale Biocatalysis. [Google Scholar]
- 99.Valladares Juárez A.G., Rost G., Heitmann U., Heger E., Müller R. Construction of a pilot plant for producing fine linen fibers for textiles. Biochem. Eng. J. 2013;71:11–18. [Google Scholar]
- 100.Anand G., Yadav S., Yadav D. Purification and characterization of polygalacturonase from Aspergillus fumigatus MTCC 2584 and elucidating its application in retting of Crotalaria juncea fiber. 3 Biotech. 2016;6:1–7. doi: 10.1007/s13205-016-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiliveri S.R., Koti S., Linga V.R. Retting and degumming of natural fibers by pectinolytic enzymes produced from Bacillus tequilensis SV11-UV37 using solid state fermentation. Springerplus. 2016:5. doi: 10.1186/s40064-016-2173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong Z.E., Ding R.Y., Zheng L., Zhang X.Q., Yu C.W. Biological scouring of Flax Rove with alkalophilic strains. J. Nat. Fibers. 2014;11:256–267. [Google Scholar]
- 103.Kaur A., Singh A., Patra A.K., Mahajan R. Cost-effective scouring of flax fibers using cellulase-free xylano-pectinolytic synergism from a bacterial isolate. J. Clean. Prod. 2016;131:107–111. [Google Scholar]
- 104.Liu M., Ale M.T., Kołaczkowski B., Fernando D., Daniel G., Meyer A.S., Thygesen A. AMB Express; 2017. Comparison of Traditional Field Retting and Phlebia Radiata Cel 26 Retting of Hemp Fibres for Fibre ‑ Reinforced Composites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yinghua T., Xiaolan L., Xiqun Z., Lu W. Production of efficient enzymes for flax retting by solid state fermentation with Aspergillus niger. Int. J. Cloth. Sci. Technol. 2014;26:212–221. [Google Scholar]
- 106.Zhao D., Liu P., Pan C., Du R., Ping W., Ge J. Flax retting by degumming composite enzyme produced by Bacillus licheniformis HDYM-04 and effect on fiber properties. J. Text. Inst. 2017;108:507–510. [Google Scholar]
- 107.Czemplik M., Korzun-Chłopicka U., Szatkowski M., Działo M., Szopa J., Kulma A. Optimization of phenolic compounds extraction from flax shives and their effect on human fibroblasts. Evid. Complement. Alternat. Med. 2017;2017 doi: 10.1155/2017/3526392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Czemplik M., Kulma A., Bazela K., Szopa J. The biomedical potential of genetically modified flax seeds overexpressing the glucosyltransferase gene. BMC Complement. Altern. Med. 2012;12:1. doi: 10.1186/1472-6882-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gredes T., Schönitz S., Gedrange T., Stepien L., Kozak K., Kunert-Keil C. In vivo analysis of covering materials composed of biodegradable polymers enriched with flax fibers. Biomater. Res. 2017;21:1–13. doi: 10.1186/s40824-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kulma A., Skórkowska-Telichowska K., Kostyn K., Szatkowski M., Skała J., Drulis-Kawa Z., Preisner M., Zuk M., Szperlik J., Wang Y.F., Szopa J. New flax producing bioplastic fibers for medical purposes. Ind. Crops Prod. 2015;68:80–89. [Google Scholar]
- 111.Skórkowska-Telichowska K., Zuk M., Kulma A., Bugajska-Prusak A., Ratajczak K., Ga̧siorowski K., Kostyn K., Szopa J. New dressing materials derived from transgenic flax products to treat long-standing venous ulcers - a pilot study. Wound Repair Regen. 2010;18:168–179. doi: 10.1111/j.1524-475x.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- 112.Styrczewska M., Kulma A., Ratajczak K., Amarowicz R., Szopa J. Cannabinoid-like anti-inflammatory compounds from flax fiber. Cell. Mol. Biol. Lett. 2012;17:479–499. doi: 10.2478/s11658-012-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charlet K., Jernot J.P., Eve S., Gomina M., Bréard J. Multi-scale morphological characterisation of flax: from the stem to the fibrils. Carbohydr. Polym. 2010;82:54–61. [Google Scholar]
- 114.Kabir M.M., Wang H., Lau K.T., Cardona F. Chemical treatments on plant-based natural fibre reinforced polymer composites: an overview. Compos. Part B Eng. 2012;43:2883–2892. [Google Scholar]
- 115.MetaCyc . 2018. MetaCyc Reaction: 4.2.2.2 Pectate Lyase [WWW Document]https://biocyc.org/META/NEW-IMAGE?type=REACTION&object=4.2.2.2-RXN# URL (accessed 8.1.18) [Google Scholar]
- 116.MetaCyc . 2018. MetaCyc Reaction: 3.2.1.8 Endoxylanase [WWW Document]https://biocyc.org/META/NEW-IMAGE?type=REACTION&object=3.2.1.8-RXN URL (accessed 8.1.18) [Google Scholar]
- 117.MetaCyc . 2018. MetaCyc Reaction: 3.2.1.37 Beta-xylosidase [WWW Document]https://biocyc.org/META/NEW-IMAGE?type=REACTION&object=3.2.1.37-RXN URL (accessed 8.1.18) [Google Scholar]
- 118.Ridley B.L., O’Neill M.A., Mohnen D. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 119.Van den Oever M., Molenveld K. 2012. Biocomposieten 2012: Natuurlijke Vezels En Bioharsen in Technische Toepassingen. DPI Value Centre. [Google Scholar]
- 120.Yadav S., Yadav P.K., Yadav D., Yadav K.D.S. Pectin lyase: a review. Process Biochem. 2009;44:1–10. [Google Scholar]